Abstract
HIV-1 infection of the brain results in a large number of behavioural defecits accompanied by diverse neuropathological signs. However,it is not clear how the virus produces these effects or exactly how the neuropathology and behavioural defecits are related. In this article we discuss the possibility that HIV-1 infection may negatively impact the process of neurogenesis in the adult brain and that this may contribute to HIV-1 related effects on the nervous system. We have previously demonstrated that the development of the dentate gyrus during embryogenesis requires signaling by the chemokine SDF-1 via its receptor CXCR4. We demonstrated that neural progenitor cells that give rise to dentate granule neurons express CXCR4 and other chemokine receptors and migrate into the nascent dentate gyrus along SDF-1 gradients. Animals deficient in CXCR4 receptors exhibit a malformed dentate gyrus in which the migration of neural progenitors is stalled. In the adult, neurogenesis continues in the dentate gyrus. Adult neural progenitor cells existing in the subgranlar zone, that produce granule neurons, express CXCR4 and other chemokine receptors, and granule neurons express SDF-1 suggesting that SDF-1/CXCR4 signaling is also important in adult neurogenesis. Because the cellular receptors for HIV-1 include chemokine receptors such as CXCR4 and CCR5 it is possible that the virus may interfere with SDF-1/CXCR4 signaling in the brain including disruption of the formation of new granule neurons in the adult brain.
Keywords: HIV-1, Chemokines, Neurogenesis, CXCR4
INTRODUCTION
It is well established that infection with HIV-1 has numerous neurological consequences (Price et al., 1988; Gabuzda and Wang, 2000; Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia 2005). In the central nervous system (CNS), replicating virus has only been found in microglial cells and macrophages (Takehashi et al., 1996; Wiley et al., 1996; Gonzalez- Scarano and Martin-Garcia, 2005). However, infection of the brain by HIV-1 results in widespread neuroinflammatory disease that is accompanied by diverse neuropathological signs, which include the activation of astrocytes and microglia, demyelination, dendritic pruning as well as neuronal death (Price et al., 1988; Kaul et al., 2001). It is therefore thought that HIV-1 compromises brain function in a number of different ways. One hypothesis posits that infected microglia, macrophages and activated astrocytes release a number of neurotoxins that then produce the observed diffuse neuronal damage (Kaul et al., 2001; Gonzalez- Scarano and Martin-Garcia, 2005). This then results in a number of motor and cognitive problems, with particularly severe consequences in children (Belman, 1997; Brouwers et al., 2000; Tamula et al., 2003). In the peripheral nervous system (PNS), HIV-1 replicates in macrophages that infiltrate peripheral nerves and is commonly associated with severe sensory neuropathies and pain (Luciano et al., 2003), and these symptoms may actually be enhanced by the antiviral drugs used for combating HIV-1 infection (HAART) (Sharma et al., 2004). Since all of these diverse neurological symptoms constitute an important part of the overall effects of HIV-1 infection, it is important to understand exactly how and why they occur, and in particular, what can be done to ameliorate them. Although there are clearly numerous adverse effects of HIV-1 in both the CNS and PNS, in this article we shall explore in particular the possibility that the virus can inhibit neurogenesis in the adult as well as the developing nervous systems, something that we postulate may contribute to the overall neurological and behavioral deficits observed.
MECHANISMS OF NEUROGENESIS
During embryonic development, neural stem cells must migrate long distances prior to undergoing terminal differentiation and appropriate integration into neuronal circuits. Different molecular mechanisms have been shown to be responsible for allowing particular groups of progenitor cells to migrate along specific routes in order to form all of the different brain nuclei and peripheral ganglia. It is now also clear that the generation of new neurons (neurogenesis) as well as glia (gliogenesis) continues to occur in the adult brain (Kempermann et al., 2004; Lie et al., 2004). Adult neurogenesis can take several forms. In the first case, there is a level of ongoing neurogenesis that normally occurs in the dentate gyrus (DG) of the hippocampus and in the olfactory bulb (OB). In the DG, the progenitor cells that continue to form new dentate granule neurons throughout life are located in the subgranular zone (SGZ), lying between the granule cell layer and the hilus. New granule neurons differentiate and migrate the short distance between the SGZ and the inner portion of the granule cell layer. This process has been extensively studied, and many intermediate stages of progenitor cell development have been identified (Kempermann et al., 2004; Seri et al., 2004). In contrast to this, the neural progenitors that form new interneurons in the OB are localized in the subventricular zone (SVZ) surrounding the lateral ventricle (Pencea and Luskin, 2003). These progenitors must migrate a relatively long distance into the OB, and this is achieved by a chain-like migration of developing neuroblasts in the Rostral Migratory Stream (RMS). Once they arrive, the progenitors migrate outwards from the core of the OB to populate the granule and periglomerular cell layers where they differentiate into interneurons (Alvarez-Buylla, 1997). As in the case of the DG, new neurons are constantly being generated throughout adult life, at least in the OB of rodents. This may occur less extensively in humans where the sense of smell is not as important (Sanai et al., 2004). Generally speaking, neurogenesis does not normally occur in other regions of the adult brain. However, this situation can change in response to brain injury or in association with brain disease. Under these circumstances neural progenitors from the SVZ or SGZ, or otherwise normally dormant progenitors throughout the neuraxis (Palmer et al., 1999), can become activated. These cells then migrate towards damaged areas of the brain, which may not be their normal destination (Kokaia and Lindvall, 2003) Once they arrive, depending on local environmental cues, progenitors differentiate into neurons and/or glia, presumably in an attempt to “repair” the brain. These new cells may either become successfully integrated into functional neuronal circuits, or else they may die (Parent, 2003). This type of increased neurogenic response has been observed in several different animal models of brain disease, including Alzheimer’s disease, stroke, and following seizure activity (Parent, 2003; Jin et al., 2004). Indeed, status epilepticus, induced by drugs such as kainate or pilocarpine, constitutes one of the most powerful known stimuli for adult neurogenesis (Parent, 2003). In an alternative paradigm, neural progenitor cells that have been prepared and propagated in cell culture, can be transplanted into the lateral ventricles or elsewhere in the brain of rodents (Lindvall et al., 2004). Normally such transplanted cells exhibit little further development. However, under some circumstances the transplanted cells can also migrate towards areas of brain damage and differentiate into neurons and glia in a similar fashion to endogenous progenitors. In a variation of this paradigm, studies have also been performed using transplanted cell lines derived from neural progenitors (Imitola et al., 2004) or else using bone marrow stromal cells (Chen et al., 2002), and these have also been shown to migrate specifically towards damaged areas of the brain where they differentiate into neurons and glia. Furthermore, it has been shown that stimulated neurogenesis occurs in the brains of rodent models of epilepsy, stroke, trauma, Alzheimer’s, Parkinson’s and Huntington’s diseases (Parent, 2003). In demyelinating disease models, such as an experimental autoimmune encephalomyelitis (EAE), an animal model of MS, progenitors primarily form oligodendrocytes and remyelinate damaged white matter (Picard-Riera et al., 2002; Ben-Hur et al., 2003; Pluchino et al., 2003).
As a result of these observations, attempts to utilize neural stem cells in brain repair have involved either stimulation of endogenous neural stem cell development using growth factors, or the use of transplanted progenitors. Under certain circumstances, transplanted progenitors may also be engineered to encourage their appropriate choice of fates, for example, to direct them to become dopaminergic neurons or oligodendrocytes (Lindvall et al., 2004). In several of these studies improvement of neurological symptoms has been reported in rodent disease models, although the basis of this improvement has not always been entirely clear (Kokaia and Lindvall, 2003; Parent, 2003; Lindvall et al., 2004). Thus, it is not necessarily the case that progenitors differentiate for the specific purpose of replacing the phenotype and neuronal connections of neurons that have been lost. Some alternative possible mechanisms for explaining some of these observations may involve the general stimulation of local neuronal survival, stimulation of transmitter release from surviving neurons, as well as other similar effects.
In all of these cases, an integral part of the neurogenic response involves the appropriate migration of developing neural progenitors in either the normal or damaged brain. What factors direct this precise migration? One group of molecules that we think may be important in this regard are the chemokines (CHEMotactic cytoKINES), which are small secreted proteins (about 100 amino acids), that exert all of their signaling effects through the activation of G protein coupled receptors (GPCRs). Chemokines fall into 4 subfamilies based upon certain structural motifs (Rossi and Zlotnik, 2000; Tran and Miller, 2003). In the β (CC) chemo-kine family, the sequence contains two contiguous cysteines near the N terminus of the molecule. In the α chemokines (CXC), the two cysteines are separated by a single amino acid. In the γ chemokines (CX3C), there are 3 amino acids between the two cysteines, and in the γ chemokines (C), there is only a single cysteine. The α and β chemokines constitute the vast majority of the chemokine family, whereas the γ and δ subfamilies only have one or two members each. Each subfamily acts on a group of related GPCRs. It has been frequently observed using in vitro studies on cloned receptors that a single chemokine can activate more than one receptor or conversely, that a single receptor can be activated by more than one chemokine, although it is likely that selectivity is actually higher in vivo. In some exceptional instances, a single chemokine receptor has been shown to be activated by only one chemokine, as in the case of the CXCR4 receptor and its agonist, the β chemokine SDF-1/CXCL12.
Chemokines have been extensively studied due to their pivotal role in the organization of the hematopoietic/lymphopoietic system, where they orchestrate the migration of different types of leukocytes and their precursors. Some chemokines are constitutively expressed at relatively high levels, and seem to be important in the normal trafficking of these cells, whereas other chemokines seem to be upregulated during inflammatory responses and to guide leukocytes to sites of inflammation (Huang et al., 2000). Of particular interest was the discovery that the cellular receptors for HIV-1, on macrophages and T-lymphocytes respectively, were the CCR5 and CXCR4 chemokine receptors. HIV-1 binds to these receptors together with the CD4 glycoprotein in order to fuse with and infect target cells (Berger et al., 1999). We now believe that chemokines may also play an important role in the direction of neural progenitor cell migration in the brain. Several studies suggest that HIV-1 infection of the brain may negatively impact these processes.
CHEMOKINES AND DEVELOPMENT
The first indication that chemokines may play a role in the migration of neuronal progenitors came from an analysis of the phenotypes of chemokine and chemokine receptor knockout (ko) mice. Deletion of the gene for the CXCR4 receptor or for its agonist SDF-1 is embryonic lethal (Ma et al., 1998; Zou et al., 1998). However, death occurs late in embryogenesis, and lack of CXCR4 was clearly accompanied by numerous developmental abnormalities in the immune and cardiovascular, as well as nervous systems. In particular, it was noted that there was a clear abnormality in the development of the cerebellum. Normally, granule cell progenitors are localized to the external granule cell layer (EGL), where they proliferate under the influence of mitogens such as sonic hedgehog (SHH) (Tran and Miller, 2003). At some point, these progenitors cease to divide and migrate through the Purkinje cell layer into the internal granule cell layer (IGL) where they develop their normal synaptic connections. In CXCR4 ko mice, granule cell progenitors migrate early and form ectopic groups of cells in the IGL at an inappropriately early time in development. This abnormality can be understood in terms of a model in which granule cell progenitors, which express CXCR4 receptors, are normally maintained within the EGL through the attractive effects of SDF-1 secreted from the overlying pia mater. Moreover, in CXCR4 null mice, this attraction is nullified and the progenitors preferentially follow migratory cues leading to their ectopic localization. A similar phenotype is observed in SDF-1 ko mice, further illustrating the specificity of the SDF-1/CXCR4 interaction. Shortly after the description of abnormal cerebellar development, it was shown that CXCR4 null mice also exhibit abnormal development of the hippocampal DG (Bagri et al., 2002; Lu et al., 2002; Tran and Miller, 2003). Normally, progenitors that give rise to dentate granule cells migrate in a migratory stream (MS) from the wall of the lateral ventricle to form a germinal matrix in the rudimentary DG. These progenitors divide as they migrate and continue to do so following their arrival in the germinal matrix. However, in CXCR4 ko mice, it was observed that many progenitors did not migrate properly and remained stalled within the MS. Once again this phenotype can be understood based on the normal localization of SDF-1 and CXCR4. During development, the granule cell progenitors express CXCR4 and the meningeal cells that surround the developing hippocampus express SDF-1. Thus, the situation is in some respects the opposite of that demonstrated during cerebellar development. In the DG, the progenitors seem to follow a gradient of secreted SDF-1 in order to migrate appropriately. More recently further developmental phenotypes have been observed in CXCR4 null mice. In the CNS, it was found that the migration of interneuron progenitors from the ganglionic eminence to the cerebral cortex was somewhat compromised (Stumm et al., 2003). Interestingly, phenotypes have also been observed in the peripheral nervous system, whereby it was observed that neural progenitors that migrated from the dorsal neural tube expressed CXCR4. These cells were found to give rise to DRG neurons including nociceptors, and SDF-1, which was expressed by cells lining the route of progenitor cell migration, exhibited a strong chemoattractant effect on these neural crest derived cells. In CXCR4 null mice, the DRG are malformed and exhibit many trailing TrkA positive cells that appear to be stalled in their migration (Belmadani et al., unpublished observations). Thus, it appears that SDF-1/CXCR4 signaling may also be important in the development of the peripheral nervous system. In addition to CXCR4, a developmental phenotype has also been detected in CXCR2 ko mice (Tsai et al., 2002; Tran and Miller, 2003). In this case, chemokines that activate CXCR2 receptors have been shown to be important in the development and migration of oligodendrocyte progenitors in the spinal cord. Interestingly, in this situation it appears that activation of CXCR2 receptors inhibits progenitor migration. During development oligodendrocyte progenitors arise in the ventral spinal cord where they proliferate in response to PDGF. The CXCR2-activating chemokine GRO-α is secreted from astrocytes in this region, and it cooperates with PDGF to produce progenitor proliferation as well as maintaining progenitors in this region by inhibiting their migration. Over time, the synthesis of GRO-α wanes, and it is then synthesized by astrocytes in the dorsal cord. Progenitors are then free to migrate into this dorsal region where they again proliferate in response to PDGF and GRO-α. It is not known exactly how activation of CXCR2 produces a decrease in migration. However, it has been speculated that it involves greater adhesion of progenitors to molecules in the extracellular environment. This would be reminiscent of the effects of CXCR2 activation on leukocytes, which enhances the capture of rolling monocytes by endothelial monolayers. It also illustrates the point that the net effect of chemokine receptor stimulation is not necessarily to increase the migration of progenitors. Thus, although the molecular mechanisms seem basically different, both in the developing spinal cord (CXCR2) and in the developing cerebellum (CXCR4), chemokines are responsible for reducing the migratory capacity of progenitors.
CHEMOKINES AND ADULT NEUROGENESIS
As was discussed above, the appropriate migration of neural progenitors is also important during adult neurogenesis. Are chemokines also important in directing the migration of adult neural progenitors? Although this has not been conclusively demonstrated, there are several reasons to believe that this is indeed the case.
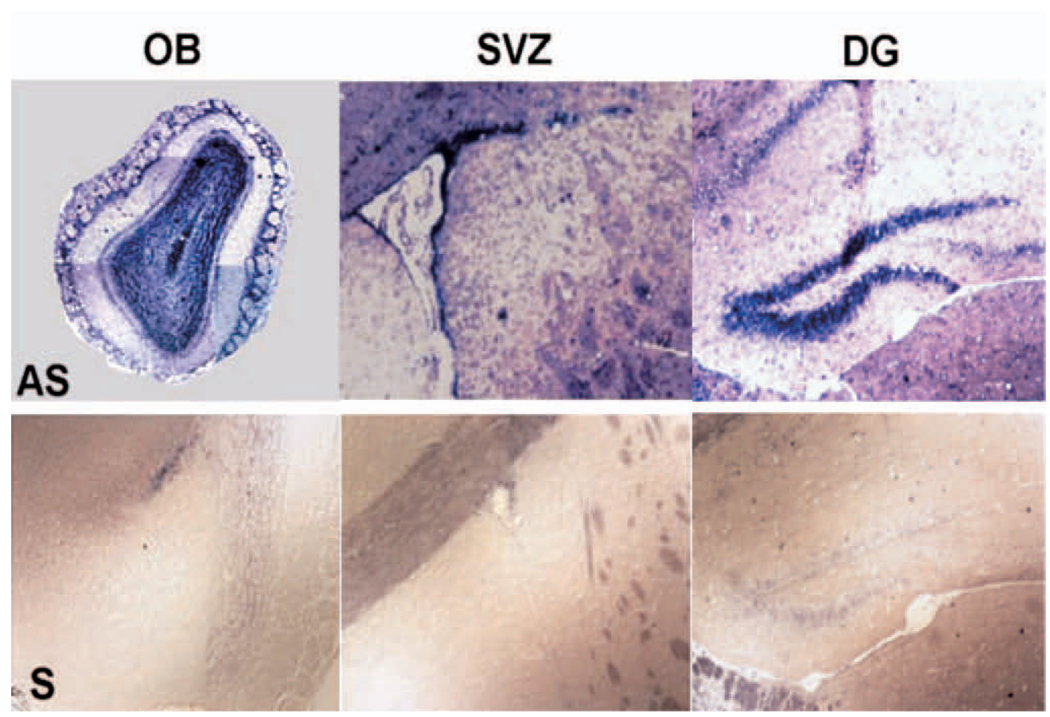
The first thing to be considered is that neural progenitor cells cultured from both embryonic and adult brain express a wide variety of chemokine receptors (Ji et al., 2003; Peng et al., 2003; Tran et al., 2004). As would be expected, the CXCR4 receptor is prominently expressed, but many other chemokine receptors are expressed as well. Indeed, we have shown that most of the known chemokine receptors are expressed by embryonic and adult neural progenitors. Furthermore, functional studies using Ca2+ imaging have demonstrated that a single adult progenitor cell can respond to multiple chemokines, suggesting that one cell expresses several receptors (Tran and Miller, 2003). One would predict from this observation that several different chemokines might act as chemoattractants for these cells, and indeed, this is the case. Our studies, as well as others, have demonstrated that many chemokines can act as chemoattractants for neural progenitor cells cultured as neurospheres (Widera et al., 2004; Tran et al., unpublished observations). Moreover, it is not just cultured progenitors that express these receptors. It would be predicted that chemokine receptors should be expressed by populations of neural progenitors in their normal environments in the developing as well as postnatal brains. Anatomical studies using in situ hybridization and immunohistochemistry to localize chemokine receptors in the brain have demonstrated that several chemokine receptors are expressed in the adult SGZ, SVZ and OB (e.g., FIG. 1), consistent with the possibility that they are normally expressed by progenitor cells in all of these areas. Attempts to colocalize the expression of the CXCR4 and CCR2 receptors with markers for progenitor cells in all of these areas have confirmed this hypothesis. For example, cells expressing these chemokine receptors have also been shown to express nestin and the TLX (tailless) gene - both markers for neural progenitors (Shi et al., 2004). Moreover, chemokine receptors are also expressed by dividing cells as assessed by their staining for the nuclear proliferation antigen, Ki67. As previously demonstrated, the patterns of chemokine receptor expression obtained in anatomical studies indicate that other types of brain cells, including neurons, also express chemokine receptors. For example, there is strong CXCR4 and CCR2 expression in the granule cell and periglomerular cell layers in the OB. Chemokine receptor expression clearly occurs in this region in some cells that also express GAD65, a marker for GABAergic interneurons. Such results are consistent with previous publications demonstrating chemokine receptor expression by populations of neurons, glia and microglia throughout the adult brain (Banisadr et al., 2002; Stumm et al., 2002; Cowell and Silverstein, 2003). In summary, it appears that chemokine receptors are normally expressed in different types of brain cells, and these include neural progenitor cells. The cell culture and anatomical data are also consistent with the possibility that a single neural progenitor may express multiple chemokine receptors, suggesting that it may be able to respond to the effects of different chemokines under different conditions.
FIGURE 1.
Localization of CCR2 receptors in neurogenic regions of mouse brain. In situ hybridization localization of CCR2 receptors in the brain of a 5 week old mouse. AS-antisense, S-sense. OB-olfactory bulb, SVZ-subventricular zone, DG-dentate gyrus.
Under what circumstances might chemokine signaling be important for progenitor cell function in the adult brain? There are two possible answers to this question. One should first consider the expression patterns of SDF-1 and CXCR4 receptors in the postnatal DG. As discussed above, progenitor cells in the SGZ express chemokine receptors including CXCR4 receptors. However, in the postnatal brain, SDF-1 is expressed by dentate granule neurons (Lu et al., 2002; Banisadr et al., 2003). The close juxtaposition of the sites of expression of SDF-1 and its receptors suggest an interaction, whereby it would be possible for SDF-1 released from granule cells to influence progenitor cells (or neurons) in the immediate vicinity. Indeed, it has been suggested that granule neurons actually form mossy-like terminations (Kaplan and Bell, 1984) as well as other types of close contacts with progenitors in the SGZ (Seri et al., 2004). It is clear that there is a connection between these two types of cells so that changes of activity in the granule cell layer can regulate the rate of neurogenesis (Kronenberg et al., 2003), what has been described as “excitation-neurogenesis” coupling (Deisseroth et al., 2004). Presumably, one or more substances can be released by granule neurons or as a consequence of their activation that might regulate the proliferation, fate potential and migration of SGZ progenitors. One candidate for this is glutamate, clearly identified as the major excitatory neurotransmitter secreted by granule neurons. The action of glutamate upon NMDA receptors has been suggested to influence the fate decisions of SGZ progenitors, making it more likely that they form neurons rather than glia (Deisseroth et al., 2004). One might imagine that the activity dependent secretion of SDF-1 and its action on CXCR4 receptors expressed by these cells might also regulate progenitor function. If we extrapolate from the effects of SDF-1 in the embryo (e.g., Bagri et al., 2002; Lu et al., 2002), one might speculate that progenitor migration and possibly proliferation could be regulated in this way. However, apart from the suggestive juxtaposition of the patterns of SDF-1 and CXCR4 expression, there is no conclusive evidence that this actually does occur. Nevertheless, it is worth noting the observations on neurogenesis in the brains of HIV-1 infected patients (Krathwohl and Kaiser, 2004). It is known that some strains of the virus interact with CXCR4 receptors and may indeed act as de facto antagonists of receptor function. Intriguingly, it has been shown that patients suffering from HIV-1 dementia have reduced rates of neurogenesis in the DG, indicating that interaction with the CXCR4 receptors expressed by progenitors may alter rates of neurogenesis in vivo. Thus, we can certainly speculate that CXCR4 function in the adult DG may play a similar role to that determined for the embryonic DG.
As discussed above, SDF-1 is constitutively expressed in diverse types of neurons in the brain. Most other chemokines are not normally expressed at such high levels. However, this situation changes substantially during the brain’s response to infection or injury. Under these circumstances, many “inflammatory” chemokines are upregulated by astrocytes as well as by microglia and neurons (Huang et al., 2000; Tran and Miller, 2003), and these chemokines serve an important function in coordinating the brain’s neuroinflammatory response. Indeed, the role of brain-derived chemokines in controlling the trafficking of leukocytes into the brain during neuroinflammation has been clearly demonstrated (Babcock et al., 2003). As a result of this, inhibition of signaling produced by some upregulated inflammatory chemokines can reduce the symptoms observed in animal models of brain disease, such as in Experimental Autoimmune Encephomyelitis (EAE), an animal model for multiple sclerosis (Fife et al., 2000; Huang et al., 2001; Mahad and Ransohoff, 2003). Although many different chemokines are upregulated under neuroinflammatory conditions, the chemokine MCP-1 is often one of the most prominent (Mahad and Ransohoff, 2003). MCP-1, and other members of the MCP family, exert their effects primarily on CCR2 receptors. Other chemokines, which have been frequently observed to be upregulated in neuroinflammatory disease include IP-10 (CXCR3), RANTES (CCR5), and MIP-1α (CCR1) (Huang et al., 2000). Nearly all types of brain infection, trauma, seizure activity or neurodegenerative disease are accompanied by neuroinflammatory phenomena, which results in the synthesis of chemokines and a variety of “proinflammatory” cytokines. If it is the case that neural progenitors express chemokine receptors, then it is reasonable to suppose that they would be attracted by the action of these chemokines. Such a hypothesis provides a potential answer to the question as to why endogenous or transplanted neural progenitors are observed to migrate towards compromised areas of the brain. However, does this actually happen?
Evidence is starting to accumulate suggesting that chemokines are responsible for directing neural progenitors to areas of brain damage. First, as discussed previously, it is clear that chemokines can act as chemoattractants for adult neural progenitors in cell culture studies. Secondly, damaged areas of the brain upregulate the synthesis of numerous chemokines. Indeed, in addition to their role in trafficking leukocytes into the brain, the synthesis of MCP-1 by infarcted areas of the brain has been shown to act as a chemoattractant for mesenchymal progenitor cells in vitro (Wang et al., 2002a,b). Thirdly, two recent publications have demonstrated that progenitor cells migrating into damaged areas of the brain express CXCR4 receptors, and that these receptors are responsible for the targeted migration of these cells (Imitola et al., 2004; Kelly et al., 2004). In both of these recent studies, neural progenitors or a neural progenitor cell line were transplanted into the brain and many cells were shown to migrate towards the site of ischemic brain injury. Migrating cells expressed CXCR4 receptors and in one of the studies it was demonstrated that interference with SDF-1/CXCR4 signaling inhibited progenitor cell migration (Imitola et al., 2004). We would therefore suggest that chemokines might generally be responsible for the targeted migration of progenitors to sites of brain damage and that the particular chemokine involved may depend on the type of progenitor or the type of damage involved as well as the major chemokines that are upregulated which accompany that response. It is worth pointing out that in general the neuroinflammatory milieu is toxic to neural progenitors (Ekdahl et al., 2003; Monje et al., 2003). This may be due to the fact that cells in these brain areas also upregulate the synthesis of numerous other cytokines such as IL-6, which may have toxic effects on progenitors. Indeed, apart from toxicity, IL-6 and other cytokines may also influencet the fate decisions made by progenitors, directing them to develop into astrocytes rather than neurons (Vallieres et al., 2002). Thus, the net effect of neuroinflammation may be mixed, particularly when viewed from the standpoint of potential effects on brain repair.
It is therefore reasonable to propose that chemokines may influence the behavior of neural progenitor cells in the adult brain in two ways. First the chemokine SDF-1 may help to regulate normal neurogenesis in the DG, and secondly, that diverse chemokines upregulated as a result of neuroinflammation may help to direct progenitor cells to the site brain injury.
HIV-1 ASSOCIATED NEUROPATHOLOGY
As discussed above, chemokines and their receptors are of particular importance in HIV-1 infection, as the chemokine receptors CCR5 and CXCR4, together with the CD4 molecule, represent the major cellular receptors for the virus allowing the virus to bind to and infect target cells. HIV-1 infection has detrimental consequences for both the CNS and PNS. How are chemokines and their receptors involved in producing these effects? For example, in the peripheral nervous system, HIV-1 associated painful neuropathies represent a special case when considering the role of chemokines in these syndromes. A very large percentage of patients infected with HIV-1 develop severe painful neuropathies, even prior to the full development of AIDS (Hall et al., 1991). There are apparently two types of causal effects involved here. One is the effect of the HIV-1 virus itself - this may include neuroinflammatory effects associated with viral infection as well as other effects of HIV-1. As the cellular receptors for HIV-1 are chemokine receptors, these could be directly involved. Gp120, the coat protein of the virus, binds to CCR5 and/or CXCR4 receptors, depending on the viral strain (Sodhi et al., 2004). It can also act as an agonist or antagonist of chemokine action depending on the circumstances, and prolonged treatment of DRG neurons in culture with gp120 produces apoptosis (Bodner et al., 2002; 2004; Keswani et al., 2003). However, as studies on the correlates of HIV-1 neuropathy have demonstrated that the virus does not infect DRG neurons, the resulting effects on sensory neuron function may be indirect and mediated by effects on glia. Indeed, cell culture experiments performed by Keswani et al. (2003) suggest this type of mechanism. A similar situation also applies to the effects of HIV-1 on the CNS, where viral infection results in deficits in cognition and motor function, although HIV-1 only productively infects macrophages and microglia at high levels (Gonzalez-Scarano and Martin-Garcia, 2005). In addition, a very similar syndrome in the PNS can be caused by the highly active antiretroviral treatment (HAART) drugs that are used to combat HIV-1 infection such as zidovudine (AZT), zalcitabine (ddC), didanosine (ddI), and stavuine (d4t), and there are indications that the painful effects of HIV-1 and HAART drugs are synergistic (Lewis et al., 2003). The most common form of this syndrome is a distal symmetric polyneuropathy (DSP), occurring in late stage HIV/AIDS. DSP typically presents during advanced immunosuppression with symptoms ranging from distal symmetric numbness to tingling and burning sensations (Cherry et al., 2003). With the onset of HAART therapy, several other neuropathologies have been noted, including inflammatory demyelinating polyneuropathy (IDP) and progressive polyradicuolopathy. IDP has an early to late onset and is marked by progressive weakness and parathesias. Progressive polyradicuolopathy is more likely to occur with advanced immunosuppression, and may be due to an opportunistic cytomegalovirus infection. Symptoms include lowered extremity weakness and parathesias (Dalakas et al., 2001). The mechanism of HAART neurotoxicity appears to be related to a metabolic toxicity, although second messenger signaling pathways have also been implicated, such as changes in Ca2+ buffering at the distal axon peripheral termination (Joseph et al., 2004). Reported neuropathological changes associated with nucleoside reverse transcriptase inhibitors (NRTI) administration include shrunken axons, axon splitting, large periaxonal spaces, macrophage infiltration and persistent myelinopathy (i.e., myelin balls ovoids and varicosities) (Schmued et al., 1996). There are also reports of the loss of small, unmyelinated fiber sometimes accompanied by large myelinated fiber loss in advanced cases (Wulff et al., 2000). Infiltration of both the DRG and peripheral nerves by macrophages has been noted in humans and rodents treated with NRTIs (Norton et al., 1996). Chemokines secreted by cells activated in association with HIV-1 infection or HAART are clearly important in the recruitment of leukocytes to the DRG and peripheral nerve (Herzberg and Sagen, 2001). These chemokines might also produce neuropathic pain by acting directly on receptors expressed by both nociceptive and non-nociceptive neurons (Oh et al., 2001). Thus, it seems that HIV-1 could trigger painful neuropathies in a number of ways, ranging from the overall consequences of neuroinflammation to the viral-induced activation of chemokine receptor induced events in neurons and glia.
In CNS HIV-1 also results in neuroinflammatory disease, as well as having direct effects on chemokine receptors expressed by the different cell types in the brain (Gonzalez-Scarano and Martin-Garcia, 2005). Different viral strains interact selectively with different chemokine receptors and may therefore elicit a different spectrum of effects. Indeed, as in the PNS, gp120 has been shown to produce apoptosis of neurons both in cell culture and in vivo. These effects may be due to direct effects of the viral protein on neurons, as well as indirect effects mediated by glia (Kaul et al., 2001). These detrimental effects will then result in deficits in development, motor and cognitive functions. As is evident from the above discussion, it is also possible that HIV-1 also targets neurogenesis either directly or indirectly, and that this may also be responsible for some of its observed effects.
HIV-1 AND NEUROGENESIS
Consideration of the above discussion indicates that there are several different ways in which infection of the brain by HIV-1 may impact neurogenesis. Virtually all strains of HIV-1 interact with cells through the use of either CXCR4 or CCR5 receptors. Many viruses that replicate in the brain use CCR5 (i.e., are M-tropic), although it is now believed that CXCR4 (T-tropic) utilizing or dual specific viral strains may also occur (Ohagen et al., 2003). It is still unclear which viral tropism is more likely to produce brain disease. Viruses that replicate in microglia can clearly lead to the activation of these cells and also of astrocytes (Gonzalez-Scarano and Martin-Garcia, 2005). Activation of these two types of cells will produce increased synthesis of proinflammatory cytokines and chemokines, and this will subsequently lead to widespread inflammatory disease (HIV-1 encephalitis) (Kaul et al., 2001). We would expect that chemokines produced during this process would act to attract neural progenitors to inflamed areas of the brain. Indeed, activated microglia have been shown to attract neural progenitors, although the mechanism for this effect has not been established (Aarum et al., 2003). The fate of such cells would depend on other factors in the inflammatory environment. However, some inflammatory cytokines produced during neuroinflammation such as IL-6 are clearly toxic to neural progenitors. Thus, many of these cells may not survive. Secondly, in the case of CXCR4-utilizing viruses, we might expect additional effects. If CXCR4 is of particular importance in regulating adult neurogenesis in the DG, then viruses that interfere with this process may negatively impact neurogenesis in that region of the brain. Cell culture studies have indicated that T-tropic versions of the HIV-1 coat protein gp120 can interact with CXCR4 receptors expressed by adult neural progenitors. Moreover, gp120 can inhibit the chemoattractant and proliferative effects of SDF-1 on these cells (Tran et al., 2005). As discussed above, examination of the brains of patients suffering from AIDS dementia indicate a deficit of neurogenesis in the DG (Krathwohl and Kaiser, 2004). Thus, it appears that HIV-1 may inhibit DG neurogenesis as a direct result of its ability to interact with chemokine receptors expressed by neural progenitors. Due to the fact that new adult granule neurons are important for cognitive function in adults, interference with this process may result in adult cognitive deficits. Moreover, the development of granule neurons is clearly important in early childhood, and as a result, interference with this process during early brain development may have particularly devastating consequences (Kao and Price, 2004).
Acknowledgment
This work was supported by grants from the NIH. The authors would like to thank Dr. Dongjun Ren for helping provide the data shown in figure 1.
References
- Aarum M, Sandberg K, Haeberlin SLB, Persson MAA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl. Acad. Sci USA. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Mechanism of migration of olfactory bulb interneurons. Semin. Cell. Biol. Dev. 1997;8:207–213. doi: 10.1006/scdb.1996.0134. [DOI] [PubMed] [Google Scholar]
- Babcock A, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz M. Neuroanatomical distribution of CXCR4 in adult brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrydzelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell derived factor 1/CXCL12 in adult rat brain an constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur. J. Neurosci. 2003;18 doi: 10.1046/j.1460-9568.2003.02893.x. 15903-1606. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YE, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF-1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Belman AL. Infants, children and adolescents. In: Berger JR, Levy RM, editors. AIDS and the Nervous System. Lippincott-Raven; 1997. pp. 223–253. [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry tropism and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bodner A, Maroney AC, Finn JP, Ghadge G, Roos RP, Miller RJ. Mixed lineage kinase 3 mediates gp120IIIB-induced neurotoxicity. J. Neurochem. 2002;82:1424–1434. doi: 10.1046/j.1471-4159.2002.01088.x. [DOI] [PubMed] [Google Scholar]
- Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp. Neurol. 2004;188:246–253. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brouwers P, Civitello L, DeCarli C, Wolters P, Sei S. Cerebrospinal fluid vital load is related to cortical atrophy and not to intracerebral calcifications in children with symptomatic HIV disease. J. Neurovirol. 2000;6:390–397. doi: 10.3109/13550280009018303. [DOI] [PubMed] [Google Scholar]
- Chen Chen J, Li Y, Wang L, Lu M, Chopp M. Caspase inhibition by ZVAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAO in rats. J. Neurol. Sci. 2002;199:17–24. doi: 10.1016/s0022-510x(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. J. Clin. Virol. 2003;26:195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J. Comp. Neurol. 2003;457:7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J. Neurovirol. 2000;6 Suppl. 1:S29–S32. [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hall CD. Peripheral neuropathy in a cohort of human immunodeficiency virus-infected patients. Incidence and relationship to other nervous system dysfunction. Arch. Neurol. 1991;48:1273–1274. doi: 10.1001/archneur.1991.00530240077026. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J. Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O’Bryan S, Bielecki B, Zhou ZL, Majumder S, Ransohoff RM. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol. Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by stromal cell derived factor 1/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci. Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimers disease transgenic mice. Proc. Nat. Acad. Sci. USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kao AW, Price RW. Chemokine receptors, neural progenitor cells, and the AIDS dementia complex. J. Infect. Dis. 2004;190:211–215. doi: 10.1086/422012. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9 day old and 11 month old rodent hippocampus. J. Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FL. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends. Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischemic brain injury. Curr. Opin. Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J. Infect. Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders –how to make it work. Nat. Med. 2004;10 Suppl.:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu. Rev. Pharmacol. Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr. Opin. Neurol. 2003;16:403–409. doi: 10.1097/01.wco.0000073943.19076.98. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis and derailed cerebellar neuronal migration in CXCR4 and SDF-1 deficient mice. Proc. Natl. Acad. Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin. Immunol. 2003;15(1):23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Norton GR, Sweeney J, Marriott D, Law MG, Brew BJ. Association between HIV distal symmetric polyneuropathy and Mycobacterium avium complex infection. J. Neurol. Neurosurg. Psychiatr. 1996;61:606–609. doi: 10.1136/jnnp.61.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SB Oh, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, Taylor J, Levy R, Murphy RL, Wolinsky SM, Gabuzda D. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J. Virol. 2003;77(22):12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor –2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury induced neurogenesis in the adult mammalian brain. The Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Pencea V, Luskin MB. Prenatal development of the rodent rostral migratory stream. J. Comp. Neurol. 2003;463:402–418. doi: 10.1002/cne.10746. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen S, Herek N, Fujii H, Tamamura J, Zheng J. Stromal cell derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J. Neurosci. Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A. Experimental autoimmune encephomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. USA. 2002;89:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and the AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson CM, Andrews A, Sandberg JA, Nickols J, Slikker W., Jr Evaluation of brain and nerve pathology in rats chronically dosed with ddI or isoniazid. Neurotoxicol. Teratol. 1996;18:555–563. doi: 10.1016/0892-0362(96)00088-8. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwan BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Sharma PL, Nurpeisov V, Hernandez-Santiago B, Beltran T, Schinazi RF. Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Curr. Topics Med. Chem. 2004;4:895–919. doi: 10.2174/1568026043388484. [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung LD, Taupin P, Nakashima R, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat. Rev. Mol. Cell Biol. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schultz SA. Dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform selective regulation of SDF-1 expression modulates CXCR4 dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini R, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J. Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamula MAT, Wolters PL, Walsek C, Zeichner S, Civitello L. Cognitive decline with immunologic and virologic stability in four children with human immunodeficiency virus disease. Pediatrics. 2003;112:679–684. doi: 10.1542/peds.112.3.679. [DOI] [PubMed] [Google Scholar]
- Takehashi K, Wessenlingh SL, Griffin DE, McArthur JC, Johnston RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction in situ hybridization and immunocytochemistry. Ann. Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors;signposts to brain development and disease. Nat. Rev. Neuroscienc. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J. Neurosci. Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren DJ, Miller RB. The HIV-1 coat protein gp120 regulates CXCR4 mediated signaling in neural progenitor cells. J. Neuroimmunol. 2005;160(1–2):68–76. doi: 10.1016/j.jneuroim.2004.11.001. Epub 2004 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Frost E, To V, Robinson S, Ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in the developing spinal cord by arresting their migration. Cell. 2002;110(3):373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell LL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of IL-6. J. Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Y Li, Chen X, Gautman SC, Xu Y, Chopp M. MCP-1, MIP-1 and IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Haematology. 2002a;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Gautam SC, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 2002b;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur. J. Cell. Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the cental nervous system. AIDS. 1996;10:843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Wulff EA, Wang AK, AK, Simpson DM. HIV-asssociated neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of chemokine receptor CXCR4 in haematopoiesis and cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]