Abstract
The functional activity of Six2, a member of the so/Six family of homeodomain-containing transcription factors, is required during mammalian kidney organogenesis. We have now determined that Six2 activity is also necessary for the formation of the pyloric sphincter, the functional gate at the stomach-duodenum junction that inhibits duodenogastric reflux. Our data reveal that several genes known to be important for pyloric sphincter formation in the chick (e.g., Bmp4, Bmpr1b, Nkx2.5, Sox9, and Gremlin) also appear to be required for the formation of this structure in mammals. Thus, we propose that Six2 activity regulates this gene network during the genesis of the pyloric sphincter in the mouse.
Keywords: Six2, pyloric sphincter, stomach development, mouse
INTRODUCTION
The function of the vertebrate digestive system is to ingest food into the body, digest and absorb nutrients from the food, and excrete waste products. The gut develops soon after gastrulation as a simple tube of endoderm encircled by splanchnic mesoderm (Hogan, 2002; Lawson et al., 1986; Roberts, 2000). Beginning at embryonic day (E) 8.5, the mouse gut tube is patterned on the anterior-posterior (A– P), dorsal-ventral, left-right, and radial axes by reciprocal mesenchymal-epithelial interactions (Franklin et al., 2008; Lawson et al., 1986; Levin, 1997; Lowe et al., 1996; Lyons et al., 1995b; Mendelsohn, 2006; Roberts, 2000; Ryan et al., 1998; Sukegawa et al., 2000).
Along the A–P axis, endodermally derived signals pattern the tube into distinct regions including the foregut, which will give rise to the esophagus, liver, lungs, pancreas, and stomach; the midgut, which will form the small intestine (SI); and the hindgut, which is the precursor to the large intestine (Aufderheide and Ekblom, 1988; Duluc et al., 1994; Haffen et al., 1987; Lawson et al., 1986; Roberts, 2000; Wells and Melton, 1999; Yasugi, 1993). The A–P patterning of the gut tube into organ primordia is evident by E10.5, as indicated by the spatially restricted expression of different transcription factors and signaling molecules that participate in mesenchymal-epithelial interactions (Aufderheide and Ekblom, 1988; Kedinger et al., 1990; Lyons et al., 1995b; Roberts, 2000; Wells and Melton, 1999; Yasugi, 1993). Shh and Bmp signaling pathways, as well as Hox transcription factors, participate in these mesenchymal-epithelial interactions (Beck et al., 2000; Grapin-Botton and Melton, 2000; Litingtung et al., 1998; Marigo et al., 1996; Narita et al., 2000; Pepicelli et al., 1998; Pitera et al., 1999; Roberts et al., 1995; Roberts et al., 1998; Sekimoto et al., 1998; Smith and Tabin, 1999; Yokouchi et al., 1995; Zakany and Duboule, 1999). Each region of the gut tube is separated by sphincters, which are thick circular muscles that control the passage of matter through the digestive system.
One of the key organs that form along the gut tube is the stomach, which initially digests the food bolus and converts it into acidic chyme. Impulses from the nerve plexuses of the enteric nervous system coordinate peristaltic waves of contraction that grind and thrust the contents of the stomach posteriorly. As the peristalsis reaches the pylorus, the pyloric sphincter (PS) reacts by closing, thereby causing retropulsion of the contents and creating shearing forces that grind the food. Once the food bolus is converted into acidic chyme, the PS opens and delivers it to the SI.
The PS consists of a thickened smooth muscle layer covered by mucous-secreting glands at the narrow posterior boundary of the stomach. The mechanisms that underlie the formation of the mammalian PS are not yet known. The only available working model describing the formation of the PS was proposed for chicken embryos. In these animals and in response to Shh signaling, Bmp4 is expressed in the mesoderm of the SI (Roberts et al., 1995; Roberts et al., 1998; Smith et al., 2000a; Smith et al., 2000b; Smith and Tabin, 1999), whereas the Bmp receptor 1b (Bmpr1b) is expressed in the mesoderm of the gizzard, the chick’s posterior stomach (Smith et al., 2000a; Smith et al., 2000b; Smith and Tabin, 1999). Bmp signaling from the SI specifies the PS in the mesoderm located at the junction of the gizzard and SI (the region where Bmp4 and Bmpr1b expression overlaps) by inducing the expression of the transcription factors Nkx2.5 and Sox9 in the posterior gizzard mesoderm (Moniot et al., 2004; Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005).
Loss- and gain-of-function approaches have shown that Nkx2.5 and Sox9 are necessary and sufficient to specify the typical bleb-like microvilli of the PS epithelium (Moniot et al., 2004; Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005). In the case of Sox9, this functional role could be accomplished by inducing Gremlin expression, which in turn, modulates Bmp activity (Moniot et al., 2004). Briefly, it has been argued that in the chick, Bmp signaling controls the localization of PS formation, as well as the expression of Sox9 and Nkx2.5, two genes that determine the characteristic epithelium of the PS (Moniot et al., 2004; Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005).
In the mouse, Bmp4 is expressed in the mesenchyme of the stomach and anterior SI (Bitgood and McMahon, 1995; Jones et al., 1991; Smith et al., 2000a); Bmpr1b is expressed in the posterior stomach (Smith et al., 2000a); and Nkx2.5 is expressed in the mesoderm of the PS (Chi et al., 2005; Lints et al., 1993; Smith et al., 2000a). These relatively similar expression patterns of Bmp4, Bmpr1b, and Nkx2.5 in the chick and mouse digestive tracts suggest that the mechanisms involved in the formation of the PS may be conserved between the two species.
Six2 belongs to the so/Six family of homeobox-containing genes (Oliver et al., 1995). Initial characterization of its expression profile revealed that Six2 was expressed in tissues such as the developing head, kidneys, limbs, and stomach (Oliver et al., 1995). Further work has shown that this gene’s expression in the stomach is also conserved in frog and chick (Smith et al., 2000a). Functional characterizations have determined that Six2 plays crucial roles during the development of the kidney and branchial arches (Kutejova et al., 2008; Self et al., 2006). Those initial analyses also identified defects in the development of certain parts of the digestive tract in animals lacking Six2 activity (our unpublished observations).
Here we have investigated the functional role of Six2 in the development of the murine digestive tract, particularly in the formation of the PS during stomach organogenesis. We identified Six2 as a key gene required for the formation of the mammalian PS. Six2 functions in this developmental process by regulating a genetic network that is conserved between mouse and chick.
MATERIALS AND METHODS
Functional Inactivation of Six2
The strategy for the functional inactivation of Six2 has been previously described (Self et al., 2006).
In Situ Hybridization
Embryos were fixed in 4% paraformaldehyde and processed for whole-mount in situ hybridization as reported (Wilkinson, 1995). Gelatin-embedded stained embryos were sectioned on a cryostat (Stern, 1993).
Immunohistochemistry
Embryos were fixed with 4% paraformaldehyde, cryopreserved in 30% sucrose, and sectioned (10 µm slices) on a cryostat for immunohistochemical analysis. Anti-Sox9 (Millipore, Billerica, MA) antibody staining was detected by diaminobenzidine using the VECTASTAIN® ABC kit (Vector Laboratories, Burlingame, CA), and anti–α-smooth muscle actin (α-SMA) antibody (Sigma, St. Louis, MO) was conjugated to Cy3.
RESULTS
Six2 expression in the developing stomach
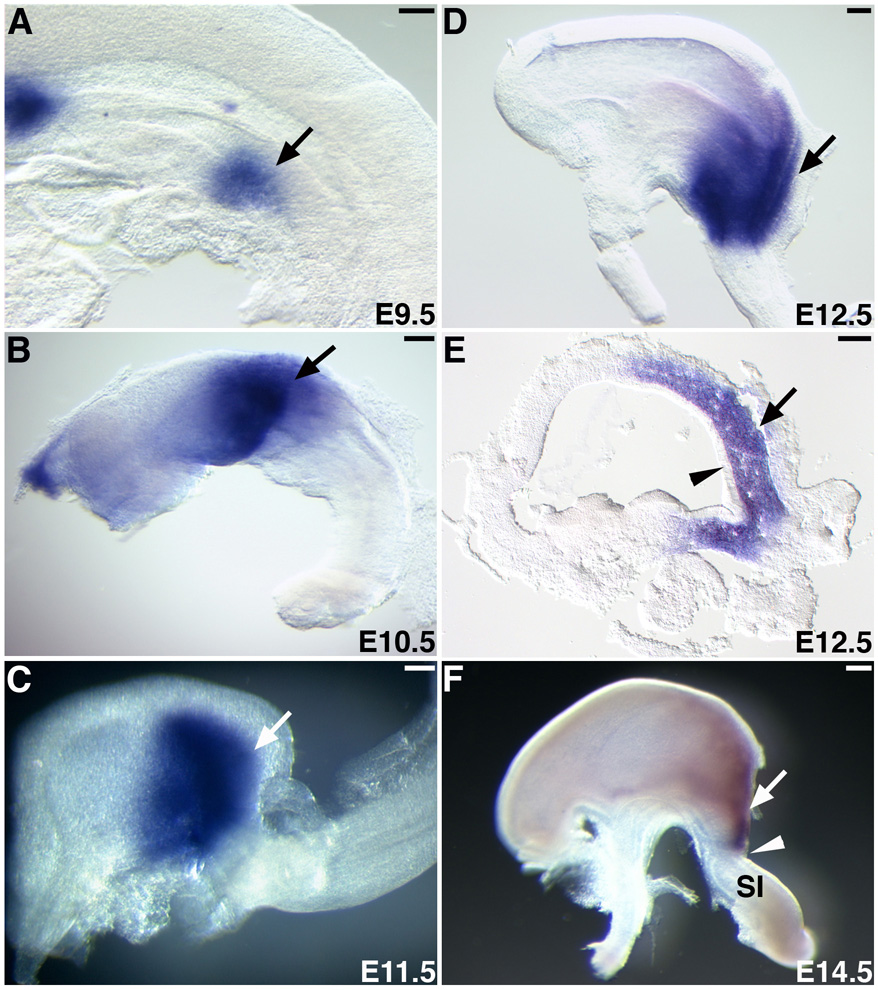
First, we performed a detailed characterization of the pattern of expression of Six2 during organogenesis of the mouse stomach. At around E9.5 and before the stomach morphologically differentiated from the gut tube, we detected Six2 expression in the region of the splanchnic mesoderm corresponding to the stomach anlage (Fig. 1A, arrow) (Oliver et al., 1995). Once the stomach became demarcated from the gut tube at around E10.5, Six2 was expressed in the posterior mesenchymal portion (Fig. 1B, arrow). By E11.5, the mesenchyme of the posterior half of the stomach continued to express Six2 (Fig. 1C, arrow). The posterior part of the mouse stomach is the glandular stomach (GS); the anterior region of the GS corresponds to the fundus, and the most posterior region corresponds to the antrum (Hogan, 2002; Karam et al., 1997; Lee, 1985; Wright, 2000). By E12.5, Six2 expression was confined to the mesenchyme of the presumptive GS (Fig. 1D, E) but was not detected in the endodermally derived epithelial lining of the stomach (Fig. 1E, arrowhead). As development of the stomach progressed, Six2 expression became more restricted, and at E14.5, it was limited to the antrum, just anterior to the PS (Fig. 1F, arrow). This expression pattern was maintained until birth (data not shown).
Fig. 1.
Six2 is expressed in the mesoderm of the posterior stomach. (A) At E9.5, Six2 is expressed in the splanchnic mesoderm of the mouse stomach anlage (arrow). (B) By E10.5, Six2 is expressed in the mesoderm of the posterior stomach (arrow). (C) Expression is seen in the presumptive glandular stomach primordium at E11.5. (D, E) At E12.5, Six2 becomes restricted to the mesenchyme of the antral region of the posterior stomach (arrows); no expression is observed in the epithelial layer (arrowhead). (F) At E14.5, Six2 expression remains in the antrum, just anterior to the pyloric sphincter (arrowhead). Small intestine, SI; Scale bars, 100 µm.
Pyloric sphincter formation is defective in the Six2-null stomach
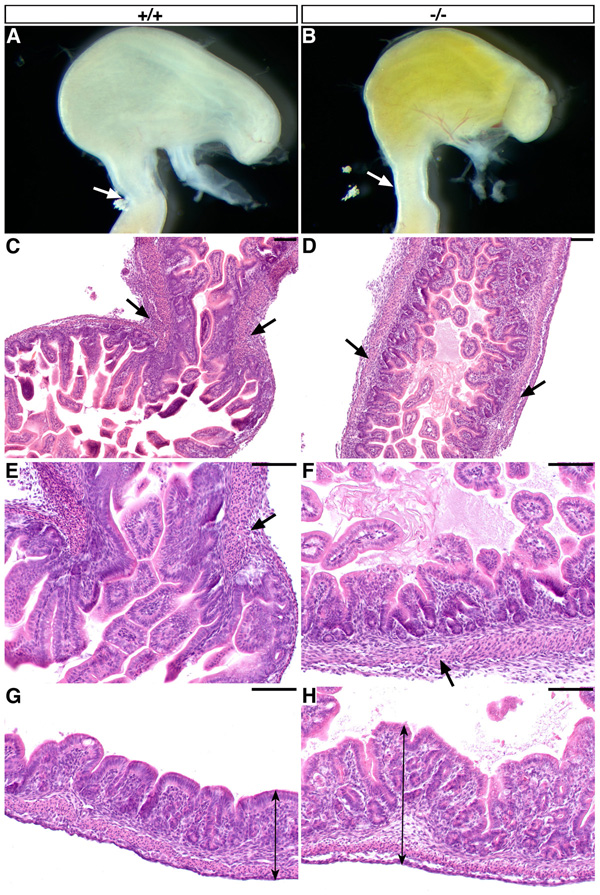
As previously reported, Six2-null embryos die at birth due to the lack of functional kidneys (Self et al., 2006). To precisely identify morphological defects resulting from the absence of Six2 activity in the digestive tube, we performed a detailed analysis of the Six2-null embryos. Visual inspection of E18.5 Six2-null embryos revealed abnormal duodenogastric reflux of amniotic fluid into the mutant stomach (Fig. 2B). The cause of the reflux could be a nonfunctional or absent PS. Normally at E18.5, a thickened smooth muscle that forms valvular flaps and a constricted region of the stomach identifies the presence of the PS at the junction of the stomach and SI (Fig. 2C, E, arrows). This thickened ring of smooth muscle and narrowing of the gut tube was not seen in Six2-null littermates (Fig. 2D, F). In addition, the mucosa of the Six2-null stomach was hypertrophic (Fig. 2H). These results indicate that during stomach organogenesis, Six2 activity controls PS formation and mucosal growth.
Fig. 2.
Six2-null embryos exhibit duodenogastric reflux and mucosal overgrowth. (A) At E18.5, the wild-type stomach contains a functional pyloric sphincter (PS; arrow) to prevent the reflux of amniotic fluid from the small intestine (SI) to the stomach. (B) The Six2-null stomach lacks a functional PS (arrow), thereby allowing reflux of the fluid (yellow content). (C, E) At this same stage, H & E staining of the wild-type stomach shows the region of the forming PS (arrows), which includes a thickened circular smooth muscle layer that constricts the gut tube at the junction of the stomach and SI. (D, F) The Six2-mutant gut tube lacks this thickened smooth muscle layer and the constriction that normally occurs at the corresponding level where the PS (arrows) should have formed. (G) At E18.5, the wild-type glandular stomach consists of primitive mucosal glands. (H) The Six2-null stomach exhibits overgrowth of the glands at this stage (compare arrows in G and H). Scale bars, 100 µm.
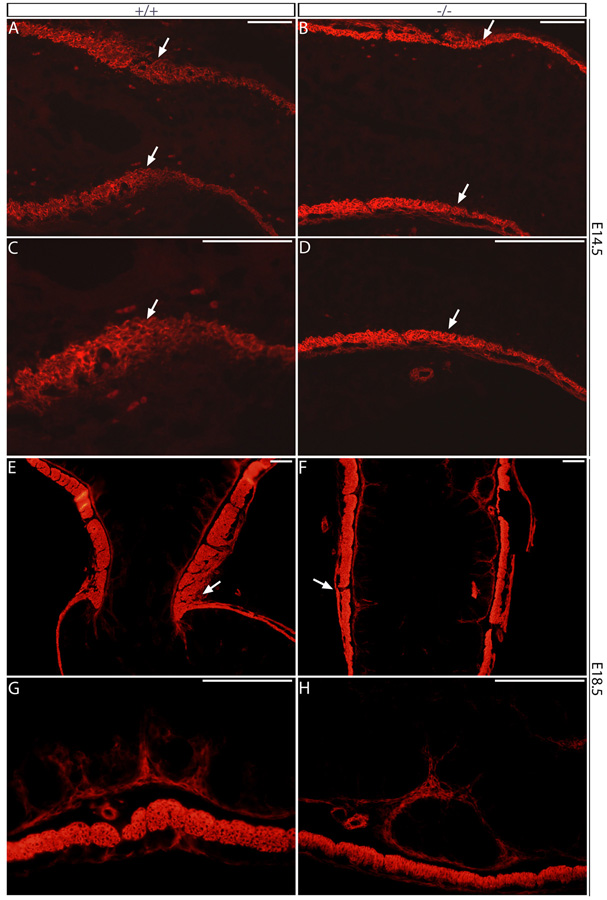
To further characterize the identified alterations in PS formation, we analyzed the expression of α-SMA, one of the earliest markers of smooth muscle differentiation (McHugh, 1995; Takahashi et al., 1998) in the Six2-null stomach. The circular smooth muscle layer begins to differentiate throughout the wild-type stomach at around E13.5, as indicated by expression of α-SMA (McHugh, 1995; Takahashi et al., 1998). By E14.5, the constricted prospective PS region expressing α-SMA was thicker than in the rest of the wild-type stomach and SI (Fig. 3A, C, arrows). Although SMA is expressed at the appropriate stage in the Six2-null smooth muscle layer, we detected no thickening or constriction in the presumptive PS region of the E14.5 Six2-null stomach (Fig. 3B, D, arrows). This result supports the proposal that PS formation is defective or absent in Six2- mutant embryos. In the E18.5 wild-type stomach, the dense muscular wall and developing valvular folds of the PS can be easily distinguished by α-SMA expression (Fig. 3E, arrow). However, at this stage the characteristic valvular folds of circular smooth muscle were not readily apparent in the presumptive PS territory of Six2-null littermates (Fig. 3F). The thickness of the circular smooth muscle layer in the remainder of the Six2-null stomach was normal (Fig. 3G, H). Analyses of apoptosis and proliferation in the PS region at E12.5 revealed no differences between wild-type and Six2-null littermates (data not shown). These data suggest that specification of the smooth muscle layer from the splanchnic mesoderm occurs normally in Six2-null stomach but lack of Six2 activity disrupts the initial steps leading to the formation of the PS, e.g., thickening of the smooth muscle layer and constriction of the gut tube in the presumptive pyloric territory. As a consequence, we observed abnormal reflux of embryonic body fluids into the stomach of the Six2-null embryos.
Fig. 3.
The lack of Six2 activity disrupts the initial steps of pyloric sphincter (PS) formation. (A, C) Expression of α-smooth muscle actin (α-SMA) in the E14.5 wild-type stomach reveals the thickening of the smooth muscle layer and the constriction of the gut tube at the level of the developing PS (arrows). (B, D) In the E14.5 Six2-null stomach, the α-SMA+ layer fails to thicken, and the constriction of the gut tube at the boundary of the stomach and small intestine (arrows) does not form. (E) At E18.5, the wild-type stomach shows the characteristic thickening of the smooth muscle layer and constriction of the gut tube in the PS territory (arrow). (F) The Six2-null stomach shows no evidence of PS formation at the stomach-SI junction (arrow). (G, H) The thickness of the smooth muscle layer (arrows) in the remainder of the Six2-null stomach (H) is similar to that of the wild-type stomach (G), as demonstrated by representative sections of the glandular stomach. Scale bars, 100 µm.
A gene expression network that regulates pyloric sphincter formation in chick is conserved in mice
As previously mentioned, not much information is available about the genes and mechanisms responsible for the development of the PS in mammals. We speculated that the functions of some genes that participate in PS formation in the chick (e.g., Bmp4, Bmpr1b, Nkx2.5, Sox9, and Gremlin) could be conserved in mice. However, in the mammalian stomach, only limited data about the expression patterns of some of these genes have been reported (Bitgood and McMahon, 1995; Chi et al., 2005; Jones et al., 1991; Lints et al., 1993; Smith et al., 2000a). Therefore, we first analyzed the expression of those PS markers during mouse stomach development.
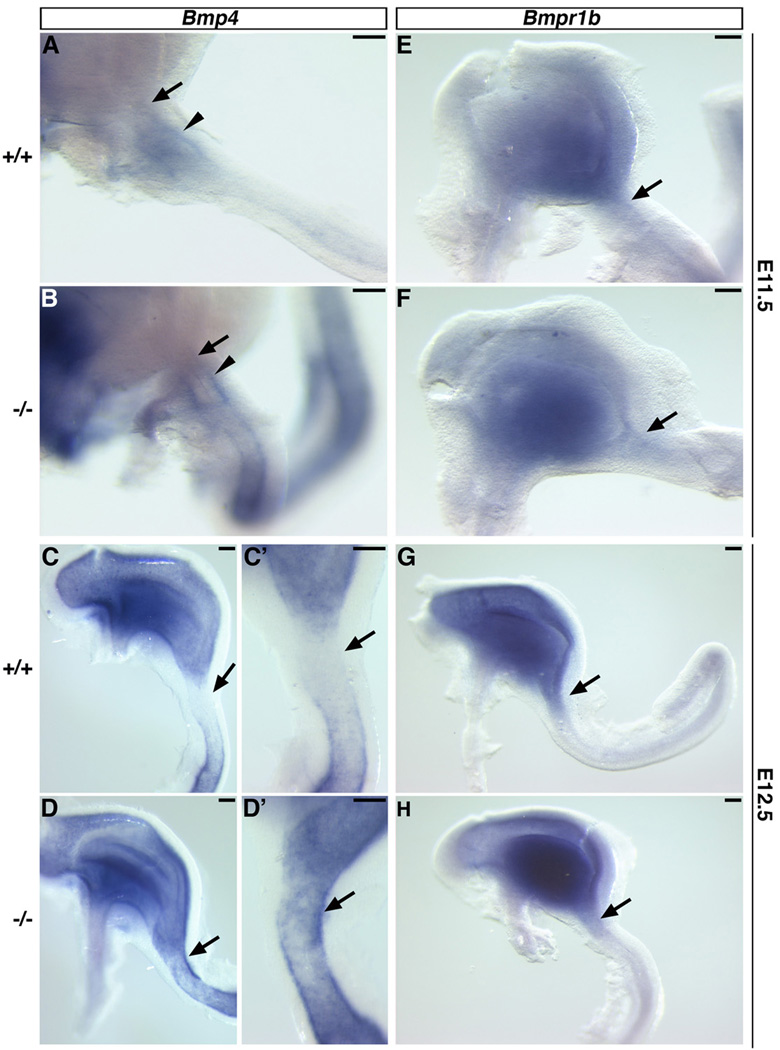
As previously described (Bitgood and McMahon, 1995; Jones et al., 1991; Smith et al., 2000a), at E11.5 Bmp4 is expressed in the mesenchyme of the wild-type forestomach, which is located anterior to the GS (data not shown), and duodenum (Fig. 4A, arrow), which is located posterior to the presumptive PS territory (Fig. 4A, arrowhead). In the Six2−/− stomach, we observed no obvious changes in the expression of Bmp4 in these regions (Fig. 4B and data not shown). At E12.5, Bmp4 was expressed in the mesenchyme throughout the wild-type stomach and in the mesenchyme of the SI, but it was absent from the mesenchyme of the PS region (Fig. 4C, C’, arrows). However, in the Six2−/− stomach, Bmp4 expression was ectopically expanded into the presumptive PS territory at E12.5 (Fig. 4D, D’, arrow). At these same stages in wild-type embryos, we detected Bmpr1b expression in the GS mesenchyme that extended into the presumptive PS area (Smith et al., 2000a) (Fig. 4E, G). The pattern of Bmpr1b expression was similar in the Six2-null stomach (Fig. 4F, H). These results suggest that Six2 is required to maintain a Bmp4-free PS territory for proper morphogenesis of the PS in the mouse stomach.
Fig. 4.
Six2 is required to maintain a Bmp4-free territory in the prospective pyloric sphincter (PS) region. (A) Normally at E11.5, Bmp4 is expressed in the mesenchyme surrounding the epithelium of the presumptive duodenum (arrowhead) and is absent from the mesoderm of the presumptive PS region (arrow). (B) This expression pattern is not obviously affected in the Six2-null gut tube. (C, C’) At E12.5, Bmp4 expression is excluded from the PS territory (arrow) in wild-type embryos. (D, D’) In Six2−/− littermates, expression of Bmp4 has ectopically expanded into the prospective PS region (arrow). (E) Expression of Bmpr1b is detected in the wild-type mesenchyme of the developing glandular stomach and PS region (arrow). (F) This expression pattern appears unaffected in the Six2-null stomach. Bmpr1b expression remains in the PS territory (arrows) of E12.5 wild-type (G) and Six2-null (H) stomachs. Scale bars, 100 µm.
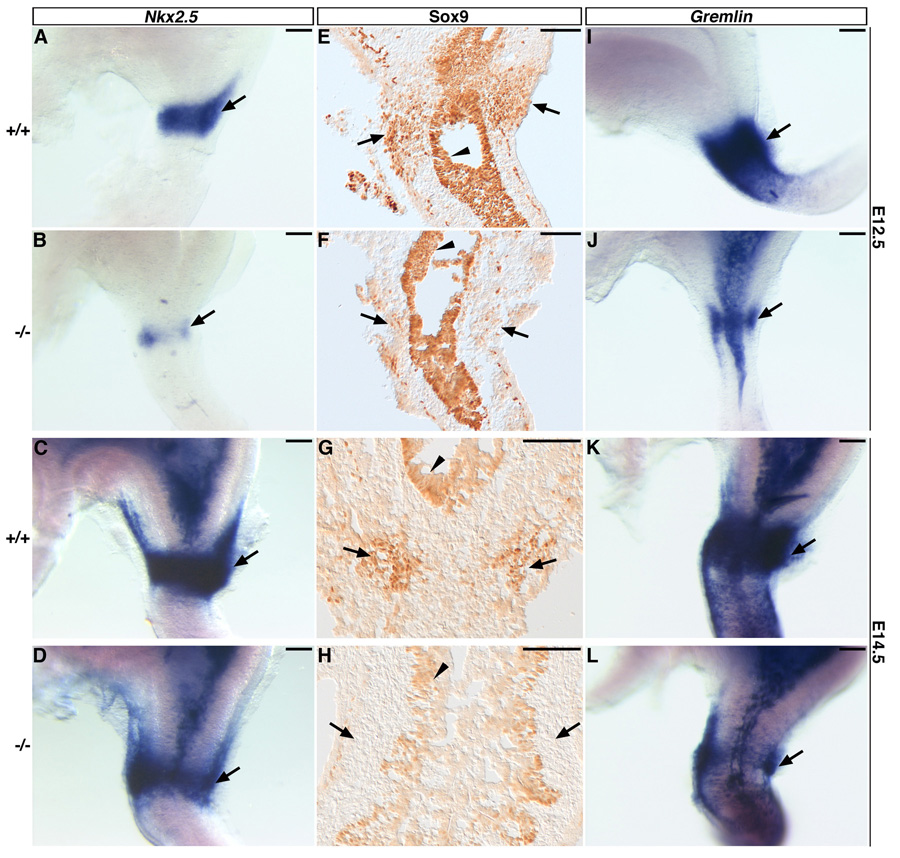
The chick model argues that specification of the PS-like epithelial phenotype requires Bmp-mediated mesodermal expression of Nkx2.5 and Sox9 (Moniot et al., 2004; Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005). In the mouse, we detected a ring of Nkx2.5-expressing mesenchyme in the presumptive PS region at E12.5 (Fig. 5A). The expression of Nkx2.5 was weaker, and the expression domain was narrower at this same stage in the Six2−/− stomach (Fig. 5B). At E14.5, we detected no obvious differences in Nkx2.5 expression in the PS regions of wild-type and Six2-null stomachs (Fig. 5C, D). At the same stages, Sox9-expressing cells were detected in the mesenchyme of the presumptive PS territory in wild-type embryos (Fig. 5E, G, arrows). Instead, we observed just a few Sox9+ cells in the Six2-null presumptive PS region at E12.5 (Fig. 5F, arrow). At E14.5, no Sox9-expressing cells could be identified in the mesenchyme of the Six2−/− presumptive PS territory (Fig. 5H, arrow). However, Sox9 remained at normal levels in the stomach epithelium at both of these stages (Fig. 5E–H, arrowheads).
Fig. 5.
Expression of pyloric sphincter (PS) markers is aberrant in the Six2-null stomach. (A) Nkx2.5 is expressed in the E12.5 wild-type PS territory (arrow). (B) However, in the prospective PS territory of E12.5 Six2−/− littermates, the domain of Nkx2.5 expression (arrow) is smaller. (C) At E14.5, Nkx2.5 expression remains in the wild-type presumptive PS territory (arrow). (D) Nkx2.5 expression appears normal in the Six2-null stomach at this stage. Sox9 is expressed in the mesenchyme of the prospective PS region (arrows) and in the epithelium of the stomach (arrowheads) at E12.5 (E) and E14.5 (G). In the absence of Six2, the domain of mesodermal cells (arrows) expressing Sox9 is smaller at E12.5 (F) and absent at E14.5 (H); its pattern of expression is normal in the stomach epithelium at both stages (arrowheads). Gremlin is also expressed in the mesenchyme of the presumptive wild-type PS region (arrows) at E12.5 and E14.5 (I, K). The territory of mesenchymal cells expressing Gremlin (arrows) is smaller in the Six2−/− stomach at E12.5 (J) and E14.5 (L). Scale bars, 100 µm.
The mesenchymal layer of the presumptive PS territory of the E11.5 (data not shown) and E12.5 (Fig. 5I) wild-type stomach also expressed Gremlin. Similar to that of Nkx2.5 and Sox9, the expression domain of Gremlin was smaller in the mesodermal layer of Six2-null littermates at E11.5 (data not shown) and E12.5 (Fig. 5J). By E14.5, the level of Gremlin expression appeared normal in the Six2−/− stomach but its expression domain was smaller than that of the wild-type stomach (Fig. 5K, L). These data suggest that the expression and function of certain genes that are essential for PS formation in chick are also important during PS formation in mammals.
DISCUSSION
Based on our results and those from Smith et al. (2000a), we suggest that in mammals the formation of the PS begins as a thickened region of circular smooth muscle forms at the boundary of the antrum and duodenum at E14.5. This thickened area of smooth muscle physically causes the gut tube to constrict at this region. At later stages of development (E18.5) the smooth muscle shapes into valvular flaps or folds of smooth muscle tissue that form a physical boundary between the stomach and SI and functions to prevent the backflow of gastric juices from the SI to the stomach. The mechanisms that control the formation of the mammalian PS are poorly understood. Conservation of Six2 expression in the posterior mesodermal compartment of the developing stomach of frog, chick, and mouse embryos suggests that its activity is required for the genesis of a functional PS. Our results shed some light on this process as they identify Six2 as a gene whose activity is required for the formation of a functional PS, possibly by regulating a gene network conserved between chick and mouse (Fig. 6). Our data suggest that the abnormal ectopic expansion of mesodermal Bmp4 expression into the presumptive PS territory and the decreased expression domains of Nkx2.5, Sox9, and Gremlin are responsible for the lack of PS formation observed in the Six2-null stomach.
Fig. 6.
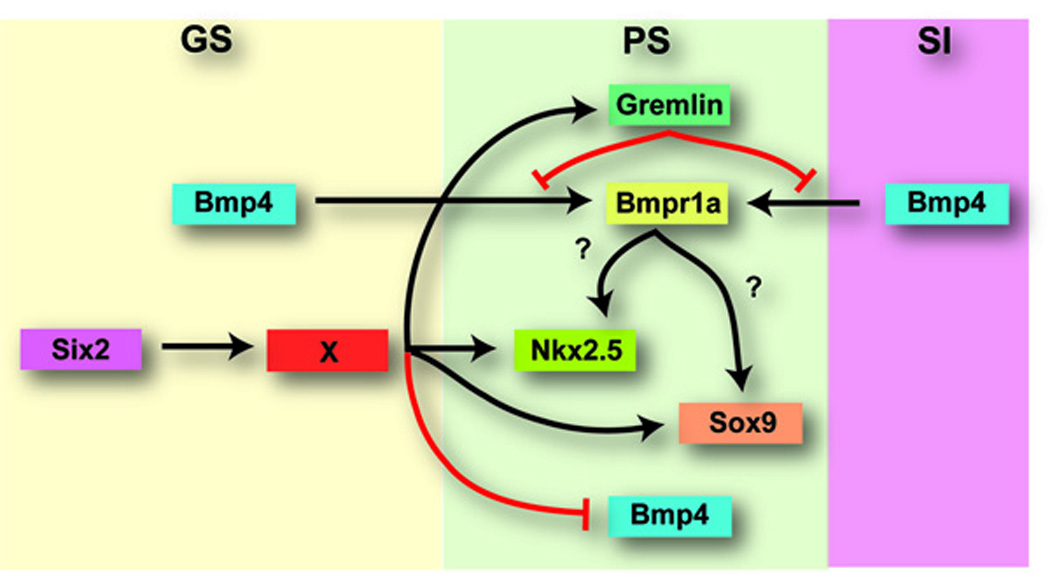
Model of Six2 function during mouse pyloric sphincter development. Based on the results presented here and those published for chick PS development, we propose a model for PS formation in the mouse. Six2 activity in the GS region of the developing stomach functions to suppress expression of Bmp4 in the PS territory. It is possible that this regulation of the Bmp4 pathway is accomplished through transcriptional regulation of another unidentified factor. Other PS markers (Gremlin, Nkx2.5, and Sox9) are regulated downstream of Six2 activity either by an unidentified factor or by the Bmp4 pathway. Gremlin can further abrogate Bmp signaling to control smooth muscle thickness.
In chick embryos, Bmp4 is expressed throughout the early gut tube, except for the stomach, where its expression is detected in the submucosal layer of the gizzard only at later stages of development (Moniot et al., 2004; Roberts et al., 1995; Roberts et al., 1998; Smith et al., 2000a; Smith et al., 2000b; Smith and Tabin, 1999). Bmpr1a and Bmpr1b exhibit complementary expression patterns: Bmpr1a is located in the mesoderm of the SI, and Bmpr1b, in the mesoderm of the gizzard (Smith et al., 2000a; Smith et al., 2000b; Smith and Tabin, 1999). Interestingly, in the Bmp4-free region of the chick stomach, the smooth muscle layer is thicker than it is in the rest of the gut tube, a result suggesting that Bmp4 limits the growth of the mesodermal layer along the radial axis during gut regionalization (Roberts et al., 1998). This proposal is supported by results showing that misexpression of Bmp4, Bmpr1a, or Bmpr1b in the chick stomach results in smaller thin-walled stomachs with altered rates of apoptosis and proliferation (Moniot et al., 2004; Roberts et al., 1998; Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005). Consistent with these results, in the developing mouse stomach Bmp4 expression is restricted from the presumptive PS region (Fig. 6). In the Six2-null stomach, ectopic Bmp signaling and decreased expression of the Bmp signal modulator Gremlin results in a thinner muscle layer; a result suggesting that in mammals, Bmp signaling also negatively regulates smooth muscle development.
In the chick, the expression of Nkx2.5 in a precisely delimited region of the gut mesoderm (i.e., located at the boundary between the gizzard and the SI) is one of the first indicators of the territory where the PS will develop (Buchberger et al., 1996; Smith et al., 2000a; Smith and Tabin, 1999; Theodosiou and Tabin, 2005). Injection of constitutively active Bmp receptors or Bmp4 constructs into the embryonic gizzard activated Nkx2.5 expression in the gizzard mesoderm followed by a morphologic change in the endoderm of the gizzard that acquires the bleb-like microvilli that are characteristic of the PS epithelia (Smith et al., 2000b; Smith and Tabin, 1999; Theodosiou and Tabin, 2005). On the other hand, blocking Nkx2.5 activity in the PS region resulted in the loss of the PS endodermal phenotype (Smith and Tabin, 1999). Together, these results argued that in the chick, Bmp signaling is involved in the specification of the PS in the mesoderm located at the junction of the gizzard and the SI and that Nkx2.5 activity is sufficient and necessary to specify some aspects of the PS phenotype (Smith et al., 2000b; Smith and Tabin, 1999).
In the chick, Sox9 is expressed in the endoderm throughout the GI tract, except for the gizzard; it is also expressed in the mesoderm of the PS (Moniot et al., 2004; Theodosiou and Tabin, 2005). Similar expression has been observed in human embryos (Moniot et al., 2004), as well as in the mouse (Fig. 5). Misexpression of Bmp4 in the chick stomach caused the anterior expansion of the Sox9 domain (Moniot et al., 2004); however, not all cells in this expanded domain expressed Sox9. This result suggests that the mesodermal cells in the stomach differentially respond to Bmp4 activation. Abrogated Bmp signaling in the stomach by misexpression of Noggin caused muscular hypertrophy, downregulation of Sox9, and PS defects (Moniot et al., 2004; Theodosiou and Tabin, 2005). These results suggest that Bmp signaling is both necessary and sufficient for Sox9 expression in the gizzard mesoderm (Moniot et al., 2004; Theodosiou and Tabin, 2005). Ectopic expression of Sox9 in the gizzard mesoderm promoted the ectopic induction of Gremlin expression in the mesoderm followed by the transformation of the gizzard epithelium into a PS-like epithelium (Moniot et al., 2004). In summary, results in chick embryos suggest a model in which Bmp4 signaling via Bmpr1b at the junction of the gizzard and the SI directs PS formation by inducing the expression of Nkx2.5 and Sox9 in the presumptive PS territory. Both of these genes specify the pyloric epithelium, and Sox9, in turn, induces the expression of Gremlin, which participates in a negative feedback loop to abrogate Bmp signaling.
On the basis of our results, we propose that a gene cascade similar to that proposed for chick also participates in the development of the mammalian PS (Fig. 6). Unfortunately not much information is yet available regarding the functional roles of these same genes during stomach development in the mouse. Bmp4- and Nkx2.5-mutant mice exhibit early embryonic lethality precluding any analysis of these genes’ roles in stomach development (Lyons et al., 1995a; Winnier et al., 1995). Our results confirmed that the expression of genes that are important in chick PS formation is at least partially conserved in the mouse and that the expression patterns of those genes are affected in the defective pyloric region of the Six2-null stomach. A major difference between chick and mouse PS formation is that in chick, the PS forms in the region where Bmpr1b and Bmp4 expression overlaps at the junction of the gizzard and the SI. Normally in the mouse, Bmp4 expression is specifically absent from the prospective PS territory during early stages of stomach development. However, in the Six2-null stomach, Bmp4 expression is ectopically expanded into this region, and the smooth muscle layer fails to thicken. In contrast with data from chick studies, the ectopic expansion of Bmp signaling into the prospective PS territory of the Six2-null mouse stomach was not followed by the induction or expansion of the Sox9- or Nkx2.5-expression domains; the sizes of the Sox9-, Nkx2.5-, and Gremlin-expressing domains were reduced during early stages of stomach development. Therefore, although expression of the genes that are important for PS formation in chick is conserved during mouse PS development, regulation of this network appears to differ between these species. Another minor difference between chick and mouse PS formation is that, as far as we are aware, the embryonic mouse PS does not possess the epithelial microvilli characteristic of the chick PS.
Six2 is expressed throughout the antral mesenchyme of the developing stomach suggesting that Six2 may regulate development of this entire region of the gut tube. This hypothesis is supported by the evidence of hypertrophy of the stomach mucosa in Six2-null embryos at later stages of development. However, it is likely that Six2 plays a distinct role in regulating PS development since changes in specific PS markers are detected in the Six2-null stomach. Extensive analysis of a battery of known antrum markers revealed no striking differences in the Six2-null stomach compared to wild-type littermates (data not shown). Therefore, in this paper we restricted our studies to the specific PS phenotype. We envision two possible roles of Six2 during mammalian PS formation. Six2 could be required to provide PS competence to a broad region of the antral mesenchyme. In this case, Six2 activity could be required for some of the aforementioned PS markers to reach a certain expression threshold or for the cells expressing the PS markers to reach their proper cell number. In the absence of Six2 activity, the expression levels of the genes discussed above are reduced, and their expression domains and smaller. Therefore, PS specification does not take place. Alternatively, Six2 may be required to maintain a Bmp4-free territory in the prospective PS region so that proper mesodermal differentiation will result in a thicker smooth muscle layer and constriction at this site of the gut tube.
A better understanding of the cellular and molecular mechanisms regulating the development of the mammalian digestive tract may shed light on human metaplasias and congenital disorders. For example, excessive duodenogastric reflux is caused by incomplete closure of the PS, ablation of the pylorus, or imperfect timing of peristalsis, and it can be damaging to the gastric mucosa (DuPlessis, 1960; DuPlessis, 1965; Lawson, 1964; Schrager and Oates, 1978; Vaezi and Richter, 1996; Vaezi et al., 1995). This reflux is also associated with an increased risk of gastric carcinoma (Lundegardh et al., 1988; Miwa et al., 1992; Yasuda et al., 2005). Primary duodenogastric reflux is rare in children, and the origin is unknown (Hermans et al., 2003). Infantile hypertrophic pyloric stenosis (IHPS) is a human condition in which the gastric outlet is obstructed by hypertrophy of the PS muscle, which fills the lumen. IHPS occurs in only two to four of every 1000 infants born, and symptoms arise within the first 2 to 12 weeks of life (Applegate and Druschel, 1995; Hernanz-Schulman et al., 2001; Rollins et al., 1989). This represents a phenotype opposite of that of the Six2-null stomach. The generated Six2−/− mice could become a useful animal model in which to study the expression of genes known to be crucial in smooth muscle hypertrophy in patients with IHPS.
Acknowledgements
We thank R. Zeller, Z. E. Yutzey, and B. Hogan for plasmids. This project was supported in part by Cancer Center Support CA-21765 and the American Lebanese Syrian Associated Charities (ALSAC).
References
- Applegate MS, Druschel CM. The epidemiology of infantile hypertrophic pyloric stenosis in New York State, 1983 to 1990. Arch Pediatr Adolesc Med. 1995;149:1123–1129. doi: 10.1001/archpedi.1995.02170230077011. [DOI] [PubMed] [Google Scholar]
- Aufderheide E, Ekblom P. Tenascin during gut development: appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol. 1988;107:2341–2349. doi: 10.1083/jcb.107.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F, Tata F, Chawengsaksophak K. Homeobox genes and gut development. Bioessays. 2000;22:431–441. doi: 10.1002/(SICI)1521-1878(200005)22:5<431::AID-BIES5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Buchberger A, Pabst O, Brand T, Seidl K, Arnold HH. Chick NKx-2.3 represents a novel family member of vertebrate homologues to the Drosophila homeobox gene tinman: differential expression of cNKx-2.3 and cNKx-2.5 during heart and gut development. Mech Dev. 1996;56:151–163. doi: 10.1016/0925-4773(96)00521-7. [DOI] [PubMed] [Google Scholar]
- Chi X, Chatterjee PK, Wilson W, 3rd, Zhang SX, Demayo FJ, Schwartz RJ. Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules. Proc Natl Acad Sci U S A. 2005;102:13490–13495. doi: 10.1073/pnas.0504295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc I, Freund JN, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPlessis DJ. Some aspects of the pathogenesis and surgical management of peptic ulcers. South Afr Med J. 1960;34:101–108. [PubMed] [Google Scholar]
- DuPlessis DJ. Pathogenesis of gastric ulceration. Lancet. 1965;1:974–978. doi: 10.1016/s0140-6736(65)91214-6. [DOI] [PubMed] [Google Scholar]
- Franklin V, Khoo PL, Bildsoe H, Wong N, Lewis S, Tam PP. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech Dev. 2008;125:587–600. doi: 10.1016/j.mod.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. doi: 10.1016/s0168-9525(99)01957-5. [DOI] [PubMed] [Google Scholar]
- Haffen K, Kedinger M, Simon-Assmann P. Mesenchyme-dependentdifferentiation of epithelial progenitor cells in the gut. J Pediatr Gastroenterol Nutr. 1987;6:14–23. doi: 10.1097/00005176-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Hermans D, Sokal EM, Collard JM, Romagnoli R, Buts JP. Primary duodenogastric reflux in children and adolescents. Eur J Pediatr. 2003;162:598–602. doi: 10.1007/s00431-003-1259-y. [DOI] [PubMed] [Google Scholar]
- Hernanz-Schulman M, Lowe LH, Johnson J, Neblett WW, Polk DB, Perez R, Jr., Scheker LE, Stein SM, Heller RM, Cywes R. In vivo visualization of pyloric mucosal hypertrophy in infants with hypertrophic pyloric stenosis: is there an etiologic role? AJR Am J Roentgenol. 2001;177:843–848. doi: 10.2214/ajr.177.4.1770843. [DOI] [PubMed] [Google Scholar]
- Hogan BLM, Zaret KS. Development of the Endoderm and Its Tissue Derivatives. In: Rossant J, Tam PPL, editors. Mouse Development: Patterning, Morphogenesis, and Organogenesis. San Diego: Academic Press; 2002. pp. 301–330. [Google Scholar]
- Jones CM, Lyons KM, Hogan BL. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–542. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol. 1997;272:G1209–G1220. doi: 10.1152/ajpgi.1997.272.5.G1209. [DOI] [PubMed] [Google Scholar]
- Kedinger M, Simon-Assmann P, Bouziges F, Arnold C, Alexandre E, Haffen K. Smooth muscle actin expression during rat gut development and induction in fetal skin fibroblastic cells associated with intestinal embryonic epithelium. Differentiation. 1990;43:87–97. doi: 10.1111/j.1432-0436.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kutejova E, Engist B, Self M, Oliver G, Kirilenko P, Bobola N. Six2 functions redundantly immediately downstream of Hoxa2. Development. 2008;135:1463–1470. doi: 10.1242/dev.017624. [DOI] [PubMed] [Google Scholar]
- Lawson HH. Effect of Duodenal Contents on the Gastric Mucosa under Experimental Conditions. Lancet. 1964;1:469–472. doi: 10.1016/s0140-6736(64)90800-1. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev Biol. 1986;115:325–339. doi: 10.1016/0012-1606(86)90253-8. [DOI] [PubMed] [Google Scholar]
- Lee ER. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am J Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in vertebrate embryogenesis. Bioessays. 1997;19:287–296. doi: 10.1002/bies.950190406. [DOI] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–161. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Lundegardh G, Adami HO, Helmick C, Zack M, Meirik O. Stomach cancer after partial gastrectomy for benign ulcer disease. N Engl J Med. 1988;319:195–200. doi: 10.1056/NEJM198807283190402. [DOI] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995a;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995b;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Marigo V, Scott MP, Johnson RL, Goodrich LV, Tabin CJ. Conservation in hedgehog signaling: induction of a chicken patched homolog by Sonic hedgehog in the developing limb. Development. 1996;122:1225–1233. doi: 10.1242/dev.122.4.1225. [DOI] [PubMed] [Google Scholar]
- McHugh KM. Molecular analysis of smooth muscle development in the mouse. Dev Dyn. 1995;204:278–290. doi: 10.1002/aja.1002040306. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. Going in circles: conserved mechanisms control radial patterning in the urinary and digestive tracts. J Clin Invest. 2006;116:635–637. doi: 10.1172/JCI27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Hasegawa H, Fujimura T, Matsumoto H, Miyata R, Kosaka T, Miyazaki I, Hattori T. Duodenal reflux through the pylorus induces gastric adenocarcinoma in the rat. Carcinogenesis. 1992;13:2313–2316. doi: 10.1093/carcin/13.12.2313. [DOI] [PubMed] [Google Scholar]
- Moniot B, Biau S, Faure S, Nielsen CM, Berta P, Roberts DJ, de Santa Barbara P. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. 2004;131:3795–3804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T, Saitoh K, Kameda T, Kuroiwa A, Mizutani M, Koike C, Iba H, Yasugi S. BMPs are necessary for stomach gland formation in the chicken embryo: a study using virally induced BMP-2 and Noggin expression. Development. 2000;127:981–988. doi: 10.1242/dev.127.5.981. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Pitera JE, Smith VV, Thorogood P, Milla PJ. Coordinated expression of 3' hox genes during murine embryonal gut development: an enteric Hox code. Gastroenterology. 1999;117:1339–1351. doi: 10.1016/s0016-5085(99)70284-2. [DOI] [PubMed] [Google Scholar]
- Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–120. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Rollins MD, Shields MD, Quinn RJ, Wooldridge MA. Pyloric stenosis: congenital or acquired? Arch Dis Child. 1989;64:138–139. doi: 10.1136/adc.64.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, Norris DP, Robertson EJ, Evans RM, Rosenfeld MG, Izpisua Belmonte JC. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Schrager J, Oates MD. Relation of human gastrointestinal mucus to disease states. Br Med Bull. 1978;34:79–82. doi: 10.1093/oxfordjournals.bmb.a071463. [DOI] [PubMed] [Google Scholar]
- Sekimoto T, Yoshinobu K, Yoshida M, Kuratani S, Fujimoto S, Araki M, Tajima N, Araki K, Yamamura K. Region-specific expression of murine Hox genes implies the Hox code-mediated patterning of the digestive tract. Genes Cells. 1998;3:51–64. doi: 10.1046/j.1365-2443.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Grasty RC, Theodosiou NA, Tabin CJ, Nascone-Yoder NM. Evolutionary relationships between the amphibian, avian, and mammalian stomachs. Evol Dev. 2000a;2:5214–5228. doi: 10.1046/j.1525-142x.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development. 2000b;127:3671–3681. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- Smith DM, Tabin CJ. BMP signalling specifies the pyloric sphincter. Nature. 1999;402:748–749. doi: 10.1038/45439. [DOI] [PubMed] [Google Scholar]
- Stern CD. Immunocytochemistry of embryonic material. In: Stern CD, Holland PWH, editors. Essential Developmental Biology, A Practical Approach. New York: Oxford University Press; 1993. pp. 193–212. [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Imanaka T, Takano T. Spatial pattern of smooth muscle differentiation is specified by the epithelium in the stomach of mouse embryo. Dev Dyn. 1998;212:448–460. doi: 10.1002/(SICI)1097-0177(199807)212:3<448::AID-AJA12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Tabin CJ. Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Dev Biol. 2005;279:481–490. doi: 10.1016/j.ydbio.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192–1199. doi: 10.1053/gast.1996.v111.pm8898632. [DOI] [PubMed] [Google Scholar]
- Vaezi MF, Singh S, Richter JE. Role of acid and duodenogastric reflux in esophageal mucosal injury: a review of animal and human studies. Gastroenterology. 1995;108:1897–1907. doi: 10.1016/0016-5085(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. RNA detection using non-radioactive in situ hybridization. Curr Opin Biotechnol. 1995;6:20–23. doi: 10.1016/0958-1669(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol. 2000;81:117–143. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Elevated cyclooxygenase-2 expression in patients with early gastric cancer in the gastric pylorus. J Gastroenterol. 2005;40:690–697. doi: 10.1007/s00535-005-1612-1. [DOI] [PubMed] [Google Scholar]
- Yasugi S. Role of Epithelial-Mesenchymal Interactions in Differentiation of Epithelium of Vertebrate Digestive Organs. Develop. Growth & Differ. 1993;35:1–9. doi: 10.1111/j.1440-169X.1993.00001.x. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sakiyama J, Kuroiwa A. Coordinated expression of Abd-B subfamily genes of the HoxA cluster in the developing digestive tract of chick embryo. Dev Biol. 1995;169:76–89. doi: 10.1006/dbio.1995.1128. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. Hox genes and the making of sphincters. Nature. 1999;401:761–762. doi: 10.1038/44511. [DOI] [PubMed] [Google Scholar]