Abstract
Chronic (neuropathic) pain is one of the most widespread and intractable of human complaints, as well as being one of the most difficult syndromes to treat successfully with drugs or surgery. The development of new therapeutic approaches to the treatment of painful neuropathies requires a better understanding of the mechanisms that underlie the development of these chronic pain syndromes. It is clear that inflammatory responses often accompany the development of neuropathic pain, and here we discuss the idea that chemokines might be key to integrating the development of pain and inflammation and could furnish new leads in the search for effective analgesic agents for the treatment of painful neuropathies.
Pain serves obvious physiological functions, such as warning of potentially dangerous stimuli or drawing attention to inflamed tissue. Messenger molecules released from these inflamed areas can lead to the activation of a hypersensitive pain response, which is essential for tissue healing and regeneration. However, sometimes pain can linger well after the time that the original stimulus has vanished — this type of pain, termed ‘neuropathic’ pain1, is an inappropriate or pathological response. Neuropathic pain can present as spontaneous pain sensations, hypersensitivity to mild pain (hyperalgesia) and/or pain resulting from a stimulus that ordinarily does not elicit a painful response, such as light touch (allodynia). In the broadest context, neuropathic pain can be due to direct peripheral nerve damage, the toxic side effects of drugs2, diseases such as diabetes or HIV-1 infection3, or any combination of these factors.
Clinical cases of neuropathic pain are relatively insensitive to morphine or other opioid drugs, which are normally the most powerful painkillers available4. This necessitates the development of new drug strategies for treating painful neuropathies. Examples of newer, non-opioid drugs with novel mechanisms of action include gabapentin, pregabalin and ziconotide5,6. The effectiveness of these treatments is largely based on the premise that damage to the peripheral nervous system (PNS) produces chronic changes in neuronal excitability within the dorsal root ganglia (DRG) and/or the spinal cord dorsal horn7–11. This neuropathic neuronal activity can present as spontaneous discharge and ectopic activity in sensory neurons that are directly compromised by injury and adjacent uninjured sensory neurons12–16 and/or heightened synaptic transmission in the somatosensory neurons in the spinal cord dorsal horn17. It is likely that these drugs act by blocking voltage-dependent Ca2+ channels and neurotransmitter release from affected neurons18–20. Nevertheless, these new agents are only a start and more effective drugs or therapies still need to be developed. Indeed, ziconotide, for example, is a peptide that is based on the structure of a cone snail toxin that blocks Ca2+ channels21. Although it is effective, ziconotide can only be administered by intrathecal injection, which limits its application22.
The development of new drugs for neuropathic pain necessitates an in-depth understanding of the cellular and molecular mechanisms involved in the development of the chronic pain that follows peripheral nerve injury. Despite the discovery of numerous molecules thought to be essential for the development and maintenance of neuropathic pain, few therapies based on these molecules have been successful. One reason for this could be a lack of appreciation of the neuroinflammatory response that frequently accompanies peripheral nerve injury and the resultant neuropathic pain syndromes23, as the cascade of inflammatory events that accompanies most nervous tissue injury is a major contributor to the establishment and maintenance of neuropathic pain.
Neuroinflammation
Damage to the PNS (FIG. 1) and the surrounding nonnervous tissue elicits an inflammatory response that includes increased blood supply at the site of injury, changes in capillary permeability and migration of peripheral blood leukocytes out of post-capillary venules into the surrounding tissue24. The migration of different types of leukocytes to the compromised area facilitates microbicidal activity, immunological defence mechanisms and wound healing, and is invariably accompanied by pain and tenderness23. This complex inflammatory response is orchestrated by the action of groups of cytokines and other molecules, including eicosanoids, free radicals and transcription factors such as those in the nuclear factor-κB (NF-κB) family. Of particular interest are the chemokines (chemotactic cytokines)25 (TABLE 1, TABLE 2). These small, secreted proteins are vital for organizing the directed trafficking of leukocytes under normal conditions and in response to tissue damage, when they target cells to sites of inflammation. In keeping with these observations, chemo kines have been shown to be intimately involved in the inflammatory responses that accompany damage to the PNS26–29.
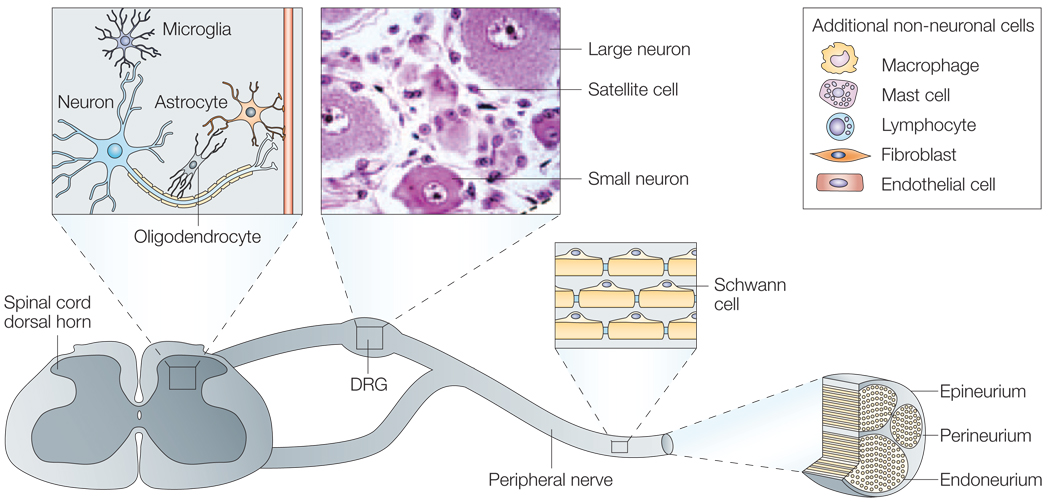
Figure 1. Schematic diagram of peripheral nervous sytem.
The soma of the general somatic primary afferent neurons are housed in the dorsal root ganglia (DRG). These pseudo-unipolar neurons with a peripheral process that contacts with target tissues and a central process in the spinal cord dorsal horn are closely associated with numerous non-neuronal cells that are present in all three locations. Central terminations of DRG cells in the spinal cord are surrounded by central nervous system neurons, oligodendrocytes, astrocytes and microglia. Cells associated with the normal DRG include myelinating Schwann cells and non-myelinating Schwann cells (satellite cells), endothelial cells, fibroblasts, mast cells, resident endoneurial macrophages and occasional lymphoctyes. The environment of the peripheral nerve is similar to the DRG milieu, with the exception of satellite cells. These are absent in the peripheral nerve; however, non-myelinating Schwann cells are present in the form of Remak Schwann cells that surround and support nerve fibres (Remak bundles). On injury or infection, compromised neurons and/or adjacent cells produce molecules that can then be responsible for changes in sensitivity.
Table 1.
Receptor selectivity of chemokines (continued in Table 2)
| Chemokines (systematic name) | Receptor(s) activated |
|---|---|
| CC-β | |
| I309 (CCL1) | CCR8 |
| MCP1 (CCL2) | CCR2, CCR11 |
| MIP1α (CCL3) | CCR1, CCR5 |
| MIP1β (CCL4) | CCR5, CCR8 |
| RANTES (CCL5) | CCR1, CCR3, CCR4, CCR5 |
| MuC10 (CCL6) | CCR1 |
| MCP3 (CCL7) | CCR1, CCR2, CCR3 |
| MCP2 (CCL8) | CCR1, CCR2, CCR5, CCR11 |
| MIP-1γ (CCL9) | CCR1 |
| CCL10 | Unknown |
| Eotaxin (CCL11) | CCR3 |
| MCP5 (CCL12) | CCR2 |
| MCP4 (CCL13) | CCR1, CCR2, CCR3, CCR11 |
| HCC1 (CCL14) | CCR1 |
| HCC2 (CCL15) | CCR1 |
| HCC4 (CCL16) | CCR1 |
| TARC (CCL17) | CCR4, CCR8 |
| PARC (CCL18) | CCR3 |
| ELC (CCL19) | CCR7 |
| LARC (CCL20) | CCR6 |
| SLC (CCL21) | CCR7 |
| MDC (CCL22) | CCR4 |
| MPIF1 (CCL23) | CCR1 |
| Eotaxin-2 (CCL24) | CCR3 |
| TECK (CCL25) | CCR9 |
| Eotaxin-3 (CCL26) | CCR3 |
| Eskine (CCL27) | CCR10 |
Classification system of the various chemokines, including the receptors that they activate. Chemokines are classified based on their structure, with most falling into the CXC- and CC-chemokine classes. Most chemokines have a common name and a systematic name. Chemokines activate a family of receptors known as the G-protein-coupled receptors, and most are able to activate more than one. BLC, B lymphocyte chemoattractant; BRAK, breast- and kidney-expressed chemokine; CCLx, CC ligand; CCRx, CC receptor; ELC, Epstein–Barr virus-induced molecule 1 ligand chemokine; ENA, epithelial neutrophil-activating protein; GCP, granulocyte chemotactic protein; GRO, growth-related oncogene; HCC, haemofiltrate CC chemokine; IL, interleukin; IP, interferon-γ-inducible protein; ITAC, interferon-γ-inducible T cell α chemoattractant; LARC, liver and activation-regulated chemokine; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; Mig, monokine induced by interferon-γ; MIP, macrophage inflammatory protein; MPIF, myeloid progenitor inhibitory factor; NAP, neutrophil-activating peptide; PARC, pulmonary and activation-regulated chemokine; PF4, platelet factor 4; RANTES, regulated upon activation, normal T-cell expressed and secreted; SDF, stromal cell-derived factor; SLC, secondary lymphoid tissue chemokine; SRPSOX, scavenger receptor that binds phosphatidylserine and oxidized lipoprotein; TARC, thymus and activationregulated chemokine; TECK, thymus-expressed chemokine.
Table 2.
Receptor selectivity of chemokines (continued from Table 1)
| Chemokines (systematic name) | Receptor(s) activated |
|---|---|
| C-γ | |
| Lymphotactin (XCL1) | XCR1 |
| CXC-α | |
| GRO-α (CXCL1) | CXCR2 |
| GRO-β (CXCL2) | CXCR2 |
| GRO-γ (CXCL3) | CXCR2 |
| PF4 (CXCL4) | CXCR3B |
| ENA78 (CXCL5) | CXCR2 |
| GCP2 (CXCL6) | CXCR1, CXCR2 |
| NAP2 (CXCL7) | CXCR2 |
| IL8 (CXCL8) | CXCR1, CXCR2 |
| Mig (CXCL9) | CXCR3 |
| IP10 (CXCL10) | CXCR3 |
| ITAC (CXCL11) | CXCR3 |
| SDF1 (CXCL12) | CXCR4 |
| BLC (CXCL13) | CXCR5 |
| BRAK (CXCL14) | Unknown |
| Lungkine (CXCL15) | Unknown |
| SRPSOX (CXCL16) | CXCR6 |
| CXXXC-δ | |
| Fractalkine (CX3CL1) | CX3CR1 |
Classification system of the various chemokines, including the receptors that they activate. Chemokines are classified based on their structure, with most falling into the CXC- and CC-chemokine classes. Most chemokines have a common name and a systematic name. Chemokines activate a family of receptors known as the G-protein-coupled receptors, and most are able to activate more than one. BLC, B lymphocyte chemoattractant; BRAK, breast- and kidney-expressed chemokine; CCLx, CC ligand; CCRx, CC receptor; ELC, Epstein–Barr virus-induced molecule 1 ligand chemokine; ENA, epithelial neutrophil-activating protein; GCP, granulocyte chemotactic protein; GRO, growth-related oncogene; HCC, haemofiltrate CC chemokine; IL, interleukin; IP, interferon-γ-inducible protein; ITAC, interferon-γ-inducible T cell α chemoattractant; LARC, liver and activation-regulated chemokine; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; Mig, monokine induced by interferon-γ; MIP, macrophage inflammatory protein; MPIF, myeloid progenitor inhibitory factor; NAP, neutrophil-activating peptide; PARC, pulmonary and activation-regulated chemokine; PF4, platelet factor 4; RANTES, regulated upon activation, normal T-cell expressed and secreted; SDF, stromal cell-derived factor; SLC, secondary lymphoid tissue chemokine; SRPSOX, scavenger receptor that binds phosphatidylserine and oxidized lipoprotein; TARC, thymus and activation-regulated chemokine; TECK, thymus-expressed chemokine.
The inflammatory response is further amplified by the activation of glial cells of the PNS and central nervous system (CNS) (for example, satellite and Schwann cells (PNS); astrocytes and microglia (CNS)). Activation in the PNS comprises increased expression of glial fibrillary acidic protein, whereas CNS glia undergo a hypertrophic change in cell morphology that is accompanied by upregulation of the synthesis of many types of molecules, including numerous chemokines and proinflammatory cytokines23,30,31. Cytokines such as tumour-necrosis factor-α (TNFα) and interleukin-1β (IL-1β) are rapidly produced as part of the neuroinflammatory cascade32,33 and exert direct biological effects on neurons through specific cell-surface receptors. These receptors are normally present on many primary afferent neurons and their axon projections34,35, and expression of the receptor can be upregulated after nerve lesion34,36.
Neuroinflammation and pain states
During the course of a nerve injury- or disease-induced inflammatory response of the nervous system, proinflammatory mediators act synergistically to induce and maintain the development of pain and hyperalgesia by changing axonal properties of both injured and uninjured neurons37. The proinflammatory cytokines TNFα and IL-1β have proanalgesic effects that serve to either activate (generation of an action potential within the neuronal membrane) or sensitize NOCICEPTORS without directly initiating an action potential but reducing the threshold required for depolarization23,38. The first indications that proinflammatory cytokines could produce hyperalgesic effects came from studies using intraplantar injections of TNFα and IL-1β32,33. A recent study by Schafers39 and colleagues shows that TNFα directly affects both injured and uninjured DRG neurons and that these changes in neuronal properties are likely to be associated with p38 mitogen-activated protein kinase40 and/or protein kinase A41.
The influence of proinflammatory cytokines such as TNFα and IL-1β on nociception is not limited to acute inflammatory pain states, as signalling of neuronal TNFαand IL-1β receptors via the pleiotropic transcription factor NF-κB probably results in the production of many chemokines, including monocyte chemoattractant protein-1 (MCP1), by neuronal and non-neuronal cells that are associated with peripheral nerve injury42–44. Subsequent experiments showed that cytokine antagonists, inhibitors of glial cell activation or chemokine receptor gene deletion (see below) reduce nociceptive behaviour in rodents, indicating that cytokine and/or chemokine synthesis is an important step in the development of neuropathic pain45–48.
In summary, nerve injury, trauma and/or infection produces a cascade of cellular events in the PNS. These events include the activation of various cell types in peripheral nerves/DRG, a neuroinflammatory response with the release of chemical mediators, including many proinflammatory cytokines and chemokines, and ultimately increased neuronal excitability. Together, these events are likely to contribute to allodynia, hyperalgesia and the other phenomena that make up the neuropathic pain syndrome23. How are all these events coordinated? Several chemokines have recently been shown to excite primary sensory neurons44,49,50, which indicates that chemokine receptors are also expressed by sensory nociceptors44,50,51 — properties that make them ideal integrators of many aspects of the responses described. Here, we review this information and suggest how the chemokine system could afford new leads in the search for analgesic drugs.
What are chemokines?
More than 50 different chemokines have been identified in higher vertebrates52. Chemokines are small proteins consisting of about 100 amino acids, and fall into four subfamilies based on structural motifs52,53 (TABLE 1, TABLE 2). Most chemokines are members of the CC (CC motif, β-chemokine) and CXC (α-hemokine) subfamilies. Chemokines belonging to the CC subfamily contain two contiguous cysteines near the amino terminus of the molecule, whereas a single amino acid separates the two cysteines in members of the CXC subfamily. Chemokines in the CX3C (δ-chemo kine) subfamily have three amino acids between the two cysteines. The fourth subfamily comprises chemokines with a single cysteine — designated the C (γ-chemokine) subfamily. Every chemokine has two names — a name that reflects a particular aspect of its biology (for example, stromal cell-derived factor-1: SDF1), and a systematic name that reflects its structure (for example, SDF1 is also called CXCL12). Each subfamily of chemokines acts on a group of related G-protein-coupled receptors (GPCRs). It has been observed in in vitro studies that a single chemokine can activate more than one receptor, and, conversely, a single cloned receptor can frequently be activated by more than one chemokine — although it is probable that their selectivity is actually higher in vivo54. However, there are chemokines that activate only one receptor — for example, SDF1 solely activates the CXCR4 receptor.
Chemokines and the nervous system
In addition to immune and inflammatory functions, chemokines mediate several other processes throughout the body, including the development and maturation of leukocytes, angiogenesis, metastasis, wound healing and allograft rejection52. Chemokines are also abundantly expressed by neurons, glia and neural progenitor cells, the major cell types of the nervous system55. Indeed, all these cells can elaborate the synthesis of chemokines, particularly under conditions of brain injury55. Combined with the normal and/or pathological nervous system expression of chemokine receptors, these chemokines potentially initiate a cascade of events leading to neuroinflammation23. These neuroinflammatory responses can occur in both the CNS and the PNS and are clearly important in the establishment of neuropathic pain.
The chemokines generated in association with neuroinflammation are crucial for the migration of leukocytes into inflamed neural tissue, just as in other parts of the body25. However, the functions of chemokines in the nervous system extend far beyond their role as mediators of inflammation. For example, CXCR4-receptor-knockout mice show abnormalities in the development of several neuronal structures, such as the dentate gyrus of the hippocampus, the cerebellum and the DRG56,57. These phenotypes result from deficits in the chemokine-mediated migration of neural stem cells. Chemokines could also be involved in the regulation of neuronal excitability, neurotransmitter release and neuronal survival53. These possibilities are supported by the extensive expression patterns of some chemokines and their receptors throughout the developed brain58–63, and by the reported actions of chemokines on phenomena such as neuronal excitability and transmitter release in both the CNS49,64–66 and PNS43,44,50. Interestingly, although many chemokines are not normally expressed at high levels in the brain, their synthesis can be dramatically upregulated in association with neuroinflammatory responses55.
Primary afferent sensory neurons present in the DRG express many types of chemokine receptors50,51,67–69, and activation of these receptors can produce strong neuronal excitation and pain50 (FIG. 2). This raises the interesting possibility that the same molecules that orchestrate the inflammatory response might also act directly on sensory neurons to produce pain44.
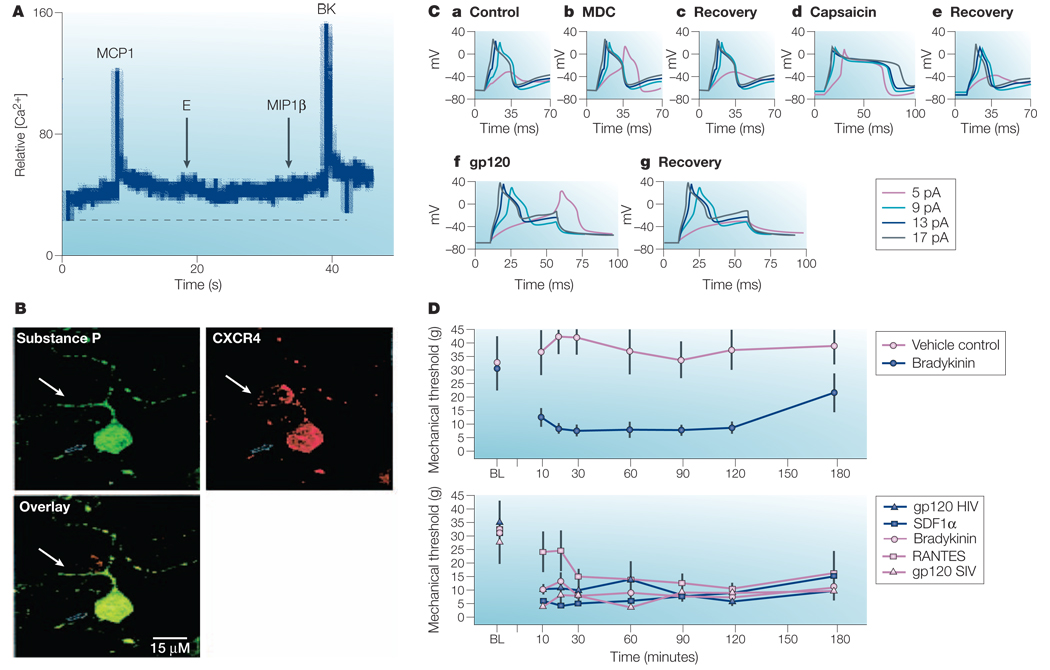
Figure 2. Chemokine receptors expressed by cultured rat neonatal dorsal rat ganglia (DRG) neurons.
A | Trace shows an increase in Ca2+ in a cultured neonatal DRG neuron in response to the chemokine monocyte chemoattractant protein-1 (MCP1)/CCL2 or to bradykinin (BK). Note that in this particular cell the chemokines macrophage inflammatory protein-1β (MIP1β) and Eotaxin (E) were both ineffective. B | Staining of rat neonatal DRG neurons for CXCR4 receptor expression. The overlay illustrates co-localization of CXCR4 with substance P in cultured DRG neurons. C | Electro physiological effects of chemokines on cultured rat DRG neurons. a–c | Current clamp recordings show that addition of macrophage-derived chemokine (MDC) (b) lowers the action potential threshold. This effect disappears following washout of the chemokine (c). The irritant pain-producing substance capsaicin (d) prolongs the action potential to the same neuron and reversal of its effect (e). Addition of the HIV-1 coat protein gp120 (f) also reversibly lowers the threshold for action potential induction in cultured neonatal DRG neurons. D | Data illustrate the threshold for paw removal from a mechanical stimulus at several time points after injection of rats with saline, bradykinin, RANTES (regulated on activation, normal T cell expressed and secreted), stromal-cell-derived factor-1α (SDF1α), MDC, gp120 SIV and gp120 HIV-1. Graphs show that the injection of chemokines, bradykinin or gp120 into the rat paw produces allodynia. Panels A–D modified, with permission, from REF. 50 © 2001 Society for Neuroscience. BL, baseline; pA, picoampere.
Chemokines and neuropathic pain
The initial observations on the effects of chemokines on sensory neurons were carried out in cell culture49,50. More recent evidence from a series of in vivo experiments in the experimental pain model known as chronic compression of the lumbar DRG (CCD) suggests that the unusual expression of the chemokine MCP1 and its cognate receptor could be central to the maintenance of neuropathic pain behaviour: CCD produces a neuroinflammatory response70 and cutaneous hyperalgesia71–73. In this rodent model of spinal stenosis, the chemokine receptor CCR2 is upregulated in both the damaged and adjacent undamaged DRG44 (FIG. 3). The chemokines that activate CCR2 receptors are members of the MCP family. Intriguingly, when the electrophysiological effects of MCP1 signalling were tested in DRG neurons using intact ganglia in vitro, MCP1 had no effect in naive control animals, but was a powerful excitant of sensory neurons previously exposed to CCD (R. LaMotte and J. Sun)44.
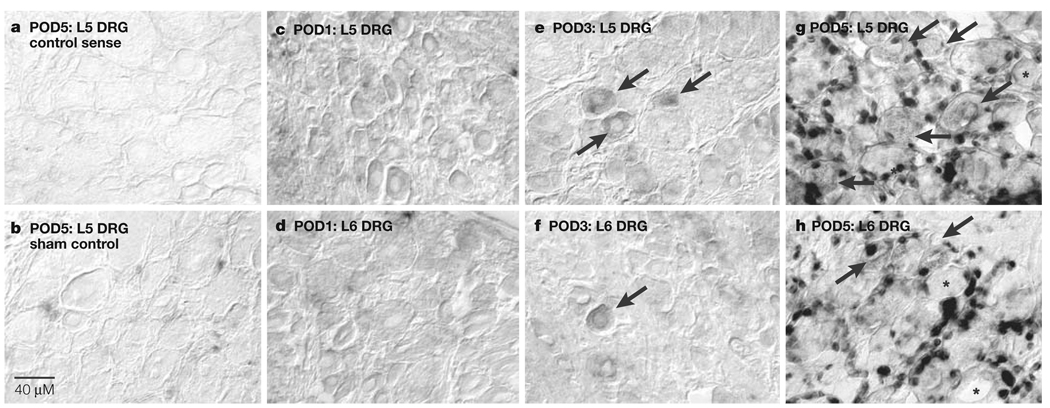
Figure 3. CCR2 mRNA expression in compressed and adjacent, non-compressed dorsal root ganglia (DRG).
a | CCR2 sense riboprobe hybridization in representative compressed L5 DRG at post-operative day (POD) 5. b | Sham-treated L5 DRG expression levels of chemokine (CC motif) receptor CCR2 mRNA at POD5. c,d | Compressed L5 DRG (c) and adjacent non-compressed L6 DRG (d) do not show CCR2 mRNA expression at POD1. e,f | Compressed L5 DRG (e) and adjacent, non-compressed L6 (f) show only neuronal CCR2 mRNA expression (indicated by arrows) at POD3. g,h | Compressed L5 DRG (g) at POD 5 also show high levels of CCR2 mRNA expression predominantly in non-neuronal cells such as satellite cells, but many more neurons are positive for CCR2 mRNA transcripts (indicated by arrows). High levels of CCR2 mRNA are present in predominantly non-neuronal cells and some neurons (indicated by arrows) of adjacent, non-compressed L6 (h) DRG at POD5. In panels g and h, asterisks indicate non-labelled neurons. Reproduced, with permission, from REF. 44 © (2005) National Academy of Science.
Interestingly, small- and medium-diameter DRG neurons subjected to CCD showed MCP1 immunoreactivity, whereas infiltrating macrophages and associated glial cells did not44. Similar findings of neuronal expression of MCP1 are evident in the rat sciatic nerve transection43. However, sciatic nerve transection is also accompanied by non-neuronal sources of MCP1 present in the distal nerve injury site. This non-neuronal MCP1 synthesis is probably the result of an inflammatory cytokine cascade that involves the synthesis of TNFα, IL-1β, IL-6 and leukaemia inhibitory factor (LIF)42,74,75. As such, the production of MCP1 after PNS injury could serve several functions. One such function could be to attract leukocytes that express CCR2 into peripheral nerve and/or ganglia injury sites. In addition, if MCP1 is released by neurons, it could either reduce the membrane threshold necessary for neuronal excitation or directly excite CCR2-expressing nociceptive neurons by autocrine or paracrine processes. In turn, a reduced membrane threshold could facilitate spontaneous action potentials in non-nociceptive neurons. One prediction from this model would be that inhibition of the cognate MCP1 receptor, CCR2, would ameliorate the development of pain in animal neuropathic pain models. Results from a recently published study using CCR2-knockout mice support this prediction — although CCR2-knockout mice appeared normal in most respects, they showed reductions in both the recruitment of leukocytes to damaged peripheral nerves and the development of neuropathic pain symptoms48. The status of chemokine synthesis by neurons is interesting. Although it seems clear that MCP1 can be synthesized by sensory neurons, the circumstances that dictate its release are not yet understood. It is possible that it is packaged and released like a neurotransmitter, or it could be constitutively secreted. However, this is still to be determined.
As data from both in vitro and in vivo studies suggest that numerous types of chemokine receptors could potentially be expressed by DRG neurons44,50,51,67,76, it is plausible that other receptors, in addition to CCR2, could have a role in mediating interactions between neuroinflammation and pain in different circumstances. In the special case of HIV-1-associated neuropathic pain, the role of the CXCR4 and CCR5 receptors could be of particular importance (discussed below). In response to focal segmental demyelination produced by local lysophosphatidylcholine-application, which is a model of Guillain–Barré syndrome, CCR5 and CXCR3 receptors are upregulated in the associated lumbar ganglia, in addition to a neuronal upregulation in CCR2 (S.K. Bhangoo, F.A. White and R.J. Miller, unpublished observations). Ligands for these receptors, such as RANTES (regulated on activation, normal T cell expressed and secreted) and interferon-inducible protein 10 (IP10), are also commonly upregulated during neuroinflammatory responses77,78 and so could also exert direct effects on nociceptive and non-nociceptive neuron excitability. Indeed, various chemokines excite cultured nociceptors and produce mechanical allodynia when injected into the inflamed rat paw50,51,67.
In addition to such direct effects on nociceptors, there are other scenarios in which chemokines could be important in the context of neuropathic pain. One of these concerns the unusual chemokine fractalkine (CX3CL1) and its receptor CX3CR1. Normally, fractalkine is expressed by neurons, including sensory neurons in the DRG68,79, and its receptor is expressed by various cell types, including astrocytes and microglia79,80. Fractalkine, in its membrane-bound conformation, can activate its receptor. However, in response to neuronal activity, fractalkine can be cleaved by matrix metalloproteinases, and soluble fractalkine can act at a distance from its source81–83. These interactions have been explored in the context of neuropathic pain68,79,84. According to one hypothesis, activation of fractalkine release, perhaps in response to an inflammatory cytokine such as TNFα, might induce local microglia migration and activation, as well as enhance aspects of the neuropathic response. Indeed, intrathecal administration of fractalkine in rats produces allodynia and injection of a CX3CR1-blocking antibody prevents both the development and maintenance of neuropathic pain, indicating that fractalkine signalling could be involved in the chronic nature of the neuropathic pain response79.
In summary, there are several points at which chemo kines could potentially be important for the development and maintenance of painful neuro pathies. Chemokines can be synthesized by nociceptive neurons and by other cells in response to injury. These chemokines can then activate receptors on macrophages and microglia, resulting in their migration and enhancing their activation. Importantly, the chemokines RANTES, SDF1α, MCP1 and fractalkine44,50,79 can act directly on nociceptive neurons to produce excitation and, in some cases, pain50,79. Taking all this evidence into consideration, drugs that inhibit chemokine receptor function would be predicted to be useful in treating painful neuropathies.
HIV-1-associated neuropathies
Chemokines and their receptors are of particular importance in HIV-1 infection3, as the chemokine receptors CCR5 and CXCR4, together with the CD4 molecule, represent the major cellular receptors for viral transfection of leukocytes, such as macrophages and microglia. Infected macrophages and/or microglial cells contribute to the neuroinflammatory response by producing chemokines85,86. Although HIV-1 does not infect neurons directly, the viral coat protein gp120 is shed by the virus (depending on the viral strain87) and can bind to both neuronal and non-neuronal CCR5 and CXCR4, facilitating receptor signalling in both the PNS and CNS87–89. This neuronal chemokine/ receptor signalling probably contributes to various neuropathologies, including severe painful neuropathies90. The aetiology of painful neuropathies associated with HIV-1 remains elusive, but up to 41% of patients infected with HIV-1 develop severe painful neuropathies, even before they develop AIDS91–93.
The viral coat protein gp120 can also act as an agonist or antagonist of chemokine action, depending on the circumstances94 (FIG 2, FIG 4)50, and prolonged treatment of DRG neurons with gp120 produces apoptosis95. However, as studies on the correlates of HIV-1 neuropathy have established that the virus does not infect DRG neurons, the resulting effects on sensory neuron function could be indirect89,96. The situation is therefore similar to the effects of HIV-1 on the CNS97,98 — viral infection results in deficits in cognition and motor function, although HIV-1 only productively infects macrophages and microglia with high viral loads3.
Figure 4. Possible chemokine receptor function in the context of HIV-1 neuropathy.
The viral coat protein gp120 can bind to the chemokine (CC motif) receptors CCR5 and CXCR4 and can also act as an agonist or antagonist of chemokine action, depending on the circumstances94. For example, gp120 can directly excite cultured dorsal root ganglia (DRG) neurons in a similar mode to chemokines and direct injection of gp120 into the rat paw produces allodynia (FIG. 2)50. In addition, prolonged treatment of DRG neurons with gp120 produces apoptosis95. Therefore, gp120 might induce some features of HIV-1 neuropathy through direct effects on chemokine receptors that are expressed by DRG neurons. It has also been suggested that gp120 could produce effects through the activation of CXCR4 receptors expressed by Schwann cells89,145. According to one hypothesis, activation of these receptors by gp120 causes Schwann cells to secrete the chemokine RANTES (regulated on activation, normal T cell expressed and secreted). RANTES can then activate CCR5 receptors expressed by DRG neurons, which could produce pain through direct excitation89. RANTES can also stimulate tumour-necrosis factor-α (TNFα) production by DRG neurons89. TNFα might then act in a cell-autonomous manner to produce excitation and pain or neuronal apoptosis120. Interestingly, TNFα also suppresses the expression of CXCR4 receptors by Schwann cells and is toxic to these cells. So, it seems that HIV-1 could trigger painful neuropathies in several ways, ranging from the overall consequences of neuroinflammation to the viral-induced activation of chemokine-receptor-induced events in neurons and glia. TNFR, tumour-necrosis factor receptor.
The development of neuropathic pain cannot be completely attributed to HIV-1 infection of cells associated with the nervous system, as similar painful peripheral neuropathies have been associated with the use of the highly active antiretroviral treatment (HAART) drugs used to combat HIV-1 infection, such as zidovudine (AZT), zalcitabine (ddC), didanosine (ddI) and stavudine (d4t)2. The combination of HIV-1 and HAART drugs could be synergistic for the development of painful neuropathies3,99.
The most common painful peripheral neuropathy — distal symmetric polyneuropathy (DSP) — occurs in late-stage HIV/AIDS. DSP typically presents during advanced immunosuppression, with symptoms ranging from distal symmetric numbness to tingling and burning sensations100. With the onset of HAART therapy, several other neuropathologies have been noted, including inflammatory demyelinating polyneuropathy (IDP) and progressive polyradiculopathy. IDP has an early to late onset and is marked by progressive weakness and PARATHESIAS. Progressive polyradiculopathy is more likely to occur with advanced immunosuppression, and could be due to an opportunistic cytomegalovirus infection: symptoms include weakness in the lower extremities and parathesias.
The mechanism of HAART neurotoxicity seems to be related to a metabolic toxicity101–103, although second messenger signalling pathways, such as changes in Ca2+ buffering at the distal axon peripheral termination104, have also been implicated105–107. Reported neuropathological changes associated with the administration of nucleoside reverse transcriptase inhibitors (NRTIs) include shrunken axons, axon splitting, large periaxonal spaces, macrophage infiltration and persistent MYELINOPATHY (that is, myelin balls, ovoids and varicosities)108. There are also reports of the loss of small, unmyelinated nerve fibre, which is sometimes accompanied by large myelinated fibre loss in advanced cases100,109,110. Infiltration of both the DRG and peripheral nerves by macrophages has been noted in humans111 and rodents108 treated with NRTIs. Notably, the amount of MCP1 in cerebral spinal fluid is substantially greater in HIV-1 patients receiving HAART treatment than it is in HIV-1-positive control patients85,86.
Chemokines secreted by macrophages, dendritic cells and microglia activated in association with HIV-1 infection are clearly important in the recruitment of other leukocytes to the DRG and peripheral nerves112–114. These chemokines might also produce neuropathic pain by acting directly on receptors expressed by both nociceptive and non-nociceptive neurons. Together with the HAART-induced neurotoxicity, activation of neuronal chemokine receptors could synergistically augment painful neuropathies in several ways, ranging from the overall consequences of neuroinflammation to the viral-induced activation of chemokine receptor-induced events in neurons and glia.
Chemokine receptor signalling and pain
Which signalling pathways are engaged by chemokines in the context of neuropathic pain and might these also be potential therapeutic targets (FIG. 5)? In some respects, the effects of chemokines resemble those of another important proinflammatory excitant — the peptide BRADYKININ. Chemokines and bradykinin both activate GPCRs and seem to signal in a similar fashion. Bradykinin signals through the bradykinin B1 and B2 receptors, which are expressed by nociceptive neurons (for B1 receptor, see REFS 115 – 118; for B2 receptor, see REF. 119). Although the activation of GPCRs by bradykinin produces numerous different effects, recent studies have identified a major mechanism for bradykinin-mediated depolarization. Many nociceptors express the transient receptor potential (TRP) vanilloid receptor 1 (TRPV1), an ion channel that is a member of the TRP family of channels120. The normal role of TRPV1 is to respond to heat in the pain-producing range (‘noxious heat’) and also to protons that are present in the neuro inflammatory milieu. TRPV1 is also the receptor for capsaicin, the irritant pain-producing substance that is synthesized by hot peppers. Normally, TRPV1 channels are tonically blocked by the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), which is situated on the inner leaflet of the cell membrane121. After activation of bradykinin receptors, phospholipase C is activated, which hydrolyses PIP2 into inositol triphosphate and diacylglycerol (DAG) and induces Ca2+ mobilization121. However, the hydrolysis of PIP2 also leads to the unblocking of TRPV1, allowing Na+ entry down its electrochemical gradient and depolarization of the neuron121. A recent publication has reported that activation of CCR1 receptors that are expressed by DRG neurons produces transactivation of the TRPV1 receptor, which is reported to be an important mechanism in the ability of this chemokine to produce pain67. Furthermore, many chemokines stimulate phospholipase C and effect Ca2+ mobilization in sensory neurons49. Therefore, this signalling pathway might be a key element in other instances of chemokine-induced sensory neuron excitation and resulting pain (FIG. 5).
Figure 5. Possible chemokine-mediated signal transduction in dorsal root ganglia neurons in association with neuropathic pain.
Neurons can synthesize the chemokine monocyte chemoattractant protein-1 (MCP1)43,44 and also respond to this chemokine via expression of its chemokine (CC motif) receptor CCR2 (REF. 50). Activation of CCR2 could effect transactivation of the transient receptor potential vanilloid receptor-1 (TRPV1) channel, as has recently been shown for the structurally related CCR1 chemokine receptor67, resulting in depolarization and action potential generation50. MCP1 can also attract leukocytes into the ganglia. Some of these can secrete endorphins129–131 that activate opiate receptors and reduce neuronal excitability. In addition, activation of CCR2 receptors by MCP1 can cross-desensitize opiate receptor function122–124.
The consequences of chemokine receptor activation have other effects on DRG function that might amplify nociceptive signalling. For example, the opiate system is a major signalling system that serves to decrease the flow of painful information from the DRG into the spinal cord. Opiate receptors are also expressed by nociceptors and their activation has powerful antinociceptive effects, as typified by the universal use of morphine as an analgesic. Why then are opiates not particularly effective in the treatment of neuropathic pain? In cells that co-express chemokine and opiate receptors, activation of chemokine receptors leads to cross desensitization of opiate receptors122–124. This could be the result of transphosphorylation of opiate receptors and/or heterodimerization of chemokine and opiate receptors125–127. Desensitization is also observed in the opposite direction — that is, downregulation of chemokine receptor function after activation of opiate receptors122,128. The interacting roles of opiates and chemokines in the regulation of pain are clearly complex. For example, during inflammation, certain infiltrating leukocytes secrete opioid peptides that act on nociceptors to ameliorate painful effects129–131. As chemokines have an important role in attracting leukocytes into the nerves and ganglia, they encourage migration of cells that secrete antinociceptive opioid peptides, and also serve to desensitize opiate receptor function in the DRG through a process of cross desensitization. The overall consequences of these apparently contradictory effects for pain perception presumably depend on the exact context.
Chemokine receptors as therapeutic targets
The data discussed here indicate that chemokines and their receptors have a previously unappreciated role in the genesis and maintenance of neuropathic pain. Therefore, agents that block chemokine receptors could constitute a new class of drugs for the treatment of this condition. Indeed, targeting chemokine receptors might have some particular advantages. As we have discussed above, chemokines such as MCP1 are involved in organizing inflammatory reactions and in directly producing pain by activating CCR2 receptors that are expressed by the DRG. So, inhibiting these receptors could potentially have two beneficial effects — a reduction in inflammatory responses and an analgesic effect. The results of studies using CCR2-knockout animals support this assertion. However, as yet, there is no real proof-of-principle study using a small-molecule CCR2 antagonist, although an inhibitor of the CCR2 receptor has been reported that seems to have anti-pain activity in the neuropathic pain model of partial sciatic nerve ligation, which would support the proposed benefits of targeting these receptors132. These results suggest that chemokine receptor antagonists could have antinociceptive/analgesic properties of the type discussed here and should be considered as a potentially new therapeutic approach to intractable neuropathic pain. However, there is clearly a great deal to be done to test this hypothesis properly. Producing small-molecule inhibitors for chemokine receptors has been quite an active field of medicinal chemistry, particularly because antagonists of the CCR5 and CXCR4 receptors inhibit viral replication of HIV-1 and several archetypal drugs of this type have been produced133. The most thoroughly investigated of these is the bicyclam AMD3100, an agent that is efficacious in blocking CXCR4 receptors and inhibiting the infection of cells by CXCR4-specific strains of HIV-1 (REF. 134). Other agents, such as TAK-779, that block CCR5 receptors133,135 have been synthesized. In addition, some agents have been produced that block other chemokine receptors, such as CCR1, CXCR1 and CXCR2, in the search for new anti-inflammatory leads136,137, as well as for therapeutic treatments for rheumatoid arthritis, multiple myeloma, graft rejection and numerous other indications138. Although several of these agents are currently in advanced clinical trials and seem to be generally safe, the involvement of chemokine receptors in the development of neuropathic pain is a relatively new concept, and so none of these agents has been tested for this indication. However, because several good models for painful neuropathies now exist, for example, for neuropathies associated with cancer139, drug toxicity89,140, diabetes141, spinal cord trauma142 and demyelinating disease143,144, it should be possible to obtain preclinical indications as to their usefulness in this regard. The realization that chemokine receptors have such a key role in integrating neuropathic pain and inflammation underlines that this will be a fruitful approach to identifying new therapies for these extremely widespread and intractable syndromes.
DATABASES
The following terms in this article are linked online to:
Entrez Gene
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
B1 receptor| B2 receptor| CCR1 | CCR2 | CCR5 | CX3CL1 | CX3CR1 | CXCR1 | CXCR2 | CXCR3 | CXCR4 | IL-1β| IL-6 | LIF | MCP1 | RANTES | SDF1 | TNFα | TRPV1
OMIM
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
Guillain–Barré syndrome | HIV-1
Access to this interactive links box is free online.
Glossary
- GLIAL FIBRILLARY ACIDIC PROTEIN
(GFAP). Principle astrocyte intermediate filament that is upregulated in Schwann cells following injury. It is likely to play a direct role in the subsequent inflammatory response.
- NOCICEPTORS
Sensory neurons that respond to pain and noxious stimulation.
- PARATHESIAS
Abnormal or unpleasant sensations that result from injury to one or more nerves, often described by patients as numbness or as prickly, stinging or burning feelings.
- MYELINOPATHY
The degeneration of myelin sheaths of neurons.
- BRADYKININ
Small peptide of the kinin family that excites peripheral nerves and regulates the contraction of blood vessels and fluid transport by epithelia.
Footnotes
Competing interests statement
The authors declare no competing financial interests
References
- 1.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 2.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature Rev. Drug Discov. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 4.Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J. Pharmacol. Exp. Ther. 2001;299:811–817. [PubMed] [Google Scholar]
- 5.Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- 6.Frampton JE, Scott LJ. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–2820. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- 7.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai A, Sarantopoulos C, McCallum JB, Hogan Q. Painful neuropathy alters the effect of gabapentin on sensory neuron excitability in rats. Acta Anaesthesiol. Scand. 2004;48:507–512. doi: 10.1111/j.1399-6576.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 9.Passmore GM, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo ZD, et al. Injury type-specific calcium channel α 2 δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 11.Bayer K, Ahmadi S, Zeilhofer HU. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–749. doi: 10.1016/j.neuropharm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Ma C, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, et al. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, et al. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur. J. Pain. 2004;8:135–143. doi: 10.1016/S1090-3801(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 16.Obata K, et al. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 17.Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–140. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–414. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 19.Moore KA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sluka KA. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J. Pharmacol. Exp. Ther. 1998;287:232–237. [PubMed] [Google Scholar]
- 21.Lewis RJ, et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- 22.Smith MT, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- 23. Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. Excellent review linking the failure of drugs to control neuropathic pain to our ignorance of neuroimmune interactions.
- 24.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 25.Ransohoff RM. The chemokine system in neuroinflammation: an update. J. Infect. Dis. 2002;186 Suppl. 2:S152–S156. doi: 10.1086/344266. [DOI] [PubMed] [Google Scholar]
- 26.Carroll SL, Frohnert PW. Expression of JE (monocyte chemoattractant protein-1) is induced by sciatic axotomy in wild type rodents but not in C57BL/Wld(s) mice. J. Neuropathol. Exp. Neurol. 1998;57:915–930. doi: 10.1097/00005072-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Rutkowski JL, et al. Signals for proinflammatory cytokine secretion by human Schwann cells. J. Neuroimmunol. 1999;101:47–60. doi: 10.1016/s0165-5728(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 28.Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J. Neuroimmunol. 2000;110:177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- 29.Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α nd interleukin-1β in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- 30.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 31.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 32. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. Established that proinflammatory cytokines have an early and crucial role in the development of inflammatory hyperalgesia.
- 33.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-α and TNF-α receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 35.Holmes GM, Hebert SL, Rogers RC, Hermann GE. Immunocytochemical localization of TNF type 1 and type 2 receptors in the rat spinal cord. Brain Res. 2004;1025:210–219. doi: 10.1016/j.brainres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Shubayev VI, Myers RR. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- 37.Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-α in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 38.Sommer C. Painful neuropathies. Curr. Opin. Neurol. 2004;16:623–628. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- 39.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-α after spinal nerve ligation. J. Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu B, Li H, Brull SJ, Zhang J-M. Increased sensitivity of sensory neurons to tumor necrosis factor α in rats with chronic compression of the lumbar ganglia. J. Neurophysiol. 2002;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- 42.Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur. J. Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci. Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 44.White FA, et al. MCP-1/CCR2 signaling is upregulated in a subset of sensory neurons subjected to chronic compression injury. Proc. Natl Acad. Sci. USA. (in the press) [Google Scholar]
- 45.Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-α on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- 46.Sommer C, et al. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- 47.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 48. Abbadie C, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl Acad. Sci. USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. Showed that genetic deletion of CCR2 diminishes injury-induced neuropathic pain.
- 49.Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. Regulation of calcium currents by chemokines and their receptors. J. Neuroimmunol. 2002;123:66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- 50. Oh SB, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. The first paper describing the expression of diverse chemokine receptors by DRG neurons and their potential role in the generation of pain.
- 51.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J. Neurosci. Res. 2005 July 26; doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 52.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 53.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nature Rev. Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 54.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 55.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res. Brain Res. Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 56. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. Noteworthy as the first report of a role for a chemokine receptor in neuronal cell migration and development of the CNS.
- 57.Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl Acad. Sci. USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stumm RK, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur. J. Neurosci. 2003;18:1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- 60.Banisadr G, et al. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- 61.Banisadr G, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J. Neurochem. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 62.Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J. Comp. Neurol. 2003;457:7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- 63.Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res. Dev. Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Ragozzino D. CXC chemokine receptors in the central nervous system: role in cerebellar neuromodulation and development. J. Neurovirol. 2002;8:559–572. doi: 10.1080/13550280290100932. [DOI] [PubMed] [Google Scholar]
- 65.Nelson TE, Gruol DL. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J. Neuroimmunol. 2004;156:74–87. doi: 10.1016/j.jneuroim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Puma C, Danik M, Quirion R, Ramon F, Williams S. The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 2001;78:960–971. doi: 10.1046/j.1471-4159.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 67. Zhang N, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl Acad. Sci. USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. Showed that a chemokine acting on its cognate chemokine receptor can cross-sensitize TRPV1 and contribute to hyperalgesia during inflammation.
- 68. Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur. J. Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. Provides evidence that the presence of chemokine receptors on spinal microglia is associated with nociceptive transmission and potentially neuropathic pain mechanisms.
- 69. Bolin LM, et al. Primary sensory neurons migrate in response to the chemokine RANTES. J. Neuroimmunol. 1998;81:49–57. doi: 10.1016/s0165-5728(97)00158-6. Suggests that the presence of the chemokine RANTES is essential for neuronal migration and differentiation of nociceptive neurons in the DRG.
- 70.Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor α to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:239–246. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 71. Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J. Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. Showed that chronic compression of the ganglia produces enhanced excitability in the DRG.
- 72.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J. Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- 73.Hu S-J, Song X-J, Greenquist KW, Zhang J-M, LaMotte RH. Protein kinase A modulates spontaneous activity in chronically compressed dorsal root ganglion neurons in the rat. Pain. 2001;94:39–46. doi: 10.1016/S0304-3959(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 74.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glabinski AR, et al. TNF-α microinjection upregulates chemokines and chemokine receptors in the central nervous system without inducing leukocyte infiltration. J. Interferon Cytokine Res. 2003;23:457–466. doi: 10.1089/107999003322277874. [DOI] [PubMed] [Google Scholar]
- 76.Belmadani A, et al. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J. Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujioka T, Purev E, Rostami A. Chemokine mRNA expression in the cauda equina of Lewis rats with experimental allergic neuritis. J. Neuroimmunol. 1999;97:51–59. doi: 10.1016/s0165-5728(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 78.Sorensen TL, et al. Expression of specific chemokines and chemokine receptors in the central nervous systemof multiple sclerosis patients. J. Clin. Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milligan ED, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 80.Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J. Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 81.Garton KJ, et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J. Biol. Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 82.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-α-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 83.Ludwig A, Berkhout T, Moores K, Groot P, Chapman G. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-α and is modulated by metalloproteinase activity. J. Immunol. 2002;168:604–612. doi: 10.4049/jimmunol.168.2.604. [DOI] [PubMed] [Google Scholar]
- 84.Johnston IN, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marzocchetti A, et al. Macrophage chemoattractant protein-1 levels in cerebrospinal fluid correlate with containment of JC virus and prognosis of acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. J. Neurovirol. 2005;11:219–224. doi: 10.1080/13550280590924539. [DOI] [PubMed] [Google Scholar]
- 86.Avison MJ, et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J. Neurovirol. 2004;10:223–232. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- 87.Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nature Rev. Mol. Cell Biol. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y, et al. Lentivirus infection causes neuroinflammation and neuronal injury in dorsal root ganglia: pathogenic effects of STAT-1 and inducible nitric oxide synthase. J. Immunol. 2005;175:1118–1126. doi: 10.4049/jimmunol.175.2.1118. [DOI] [PubMed] [Google Scholar]
- 89.Keswani SC, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 90.Milligan ED, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 91.Hall CD, et al. Peripheral neuropathy in a cohort of human immunodeficiency virus-infected patients. Incidence and relationship to other nervous system dysfunction. Arch. Neurol. 1991;48:1273–1274. doi: 10.1001/archneur.1991.00530240077026. [DOI] [PubMed] [Google Scholar]
- 92.Bacellar H, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: multicenter AIDS cohort study, 1985–1992. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 93. Snider WD, et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann. Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. First observation of painful peripheral neuropathies in HIV-positive individuals.
- 94.Lee BJ, Koszinowski UH, Sarawar SR, Adler H. A γ-herpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J. Immunol. 2003;170:243–251. doi: 10.4049/jimmunol.170.1.243. [DOI] [PubMed] [Google Scholar]
- 95.Bodner A, et al. Mixed lineage kinase 3 mediates gp120IIIB-induced neurotoxicity. J. Neurochem. 2002;82:1424–1434. doi: 10.1046/j.1471-4159.2002.01088.x. [DOI] [PubMed] [Google Scholar]
- 96.Ho DD, et al. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N. Engl. J. Med. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 97.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 98.An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Keswani SC, et al. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann. Neurol. 2003;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- 100.Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. J. Clin. Virol. 2003;26:195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 101.Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- 102.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 103.Keilbaugh SA, Hobbs GA, Simpson MV. Effect of 2′,3′-dideoxycytidine on oxidative phosphorylation in the PC12 cell, a neuronal model. Biochem. Pharmacol. 1997;53:1485–1492. doi: 10.1016/s0006-2952(97)82442-2. [DOI] [PubMed] [Google Scholar]
- 104.Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111:389–397. doi: 10.1016/s0306-4522(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 106.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J. Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dina OA, et al. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J. Neurosci. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmued LC, et al. Evaluation of brain and nerve pathology in rats chronically dosed with ddI or isoniazid. Neurotoxicol. Teratol. 1996;18:555–563. doi: 10.1016/0892-0362(96)00088-8. [DOI] [PubMed] [Google Scholar]
- 109.Tyor WR, Wesselingh SL, Griffin JW, McArthur JC, Griffin DE. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995;9:379–388. [PubMed] [Google Scholar]
- 110.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 111.Norton GR, Sweeney J, Marriott D, Law MG, Brew BJ. Association between HIV distal symmetric polyneuropathy and Mycobacterium avium complex infection. J. Neurol. Neurosurg. Psychiatry. 1996;61:606–609. doi: 10.1136/jnnp.61.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J. Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 113.Milligan ED, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Foley JF, et al. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J. Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 115.Ma Q-P. The expression of bradykinin B1 receptors on primary sensory neurones that give rise to small caliber sciatic nerve fibres in rats. Neuroscience. 2001;107:665–673. doi: 10.1016/s0306-4522(01)00387-6. [DOI] [PubMed] [Google Scholar]
- 116.Ma QP, Hill R, Sirinathsinghji D. Basal expression of bradykinin B1 receptor in peripheral sensory ganglia in the rat. Neuroreport. 2000;11:4003–4005. doi: 10.1097/00001756-200012180-00020. [DOI] [PubMed] [Google Scholar]
- 117.Wotherspoon G, Winter J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 2000;294:175–178. doi: 10.1016/s0304-3940(00)01561-5. [DOI] [PubMed] [Google Scholar]
- 118.Davis CL, et al. B1 bradykinin receptors and sensory neurones. Br. J. Pharmacol. 1996;118:1469–1476. doi: 10.1111/j.1476-5381.1996.tb15562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davis AJ, Perkins MN. Substance P and capsaicin-induced mechanical hyperalgesia in the rat knee joint; the involvement of bradykinin B1 and B2 receptors. Br. J. Pharmacol. 1996;118:2206–2212. doi: 10.1111/j.1476-5381.1996.tb15664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 121.Chuang H-H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 122. Grimm MC, et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J. Exp. Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. Showed that opioids could directly interact with chemokine receptors, thereby effectively desensitizing them.
- 123. Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J. Immunol. 2004;173:594–599. doi: 10.4049/jimmunol.173.1.594. Describes the observation that proinflammatory chemokines could interact with opioid receptors, thereby effectively desensitizing the neuronal receptors.
- 124.Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 125.Chen C, et al. Heterodimerization and cross-desensitization between the μ-opioid receptor and the chemokine CCR5 receptor. Eur. J. Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of μ, κ and δ with CCR5 on immune cells. Exp. Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- 127.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1α and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J. Pharmacol. Exp. Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 128.Szabo I, et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J. Leukoc. Biol. 2003;74:1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- 129.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brack A, et al. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain. 2004;112:229–238. doi: 10.1016/j.pain.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 131.Mousa SA. Morphological correlates of immune-mediated peripheral opioid analgesia. Adv. Exp. Med. Biol. 2003;521:77–87. [PubMed] [Google Scholar]
- 132.Neilan CL, et al. Experimental neuropathic pain in mice. Soc. Neurosci. Abstr. 2004:17.4. [Google Scholar]
- 133.Horuk R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods. 2003;29:369–375. doi: 10.1016/s1046-2023(02)00361-4. [DOI] [PubMed] [Google Scholar]
- 134.De Clercq E. The bicyclam AMD3100 story. Nature Rev. Drug Discov. 2003;2:581. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 135.Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol. Sci. 2002;23:459–467. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- 136.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10:247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 137.Bertini R, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc. Natl Acad. Sci. USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ribeiro S, Horuk R. The clinical potential of chemokine receptor antagonists. Pharmacol. Ther. 2005;107:44–58. doi: 10.1016/j.pharmthera.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 139.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: from model to mechanism to therapy. J. Pain Symptom Manage. 2005;29:S32–S46. doi: 10.1016/j.jpainsymman.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 140.Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 141.Calcutt NA. Experimental models of painful diabetic neuropathy. J. Neurol. Sci. 2004;220:137–139. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 142.Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J. Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- 143.Aicher SA, Silverman MB, Winkler CW, Bebo BF., Jr Hyperalgesia in an animal model of multiple sclerosis. Pain. 2004;110:560–570. doi: 10.1016/j.pain.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 144.Wallace VCJ, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kury P, Greiner-Petter R, Cornely C, Jurgens T, Muller HW. Mammalian achaete scute homolog 2 is expressed in the adult sciatic nerve and regulates the expression of Krox24, Mob-1, CXCR4, and p57kip2 in Schwann cells. J. Neurosci. 2002;22:7586–7595. doi: 10.1523/JNEUROSCI.22-17-07586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]