Abstract
Startle reflex modulation by affective pictures is a well-established effect in human emotion research. However, much less is known about startle modulation by affective faces, despite the growing evidence that facial expressions robustly activate emotion-related brain circuits. In this study, acoustic startle probes were administered to 33 young adult participants (16 women) during the viewing of slides from the Pictures of Facial Affect set including neutral, happy, angry, and fearful faces. The effect of expression valence (happy, neutral, negative) on startle magnitude was highly significant (p<.001). Startle reflex was strongly potentiated by negative expressions (fearful and angry), however, no attenuation by happy faces was observed. A significant valence by gender interaction suggests stronger startle potentiation effects in females. These results demonstrate that affective facial expressions can produce significant modulation of the startle reflex.
Keywords: startle reflex, facial expression, emotion
1. Introduction
Startle reflex modulation by an affective foreground is a well-established experimental phenomenon in human emotion research. Acoustic startle reflex is an automatic, obligatory defensive response triggered by abrupt and loud noise. Studies of animals (Davis, 1989) and humans (Grillon, 2002) have shown that the startle reflex is potentiated in the presence of a conditioned fear stimulus. Converging evidence suggests that the intensity of the startle reflex depends on the ongoing motivational and affective state of the subject, such that the reflex is facilitated by aversive/defensive motivational states and attenuated by appetitive states (Grillon & Baas, 2003; Lang, Bradley, & Cuthbert, 1998; Vrana, Spence, & Lang, 1988).
The neurobiological substrates of startle modulation by emotion have been extensively studied in animals using fear-potentiated startle paradigms (reviewed in Koch & Schnitzler, 1997). These studies showed that the primary acoustic startle pathway in the brain stem is modulated through direct projections by the secondary pathway, in which the amygdala plays the central role. Thus, the degree of startle reflex modulation by various stimuli can serve as an objective measure of the extent to which particular stimuli activate (or suppress) the neural circuitry underlying the two basic motivational systems, aversive and appetitive, and two emotional states, pleasant and unpleasant (Koch & Schnitzler, 1997; Lang et al., 1998).
Human startle experiments have largely relied on affective pictures as stimulus material for emotion induction, particularly images from the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 1999). The IAPS pictures have been shown to produce a robust modulation of the startle reflex, such that reflex magnitude was the largest during unpleasant, intermediate during neutral, and smallest during pleasant pictures (Cuthbert, Bradley, & Lang, 1996; Lang et al., 1998; Vrana et al., 1988). This valence effect has been replicated using other kinds of stimuli such as emotional sounds (Bradley & Lang, 2000) as well as conditioned stimuli in fear conditioning paradigms (Grillon, 2002). Individual differences in affective modulation of startle have been associated with personality characteristics and psychopathology (Corr, Kumari, Wilson, & Gray, 1996; Grillon & Baas, 2003; Vaidyanathan, Patrick, & Bernat, 2009). In contrast to the extant literature on startle modulation by affective pictures, affective facial expressions have been little used in startle experiments, although several important features of facial images such as structural homogeneity and uniform perceptual complexity over a range of emotional expression, as well as low novelty can provide a more rigorous control for picture characteristics that are unrelated to emotional content.
The neural mechanisms of face perception are being increasingly understood (reviewed in Haxby, Hoffman, & Gobbini, 2002; Posamentier & Abdi, 2003). Studies of amygdala lesions (Adolphs et al., 2005; Adolphs, Tranel, Damasio, & Damasio, 1995) and direct electrical stimulation of the amygdala suggest that this structure plays an important role in the processing of facial emotional expressions by humans and non-human primates. Neuroimaging studies have demonstrated a robust activation of the amygdala by emotional facial expressions, especially by fearful faces (Breiter et al., 1996; Morris et al., 1996). Two functional neuroimaging studies directly compared brain activation patterns induced by facial expressions and affective pictures from the IAPS. Hariri et al. (2002) found that fearful and angry facial expressions produce a significantly stronger response in the amygdala compared to the IAPS pictures. Moreover, facial stimuli also produced a greater autonomic (skin conductance) response than affective pictures in the same study. Another study found that both affective faces and IAPS pictures recruit similar brain regions but also noted a greater activation of some regions by affective faces (Britton, Taylor, Sudheimer, & Liberzon, 2006).
It should be noted, however, that the relation between threatening facial expressions and amygdala activation is not universal: amygdala activation has also been reported for other facial expressions and by faces in general (Breiter et al., 1996), and reduced amygdala responses were observed in tasks requiring explicit emotion recognition, in contrast to increased amygdala activation in tasks involving implicit processing of facial expressions (Critchley et al., 2000). These exceptions notwithstanding, available evidence indicates that affective facial expressions, particularly fearful expressions produce a robust activation of the amygdala during passive viewing (i.e., in the absence of explicit processing demands).
The few studies that have examined the effects of the valence of facial expressions on startle modulation have reported mixed results. In a study by Balaban (1995), a startle probe was administered while 5-month-old infants were shown photographic slides of unfamiliar adult faces with happy, neutral, and angry expressions. There was a linear relationship between startle response magnitude and slide valence: the response was augmented during exposure to the angry faces and was reduced during exposure to the happy faces relative to neutral faces. However, a study of 4–8-year-old children did not find differences in startle responses during viewing of angry and neutral faces (Waters, Neumann, Henry, Craske, & Ornitz, 2008). The few studies that used adult samples have also provided mixed findings. One abstract (Alpers & Adolph, 2006) reported no effect of expression valence (i.e., angry and happy vs. neutral) on startle modulation. In another study, pictures of smiling and crying infants failed to produce startle modulation in young adults (Spangler, Emlinger, Meinhardt, & Hamm, 2001). A recent study by Hess et al. (2007) in which happy, neutral, and angry faces were administered to a group of young adults, also produced mixed results. No main effect of facial expression was found, but there was a significant interaction between expression and the actor's sex. However, possible effects of viewer's gender were not reported.
We are aware of only one published startle reflex study in which both angry and fearful faces were administered (Springer, Rosas, McGetrick, & Bowers, 2007). In one experiment, the authors found a significant effect of facial expression on startle magnitude, where angry but not fearful faces produced an increased eyeblink response compared to all other expressions. A replication experiment using the same paradigm with different facial material failed to show a significant main effect of facial expression on startle, but in pairwise comparisons, angry faces still showed significant differences from other expressions. It should be noted, however, that the startle stimuli were administered on every trial and thus were fully predictable to the subjects, which is not typical for startle modulation studies (Springer et al., 2007).
Taken together, the available evidence does not seem to support the notion that affective facial expression can modulate the startle response in adults as consistently as affective pictures. However, it is important to note that only one study included faces with fearful expression.
The goal of this study was to examine startle reflex modulation by affective faces in a community-based sample of young adults. We hypothesized that affective facial expression would influence the startle magnitude in the direction predicted by the theory of motivation and emotion proposed by Lang et al. (1993), i.e. that the startle response will be potentiated by expressions with negative emotional valence (fearful and angry) and attenuated by positively valenced (happy) faces. In addition, we intended to examine possible effects of the observer's sex on startle modulation by affective faces.
2. Methods
2.1. Participants
Thirty-nine individuals including 18 men (18–22 years; M age ± SD: 19.4±1.2 years) and 21 woman (18–21 years; M age ± SD: 19.0±1.3 years) participated in the study. Participants were recruited through state birth records as part of a larger population-based epidemiological study of twins and families and were included in the present study after screening for exclusion criteria. The criteria included a history of head trauma with loss of consciousness for more than 5 min, known history of epilepsy, currently taking a psychoactive medication, as well as hearing, visual and other physical and mental impairments that could prevent the participants from understanding and following the experimental instructions. Apart from these exclusion criteria, participants were not selected, and the sample is thus well representative of the general population. The study was approved by Washington University Institutional Review Board, and the subjects provided written informed consent.
2.2. Stimuli and Procedure
The participants were administered photographs of faces from Ekman’s and Friesen's Pictures of Facial Affect set (Ekman, 1976) depicting basic emotional expressions. The procedure was kept very close to procedures commonly used in previous studies employing affective pictures as stimulus material. Each slide was presented on a computer monitor for 6s, with 12–24s (average 15s) intervals between pictures. A total of 55 images were presented, including 18 happy faces, 19 neutral faces, and 18 faces with negative emotional expression (9 angry and 9 fearful). Faces with positive, neutral, and negative emotional expression were presented in a fixed pseudorandom order. Each image consisted of a black-and-white face oval on a black background; the dimensions were 20 cm by14 cm, i.e. close to a life-size. The monitor was placed at 110 cm in front of the subject's face; thus the visual angular dimensions of the image were 10.42° by 7.29°. A fixation cross was presented in the center of the screen during the inter-picture intervals. Auditory stimuli were administered through calibrated foam insert earphones (Etymotic Research). A 70 dB white noise background was present throughout the experiment. The startle stimuli were 105dB, 40 ms white noise bursts with near instantaneous rise time. Startle stimuli were administered during two thirds of the pictures at 3, 4, or 5 seconds after the picture onset. In addition, 10 startle stimuli were presented during inter-picture intervals (blank screen with a fixation cross). As in previous studies using affective pictures, this presentation schedule was used to minimize the predictability of the startle stimuli.
The first startle stimulus was presented during a neutral face picture and was not scored. Of the remaining 36 startle stimuli presented during the viewing of faces, 12 were administered during neutral faces (5 male and 7 female), 12 during happy faces (6 male and 6 female), and 12 during emotionally negative faces (6 angry and 6 fearful, including 8 male and 4 female faces). The average serial position of the positive, neutral, and negative expressions was 25.7, 23.7, and 22.2 (differences were non-significant: F(2,34)=.31, p=.74; all pairwise comparisons: p>.8).
2.3. Startle EMG recording and quantification
Electromyographic (EMG) activity was recorded from two miniature Ag/AgCl electrodes placed 1 cm apart over the orbicularis oculi muscle beneath the left eye. The EMG data were digitized online with 1000 Hz sampling rate using a Synamps amplifier and were analyzed off-line using Scan 4.3 software (Compumedics-Neuroscan). The EMG recordings were visually inspected, and trials were removed from the analysis if the startle stimulus overlapped with spontaneous eye blinks or there was excessive baseline EMG activity in the startle channel. This resulted in the exclusion of 11.8 % of trials on the average. Quantification of the startle response magnitude included bandpass filtering (10–200 Hz), signal rectification, smoothing over 5 adjacent data points with 3 consecutive passes, baseline correction using a 70 ms baseline (from 50 ms before to 20 ms after the stimulus onset) and detection of the peak value in the time window 20–120 ms after the stimulus onset. Startle blink magnitude in individual trials was measured as the peak magnitude relative to the baseline. Two participants were excluded due to noisy baseline EMG recording and/or lack of distinct responses to the startle stimuli (less than 1µV above baseline).
Individual startle trials were sorted into three emotional valence categories according to the foreground picture (happy, neutral, or negative emotional expression) with equal number of trials (n=12) in each category. Angry and fearful expression were collapsed into a single “negative expression” category. Next, startle responses in individual trials were averaged separately within each facial expression category. Kolmogorov-Smirnov tests indicated that distributions of the average startle response magnitudes from each expression category did not depart significantly from normality.
2.4. Statistical analysis
First, we examined the effect of startle habituation over 46 trials and corrected for habituation using regression analysis. Habituation can bias results because trials occurring earlier in the experiment can make a disproportionally large contribution to the overall effect compared to trials occurring later in the experiment. Furthermore, individual differences in the degree and time course of habituation can also bias results. To control for habituation effects, we fit different regression models and found that habituation was best described by a quadratic model that accounted for the largest percentage of startle magnitude variance (R2 = .70, p<.001). This model was fit to individual subject data and residual values were computed for each trial. The correlation between startle magnitude in individual trials (averaged across participants) and the trial number was near-zero (r=.009, p=.95) indicating that habituation effect was effectively removed. The habituation-adjusted values were used in all subsequent analyses.
To examine the effect of facial affect on the magnitude of the startle reflex, we used repeated measures analysis of variance (RM ANOVA) as implemented in the general linear models (GLM) procedure in SPSS17 with habituation-corrected startle magnitude as the dependent variable, a 3-level within-subject (repeated measures) factor "Valence" (Positive, Neutral, and Negative emotional expression), and Gender as between-subject factor.
3. Results
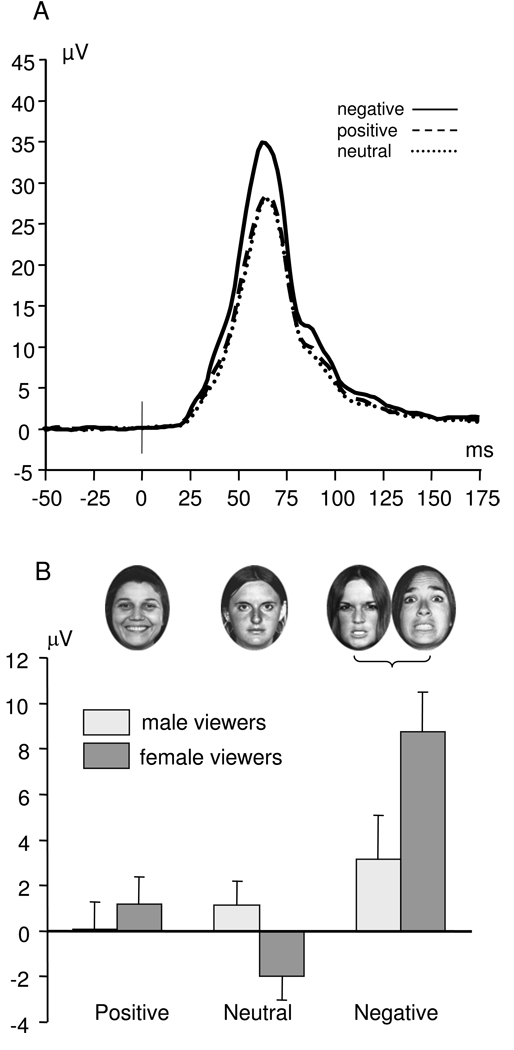
Grand-averaged startle EMG responses, as well as average startle magnitudes for different expression conditions are shown on Fig. 1. RM ANOVA indicated that the main effect of Valence was highly significant (F(2,70)=11.6, p<.001, effects size η2=.249, Greenhouse-Geisser epsilon (ε) =0.97; here and in the following analyses p-values are Greenhouse-Geisser corrected.
Fig. 1.
A. Acoustic eyeblink startle response elicited during the viewing of affective faces (rectified, smoothed, and baseline-corrected raw EMG signal averaged across participants within each facial expression category). B. Magnitude of the startle response (M±SE) elicited in male and female observers as a function of facial expression category. Data corrected for habituation effects are shown (residual values from regression analysis over 46 startle trials).
There was no significant main effect of Gender on the magnitude of startle responses (F(1,35)=1.22, η2=.03, p=.28). However, there was a significant Valence by Gender interaction: F(2,70)=4.68, η2=.12, p=.013, suggesting sex differences in startle modulation by affective faces. Fig. 1(B) suggests that in both genders, emotionally negative expressions tended to facilitate startle response, but the effect was more pronounced in females. Follow-up analyses conducted separately by gender revealed that in males the main effect of Valence did not reach significance, although there was a trend in the expected direction (F[2,32)=1.75, p=.19, ε=0.87). In contrast, in females the effect of Valence was highly significant: F(2,38)=12.68, p=<.001, ε=0.86. Pair-wise comparisons between valence conditions were non-significant in males: neutral-happy: t(16)=.55, p=.92; negative-neutral: t(16)=1.43, p=.74; negative-happy: t(16)=1.9, p=.40 (here and below p-values are Sidak-adjusted for multiple comparisons). In females, negative valence condition showed highly significant difference from both neutral and happy conditions (negative-neutral: t(19)=4.31, p<.001; negative-happy: t(19)=3.29, p=.002), but neutral and happy conditions did not differ significantly (t(19)=1.85, p=.22).
4. Discussion
The results of the present study demonstrate that affective facial expressions can modulate the startle reflex in young adults. However, the data only partially support our original hypothesis, because modulation effects were limited to startle potentiation by emotionally negative expressions, and startle was not suppressed by positive emotional expression. Furthermore, this effect was significant in female participants only.
The present finding differs from previous studies that did not find a main effect of facial expression on startle magnitude in adults. It is noteworthy that, with the only exception of Springer et al. (2007), none of the previously published studies included fearful faces. Given that fearful faces consistently activated emotion-related neural circuits in functional neuroimaging studies, the failure to include fearful expressions might be a possible explanation for the mixed results reported in previous studies. However, there is also a discrepancy between our findings and the study by Springer et al. (2007). They reported a significant startle potentiation by angry but not fearful faces in their first experiment and no significant effect of expression in their second experiment that used a different set of faces. One reason for this might be due to differences in the experimental procedures. In our study, startle was administered during only 66% of the images, and additional startle stimuli were administered during the intervals between images. This type of procedure has been commonly used in startle modulation studies in order to minimize the predictability of the startle probe. In contrast, startle stimuli in the Springer et al. study were delivered during every picture and were never delivered in the absence of a picture, which rendered the startle stimuli fully predictable. The extent to what the predictability of the startle stimulus might affect startle modulation by emotional foreground is not clear and should be clarified in future research.
The significant interaction between facial expression and the observer's sex found in the present study suggests that females may be more sensitive to the facial cues of emotion compared to males. This is consistent with the extant evidence for sex differences in the processing of facial information. Females have been shown to outperform males on both facial emotion recognition and facial identity discrimination tasks (McBain, Norton, & Chen, 2009). Females showed larger event-related neuroelectric activity during the processing of facial affect (Knyazev, Bocharov, & Slobodskoj-Plusnin, 2009) and facial discrimination (Orozco & Ehlers, 1998). Finally, neuroimaging studies revealed different patterns of activation of the emotion processing network, particularly the amygdala, in males and females, suggesting greater lateralization of responses in males (Derntl et al., 2009; Killgore & Yurgelun-Todd, 2001).
Several limitations of the present study need to be acknowledged. First, the study did not include other categories of facial emotions such as disgust or surprise. Next, a fixed pseudorandom sequence of facial stimuli was used, rather than randomization of the stimulus sequence across participants. Although correction for habituation can alleviate any potential effects of serial position of specific stimuli in the sequence, the present findings need to be replicated using a completely randomized design. Finally, the extent to which facial expressions used in the present study (Ekman, 1976) could elicit emotion is unclear due to the lack of normative valence and arousal ratings. Although studies using other sets of affective faces have demonstrated that facial expressions can induce emotion in viewers (e.g. Britton et al., 2006; Wild, Erb, & Bartels, 2001), such effects have not yet been systematically evaluated for the set of faces used in the present study.
In conclusion, the present study suggests that facial expressions of negative affect (fear and anger) can produce significant potentiation of the acoustic startle reflex, at least in female viewers, whereas emotionally positive (happy) faces fail to produce significant startle attenuation.
Acknowledgements
This work was supported by the grants DA00421 and DA018899 from the National Institute on Drug Abuse. The authors thank Dr. Sean Kristjansson and two anonymous reviewers for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers GW, Adolph D. Startle and autonomic nervous system modulation while viewing emotional scenes or emotional facial expressions. Psychophysiology. 2006;43 Supplement 1:S7. [Google Scholar]
- Balaban MT. Affective influences on startle in five-month-old infants: reactions to facial expressions of emotions. Child Development. 1995;66(1):28–36. doi: 10.1111/j.1467-8624.1995.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–215. [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. NeuroImage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Corr PJ, Kumari V, Wilson GD, Gray JA. Personality and affective modulation of the startle reflex. Journal of Psychophysiology. 1996;10(1):87–87. [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: activation and emotion. Psychophysiology. 1996;33(2):103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Annals of the New York Academy of Sciences. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, et al. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci. 2009;10:91. doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001;12(11):2543–2547. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Bocharov AV, Slobodskoj-Plusnin JY. Hostility- and gender-related differences in oscillatory responses to emotional facial expressions. Aggress Behav. 2009 doi: 10.1002/ab.20318. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. University of Florida: The Center for Research in Psychophysiology; 1999. International affective picture system (IAPS):instruction manual and affective ratings. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, Patrick CJ. Emotion and psychopathology: a startle probe analysis. Progress in Experimental Personality and Psychopathology Research. 1993;16:163–199. [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y. Females excel at basic face perception. Acta Psychologica. 2009;130(2):168–173. doi: 10.1016/j.actpsy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biological Psychiatry. 1998;44(4):281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychology Review. 2003;13(3):113–143. doi: 10.1023/a:1025519712569. [DOI] [PubMed] [Google Scholar]
- Spangler G, Emlinger S, Meinhardt J, Hamm A. The specificity of infant emotional expression for emotion perception. International Journal of Psychophysiology. 2001;41(2):155–168. doi: 10.1016/s0167-8760(01)00127-1. [DOI] [PubMed] [Google Scholar]
- Springer US, Rosas A, McGetrick J, Bowers D. Differences in startle reactivity during the perception of angry and fearful faces. Emotion. 2007;7(3):516–525. doi: 10.1037/1528-3542.7.3.516. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46(1):75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, Ornitz EM. Baseline and affective startle modulation by angry and neutral faces in 4–8-year-old anxious and non-anxious children. Biological Psychology. 2008;78(1):10–19. doi: 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Wild B, Erb M, Bartels M. Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: quality, quantity, time course and gender differences. Psychiatry Research. 2001;102(2):109–124. doi: 10.1016/s0165-1781(01)00225-6. [DOI] [PubMed] [Google Scholar]