Abstract
The way in which sex hormones influence cognitive and affective brain development is poorly understood. Despite increasing knowledge in the area of pediatric mood disorders, little is known about the influence of sex hormones on the regulation of emotion. Animal studies and preliminary human studies suggest a strong impact of testosterone on limbic structures such as the hippocampus and amygdala. We used functional magnetic resonance imaging (fMRI) to examine emotional processing in familial male-precocious puberty (FMPP), an extremely rare gonadotropin-independent form of precocious puberty characterized by early excess testosterone secretion. We compared this group (n = 7, mean age = 13 ± 3.3 years) to healthy age and sex-matched controls (n = 14, mean age = 13 ± 2.3 years). Participants were presented with emotional and neutral face stimuli and were required either to judge the hostility of the presented face, their subjective level of anxiety, or the width of the nose of the presented faces (nonemotional condition). In a fourth, passive viewing condition, no responses were required. Boys with FMPP responded faster to fearful faces during perception of threat compared to unaffected controls. Concurrently, fMRI data revealed significant differences in hippocampus activation in response to fearful faces relative to baseline whereas controls showed no differences. In contrast, no significant activation of the amygdala was found. These data are consistent with previous studies of the effects of sex hormones on brain function and support the role of testosterone on emotional development.
Introduction
The impact of sex hormones on brain and behavior are well documented (Rubinow and Schmidt 1996). During development, hormonal effects can manifest as long-term effects that affect the organization of the neural circuitry or as short-term changes occurring only when hormones are present (Sisk and Foster 2004). Cognitive (Mueller et al. 2008) and affective (Su et al. 1993; Ernst et al. 2007) functions are influenced by sex hormone perturbations during development and in adulthood. In particular, given the high prevalence of mood disorders in children and adolescents (Wittchen et al. 1998), a thorough understanding of the effect of sex hormones on emotional brain development is crucial to a more comprehensive appreciation of the neurobiology underlying developmental disorders and sex-appropriate therapeutic interventions. Unfortunately, most work directly examining the link between sex hormones and brain development has been conducted in animals (Edwards 1968; Dohler et al. 1982; Romeo 2003), while studies in humans have been restricted to the elderly population (Stevens et al. 2005; Maki et al. 2007).
With the advent of functional magnetic resonance imaging (fMRI) and the use of natural models of steroid dysfunction early in life, it may be possible to begin to examine the neurobiological development of emotion. Familial male precocious puberty (FMPP), or testotoxicosis, may represent a prototypical example of such a model because it involves isolated dysfunction of androgen secretion. In FMPP, an activating mutation of the luteinizing hormone (LH) receptor gene results in continuous unregulated secretion of testosterone from the testicular Leydig cells (Shenker et al. 1993). Boys with FMPP typically present as toddlers with signs and symptoms of hyperandrogenism, including growth acceleration, skeletal advancement, pubic hair, acne, and phallic enlargement (Leschek 2004). However, because FMPP is an extremely rare genetic disorder (1–9/1,000,000, Orphanet), studies of FMPP are difficult to implement (Office of Rare Diseases, National Institutes of Health), and, in particular, the cognitive profile of children with this disorder has not been described.
Previous studies in other endocrinological disorders of steroid hormones support the involvement of testosterone in cognitive/affective function. For instance, females with severe forms of congenital adrenal hyperplasia (CAH), a disorder involving adrenal insufficiency and testosterone excess, exhibit increased spatial memory (Mueller et al. 2008). Other studies have reported changes in the ability to aim objects accurately at specific points in space (targeting ability) (Hines et al. 2003), verbal expression (Resnick et al. 1986), and predisposition to reward dependence (Charmandari et al. 2004). Additionally, due to excess androgens, children with precocious puberty commonly exhibit increased levels of aggression and other behavioral problems (Sonis et al. 1986; Laue et al. 1989; Mazur and Clopper 1991; Weissenberger et al. 2001; Pasterski et al. 2007). Testosterone has also been associated with the regulation of affect. In elderly adults, reduced testosterone levels have been proposed as one of the major mechanisms associated with the dysphoria that is often observed during the late stages of life (Booth et al. 1999). In addition, testosterone is invoked in the regulation of fear and anxiety. The administration of small doses of testosterone to healthy female volunteers has been found to reduce automatic responses to fear in a masked emotional Stroop task without affecting self-reports of anxiety severity (van Honk et al. 2005).
These behavioral findings point to loci of action in the brain that mediate cognitive and affective processes affected by testosterone levels. With regard to affective processes, two lines of evidence support the idea that sex hormones alter the neural substrates of the circuitry involved in emotion regulation. First, sex steroid receptors are abundant in limbic regions, particularly the amygdala and the hippocampus (Simerly et al. 1990; Abdelgadir et al. 1999; Beyenburg et al. 2000). Both regions are well recognized for their role in affective coding (Papez 1937; Gray and McNaughton 2000; LeDoux2000; Phelps 2004; Reinders et al. 2006). Second, recent functional neuroimaging studies have demonstrated that increasing testosterone levels in older adults alter neural activation in the ventromedial temporal cortex (Maki et al. 2007). Although no such studies have been conducted in healthy children, early alteration of sex steroids, including androgens, has been studied in CAH adolescents who are characterized by prenatal deficits in cortisol and excessive androgen formation resulting from lack of negative feedback in the hypothalamic–pituitary–adrenal (HPA) axis (Merke and Bornstein 2005). In these studies, the amygdala was decreased in volume (Merke et al. 2003) and more active in response to negative facial expressions (Ernst et al. 2007) in adolescents with CAH compared to healthy adolescents. Although the pattern of disruption in the amygdala was suggestive of a sex effect, separation of corticosteroid from sex steroid effects was not possible.
To assess the early influence of androgens on brain development specifically, particularly on circuits mediating emotion processing, we compared adolescent males with congenital testosterone elevation due to FMPP to healthy age-matched adolescent males. The purpose of the present study was to examine potential lasting effects of early androgen excess on emotion processing. We hypothesized that excessive testosterone levels would be associated with abnormal responses of amygdala and hippocampus to emotional stimuli. To this aim, we used fMRI paired with a well-validated task that has previously been used successfully in pediatric psychiatric populations to probe these regions (e.g., Nelson et al. 2003; Rich et al. 2006; McClure et al. 2007).
Methods
Subjects
Seven boys with FMPP (mean age = 13.21 ± 3.3) and 13 healthy males (13.72 ± 2.4) completed the study. Age did not differ between groups. With respect to intelligence quotient (IQ), controls had on average a significantly higher IQ than children with FMPP (t(18) = 2.89, p = 0.01) (Table 1). However, this difference reflected a higher-than-average IQ in controls and an IQ within the normal range in children with FMPP. FMPP patients were initially recruited as part of an ongoing study of the Eunice Rennedy Shriver National Institute of Child Health and Human Development (NICHD)/National Institutes of Health (NIH) and subsequently consented to participate in the current National Institute of Mental Health (NIMH) study. Control subjects were recruited by advertisement in local newspapers. The institutional review boards of NIMH and NICHD approved the study. Parents and adolescents signed consent and assent forms after the study was explained in detail. All subjects completed physical, neurological, and psychiatric assessments. The psychiatric examination used a standardized, structured psychiatric interview, the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL) (Kaufman et al. 1997). One child was found to suffer from current co-morbid attention-deficit/hyperactivity disorder (ADHD) and another child had a previous, but not current, history of anxiety disorder. The full-scale IQ scores were prorated based on the Vocabulary and Block Design subtests of the Wechsler Intelligence Scales for Children (Wechsler 1999).
Table 1.
Sex, Age, IQ and Tanner Stage Characteristics for the FMPP and Control Participants Individually and as Group Means
| Subject | Gender | Age (years) | IQ | Tanner stage | Testosterone (ng/dL) | Bone age (years) |
|---|---|---|---|---|---|---|
| FMPP | ||||||
| 1 | Male | 9.86 | 82 | 1 | 174 | 14 |
| 2 | Male | 10.76 | 119 | 3 | 281 | 13 |
| 3 | Male | 15.44 | 83 | 5 | 575 | 15.4 |
| 4 | Male | 14.83 | 112 | N/A | 311 | 16.5 |
| 5 | Male | 11.61 | 93 | 4 | 428 | 12.5 |
| 6 | Male | 18.80 | N/A | 5 | 351 | 19 |
| 7 | Male | 11.01 | 98 | 3 | 387 | 10.3 |
| Mean, SD | 13.19 (3.25) | 97.83 (15.11) | 3.25 (1.52) | 358.14 (125.75) | 14.39 (2.64) | |
| Control | ||||||
| 1 | Male | 14.69 | 109 | 5 | ||
| 2 | Male | 12.28 | 127 | 2 | ||
| 3 | Male | 17.44 | 118 | 5 | ||
| 4 | Male | 9.76 | 130 | N/A | ||
| 5 | Male | 15.24 | 95 | 4 | ||
| 6 | Male | 10.68 | 122 | N/A | ||
| 7 | Male | 12.68 | 146 | 5 | ||
| 8 | Male | 10.19 | 124 | 2 | ||
| 9 | Male | 14.85 | 104 | 4 | ||
| 10 | Male | 15.38 | 109 | N/A | ||
| 11 | Male | 14.23 | 134 | 3 | ||
| 12 | Male | 15.54 | 124 | N/A | ||
| 13 | Male | 15.46 | 112 | 4 | ||
| Mean, SD | 13.72 (2.38) | 119.54 (13.68) | 3.8 (1.20) | — | — |
In addition, testosterone level and bone age are also provided for the FMPP group.
Abbreviations: FMPP = familial male-precocious puberty; IQ = intelligence quotient; SD = standard deviation; N/A = not available.
Materials
Task stimuli included faces of 56 actors. These face stimuli were derived from three standardized sets of gray-scale photographs depicting different facial expressions from Ekman and Friesen (Ekman and Friesen 1976), Gur (www.uphs.upenn.edu/bbl/pubs/downloads/nptasks.shtml), and Tottenham and Nelson (www.macbrain.org/faces/idex.htm). Each participant viewed 32 different actors. Each actor was randomly assigned to display one of four facial expressions (happy, angry, fearful, and neutral). For example, a given actor might be randomly selected to portray “anger” for one participant; this same actor might be randomly chosen to portray “fear” for another subject and “happiness” for yet another participant. This design allowed us to control for variability in nonemotional features of the actors (e.g., ethnicity, hair color). Half of the actors were female and half male. A total of 32 “null-event” fixation crosses were included to facilitate data analysis.
Participants saw each actor four times across the paradigm and each time in a different task condition. Each task condition probed a distinct attention condition. In the “threat” attention condition, participants were asked to rate the hostility of the presented face; during the “fear” attention condition, participants were asked how afraid they were of the presented face; during the “nonemotional judgment” condition, participants were asked to rate the width of the nose of the presented face; and in the passive attention condition, they were asked to simply look at the pictures without having to make a rating. Responses were on a 1- to 5-point scale, and were collected on a five-key button box developed by MRI Devices (Waukesha, WI). Each attention condition was presented in blocks of 10 randomly ordered stimuli (eight faces and two fixation crosses), and each block was presented four times. Order of presentations of condition and facial expression was randomized across participants.
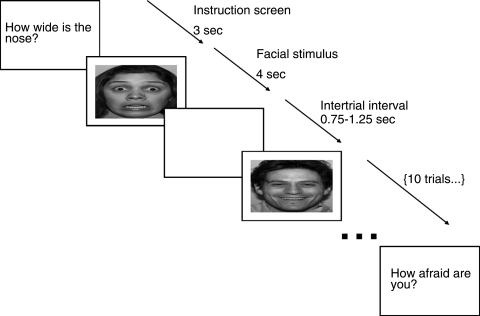
The task used a rapid event-related design presented as a 160-trial single run (four conditions × four block-repeats ×10 stimuli per block) of 14.2 minutes duration. Each of the four task conditions began with a 3,000-msec instruction screen. Following the instruction screen, the 10 randomly ordered stimulus event trials (eight faces, two fixation crosses) each appeared for 4,000 msec. The interstimulus interval was displayed as a blank screen that varied from 750 to 1,250 msec (averaging 1,000 msec within a 10-trial block) (Fig. 1).
FIG. 1.
Presentation of individual stimuli and blocks during the experiment.
Prior to scanning, participants were trained in an MRI simulator to become familiar with the environment and response device. Participants were trained in a practice block prior to scanning and were shown neutral expressions not presented during the MRI version of the task. To have adequate baseline comparisons, we chose to analyze contrasts relative to happy faces rather than neutral faces, because happy stimuli are considered less ambiguous in youth and have been successfully used in previous fMRI studies (Surguladze et al. 2005; McClure et al. 2007).
Behavioral data analysis
Face ratings and response times were each analyzed with a separate repeated-measures analysis of variance (ANOVA). Each ANOVA used Emotion (angry, fearful, happy, and neutral) and Attention (hostility rating vs. fear rating vs. nose width rating) as the two within-subject factors, Group (FMPP vs. control) as the between-subject factor resulting in a 4 × 3 × 2 ANOVA. Significant three-way interactions were followed up with a 2 (Group) × 4 (Emotion) ANOVA for each attention condition separately. Significant two-way interactions involving the Group factor were decomposed using independent t-test comparisons (two-tailed) and a measure of effect size was calculated for every significant effect (Cohen 1992).
FMRI data acquisition
Whole brain oxygen level-dependent (BOLD) fMRI data were acquired using a 3 Tesla General Electric Signa Scanner (Waukesha, Wisconsin). Head movement was restricted using foam padding. Subjects viewed stimuli through goggles (Avotec Silent Vision Glasses Stuart, FL). Following sagittal localization and manual shimming, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix size of 64 × 64 mm, repetition time (TR) of 2,000 msec, echo time (TE) of 40 msec, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 5 mm providing whole brain coverage. Images were acquired in 23 contiguous 5-mm axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC–PC) line. All functional data were gathered in a single 14.2-min run for each subject.
After echo-planar imaging (EPI) acquisition, a high-resolution T1-weighted anatomical image was acquired to aid with spatial normalization. A standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV = 256, number of excitations (NEX) = 1, TR = 11.4 msec, TE = 4.4 msec, matrix = 256 × 256, time to inversion (TI) = 300, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels) msec was used to facilitate spatial normalization.
fMRI processing
All subsequent analyses were conducted with SPM software (SPM99, Wellcome Department of Imaging Neuroscience, University College of London, London, UK) and Matlab 6.1 (The Mathworks Inc., Natick, MA) routines. Functional data were corrected for slice timing, motion corrected, co-registered to the anatomical data, and spatially normalized to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99. After preprocessing, fMRI images were inspected visually to evaluate the quality of the normalization procedure. Head movement of subjects was analyzed with MedX software (Medical Numerics, Sterling, VA), and subjects who moved more than 4.0 mm in any direction were removed from further analysis.
Individual subject-level, event-related response amplitudes were estimated using a general linear model (GLM) for each event type. Event types were defined based on each face type crossed by each viewing condition. Specific contrasts of interest were selected after conducting an initial analysis of behavioral performance, which provided the set of contrasts most sensitive to group status (see below). This approach was dictated by the limitation of the sample size. Indeed, an omnibus analysis of all trial types, as employed in McClure et al. (2007), that tests for three-way (Group-by-face-Emotion-by-Attention-state) interactions, would minimize the potential for type I errors. However, the extreme rarity of FMPP resulted in a small sample size. With this sample size, effect sizes large enough to produce three-way interactions would be physiologically implausible. Thus, we used the analysis of behavioral data to guide the analysis of imaging data. This strategy provided a better balance of type I and type II errors than an omnibus statistical approach.
The behavioral analysis revealed that the boys with FMPP responded faster to fearful faces during threat rating than controls. Therefore, the FMPP group was associated with a specific perturbation in the affective response to fearful faces. Accordingly, the contrast chosen for the fMRI analysis was the comparison of fearful faces versus happy faces during the threat rating condition (i.e., “How hostile is the face?”). Fixation trials served as an implicit baseline. The waveform used to model event-related responses was a rectangular pulse (4-second duration) convolved with the hemodynamic response function specified in SPM99. Contrast images were created for each subject using pairwise comparisons of the different event-related BOLD response amplitudes. Before performing group-level analyses, each contrast image was divided by the subject-specific voxel time series mean, generating values proportional to percentage fMRI signal change (Zarahn et al. 1997). These normalized contrast images were then smoothed with an isotropic Gaussian kernel (full-width half-maximum = 11.4 mm) to reduce nonstationarity in the spatial autocorrelation structure produced by the previous step (Friston et al. 2000).
For all group-level analyses, the contrast images produced for each participant were fit to a second-level random effects model. On the basis of our a priori hypotheses, the primary statistical analyses were regions of interest (ROI) based. A Gaussian random field threshold was used to determine significance of statistical comparisons. Activation had to survive the small-volume correction (SVC) Gaussian random field threshold (α = 0.05) within prespecified ROIs. The primary analyses focused on three bilateral ROIs: The amygdala, the anterior hippocampus, and the posterior hippocampus. ROIs were defined using standard, previously validated, anatomical criteria. They were hand-traced on the single MNI template to which fMRI data were normalized, and then applied to all normalized brains at the group level (Szeszko et al. 1999). MNI x, y, and z coordinates are reported for significant results.
Significant findings were further assessed using SPSS 13.0. Percent BOLD signal changes relative to baseline during threat rating were extracted for each component of the main contrast, i.e., fearful face versus baseline, and happy face versus baseline, and submitted to two-way ANOVAs with Group (FMPP, control) as the between-subjects factor and Emotion (fearful face, happy face) as the within subjects factor.
Results
Performance scores
Reaction times
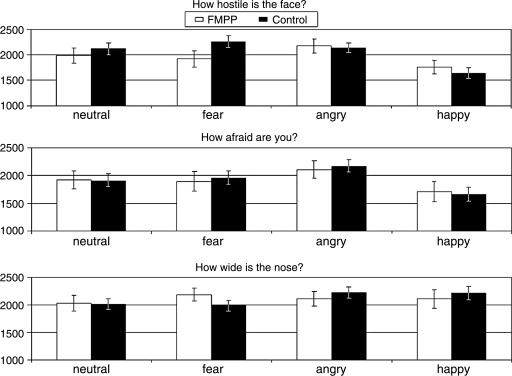
The ANOVA showed a significant three-way interaction among Group, Attention, and Emotion (F(6,108) = 2.56, p = 0.02) This interaction reflected that, during the threat-rating condition, response times to fearful faces were significantly shorter for the FMPP group than for the control group (t(16.03) = −2.07, p = 0.05, Cohen d = 0.85). By contrast, reaction times did not differ between groups when rating the other face emotions in either the threat attention condition, the fear attention condition, or the nonemotional judgement condition (Fig. 2). As expected, main effects of Emotion (F(3,54) = 9.81, p < 0.001) and Attention (F(2,36) = 4.14, p = 0.02) were also present. Moreover, Emotion interacted with Attention (F(6,108) = 5.72, p < 0.001).
FIG. 2.
Behavioral performance: Reaction times (in msec) in each attention condition for the FMPP group (white bars) and the control group (black bars). Error bars denote standard error of the mean.
Face rating scores
The three-way ANOVA failed to show any evidence of Group effects, alone or in interaction with Emotion and/or Attention. A main effect of Emotion indicated that angry faces received the highest rating scores (F(3,54) = 40.64, p < 0.001). A main effect of Attention reflected higher scores to the rating of nose width than to the other two rating conditions (F(2,38) = 38.36, p < 0.001). As with reaction times, Emotion and Attention interacted significantly (F(6,108) = 27.39, p < 0.001).
fMRI BOLD signal changes
Table 2 presents the results from the SPM ROI voxel-wise analyses. These SPM analyses compared bilateral activations of amygdala, anterior hippocampus and posterior hippocampus to the (fearful face vs. happy face) contrast during the threat condition between the FMPP group and the control group. Both anterior and posterior hippocampi showed significant clusters of activation. No significant activation was detected in the amygdala.
Table 2.
Group Differences (FMPP vs. Controls) in Peak Activations of Regions of Interest in Response to [Ffearful Faces vs. Happy Faces] in the “Threat” Attention Condition.
| cluster P (cor) | cluster equivk | cluster P (uncor) | voxel P (cor) | voxel T | voxel equivZ | voxel P (uncor) | x,y,z (mm) | |
|---|---|---|---|---|---|---|---|---|
| R anterior hippocampus | 0.006 | 129 | 0.018 | 0.06 | 2.78 | 2.51 | 0.006 | 38 −18 −18 |
| R posterior hippocampus | 0.121 | 6 | 0.237 | 0.13 | 2.52 | 2.30 | 0.011 | 24 −20 −18 |
| L anterior hippocampus | 0.012 | 96 | 0.040 | 0.08 | 2.63 | 2.39 | 0.008 | −26 −18 −14 |
| L posterior hippocampus | 0.030 | 22 | 0.072 | 0.13 | 2.50 | 2.29 | 0.011 | −26 −20 −16 |
Cor = corrected; uncor = uncorrected; FMPP = familial male precocious puberty.
Values for each component of the main contrast, i.e., fearful versus baseline and happy versus baseline, were extracted and submitted to two-way ANOVAs with Group as the between-subjects factor and Emotion as the within-subjects factor.
Hippocampus
Similar to the SPM analysis, the two-way ANOVAs of Group (FMPP, control) by Emotion (fearful, happy) conducted on extracted BOLD signal changes revealed significant Group by Emotion interactions for the right anterior hippocampus (38 mm, −8 mm, − 18 mm), F(1,18) = 7.16, p < 0.02, the right posterior hippocampus (24 mm, − 20 mm, −18 mm), F(1,18) = 6.61, p < 0.02, left anterior hippocampus (−26 mm, −18 mm, −14 mm) (F(1,18) = 7.46, p = 0.01) and left posterior hippocampus (−26 mm, − 20 mm, − 16 mm) (F(1,18) = 7.04, p < 0.02).
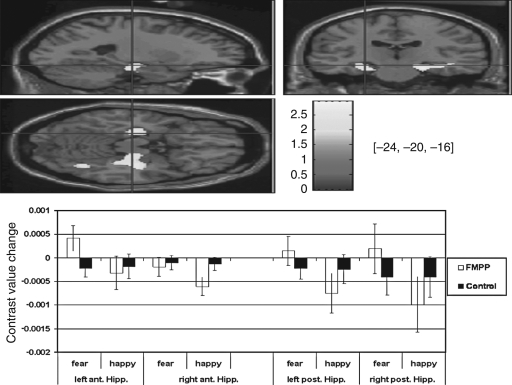
These interactions reflected that, while activations for the control group did not differ between fearful and happy faces, the FMPP group showed significantly stronger activations for fearful relative to happy faces in the “threat” condition (Fig. 3).
FIG. 3.
Functional imaging data showing bilateral hippocampal activation in the FMPP group in the fearful versus happy faces contrast during the threat attention condition. (Upper panel) A high-resolution anatomical overlay T1 image in MNI space, provided by SPM, was used to render the hippocampal activation. The figure displays sagittal, (upper left), coronal (upper right), and transaxial (middle left panel) slices. Cross-hairs are positioned at the MNI coordinates x = − 24, y = − 20, z = − 16. The threshold was set at p = 0.05. (Lower panel) BOLD signal changes from baseline are shown for the peak hippocampal activations for each of the conditions and by group (FMPP, white bars; control, black bars). Error bars denote standard error of the mean.
Whole brain analysis
For completeness, an additional whole brain analysis for regions not hypothesized was carried out at p < 0.001 and did not reveal any significant effects.
Correlations with IQ
Whereas the FMPP group exhibited IQs within the normal range, a significantly higher-than-average IQ was found in healthy controls (FMPP, 97.8, vs. control, 119.5). To examine whether general intelligence level should be controlled for in subsequent analyses, IQs were correlated with individual peak hippocampal activations (Table 2). There were no significant effects of IQ on hippocampal activations (all r2(19) < 0.32, p > 0.18).
Discussion
The aim of the present study was to examine the effects of androgens on the development of the neural circuitry underlying emotion regulation. To this aim, we used an endocrinological disorder as a natural model of early disruption of androgen activity. This approach represents an extension to previous work with children suffering of CAH, a genetic disorder of steroid dysfunction involving both testosterone and cortisol abnormalities (Merke et al. 2003; Ernst et al. 2007; Mueller et al. 2008). Here, we studied a sample of boys with FMPP, an extremely rare genetic disorder of early and sustained isolated testosterone excess (Shenker et al. 1993; Leschek 2004). Patients and controls were compared using fMRI during performance on a well-validated emotional task (e.g., Nelson et al. 2003; Rich et al. 2006; McClure et al. 2007). On the basis of behavioral and neurophysiological evidence of the role of testosterone in the modulation of emotional processes, we predicted perturbed amygdala and hippocampal function. As expected, we found greater hippocampal activation in the FMPP group compared to the control group. This finding suggested that early androgen excess was associated with later (adolescent) deficits in hippocampal function in response to an emotional processing task. Behaviorally, boys with FMPP responded faster to fearful stimuli when evaluating threat compared to their unaffected peers. In contrast to predictions, amygdala activation did not differ between groups.
Behaviorally, group differences in performance were restricted to the attention condition of threat evaluation and the fearful emotion. Relative to unaffected healthy boys, adolescents with FMPP showed a bias toward fearful faces by responding faster to these stimuli when rating threat. In contrast, no group differences were found in ratings of “how afraid” subjects felt, or “how large is the nose.” These last two attention conditions required that concentration be directed toward internal affective states, and a physical, nonaffective feature of the presented stimuli. In contrast, the threat condition, “how hostile is the face?” required subjects to focus on the external affective context of threat. This type of condition, which was sensitive to FMPP pathology, points to a locus of dysfunction that affects the processing of negative affective information conveyed by external stimuli. This finding is consistent with recent work showing that pharmacological manipulation of testosterone levels in healthy adults speeded response times to fearful faces (van Honk et al. 2005).
On the basis of these behavioral findings, the neuroimaging analysis focused on the contrast (fearful faces − happy faces) during the “how hostile” attention condition. The happy expression was used as a control emotion to avoid the well-documented ambiguity factor associated with neutral expressions (Gur et al. 1992). In this contrast, the hippocampus was significantly more activated in the FMPP group compared to the control group. This result reflected greater hippocampal activation in response to fearful faces compared to happy faces in the FMPP group. This group difference predominantly affected the anterior hippocampus (see Table 2, clusters of activations involved), a region tightly linked to the amygdala (Pitkanen et al. 2000).
In humans (Beyenburg et al. 2000) and nonhuman primates (Abdelgadir et al. 1999), both the amygdala and particularly the hippocampus contain relatively high concentrations of androgen receptors, making these structures critical targets to androgen action. Furthermore, the anterior hippocampus has strong reciprocal projections to the HPA axis (Swanson and Cowan 1977; Poletti and Sujatanond 1980; Jacobsen and Sapolsky 1991). These hippocampal connections to both amygdala and HPA axis underline the hippocampal role in emotion regulation.
In line with our findings involving fearful emotion, the anterior hippocampus has been found to be implicated specifically in anxiety-related processes. Selective hippocampal lesions in rodents have been shown to produce behavioral deficits that can be likened to anxiolytic effects in the context of social interaction (Bannerman et al. 2002). Another link between hippocampal function and anxiety has recently emerged in a study of anxious adolescents. This study examined spatial navigation, a cognitive process highly dependent on the functional integrity of the hippocampus. Findings suggested deficits in spatial navigation in the anxious group compared to a control group, pointing to an association between hippocampal dysfunction and anxiety disorders (Muller et al., submitted).
In the present study, 1 boy with FMPP had a previous history of an affective disorder while another had a current diagnosis of ADHD. Testosterone has been associated with both depressive symptoms (Booth et al. 1999) and with aggressive tendencies (Pasterski et al. 2007), both of which reflect emotional disturbances. The present findings may shed some light on the mechanisms underlying the association between increased levels of aggression and elevated levels of testosterone. Increased aggression has been reported in patients with FMPP (Laue et al. 1989; Weissenberger et al. 2001). The amygdalo-hippocampal system has been shown to play a role in aggressive behavior (Halasz et al. 2008; Nakamura et al. 2008). For example, Sterzer (2005) found perturbed amygdala activation to negatively valenced pictures in adolescents with conduct disorder. Additionally, testosterone has also been implicated in pervasive developmental disorders, including autism/Asperger syndrome (Baron-Cohen 2002) and Tourette syndrome (Peterson et al. 1992). Interestingly, this spectrum of disorders affects males significantly more frequently than females and is characterized by severe social deficits and mood disorders.
Taken together, these findings suggest that early (perhaps even prenatal) testosterone excess affects the development of limbic function, particularly with respect to its role in negative emotions. The extent to which ongoing androgen imbalance, rather than prenatal exposure, affects these outcomes is not known. Despite the fact that all of the FMPP patients were treated since toddlerhood with adequate pubertal suppression starting very early in life (generally by age 2–3 years), androgen effects were blocked at the receptor level rather than at the synthetic level, so normal physiology was not truly restored in these boys.
A secondary goal of the study was to clarify our previous findings of emotional and spatial processing differences in CAH. Similar to adolescents with FMPP, adolescents with CAH were found in prior work to have reduced sensitivity to negatively valenced stimuli (Ernst et al. 2007). Moreover, adolescents with CAH were also found to perform differently from healthy adolescents on a spatial cognitive task, consistent with hippocampal dysfunction (Mueller et al. 2008). Both findings were mainly present in the adolescent females with CAH, suggesting a prominent role of androgen dysfunction rather than corticosteroid dysfunction because the impact of testosterone excess is more significant in females with CAH compared to males with CAH. These prominent sex differences would not be expected in the case of corticosteroid abnormalities. The present findings support the notion that sex steroids are a major contributor to the previous CAH results.
A number of limitations need to be mentioned. Most importantly, the small sample size of the FMPP group seriously limits the statistical power of this study. However, we were able to evidence abnormalities, both behaviorally and on neural measures, suggesting that these findings are robust. Moreover, prior work on extremely rare conditions suggests the importance of using so-called “experiments of nature” to generate novel insights in areas where research using other methods are not ethically justified. For example, considerable prior work implicating the hippocampus in memory emerged from extensive research on a single patient, H.M., with a hippocampal lesion (Scoville and Milner 1957). The current example of FMPP provides a similarly unique opportunity to examine a rare, genetic, specific perturbation in steroid hormone metabolism arising early in development.
Second, because of the low statistical power, any negative findings must be viewed with caution. The fact that no other regions than the medial-temporal lobe differed between groups in the whole brain analysis does not rule out the possibility of the involvement of other neural networks in FMPP. Third, two FMPP adolescents had a history of a psychiatric disorder, one current and one past. Given the small sample size, it was impossible to examine the contribution of these diagnoses to our findings. Fourth, as was mentioned earlier, this study cannot discriminate between the prenatal effects of testosterone and the ongoing impact during postnatal development. Similarly, it cannot differentiate between permanent organizational and temporary activational effects. Fifth, a potential influence of general intelligence cannot be ruled out given that controls evidenced a higher-than-average IQ. However, no significant correlations of hippocampal peak activations with IQ were found.
In summary, the current study presents some preliminary evidence for the role of early testosterone imbalance on hippocampal function in the context of negative emotions. A fuller understanding of the clinical implications of this finding requires additional research. However, a priori hypotheses involving the relationship between sex steroid abnormalities and other hippocampal functions, such as spatial navigation or implicit memory, should be tested.
Disclosures
Dr. Merke received research funds from Phoqus Pharmaceuticals in 2007–2008. Drs. Mueller, Leschek, Pine, and Ernst and Ms. Mandell have no financial ties or conflicts of interest to disclose.
Footnotes
The research was supported by the Intramural Program of the National Institute of Mental Health and Eunice Rennedy Shriver National Institute of Child Health and Human Development.
Acknowledgments
D.P.M. is a Commissioned Officer in the U.S. Public Health Service.
References
- Abdelgadir SE. Roselli CE. Choate JV. Resko JA. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: Studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biol Reprod. 1999;60:1251–1256. doi: 10.1095/biolreprod60.5.1251. [DOI] [PubMed] [Google Scholar]
- Bannerman DM. Deacon RM. Offen S. Friswell J. Grubb M. Rawlins JN. Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Beyenburg S. Watzka M. Clusmann H. Blumcke I. Bidlingmaier F. Elger CE. Stoffel-Wagner B. Androgen receptor mRNA expression in the human hippocampus. Neurosci Lett. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- Booth A. Johnson DR. Granger DA. Testosterone and men's depression: The role of social behavior. J Health Soc Behav. 1999;40:130–140. [PubMed] [Google Scholar]
- Charmandari E. Merke DP. Negro PJ. Keil MF. Martinez PE. Haim A. Gold PW. Chrousos GP. Endocrinologic and psychologic evaluation of 21-hydroxylase deficiency carriers and matched normal subjects: Evidence for physical and/or psychologic vulnerability to stress. J Clin Endocrinol Metab. 2004;89:2228–2236. doi: 10.1210/jc.2003-031322. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psycholog Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dohler KD. Coquelin A. Davis F. Hines M. Shryne JE. Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- Edwards DE. Mice: Fighting by neonatally androgenized females. Science. 1968;161:1027–1028. doi: 10.1126/science.161.3845.1027. [DOI] [PubMed] [Google Scholar]
- Ekman P. Friesen W. Pictures of facial affect. Palo Alto (California): Consulting Psychologists Press; 1976. [Google Scholar]
- Ernst M. Maheu FS. Schroth E. Hardin J. Golan LG. Cameron J. Allen R. Holzer S. Nelson E. Pine DS. Merke DP. Amygdala function in adolescents with congenital adrenal hyperplasia: A model for the study of early steroid abnormalities. Neuropsychologia. 2007;45:2104–2113. doi: 10.1016/j.neuropsychologia.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Josephs O. Zarahn E. Holmes AP. Rouquette S. Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Gray J. McNaughton N. The Neuropsychology of Anxiety. Oxford: Oxford University Press; 2000. [Google Scholar]
- Gur RC. Erwin RJ. Gur RE. Zwil AS. Heimberg C. Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Halasz J. Toth M. Mikics E. Hrabovszky E. Barsy B. Barsvari B. Haller J. The effect of neurokinin1 receptor blockade on territorial aggression and in a model of violent aggression. Biol Psychiatry. 2008;63:271–278. doi: 10.1016/j.biopsych.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Hines M. Fane BA. Pasterski VL. Mathews GA. Conway GS. Brook C. Spatial abilities following prenatal androgen abnormality: Targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Jacobsen L. Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic- pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Laue L. Kenigsberg D. Pescovitz OH. Hench KD. Barnes KM. Loriaux DL. Cutler GB., Jr Treatment of familial male precocious puberty with spironolactone and testolactone. N Engl J Med. 1989;320:496–502. doi: 10.1056/NEJM198902233200805. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leschek EW. Familial male-limited precocious puberty. The Endocrinologist. 2004;14:148–151. [Google Scholar]
- Maki PM. Ernst M. London ED. Mordecai KL. Perschler P. Durso SC. Brandt J. Dobs A. Resnick SM. Intramuscular testosterone treatment in elderly men: Evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- Mazur T. Clopper RR. Pubertal disorders. Psychology and clinical management. Endocrinol Metab Clin North Am. 1991;20:211–230. [PubMed] [Google Scholar]
- McClure EB. Monk CS. Nelson EE. Parrish JM. Adler A. Blair RJ. Fromm S. Charney DS. Leibenluft E. Ernst M. Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Merke DP. Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- Merke DP. Fields JD. Keil MF. Vaituzis AC. Chrousos GP. Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: Potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Mueller SC. Temple V. Oh E. VanRyzin C. Williams A. Cornwell B. Grillon C. Pine DS. Ernst M. Merke D. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. Kikusui T. Takeuchi Y. Mori Y. Changes in social instigation- and food restriction-induced aggressive behaviors and hippocampal 5HT1B mRNA receptor expression in male mice from early weaning. Behav Brain Res. 2008;187:442–448. doi: 10.1016/j.bbr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nelson EE. McClure EB. Monk CS. Zarahn E. Leibenluft E. Pine DS. Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: An event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neurol Pathol. 1937;38:725–743. [Google Scholar]
- Pasterski V. Hindmarsh P. Geffner M. Brook C. Brain C. Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH) Horm Behav. 2007;52:368–374. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS. Leckman JF. Scahill L. Naftolin F. Keefe D. Charest NJ. Cohen DJ. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette's syndrome. Psychoneuroendocrinology. 1992;17:553–563. doi: 10.1016/0306-4530(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Pikkarainen M. Nurminen N. Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann NY Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poletti CE. Sujatanond M. Evidence for a second hippocampal efferent pathway to hypothalamus and basal forebrain comparable to fornix system: A unit study in the awake monkey. J Neurophysiol. 1980;44:514–531. doi: 10.1152/jn.1980.44.3.514. [DOI] [PubMed] [Google Scholar]
- Reinders AA. Glascher J. de Jong JR. Willemsen AT. den Boer JA. Buchel C. Detecting fearful and neutral faces: BOLD latency differences in amygdala-hippocampal junction. Neuroimage. 2006;33:805–814. doi: 10.1016/j.neuroimage.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Resnick SM. Berenbaum SA. Gottesman II. Bouchard TJ. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Dev Psychol. 1986;22:191–198. [Google Scholar]
- Rich BA. Vinton DT. Roberson-Nay R. Hommer RE. Berghorst LH. McClure EB. Fromm SJ. Pine DS. Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR. Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Scoville WB. Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker A. Laue L. Kosugi S. Merendino JJ Jr. Minegishi T. Cutler GB:, Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Chang C. Muramatsu M. Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sisk CL. Foster DL. The neural basis of puberty and adolescence. Nature Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sonis WA. Comite F. Pescovitz OH. Hench K. Rahn CW. Cutler GB Jr. Loriaux DL. Klein RP. Biobehavioral aspects of precocious puberty. J Am Acad Child Psychiatry. 1986;25:674–679. doi: 10.1016/s0002-7138(09)60293-4. [DOI] [PubMed] [Google Scholar]
- Sterzer P. Stadler C. Krebs A. Kleinschmidt A. Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stevens MC. Clark VP. Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Res. 2005;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Su TP. Pagliaro M. Schmidt PJ. Pickar D. Wolkowitz O. Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- Surguladze S. Brammer MJ. Keedwell P. Giampietro V. Young AW. Travis MJ. Williams SC. Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Szeszko PR. Robinson D. Alvir JM. Bilder RM. Lencz T. Ashtari M. Wu H. Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- van Honk J. Peper JS. Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio (Texas): Harcourt Assessment; 1999. [Google Scholar]
- Weissenberger AA. Leschek EW. Zametkin AJ. Case study: Sexual hyperactivity treated with psychostimulants in familial male precocious puberty. J Am Acad Child Adolesc Psychiatry. 2001;40:373–376. doi: 10.1097/00004583-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Nelson CB. Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28:109–126. doi: 10.1017/s0033291797005928. [DOI] [PubMed] [Google Scholar]
- Zarahn E. Aguirre GK. D'Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]