Abstract
Phenolic β2-adrenoreceptor agonists salbutamol, fenoterol and terbutaline relax smooth muscle cells that relieve acute airway bronchospasm associated with asthma. Why their use sometimes fails to relieve bronchospasm, and why the drugs appear to be less effective in patients with severe asthma exacerbations, remains unclear. We show that in the presence of hydrogen peroxide, both myeloperoxidase, secreted by activated neutrophils present in inflamed airways, and lactoperoxidase, which is naturally present in the respiratory system, catalyze oxidation of these β2-agonists. Azide, cyanide, thiocyanate, ascorbate, glutathione, and methimazole inhibited this process, while methionine was without effect. Inhibition by ascorbate and glutathione was associated with their oxidation to corresponding radical species by the agonists’-derived phenoxyl radicals. Using electron paramagnetic resonance (EPR), we detected free radical metabolites from β2-agonists by spin trapping with 2-methyl-2-nitrosopropane (MNP). Formation of these radicals was inhibited by pharmacologically-relevant concentrations of methimazole and dapsone. In alkaline buffers radicals from fenoterol and its structural analog, metaproteronol, were detected by direct EPR. Analysis of these spectra suggests that oxidation of fenoterol and metaproterenol, but not terbutaline, causes their transformation through intramolecular cyclization by addition of their amino nitrogen to the aromatic ring. Together, these results indicate that phenolic β2-agonists function as substrates for airway peroxidases and that the resulting products differ in their structural and functional properties from their parent compounds. They also suggest that these transformations can be modulated by pharmacological approaches using appropriate peroxidase inhibitors or alternative substrates. These processes may affect therapeutic efficacy and also play a role in adverse reactions of the β2-agonists.
Keywords: asthma, β2-agonists, EPR, fenoterol, phenoxyl radicals, lactoperoxidase, methimazole, myeloperoxidase, oxidation, salbutamol, terbutaline
Introduction
Asthma is a chronic inflammatory disease characterized by bronchial smooth muscle contraction and episodic narrowing of the airway. This, along with edema of the bronchial wall and accumulation of airway mucus, limits airflow and gas exchange. Standard treatment of acute asthma exacerbations includes inhalation of β2-adrenergic agonists, which activate β2-adrenergic receptors (β2AR)1 on bronchial smooth muscle cells, triggering an increase in cyclic AMP that leads to smooth muscle relaxation. Short-acting β2-adrenergic agonists used to relieve acute airway bronchospasm have included salbutamol, fenoterol and terbutaline. Why their use sometimes fails to relieve bronchospasm and why the drugs appear to be much less effective in relieving bronchoconstriction in patients with severe asthma exacerbations (status asthmaticus), or increases risk of death (1-3), is largely unknown.
It is now recognized that inflammation is an important component of asthma (4-9). Elevated levels of inflammatory cells, particularly neutrophils (PMN) and eosinophils (EOS) and their secretory products, are present in asthmatic airways and increase during clinical exacerbations of the disease. Upon activation, EOS and PMN generate superoxide (O2•–), hydrogen peroxide (H2O2) and secrete unique peroxidases: eosinophil peroxidase (EPO) by EOS and myeloperoxidase (MPO) by PMN. These heme enzymes functionally resemble lactoperoxidase (LPO) that is normally present in lung lining fluid, where it plays a protective role against pathogens. It is now believed that oxidative processes supported by these enzymes contribute to tissue injury (4-9). MPO, EPO, and LPO commonly utilize endogenously generated H2O2 to convert substrates such as tyrosine (TyrOH), SCN–, NO2–, Br– and Cl– (only MPO) to reactive metabolites that interact with cell components causing their modification and resulting in loss of normal physiological functions. Studies in vitro showed that β2-agonists affect the function of granulocytes. Treatment of PMN and EOS with salbutamol and fenoterol inhibited superoxide production and degranulation (10,11). Antioxidant activity with respect to superoxide, hydrogen peroxide, hypochlorous acid and hydroxyl radicals was reported for a number of β2-agonists (12). It was speculated that the antioxidant properties of the agonists are due to their scavenging of oxidants (13).
Phenols are typical peroxidase substrates and their oxidation can be described by reactions given by Eqs 1-3 with MPO as a representative peroxidase and TyrOH as a substrate. The immediate metabolite of TyrOH is the tyrosyl radical (TyrO•).
| (1) |
| (2) |
| (3) |
| (4) |
In this mechanism H2O2 first oxidizes the enzyme in the resting (ferric) state to a highly reactive compound I (MPO-I) (Eq 1), with two oxidizing equivalents above the ground level. Compound I is then reduced back to the ferric state through interaction with 2 molecules of TyrOH. This occurs through the intermediacy of compound II (MPO-II), which is the product of one-electron reduction of MPO-I by the substrate (Eq 2). During the reaction two TyrO• radicals are formed. In the absence of other substrates phenoxyl radicals typically form dimers through o,o′-biphenyl or p-phenoxyphenyl ether linkages (Eq 4) (14,15). Specifically, tyrosyl radicals recombine to form o,o′-dityrosine as the major product (16-18). The TyrO• radicals also react with other targets such as tyrosine residues in proteins (19) or react with reduced glutathione causing its oxidation (20). Oxidation of phenolics by LPO and EPO systems occurs, in principle, according to the same mechanism (Eqs 1-4) (21-23).
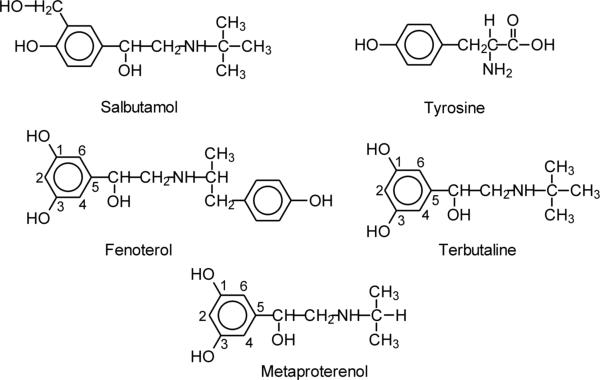
Given that β2-agonists (salbutamol, fenoterol, terbutaline) also possess the phenolic character (Fig. 1) and that, by necessity, they must function in the oxidizing environment of inflamed airways, we hypothesized that they too can be metabolized by airway peroxidases. This metabolic pathway of β2-agonists has never been explored. Such a peroxidase-mediated oxidation would be expected to cause structural modifications of the drugs similar to those reported for tyrosine, rendering them less active. Consequently, their therapeutic activity might decrease during times of increased airway inflammation that characterize acute asthma exacerbations.
Figure 1.
Structures of β2-agonists (salbutamol, fenoterol, terbutaline) and related compounds investigated.
In this report we show that salbutamol, fenoterol and terbutaline (Fig. 1) are oxidatively modified in vitro by peroxidases likely to be present in asthmatic airways, MPO and LPO. It is also shown that these drugs differ markedly in their capacity to undergo oxidation and that their oxidation products are highly reactive. Our in vitro data also suggest that it may be possible to minimize the oxidative transformation of β2-agonists by peroxidase inhibitors and antioxidants, thus preserving their bronchodilation capacity. Therefore, these observations may be pertinent to therapeutic and toxicological functions of β2-agonists.
Experimental Procedures
Materials
Lactoperoxidase (LPO) from bovine milk (EC 1.11.1.7), catalase from bovine liver (EC 1.11.1.6; 2,350 U/mg), horseradish peroxidase (HRP), terbutaline hemisulfate, metaproterenol hemisulfate, L-tyrosine, and all other chemicals (hydrogen peroxide (30%), L-GSH, ascorbic acid, methimazole, dapsone, L-methionine, NaSCN, NaCN, NaN3, diethylenetriamine pentaacetic acid (DTPA), 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) (ABTS), 5,5-dimethyl pyrroline N-oxide (DMPO)), 2-methyl-2-nitrosopropane (MNP) and albumin (bovine serum, BSA)) were obtained from Sigma-Aldrich Co. (St. Louis, MO). LPO concentration was determined using ε412 of 1.12 × 105 M−1 cm−1 (24). Myeloperoxidase (MPO) from human leucocytes (lyophilized powder, 25 U, RZ (A429/A280) of 0.61) and SOD from bovine liver (5000 U/mg) were obtained from Axxora, LLC (San Diego, CA). MPO was reconstituted with 0.25 mL of distilled water before use. Salbutamol hemisulfate, fenoterol hydrobromide and glucose oxidase type X were from MP Biochemicals, Inc. (Solon, OH). α-D(+)-Glucose was from Across Organic (Belgium). All chemicals were used as received. H2O2 concentration was determined using ε240 of 39.4 M−1 cm−1 (25) and that of DMPO using ε277 of 8 × 103 M−1 cm−1 in water (26). Stock solution of MNP (10 mM in dimers) was prepared in 0.1 M phosphate buffers (pH 7.0 and 8.0) containing DTPA (0.1 or 0.2 mM) by stirring overnight in a vessel protected from light. This procedure generates a significant amount of MNP monomers capable of trapping radicals. Stock solutions of other reagents were prepared in glass-distilled water.
Spectrophotometric Measurements
Spectra were measured using an Agilent diode array spectrophotometer model 8453 (Agilent Technologies, Inc., Santa Clara, CA). Oxidation of β2-agonists was studied by measuring absorption spectra at designated time points following the start of the reaction. Samples were prepared in 50 mM acetate buffer (pH 5.0), 50 and 100 mM phosphate buffers (pH 7.0 and 8.0) and 100 mM Tris/HCl (pH 9.19). All buffers contained DTPA (100 and 200 μM) and measurements were performed at ambient temperature of 20°C. Typically the reaction was started by the addition of a small aliquot of H2O2 (2, 5 or 10 μL), or glucose oxidase (1 μL), if glucose/glucose oxidase was used to generate H2O2, to a sample containing a studied compound, peroxidase and, if required, an inhibiting co-factor. Time course measurements were carried out following changes in absorbance at 315 nm in 15 s intervals versus absorbance at 800 nm, where none of the compounds absorb. The 315 nm wavelength was chosen because β2-agonists’ oxidation products absorb intensely near 315 nm, and because it is close to the absorption maximum of tyrosine dimers.
In certain experiments oxidation of β2-agonists by peroxidases was carried out using H2O2 generated by the reaction of glucose (1 mM) with glucose oxidase (0.2 μg/mL). The rate of H2O2 generation in these systems was estimated based on the rate of oxidation of ABTS (1 mM) to the green ABTS radical cation (ABTS•+) by HRP, at increasing concentrations of the enzyme. Concentrations of glucose and glucose oxidase were the same as those used in experiments with β2-agonists. The plot of the rate of ABTS•+ oxidation at 420 nm (determined from the linear portion of kinetic runs) versus [HRP] is a curve, which plateaus above a certain threshold value [HRP]. The mean value of the rate from the plateau region (dA420/dt = ε420 × d[ABTS•+]/dt), was taken as the rate, at which all H2O2 produced by glucose/glucose was immediately used up by the enzyme to oxidize ABTS. Calculations were performed using ε420 (ABTS•+ of 3.6 × 104 M−1 cm−1) (27) and assuming that stoichiometry for the reaction is 1 mole of H2O2 to 2 moles of ABTS. The rate of H2O2 generation determined in this way was 3.33 μM/min, based on two separate determinations.
Because the commercially available fenoterol exists in the form of hydrobromide, and because bromide anion (Br–) is converted by peroxidases to brominating hypobromous acid (HOBr), there was the possibility that Br– might interfere with enzymatic oxidation of the drug. However, experiments performed in the presence of taurine and L-methionine (traps for HOBr), as well as additional doses of bromide (as NaBr) added to the sample, did not reveal any meaningful changes in the oxidation kinetics of fenoterol. In contrast to taurine, L-methionine is considered to afford a less reactive product upon reaction with HOBr. However 2 mM L-methionine only minimally inhibited oxidation of fenoterol (95 % of the control (see Table 2)). Therefore it was concluded that Br– that is naturally present in the sample does not influence significantly the metabolism of fenoterol. To evaluate the role of oxygen in oxidative processes spectrophotometric experiments were performed after bubbling N2 gas through the sample (1 mL volume) for 5 min before start of the reaction (H2O2 addition) and then between readouts, which were collected every 1 min.
Table 2.
Oxidation of fenoterol and salbutamol by LPO/H2O2 - Effect of inhibitors. H2O2 was generated by glucose (1 mM) glucose oxidase (0.2 μg/mL). Oxidation of fenoterol (50 μM) and salbutamol (100 μM) was carried out in 0.1 M potassium phosphate buffer (pH 7.0) containing 0.1 mM DTPA in the presence of LPO (158 nM LPO for fenoterol and 216 nM for salbutamol). The extent of inhibition was determined by measuring ΔA315 during 30 min reaction and is expressed as % of control (mean ± SE from at least two determinations).
| Amount of metabolite formed ΔA315(%) | ||
|---|---|---|
| Fenoterol |
Salbutamol |
|
| Control | 100 | 100 |
| NaN3 (1 mM) | 45.5 ± 3.9 | 17.4 ± 7.1 |
| NaCN (1 mM) | 95.2 ± 5.3 | 80.0 ± 3.2 |
| NaSCN (0.1 mM) | 22.8 ± 3.8 | 5.5 ± 7.3 |
| GSH (0.1 mM) | 38.1 ± 3.2 | 42.7 ± 3.4 |
| Methionine (0.1 mM) | 103.7 ± 2.0 | 105.5 ± 16.2 |
| Methionine (2 mM) | 95.0 ± 3.5 | ----------- |
| Methimazole (20 μM) | 30.0 ± 2.9 | 16.7 ± 4.6 |
EPR Measurements
EPR spectra were recorded using a Brüker EMX EPR spectrometer (Brüker Biospin Co., Billerica, MA), operating in X band and equipped with a high sensitivity resonator ER 4119HS. Formation of free radicals from β2-agonists was studied in samples prepared in 100 mM phosphate buffer (pH 7.0 and 8.0)/DTPA (0.2 mM) (total volume 250 μL) containing MNP, MPO (or LPO) and the agonists. The reaction was initiated by the addition of H2O2 as the last component. In experiments, in which H2O2 was generated using glucose (1 mM) and glucose oxidase (3.9 μg/mL), glucose oxidase was added as a last component. The sample was transferred to a flat aqueous EPR cell and recording was started 1 min after initiation of the reaction. Typically, spectra of MNP adducts were recorded using microwave power 20 mW, modulation amplitude 0.1 mT, receiver gain 2 × 105, conversion time 40.96 ms, time constant 81.92 ms and scan rate of 10 mT/41.92 s. EPR spectra are average of 5 scans and represent results of typical experiments. Unless stated otherwise, direct EPR measurements (spin traps omitted) of free radicals derived from β2-agonists were performed using conditions similar to those for the detection of MNP adducts. EPR spectra were simulated using WINSIM software developed at NIEHS/NIH (RTP, NC).
The effect of methimazole and dapsone on the formation of free radicals from drugs was studied in phosphate buffers (pH 7.0 and 8.0) containing DTPA (0.2 mM) and MNP (10 mM in dimers). Oxidation was carried out by MPO (0.43 units/mL)/H2O2 (37 μM) and LPO (0.39 μM)/glucose (1 mM)/glucose oxidase (0.8 μg/mL). In experiments involving methimazole, the concentrations of fenoterol and terbutaline were 0.47 mM, while in those involving dapsone, their concentrations were 0.047 mM.
The effects of AscH– and GSH on the metabolism of drugs were investigated using samples in 100 mM phosphate buffer (pH 7.0)/DTPA (0.1 mM) (total volume 250 μL) containing salbutamol or fenoterol and MPO, and the reaction was initiated by addition of H2O2 as the last component. When the effect of GSH was studied, the spin trap DMPO (18 mM) was also present. The sample was transferred to a flat aqueous EPR cell and recording was started 1 min after initiation of the reaction. The spectra of DMPO adducts were recorded using the same parameters as above but the sweeping rate was 8 mT/41.92 s. The EPR spectra of ascorbate radicals were obtained using microwave power 5 mW, modulation amplitude 0.05 mT, scan rate 4 mT/41.92 s. EPR spectra shown are average of 5 scans and represent results of a typical experiment.
Results
Interaction of β2-agonists with MPO system
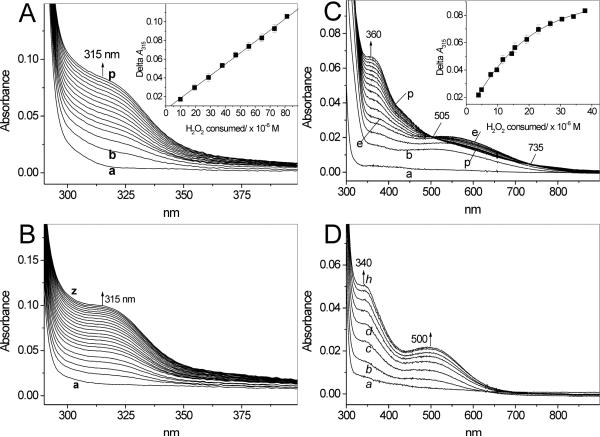
Tyrosine and other phenolics are oxidized by peroxidases to free radicals that can recombine to give rise to corresponding dimeric products (15-18,23). Because the β2-adrenergic agonists salbutamol, fenoterol and terbutaline also contain phenolic moieties (Fig. 1), we hypothesized that they too can be metabolized by peroxidases. Spectrophotometric measurements showed that these compounds have no significant absorption around 300 nm and none above this wavelength (Fig. 2A, B, D, traces a). However, when H2O2 was added to salbutamol in buffer (pH 7.0) containing MPO, a new absorption band centered around 315 nm appeared (Fig. 2A), suggesting formation of a new metabolite. The new absorption is in the range characteristic of tyrosine dimers (16-18,23). Measurements of the absorbance at 315 nm (A315) following additions of small aliquots of H2O2 (9.85 μM per dose) to salbutamol (1 mM) in buffer (pH 7.0) containing MPO (200 mU/mL) showed that in the range 0-100 μM, the plot of ΔA315 versus [H2O2] is linear (Fig. 2A, inset). Based on this relationship a molecular absorptivity, ε315, for the generated mixture of products was determined to be 1210 ± 19 M−1 cm−1 (N = 3). This value is in the range of molar absorptivities at 300 nm determined for a mixture of products derived from phenolics oxidized enzymatically at pH 5.0 (23). The time course of the reaction following a single bolus addition of H2O2 shows that A315 plateaus after ∼ 10 min reaction (Fig. 3, trace a). The lack of further increase in A315 is chiefly due to consumption of H2O2. For comparison, absorption spectra observed during oxidation of tyrosine by MPO/H2O2 are shown in Fig. 2B. Because both the spectral changes and the kinetics of oxidation of salbutamol and tyrosine (Fig. 3, traces a and b, respectively) are similar, we propose that oxidation of this β2-agonist generates the corresponding dimer, o,o′-disalbutamol, among other possible products. The efficacy of salbutamol oxidation by MPO/H2O2 is pH-dependent. After a 30 min reaction of salbutamol (1 mM) with MPO (2 mU/mL) and H2O2 (119 μM), ΔA315 increased from near zero at pH 5.0, to 0.12 at pH 7.0 and 0.178 at pH 8.0. This dependence is similar to that reported for oxidation of tyrosine (17,18).
Figure 2.
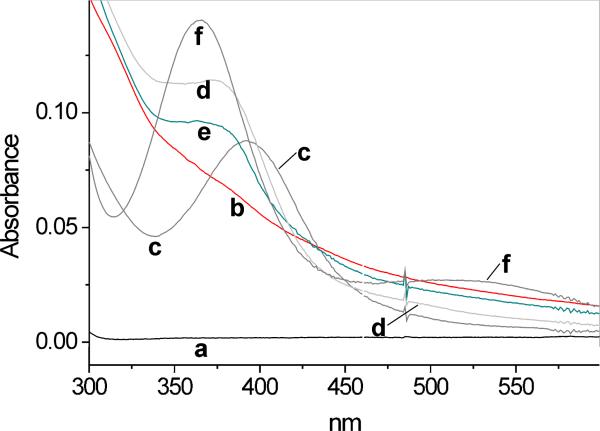
Absorption spectra observed during oxidation of salbutamol (A), tyrosine (B), fenoterol (C) and terbutaline (D) by MPO (200 mU/mL) and H2O2 in 50 mM phosphate buffer (pH 7.0) containing 100 μM DTPA. The reaction was initiated by a bolus addition addition of H2O2 to a final concentration of 50 μM (in A, B, D) and 25 μM (in C). Spectra were recorded every 30 s. Initial concentrations of β2-agonists were 1 mM. Inset in A and C: ΔA315 versus H2O2 consumed during oxidation by MPO of salbutamol and fenoterol, respectively. H2O2 (0.98 mM) was being added in small portions (5 μL to the salbutamol sample and 2 μL to the fenoterol sample) and when A315 stabilized, ΔA315 was determined after each single dose of H2O2. Other conditions were as described for the main panel.
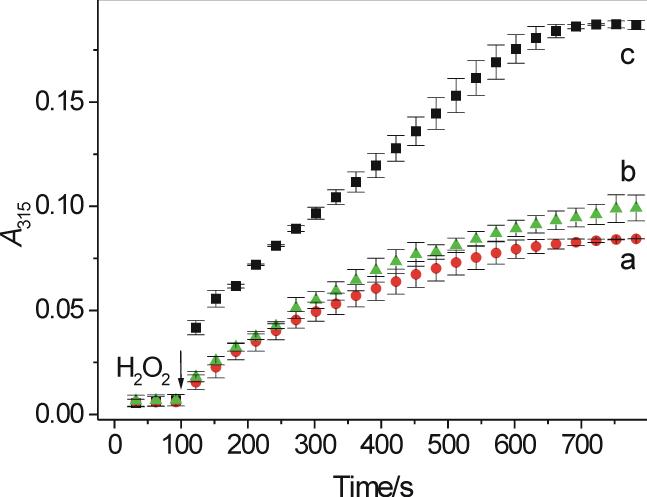
Figure 3.
Time course of absorption changes measured at 315 nm during the oxidation of salbutamol (a), tyrosine (b) and fenoterol (c). The reaction was initiated by the addition of a small aliquot of H2O2 (final concentration 60 μM) to pH 7.0 buffer (50 mM phosphate) containing a drug (1 mM each), MPO (0.2 U/mL) and DTPA (100 μM). Absorbance was read every 30 s. N = 2 (salbutamol and fenoterol) and 3 (tyrosine).
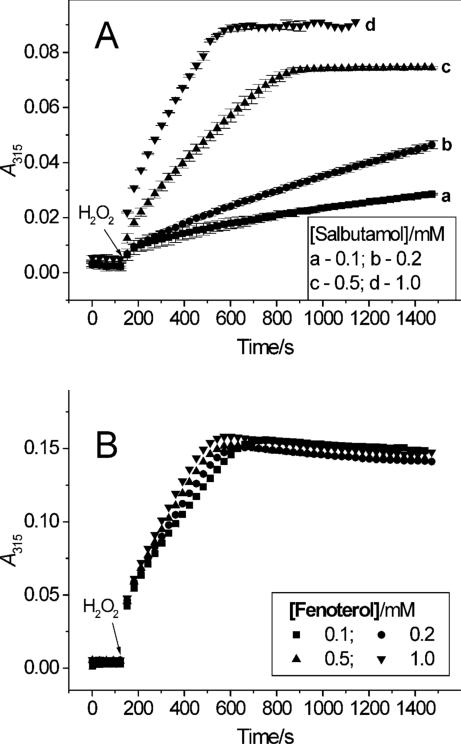
Under the conditions used, we observed that the efficacy of salbutamol oxidation was dependent upon the concentration of the agonist. At constant concentration of H2O2 of 52 μM, the rate of oxidation and the amount of the metabolite formed (measured as ΔA315 over 25 min) increased as the concentration of the drug increased from 100 μM to 1 mM (Fig. 4A, traces a – d). This type of response is suggestive of a slow reaction of salbutamol with peroxidase compound II. The yield of salbutamol oxidation was also measured at constant concentration of the agonist of 1 mM and changing [H2O2] from 25 to 200 μM. The maximum yield was found at around 100 μM of the peroxide. The decrease in yield at higher concentrations of H2O2 may be due to inhibition of the peroxidase. Cyanide and azide (1 mM each) substantially inhibited oxidation of salbutamol by MPO and H2O2 while catalase (235 units/mL) completely blocked the reaction (Table 1), confirming that the oxidation of salbutamol is a peroxidase-dependent process.
Figure 4.
Dependence of oxidation salbutamol (A) and fenoterol (B) on drug concentration. The rate of oxidation by MPO (200 mU/mL) and H2O2 (50 μM) was measured in 50 mM phosphate buffer (pH 7.0) containing 100 μM DTPA, by following changes in absorbance at 315 nm.
Table 1.
Efficacy of oxidation of fenoterol (1 mM) and salbutamol (1 mM) by MPO (200 mU/mL) and H2O2 (50 μM) in 50 mM phosphate buffer (pH 7.0) containing 0.1 mM DTPA in the presence of modulating co-factors. The extent of oxidation is expressed as ΔA315 ± SE versus control (in %) during 22 min reaction at 20° C. Values are the mean of at least duplicate determinations.
| ΔA315 (%) | ||
|---|---|---|
| Fenoterol | Salbutamol | |
| Control | 100 | 100 |
| Catalase (235 U/mL) | 0.0 | 0.0 |
| NaCN (1 mM) | 0.24 ± 0.32 | 12.5 ± 9.1 |
| NaN3 (1 mM) | 17.9 ± 4.2 | 6.6 ± 1.3 |
| GSH (0.1 mM)a | 43.3 ± 14.5 | 62.0 ± 17.3 |
| BSA (0.5 mg/mL) | 110 ± 2.0 | 99.0 ± 2.0 |
In experiments with GSH, concentrations of salbutamol and fenoterol were 100 μM each and the reaction was continued for 30 min.
Oxidation of salbutamol was compared with that of fenoterol. In contrast to salbutamol, which contains only one mono-phenolic moiety, fenoterol possesses both a mono-phenolic and a di-phenolic (1,3-dihydroxybenzene or resorcinol) moiety. It was therefore anticipated that fenoterol might behave differently from salbutamol when exposed to peroxidases, especially since resorcinols are oxidized by peroxidases (28). Figure 2C shows spectra observed during interaction of fenoterol (1 mM) with MPO (200 mU/mL) and H2O2 (25 μM) at pH 7.0. Under the condition used, and in contrast to salbutamol and tyrosine, the new species formed absorbs both in the UV and visible regions of the spectrum. The spectral lines intersect at 322, 505 and 735 nm and show new absorption maxima at 360 and 550 nm. At higher concentrations of H2O2 the absorption maxima are less pronounced and the intersection points are absent (not shown), presumably due to formation of a mixture of various dimeric and/or polymeric product(s). We confirmed that oxidation of the resorcinol moiety of the drug is responsible for these effects by showing that oxidation of a related β2-agonist, terbutaline (Fig. 1), which contains only the resorcinol group, yields spectral features (Fig. 2D) similar to those of oxidized fenoterol. However, for oxidized terbutaline, the absorption maxima (340 and 500 nm) are blue-shifted compared to those found for fenoterol. Measurements of A315 following additions of small aliquots of H2O2 (3.9 μM per dose) to fenoterol (1 mM) in buffer (pH 7.0) containing MPO (200 mU/mL) showed that the plot of ΔA315 versus [H2O2] is nonlinear (Fig. 2C, inset), presumably due to inactivation of the enzyme. Thus, under similar conditions, fenoterol behaves differently from salbutamol, for which the relationship between ΔA315 and [H2O2] was linear (Fig. 2A, inset).
The time course of absorption changes at 315 nm recorded during oxidation of fenoterol following single bolus addition of H2O2 is shown in Fig. 3 (trace c). At this wavelength the fenoterol-derived metabolite(s) absorbs approximately twice more intensely than metabolites from salbutamol or tyrosine (Fig. 3, traces a and b). This may be due to the presence of the resorcinol moiety, oxidation of which may gives rise to a more complex polyphenolic products with higher molar absorptivity. Similar to salbutamol, oxidation of fenoterol by MPO/H2O2 depends on pH. Measurements of ΔA315 during 30 min reaction of fenoterol (1 mM) with MPO (200 mU/mL) and H2O2 (119 μM) showed that ΔA315 increased from 0.0165 at pH 5.0, to 0.097 at pH 7.0 and 0.129 at pH 8.0 (corrected for autoxidation). However, in contrast to salbutamol, at pH 8 (and higher) fenoterol undergoes autoxidation, as evidenced by a slow increase in A315 without externally added H2O2 and/or MPO. When MPO was added, A315 increased faster (not shown), suggesting accumulation of H2O2 during the autoxidation phase. This was verified by the addition of catalase, which abolished the effect MPO (not shown).
The rate of fenoterol oxidation by MPO, following a single bolus addition of H2O2 (52 μM), changed minimally when the concentration of fenoterol was increased from 100 to 1000 μM (Fig. 4B). This behavior is different from that observed during oxidation of salbutamol, for which the rate of oxidation was strongly dependent on concentration of the drug (Fig. 4A). The observation that ΔA315 reached almost the same final levels at each concentration of fenoterol tested (Fig. 4B), suggests that the extent of fenoterol oxidation depends on the concentration of the peroxide. Measurements of ΔA315 versus [H2O2] at constant fenoterol concentration (1 mM) and MPO of 0.1 U/mL, showed that the maximum yield was at [H2O2] near 50 μM. The decrease at higher concentration of the peroxide is probably due to inactivation of the enzyme as was already suggested by data in Fig. 2C (inset). Addition of SOD (100 units) was without any apparent effect on oxidation of fenoterol, suggesting that free superoxide radical was not involved in the reaction. Similarly to salbutamol, oxidation of fenoterol was substantially inhibited by the peroxidase poisons cyanide and azide and completely prevented by catalase (Table 1), further supporting the peroxidative metabolism the drug's oxidation. Together, these observations confirm that salbutamol and fenoterol undergo oxidation by MPO in the presence of H2O2. They also demonstrate that the MPO metabolism of salbutamol differs from that of fenoterol both in the nature of the products formed, and in responses to changes in H2O2 and substrate concentrations.
The role of oxygen was examined using fenoterol as a substrate as this compound produced more profound changes in absorption spectra. When the sample of fenoterol was deaerated by bubbling N2 gas through the solution, the rate of the reaction and the extent of oxidation both decreased by approximately 50% (not shown), suggesting that oxygen is involved, at least partially, in the oxidative transformation of the agonist. One possible explanation of this effect may be that oxygen adds to phenoxyl radicals resulting in hydroxylation of the aromatic ring, a process known to occur during aerobic (photo)oxidation of resorcinols (29).
Effect of ascorbate and glutathione on oxidation of salbutamol and fenoterol
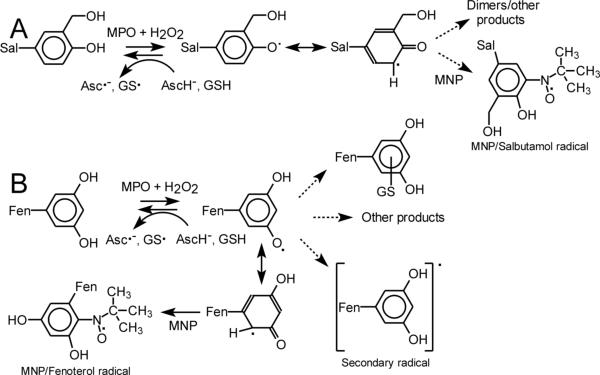
Given that the respiratory tract lining fluid contains antioxidants such as ascorbate (AscH–) and glutathione (GSH) (30), it was expected that they could affect oxidation of β2-agonists. The concentration of AscH– in airway fluid was estimated to be near 100 μM (30), and in our experiments we used concentrations within this physiological range. Figure 5 shows changes in A315 versus time for salbutamol (A) and fenoterol (B) reacting with MPO/H2O2 in the absence and presence of 10, 20, 40 and 100 μM AscH– (traces a – e, respectively). It is found that AscH– affects oxidation of salbutamol and fenoterol in a similar manner, causing delay in the net oxidation of the drugs. An increase in A315 is observed after a lag period, duration of which depends on AscH– concentration. It is concluded that only when AscH– is consumed the net oxidation of the drugs is observed. At 100 μM AscH– there is a complete inhibition of oxidation of both salbutamol and fenoterol samples. This is understandable given that the concentration of H2O2 was only 50 μM and the peroxide was used to oxidize both the drugs and AscH–. The observation that oxidation of β2-agonists is resumed after a lag period suggests that this delay is not due to inhibition/inactivation of the enzyme, but rather due interaction of AscH– with the drugs’-derived phenoxyl radicals. The proposed mechanism of the inhibition is described by the reaction given in Scheme 1A,B, which shows that recovery of the drug occurs at the expense of AscH–, which is oxidized to the ascorbate radical (Asc•–).
Figure 5.
Oxidation of salbutamol (A) and fenoterol (B) by MPO/H2O2 – effect of ascorbic acid. Salbutamol (1 mM) and fenoterol (200 μM) were exposed to MPO (200 mU/mL) and H2O2 (50 μM) in the absence and presence of 10, 20, 40 and 100 μM ascorbic acid (traces a-e, respectively) in pH 7.0 buffer (50 mM phosphate containing 100 μM DTPA) and A315 was measured in 30 s intervals following H2O2 addition.
Scheme 1.
Proposed reactions of salbutamol (A) and fenoterol (B) initiated by MPO (or LPO) and H2O2. Pathway B is also pertinent for reactions involving terbutaline and metaproterenol.
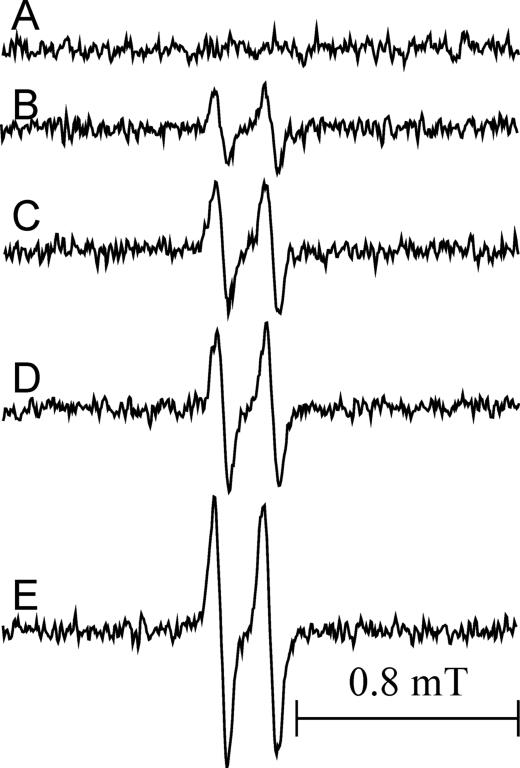
We investigated the formation of Asc•– during oxidation of β2-agonists by MPO/H2O2 in the presence of AscH– (100 μM) by EPR. Ascorbate radicals are relatively stable and produce a distinct EPR spectrum, a doublet with hyperfine splitting constant of 0.18 mT. Oxidation of AscH– by MPO/H2O2 alone is relatively inefficient, as evidenced by the weak EPR signal of Asc•– generated by the system (Fig. 6 spectrum B). In contrast, EPR spectra generated by oxidation of AscH– in the presence of salbutamol (40 and 100 μM) are more intense (Fig. 6C and D, respectively), approximately by 76% and 117%, and in the presence of fenoterol (20 μM) by 270% (Fig. 6, spectrum E), indicating that both of these agonists stimulate oxidation of AscH–, with fenoterol being substantially more effective. Spectrum in Fig. 6A shows that when MPO and H2O2 are absent, the level of Asc•– is below the detection limit.
Figure 6.
EPR spectra of ascorbate radical in pH 7.0 buffer (50 mM) containing DTPA (0.2 mM). A. Ascorbate (100 μM) in buffer, no additives. B. Same as in A in the presence of MPO (0.01 U/250 μL) and H2O2 (39 μM). C. and D. Same as B but in the presence of salbutamol (40 and 100 μM, respectively). E. Same as B but in the presence of fenoterol (20 μM). The reaction was initiated by the addition of H2O2. The recording was started 1 min after H2O2 addition. Instrumental settings: modulation amplitude 0.05 mT, receiver gain 2 × 105, time constant 40.96 ms, conversion time 40.96 ms, seep time 41.94 s. Sample volume 250 μL. The spectra shown are average of 5 scans and represent typical results.
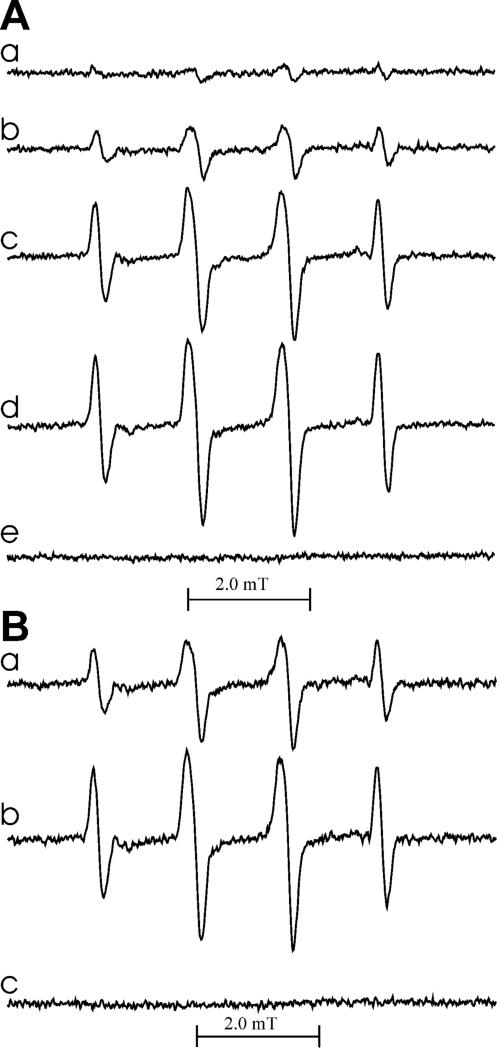
Our spectrophotometric measurements showed that GSH also inhibits oxidation of β2-agonists by MPO and H2O2 (Table 1), consistent with prior observations that oxidation of tyrosine to dityrosine by MPO/H2O2 can be inhibited by GSH (20). Because GSH is a poor peroxidase substrate, one potential mechanism of inhibition is interaction of the generated phenoxyl radical metabolites with the thiol as depicted in Scheme 1A,B. This reaction is accompanied by the formation of GS• radicals as described for tyrosine and other phenolics (31). To verify that this mechanism operates also for β2-agonists, we performed EPR experiments combined with spin trapping, in order to detect GS• radicals. When GSH was exposed to MPO/H2O2 in the presence of the spin trap DMPO and salbutamol, EPR spectra of DMPO/•SG adduct were detected. The hfsc's aN = 1.51 mT, aβH = 1.61 mT are in agreement with those determined in earlier reports for the same DMPO adduct (31-33). In Fig. 7, panel A, are shown spectra recorded in the absence (a) and presence of 80, 400, and 800 μM salbutamol (spectra b-d, respectively). They show that salbutamol in a concentration-dependent manner enhances oxidation of GSH to GS•evidenced by more intense EPR spectra of DMPO/•SG adducts. No radicals were detected when salbutamol alone (GSH omitted) was incubated with MPO and H2O2 (Fig. 7A, spectrum e), perhaps due to a low efficacy of the addition of the drug-derived phenoxyl radicals to DMPO and/or a poor stability of the resulting adduct.
Figure 7.
EPR spectra of DMPO/•SG adducts generated by oxidation of salbutamol (A) and fenoterol (B) by MPO/H2O2 in the presence of glutathione. The reaction was carried out in 50 mM phosphate buffer (pH 7.0) containing DTPA (100 μM), DMPO (18 mM), GSH (4 mM), and MPO (0.01 U/250 mL). Panel A: spectra observed in the presence of salbutamol (0, 80, 400 and 800 μM for spectra a-d, respectively). Spectrum e was obtained in the absence of GSH but with 800 μM salbutamol. Panel B: spectra observed in the presence of fenoterol (2 and 20 μM for spectra a and b, respectively). Spectrum c was obtained in the absence of GSH but with 400 μM fenoterol present. The reaction was initiated by the addition of H2O2 to a final concentration of 84 μM. The recording was started 1 min after H2O2 addition. The spectra shown are average of 5 scans and represent a typical result.
When similar experiments were conducted using fenoterol (2 and 20 μM) instead of salbutamol, EPR spectra shown in Fig. 7, panel B (spectra a and b, respectively) were detected. Although the general pattern is similar to that observed in the presence of salbutamol, namely that the intensity of the EPR spectra increases as the concentration of fenoterol increases from (spectra a and b), fenoterol appears to be markedly more efficient in stimulating oxidation of GSH. The EPR spectrum generated in the presence of 2 μM fenoterol (Fig. 7B, spectrum a) is approximately two-fold more intense than that observed in the presence of 80 μM salbutamol (Fig. 7A, spectrum b). This further confirms the higher reactivity of a metabolite(s) derived from fenoterol. When GSH was omitted, no radicals from fenoterol were detected by spin trapping with DMPO (Fig. 7B, spectrum c). The higher stimulatory action of fenoterol, when compared to that of salbutamol, implies that the compound's resorcinol moiety may play a dominating role in the interaction with GSH. The proposed cycle of redox reactions involving fenoterol, AscH– and GSH is depicted in Scheme 1B.
We found that oxidation of fenoterol in the presence of GSH generates a new species with absorption maximum at 395 nm (Fig. 8, spectrum c), which upon longer incubation shifted to 375 nm (spectrum d). The new species was tentatively ascribed to a fenoterol-SG conjugate. Because oxidation of salbutamol in the presence of GSH did not produce this spectral feature, it suggested that the fenoterol resorcine moiety might be involved (Scheme 1B). To test this possibility, we oxidized terbutaline in the presence of GSH. Terbutaline possesses resorcinol but no monophenolic moiety. As with fenoterol, terbutaline oxidized in the presence of GSH exhibited a spectrum with an intense maximum at 365 nm (Fig. 8, spectrum f) confirming that the resorcine portion of these molecules participates in the reaction with GSH. Formation of conjugates with thiols has been described for para- and ortho-quinones (34,35). Because oxidation of meta-hydroxybenzenes cannot lead to meta-quinones, formation of a fenoterol (or terbutaline) conjugate with GSH has to involve another intermediate, capable of addition the thiol, possibly a tri-hydroxybenzene, formed in situ in the system. Formation of such an intermediate is suggested by analysis of EPR spectra of radicals formed during oxidation of fenoterol and metaproterenol (vide infra).
Figure 8.
Absorption spectra of fenoterol and terbutaline oxidized by MPO/H2O2 at pH 7.0 – effect of GSH and BSA. Spectrum a – intact fenoterol (100 μM), b - oxidized fenoterol, c – fenoterol oxidized in the presence of GSH (100 μM), d – sample c after overnight incubation in the dark, e - fenoterol oxidized in the presence of BSA (0.5 mg/mL), f – same as c but with terbutaline instead of fenoterol.
As determined by measuring A315, we found that BSA (0.5 mg/mL) minimally affected oxidation of salbutamol and fenoterol by MPO/H2O2 (Table 1). Absorption spectrum of fenoterol oxidized in the presence of BSA (Fig. 8, spectrum e) closely resembles the spectrum of fenoterol oxidized in the presence of GSH (Fig. 8, spectrum d, after a prolonged incubation), suggesting that oxidized fenoterol may form a conjugate with BSA, presumably through addition to the protein thiol group.
Interaction of β2-agonists with LPO system
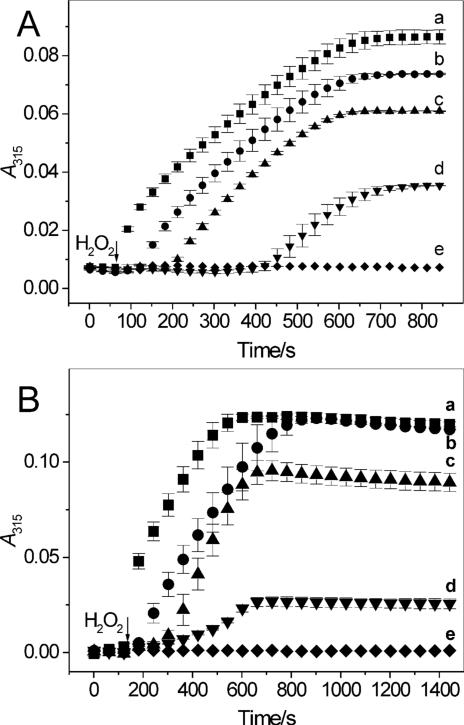
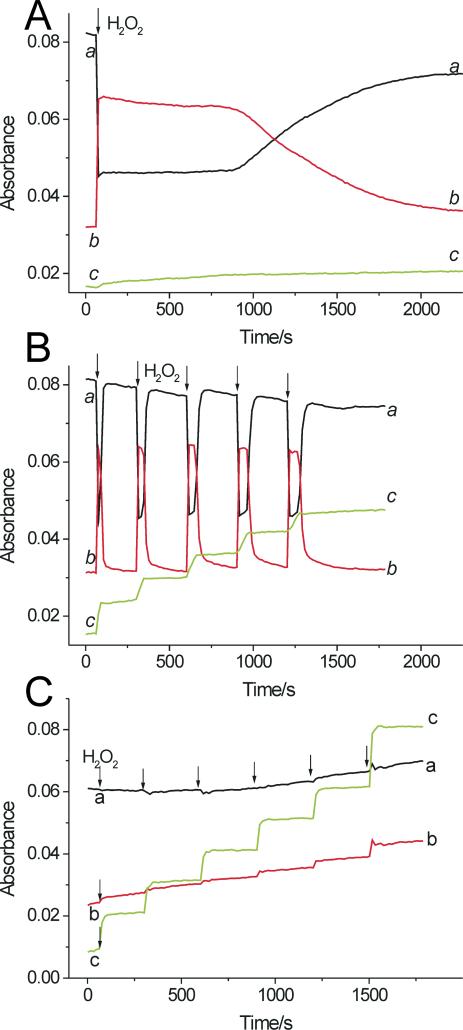
Results of preliminary spectrophotometric experiments showed that oxidation of salbutamol and fenoterol can also be accomplished by LPO and H2O2. However, the efficacy of the oxidation of salbutamol catalyzed by LPO was small, and increasing concentration of the peroxide did not improve the yield (not shown). We suspected that this inefficient oxidation could possibly be due to slow interaction of salbutamol with LPO-II, a process known to occur in the presence of poor substrates. We therefore exposed LPO to a small excess of H2O2 and measured the effect of drugs on the enzyme turnover. When H2O2 (5 μM) was added to ferric LPO (0.7 μM), the enzyme's Soret band shifted from 412 nm to 430 nm characteristic of LPO-II. Because absorbance of ferric LPO and LPO-II differ markedly at these wavelengths, changes in absorbance at 412 and 430 nm versus time rendered it possible to follow transitions between these two forms of LPO. Time course measurements shows that the addition of H2O2 caused a rapid decrease in A412 and simultaneous increase in A430 (Fig. 9A, traces a and b, respectively). The enzyme existed in the form of LPO-II for about 14 min, after which a slow return to the ferric state was observed (Fig. 9A). When salbutamol was present (100 μM), the addition of H2O2 caused similar rapid changes in A412 and A430, but the lifetime of LPO-II was dramatically shortened, to less than 45 s (Fig. 9B). Subsequent doses of H2O2 induced similar changes in A412 and A430 (Fig. 9B, arrows indicate when H2O2 was added). Absorption spectra recorded simultaneously with the kinetic runs confirmed the real formation of LPO-II (not shown). The spikes in the kinetic run at 430 nm (trace b), formed immediately after the H2O2 addition are the signatures of the transient formation and rapid decay of LPO-II. Thus, the presence of salbutamol significantly shortened the time needed for LPO-II to return to ferric LPO, suggesting completion of the peroxidative cycle after each dose of the peroxide. This required that both LPO-I and LPO-II be reduced by salbutamol, each via one-electron transfer, which implies that the reaction between H2O2 and salbutamol occurs with a 1:2 stoichiometry, in agreement with Eqs 1-3. Trace c in Fig. 9B shows that A315, which measures accumulation of salbutamol-derived metabolites, increases in a step-wise fashion after each dose of H2O2, and only during the duration of the spikes, but is invariant between the H2O2 dosing. This is consistent with oxidation of the drug only during the enzyme turnover. Trace c in Fig. 9A shows that when salbutamol was omitted, changes in A315 versus time were minimal.
Figure 9.
Oxidation of salbutamol and fenoterol by a LPO/H2O2 system. A. Time course of absorption changes at 412 nm (ferric LPO, trace a) and 430 nm (LPO compound II, trace b) following addition of H2O2 (5 μM) to LPO (0.7 μM) in 0.1 M phosphate buffer (pH 7.0) in the absence of β2-agonists. B. The same as in A but in the presence of salbutamol (100 μM). Trace c shows changes in absorbance at 315 nm due to accumulation of salbutamol oxidation products following addition of H2O2 (indicated by arrows). C. The same as in A but in the presence of fenoterol (100 μM). Data points were collected in 15 s intervals.
Responses of LPO to H2O2 depended on the concentration of salbutamol. In the presence of 10 μM salbutamol, the lifetime of LPO-II was approximately 420 s after the first dose of H2O2, markedly longer than at 100 μM salbutamol. During subsequent doses of H2O2, as the drug was consumed, the lifetime of LPO-II increased (not shown). However, also at 1 mM salbutamol, the formation of LPO-II was detected, suggesting that interaction of the drug with LPO-II is the rate-limiting step. This is consistent with the view that peroxidative activity depends on the rate with which a substrate reduces compound II to native enzyme (36). Together, these observations suggest that salbutamol undergoes one-electron oxidation both by LPO-I and LPO-II, in agreement with reactions described by Eqs 2 and 3, with the reaction involving LPO-II being the slowest step.
Under conditions similar to those in Fig. 9B, kinetic traces recorded in the presence of fenoterol (100 μM) did not show formation of LPO-II (Fig. 9C, traces a and b show that A412 and A430 are near constant). Thus, in contrast to salbutamol, fenoterol appears to readily react with both LPO-I and LPO-II, providing rapid enzyme turnover.2 The step-wise increase in A315 following every dose of the peroxide (Fig. 9C, trace c) is indicative of the formation of a fenoterol-derived metabolite. This occurs only during the brief period during which H2O2 is available. The small monotonous increase in absorbance at 412 and 430 nm over the time of observation (Fig. 9C) is due to accumulation of fenoterol metabolites, which absorb in this region (see spectra in Fig. 2C).
We next conducted experiments to find a relationship between ΔA315 and H2O2 consumed for salbutamol and fenoterol oxidized by a LPO/H2O2 system. In the H2O2 concentration range 0-50 μM, the plot of ΔA315 versus [H2O2] consumed is linear (correlation coefficient 0.99-0.999) for both salbutamol and fenoterol (not shown). Based on this relationship, the molar absorptivity for products derived from salbutamol was determined to be ε315 = 1149 ± 66 M−1 cm−1. This value is close to 1210 M−1 cm−1 determined in this study using the MPO/H2O2 system. For products derived from oxidation of fenoterol, ε315 was determined to be 1688 ± 37 M−1 cm−1. Both these values are in the range of molar absorptivities determined at 300 nm for a number of phenolics oxidized by LPO/H2O2 at pH 5 (23).
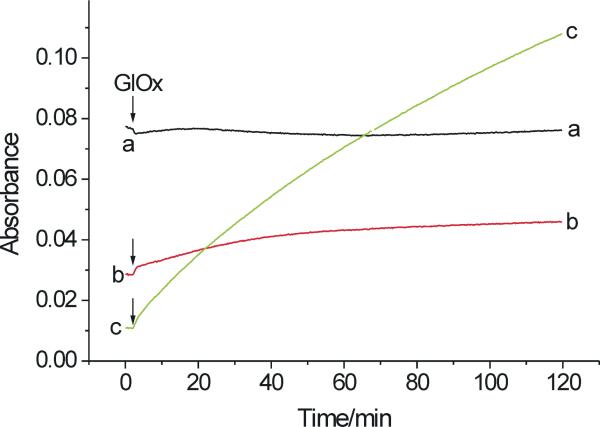
For LPO, low concentrations of H2O2 generated small amounts of metabolites and the use of higher concentrations of H2O2 did not improve the yield. We expected that more efficacious oxidation of salbutamol by LPO could be achieved by continuous generation of low H2O2 fluxes using glucose/glucose oxidase system, where inactivation of LPO could be minimized by choosing appropriately low concentrations of glucose oxidase. An additional advantage of this system is that it mimics better the generation of the peroxide in vivo. When salbutamol (100 μM) was exposed to LPO (0.7 μM), glucose (1 mM) and glucose oxidase (0.1 μg/mL) there was a continuous increase in A315 (Fig. 10, trace c), indicating oxidation of the drug. During the entire time of observation, LPO remained in its ferric form (A412 and A430 ∼ constant) (Fig. 10, traces a and b, respectively), suggesting that under the conditions of slow generation of H2O2, LPO-II does not accumulate in quantities detectable by our recording system, being rapidly reduced to ferric LPO. Oxidation of salbutamol was still observed when the concentration of glucose oxidase was increased two-fold or more, to increase the rate of H2O2 generation, but the process was less efficient, presumably due to formation of the less reactive intermediate LPO compound III (not shown). LPO-III is formed in the presence of a large excess of H2O2 over LPO and is characterized by its Soret band at 424 nm (24,37). Oxidation of salbutamol by LPO-III is in agreement with prior observations that phenols react with LPO-III converting it to native enzyme, which then re-enters the peroxidative cycle (23,38).
Figure 10.
Oxidation of salbutamol by LPO and H2O2 (generated by glucose and glucose oxidase) at pH 7.0. The sample contained salbutamol (100 μM), LPO (0.7 μM) and glucose (1 mM) and the reaction was initiated by the addition of glucose oxidase (0.1 μg/mL) as the last component (arrow). Traces a, b, and c correspond to absorption changes at 412 nm, 430 nm, and 315 nm, respectively. GlOx designates glucose oxidase. Data points were collected in 15 s intervals.
Effect of inhibitors on oxidation of β2-agonists by LPO/H2O2
Oxidation of salbutamol and fenoterol by LPO/glucose/glucose oxidase was strongly inhibited by azide, but weakly by cyanide (Table 2). Thiocyanate, the natural substrate for LPO, and GSH markedly inhibited oxidation of fenoterol and salbutamol (Table 2). In contrast, L-methionine, in which the thiol group is methylated, was inactive, emphasizing the importance of the free –SH group for effective antioxidant action. Because LPO-catalysed oxidation could be a mechanism that inactivates β2-agonists in the airways, we sought to determine whether pharmacological inhibitors of peroxidase could affect oxidation of these drugs. Methimazole, an antithyroid drug, is known to inhibit thyroid peroxidase and LPO (39,40). We observed that methimazole markedly inhibited oxidation of both agonists by the LPO system (Table 2). In the presence of methimazole the intensity of the LPO Soret band markedly decreased during the reaction indicating that inhibition of β-agonists oxidation was primarily due to inactivation of the enzyme, in agreement with previous reports (40-42). We also sought to examine the effect of dapsone, another inhibitor of LPO/H2O2 and MPO/H2O2/Cl– systems (43-46), but its inhibitory capacity could not be precisely established due to its own absorption in the analytical region of the spectrum.
EPR measurements of radicals from β2-agonists
Phenoxyl radicals are thought to be the primary metabolites of the enzymatic oxidation of phenols. Formation of these radicals has been confirmed by EPR using tyrosine as a substrate (47,48) and is consistent with the formation of tyrosine dimers through radical-radical coupling. We thus sought to verify whether peroxidative metabolism of phenolic β2-agonists also generates corresponding radicals. When fenoterol was exposed to MPO and H2O2 in pH 7.0 buffer containing the spin trap MNP, an intense EPR spectrum attributed to a MNP adduct with fenoterol-derived radical was detected (aN = 1.49 mT) (Fig. 11A). In contrast, with salbutamol only a weak EPR signal of a MNP adduct was observed after 15 min reaction (Fig. 11B). No radicals were detected at pH 7.0 when MNP was omitted in any sample.
Figure 11.
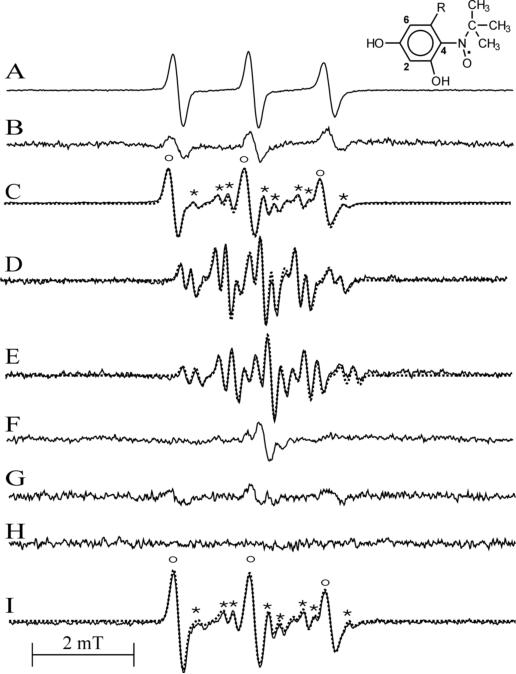
EPR spectra of radicals generated by oxidation of β2-agonists by MPO and LPO systems. A. Fenoterol (1.44 mM) oxidized by MPO (0.19 U/mL) and H2O2 (113 μM) in pH 7.0 buffer containing MNP. The structure shown is that of a MNP/fenoterol radical adduct. B. Salbutamol (2.28 mM) oxidized by MPO (0.38 U/mL) and H2O2 (113 μM) in pH 7.0 buffer containing MNP. C. Fenoterol (2 mM) oxidized by MPO (0.4 U/mL) and H2O2 (99 μM) at pH 8.0 in the presence of MNP. The spectrum is a composite of two spectra: of a MNP adduct (labeled o) and a fenoterol-derived semiquinone radical (labeled *). The dotted line is a simulated spectrum. D. Observed spectrum of a radical derived from fenoterol in the absence of MNP. Dotted line is a simulated spectrum E. Oxidation of fenoterol (2 mM) by LPO (0.425 μM) and H2O2 generated by glucose (1 mM) and glucose oxidase (4 μg/mL) at pH 8.0. F. Oxidation of salbutamol (4.7 mM) by MPO (0.94 U/mL) and H2O2 (0.43 mM) at pH 8.0. G. Oxidation of salbutamol (2 mM) by MPO (0.4 U/mL) and H2O2 (99 μM) at pH 8.0 in the presence of MNP. H. Spectrum recorded when MNP alone was exposed to MPO and H2O2 at pH 8.0. Buffers contained DTPA (0.2 mM) and, where indicated, MNP (10 mM in dimers). Instrumental settings: microwave power 20 mW (41 mW in B), modulation amplitude 0.1 mT (0.2 mT in B and F), receiver gain 2 × 104 (A), 2 × 105 (2.5 × 105 for E), time constant 40.96 ms, conversion time 40.96 ms, sweep time 10 mT/41.94 s. Sample volume 250 μL. The intensity of original spectra A and C were decreased by a factor 0.2 and spectrum E by 0.5. The spectra shown are average of 5 scans and represent typical results.
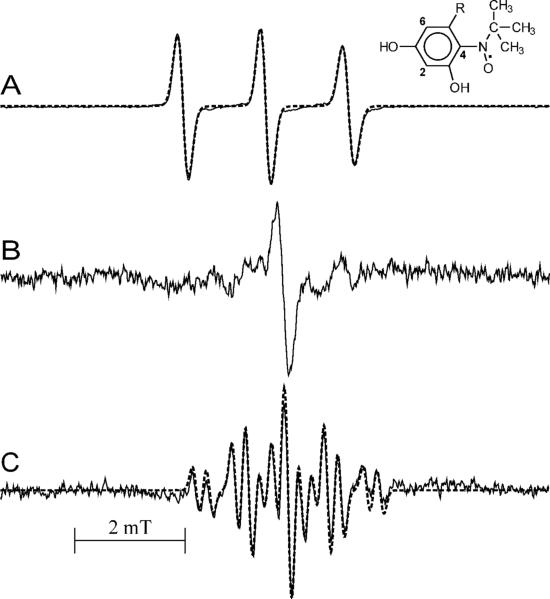
The formation of radicals from agonists shows pH dependence. When fenoterol was exposed to MPO and H2O2 in pH 8 buffer containing the spin trap MNP, the EPR spectrum shown in Fig. 11C was observed. The spectrum contains contribution from two species, one is an MNP adduct, a triplet with aN = 1.500 mT (labeled as “o”) and the other species, characterized by a multi-line EPR spectrum (labeled as “*”) has been attributed to a fenoterol-derived radical. The latter assignment was confirmed by an experiment conducted in the absence of MNP, in which an EPR spectrum of the fenoterol radical alone was observed (Fig. 11D). Initial simulations of this spectrum were performed assuming that the species is a meta-semiquinone formed via the one-electron oxidation of the resorcinol moiety of the drug. The characteristic feature of such a radical would be the presence in its EPR spectrum of a triplet of doublets due to interaction of the unpaired electron with two equivalent protons (at C4,6) and one non-equivalent proton at C2 in the ring (49). Although the spectrum calculated using this assumption reproduced well the positions of hyperfine components, amplitudes of the outermost lines did not match those of the experimental spectrum (not shown). Also the splitting on the two equivalent protons (0.67 mT) is smaller than that determined for meta-semiquinones derived from substituted resorcinols, 1.1 mT and 0.99 mT (in alkaline and acidic solutions, respectively) (49). Finally, due to their reactivity, meta-semiquinones cannot be detected using static EPR, a technique that was used in this study. These collective findings thus suggested that the spectrum detected is not that of the primary metabolite, (i.e., a fenoterol meta-semiquinone), but rather of a secondary radical product. A markedly better fit was obtained when the triplet components were calculated assuming splitting on nitrogen atom instead on two equivalent protons. The dotted line in Fig. 11D is a simulated spectrum calculated using the following hfsc's: 0.678 mT (1N), 0.190 mT (1H), 0.700 mT (1 H) and 0.889 mT (1H) (correlation coefficient 0.974). A simulated composite spectrum in Fig. 11C (dotted line) fitting well the experimental one (solid line) was obtained using hfsc's for the MNP adduct (1.495 mT (1N)) and for the fenoterol radical (0.698 mT (1N), 0.180 mT (1H), 0.6565 mT (1H), 0.903 mT (1H) (correlation coefficient 0.996)). Because there is only one nitrogen atom in the molecule, in the side chain at C5 (Fig. 1), the splitting on nitrogen is suggestive of intramolecular cyclization through the amino nitrogen adding to the resorcine moiety to form a fused 5-member ring. No radicals were detected when MNP alone was exposed to MPO and H2O2 (Fig. 11H).
To obtain better insight into the mechanism of formation and the structure of the radical formed by oxidation of fenoterol, similar experiments were conducted with its analogs, terbutaline and metaproterenol. Because terbutaline contains the resorcinol moiety, it was expected that its oxidation would generate EPR spectra similar to that of fenoterol. However, oxidation of terbutaline by MPO and H2O2 in the presence of MNP at pH 8.0, afforded only an EPR spectrum of a MNP adduct (aN = 1.505 mT) (Fig. 12A). Oxidation of terbutaline by LPO and H2O2 (generated by glucose/glucose oxidase) in the presence of MNP yielded spectrum similar to that produced by theMPO/H2O2 system (Fig. 12A). Given the similarity of the EPR spectra of MNP adducts derived from terbutaline and fenoterol, and the fact that terbutaline does not contain a monophenolic group, this result suggests that MNP adducts are formed by addition of the spin trap to a radical site located on the resorcinol ring. Because the highest electron density in meta-semiquinones has been found in positions 4 and 6 (49), addition of MNP at these positions seems to be preferred.
Figure 12.
A. EPR spectrum of radicals generated by oxidation of terbutaline (2 mM) by MPO (0.4 U/mL) and H2O2 (99 μM) in the presence of MNP (10 mM) at pH 8.0. Dotted line is a simulated spectrum using hfsc's for the MNP adducts (1.505 mT (1N)). The structure shown is that of a MNP/terbutaline radical adduct. B. EPR spectrum observed during oxidation of terbutaline (4 mM) by MPO (0.8 U/1 mL) and H2O2 (390 μM) at pH 8.0 with MNP omitted. C. EPR spectrum generated by oxidation of metaproterenol (2 mM) by MPO (0.1 U/mL) and H2O2 (360 μM) in Tris/HCl buffer (0.1 M, pH 9.19). Dotted line is a simulated spectrum.
Oxidation of terbutaline by MPO/H2O2 in the absence of MNP generated a non-characteristic EPR spectrum of low intensity (Fig. 12B). This spectrum is devoid of features typical for meta-semiquinones suggesting that it too may belong to a secondary radical product. This indicates that the secondary radicals from terbutaline are different from those produced by oxidation of fenoterol (Fig. 11D). Given this observation and the structural differences between these two agonists, we speculated involvement of the quaternary carbon (-C(CH3)3) in the terbutaline side chain, next to the amine group (-NH-), which prevents transformation of the compound to the fenoterol-like radical, since in contrast to terbutaline, fenoterol possesses a tertiary carbon, (-CH(CH3)CH2PhOH), at this position (Fig. 1). This was confirmed by the observation that oxidation of metaproterenol, a terbutaline analog containing a tertiary carbon (-CH(CH3)2) attached to the amino nitrogen (Fig. 1), generates a radical with an EPR spectrum (Fig. 12C), similar to that from oxidized fenoterol (Fig. 11D).3 Also for this radical the simulated spectrum (dotted line in Fig. 12C) fits better the experimental one, if splitting on nitrogen is included (hfsc's: 0.717 mT (N), 0.244 mT (H), 0.953 mT (H), 0.719 mT(H). These observations indicate that metaproterenol and fenoterol undergo a similar oxidative transformation. In contrast, the terminal quaternary carbon in terbutaline hinders transformation of the initial radical to the secondary, fenoterol-like radical species. Together, results obtained with fenoterol, terbutaline and metaproterenol suggest that the nature of the substituent at the amino nitrogen determines the structure of the final oxidation product.
When salbutamol was exposed to MPO/H2O2 in pH 8 buffer a weak tri-line spectrum was detected (Fig. 11F). This signal is putatively from a secondary radical derived from the drug probably from a dimer similar to the radical from oxidized di-tert-butyl-hydroxyanisole (50). When the same experiment was performed in the presence of MNP, an EPR signal of MNP adduct of low intensity was detected (Fig. 11G), suggesting that either oxidation of this agonist is slow and/or that the salbutamol radical adds inefficiently to MNP. Nonetheless, these results together confirm that oxidation of salbutamol proceeds through a free radical intermediate.
We verified by EPR that oxidation of β2-agonists by LPO/H2O2 also generates free radicals. Oxidation of fenoterol by LPO and H2O2, generated by glucose/ glucose oxidase in the presence of MNP, gives rise to both a MNP adduct (o) and the fenoterol radical (*) (Fig. 11E). A well fitted simulated spectrum (dotted line) was obtained using hfsc's for the fenoterol-derived radical 0.6836 mT (1N), 0.187 mT (1H), 0.9054 mT (1H) and 0.6927 mT (1H) and for the triplet from the MNP adduct 1.495 mT (1N) (correlation coefficient 0.992), confirming that LPO and MPO oxidize fenoterol to the same type of free radical. Also, oxidation of terbutaline by LPO/glucose/glucose oxidase in the presence of MNP afforded a MNP adduct whose EPR spectrum is similar to that generated by MPO/H2O2 (Fig. 12A).
Effect of peroxidase inhibitors on free radical formation - EPR study
We investigated the effect of methimazole and dapsone on radical formation from fenoterol and terbutaline by measuring EPR spectra of their MNP adducts. Table 3 summarizes the results obtained at pH 8.0. Methimazole (0.86 mM) and dapsone (1 mM) markedly inhibited the formation of radicals from fenoterol and terbutaline oxidized by LPO/glucose/glucose oxidase (or H2O2 reagent). When the MPO system was used, only methimazole was inhibitory. This is consistent with the known inhibitory action of both methimazole and dapsone on LPO activity and methimazole on MPO activity only (46). In contrast, dapsone has only a minimal effect on MPO activity. Results obtained at pH 7.0 qualitatively agree with those at pH 8.0 (not shown).
Table 3.
Effect of dapsone and methimazole on EPR spectra of MNP adducts with radicals from fenoterol and terbutaline generated by MPO/H2O2 and LPO/H2O2 systems at pH 8.0. Concentrations of MNP spin adducts were calculated as second integrals of the low-field component of the respective spectra and are expressed as % of control samples (no additives). Mean ± SE of at least two measurements.
| Fenoterol | ||
| + MPO + H2O2 | + LPO + H2O2 | |
| − No additives | 100a | 100b |
| + Dapsone (1 mM) | (c) | 13.9 ± 0.6a |
| + Methimazole (0.86 mM) | 22.4 ± 0.6a | 26.9 ± 8.2b |
| Terbutaline | ||
| + MPO + H2O2 | + LPO + H2O2 | |
| − No additives | 100a | 100b |
| + Dapsone (1 mM) | (c) | 54.6 ± 20.0a |
| + Methimazole (0.86 mM) | 33.5 ± 3.5a | 33.9b |
In experiments with methimazole concentrations of fenoterol and terbutaline were 0.47 mM and in experiments with dapsone 0.047 mM. The activity of MPO was 500 mU/1 mL. The concentration of LPO was 72 nM.
The oxidant was the reagent H2O2 (37 μM).
H2O2 was generated using glucose (1 mM) and glucose oxidase (0.8 μg/mL).
Not determined as dapsone does not inhibit MPO.
Discussion
This study shows that β2-agonists salbutamol, fenoterol and terbutaline undergo oxidation by MPO and LPO systems, which may be pertinent to their fate in asthmatic airways, for which significant peroxidase activities have been reported (4-9). The reaction was totally dependent on simultaneous presence of peroxidases and H2O2, was abolished by catalase and was inhibited by heme poisons (azide and cyanide) indicating that oxidation of these β2-agonists requires an active peroxidase. Because these agonists contain phenolic groups, their metabolism seems to be best described by the process that is typical for peroxidative oxidation of phenols with phenoxyl radicals as primary metabolites (Eqs 1-3). Our observations are also consistent with results of studies on oxidation of tyrosine and other phenolics by peroxidase systems (15,17,18,23,28).
For LPO, the effect of β2-agonists on the peroxidative cycle was readily observed. Thus, while in the absence of the agonists, a small dose of H2O2 induced formation of LPO-II lasting for 14 min, in the presence of salbutamol (100 μM) the lifetime of LPO-II was dramatically shortened, to less than 45 s (Fig. 9A, B). Only during the brief period, during the formation and decay of LPO-II was salbutamol oxidized, as evidenced by the momentary increase in A315 (Fig. 9B). The fact, that LPO-II was detected even in the presence of 1 mM salbutamol (i.e., when [salbutamol] >>> [H2O2]) indicates that the interaction of the drug with LPO-II is the slowest step. In contrast to salbutamol, fenoterol appeared to be a markedly more effective substrate, since in its presence LPO-II did not accumulate in detectable quantities, suggesting that it was rapidly reduced to ferric LPO (Fig. 9C).4
Oxidation of salbutamol by LPO was also observed when H2O2 was generated by glucose/glucose oxidase. In this system the rate of H2O2 generation could be regulated by the amount of glucose oxidase applied, and could be adjusted so that all H2O2 produced, was “immediately” used up for oxidation of the substrate, without apparent accumulation of LPO-II.
The formation of the agonists-derived free radicals during their oxidation by peroxidases has been verified by EPR and spin trapping with MNP, and by their stimulatory action on peroxidative oxidation of glutathione and ascorbate, a process known to be catalyzed by phenolics. The putative stable products of the oxidation of β2-agonists are, among other possible products, dimers formed by recombination of phenoxyl radicals. This transformation may be especially pertinent to salbutamol, as this agent possesses one phenolic moiety, and its metabolism seems to resemble that of tyrosine, known to produce o,o’-dityrosine dimers upon enzymatic oxidation. It has to be emphasized however, that in contrast to tyrosine, formation of salbutamol dimers may be restricted by the presence of a substituent (-CH2OH) in one ortho-position in the drug's aromatic ring, which decreases the number of possible radical sites that can participate in recombination.
Fenoterol is unique among the β-agonists investigated in that it possesses both the mono- and 1,3-dihydroxybenzene moieties and may thus be subjected to peroxidative metabolism characteristic of a simple phenolic and that of meta-hydroquinones. Using terbutaline as a model compound we found that when oxidized it forms adducts with MNP whose EPR spectra are very similar to those generated by oxidation of fenoterol. Based on this result we conclude that MNP traps the radical located on resorcinol ring, most likely by adding at C4 or C6, which are the sites of the highest spin density. Formation of MNP adducts with the radical from the fenoterol mono-phenolic group was not detected, but if formed, their contribution might be small, judging by the low intensity of EPR spectra generated by oxidation of salbutamol. It has been reported that alkyl resorcinols undergo oxidation by peroxidases and form dimeric products through addition of the corresponding phenoxyl radicals (28). Thus, theoretically, peroxidative metabolism of fenoterol may lead to dimers through recombination of two types of phenoxyl radicals: (1) those derived from mono-phenolic moiety (to form di-tyrosine type dimers) and (2) those derived from meta-semiquinone-type radicals (to form di-resorcinols). Formation of mixed type dimers may also be possible.
We found that AscH– and GSH at concentrations approximating those in airway fluids (∼100 μM) (30) inhibit oxidation of β2-agonists. During this reaction AscH– and GSH are oxidized to their respective radicals as documented by our EPR data. Absorption spectra measured during oxidation of fenoterol in the presence of albumin indicated formation of an albumin-agonist conjugate. A similar conjugate was probably formed when fenoterol and terbutaline were oxidized in the presence of GSH as judged by appearance of a characteristic absorption bands at 395 nm and 360 nm, respectively, that were not observed when GSH was omitted. Formation of such conjugates with thiols has been described for other quinonoid compounds (34,35). Thus, GSH plays a dual role during oxidation of polyphenols. First it functions as a reducing agent, converting semiquinones back to hydroquinones and secondly it may acts as an alkylating agent (Scheme 1B). It is therefore possible that if the oxidative metabolism of β2-agonists occurs in vivo it may contribute to depletion of cellular antioxidants and promote oxidative injury in tissues.
We found that oxidation of fenoterol and metaproterenol, but not terbutaline, afforded radicals that could be detected by direct EPR. Spectral analysis of these radicals suggested that these are not primary semiquinones formed by one-electron oxidation of resorcinol moieties in fenoterol and metaproterenol, but rather secondary radicals formed by oxidation of as yet not identified transient products derived from these drugs. Because the best fitted simulated spectra were obtained including splitting on nitrogen atom, it was tentatively assumed that the new radical was formed by the amino nitrogen in the side chain at C5 adding to the resorcinol ring in oxidized fenoterol and metaproterenol, forming a fused 5-member ring, followed by further oxidation. Such a transformation is known to occur for catecholamines, in which the two hydroxy groups are in ortho-position and can form electrophilic ortho-quinone. Meta-hydroquinones cannot form quinones, although formation of bi-radicals having a meta-quinone character has been considered (51). However, if formed, such a molecule would be highly electrophilic and after hydrolysis might produce 2-hydroxy-1,4-benzoquinone type chromophore, for which intramolecular cyclization would be possible. Formation of 2-hydroxy-1,4-benzoquinone from resorcinol has been reported to occur through enzymatic conversion (52,53) and reaction of the resorcinol semiquinone with oxygen (29). The observation that the rate and the extent of oxidation of fenoterol depend on oxygen (they both decrease in deaerated solutions) partially supports the latter mechanism. Hydroxylation of phenolics by MPO compound III has also been reported (54). Thus, hydroxylation of the resorcinol ring in fenoterol, terbutaline and metaproterenol through an enzymatic or chemical process seems feasible. Oxidation of terbutaline probably did not lead to the corresponding fenoterol-type radical, or the reaction was inefficient, possibly because the bulky nature of the substituent on the amino nitrogen (quaternary carbon) prevented the cyclization.
It can be hypothesized that structural modifications of β-agonists catalyzed by peroxidases, such as formation of dimers, cyclization, or formation of conjugates with GSH and albumin may impact their therapeutic activity, which is dependent on their interaction with β2-adrenergic receptors on smooth muscle cells, because their affinity to these receptors will be altered, presumably diminished. Because of this, it was important to find out whether oxidation of β2-agonists by peroxidases can be prevented or at least inhibited. For this purpose we investigate two compounds methimazole and dapsone. Methimazole acts as a suicidal inhibitor of thyroid peroxidase and LPO (40,55) and is used in the treatment of hyperthyroid conditions. Methimazole is oxidized by MPO to a free radical product without inactivation of the enzyme (56). Dapsone, an anti-inflammatory and an anti-leprotic drug, was used as a corticosteroid sparing agent in the treatment of asthma (57). Dapsone inhibits LPO activity (46), but is neutral with respect to MPO, unless chloride is also a substrate (43). Our spectrophotometric measurements revealed that methimazole inhibits oxidation of salbutamol and fenoterol by the LPO system. This was further supported by EPR studies, which showed that methimazole and dapsone inhibit generation of free radicals from fenoterol and terbutaline by LPO/H2O2, thus preventing their transformation to products with altered reactivity. Methimazole also effectively inhibited oxidation by MPO, whereas dapsone did not. However, dapsone may have potential to inhibit modifications such as chlorination and nitration, β-agonists may also be subject to. Moreover, these results suggest the intriguing possibility that peroxidase inhibitors may have potential to prevent or minimize degradation of β-agonists in asthmatic airways.
In summary, we have found that salbutamol and fenoterol are metabolized by endogenous airway peroxidases, (MPO and LPO), to free radicals, which potentially give rise to structurally modified products. Salbutamol and fenoterol differ markedly in their capacity to undergo oxidation by the peroxidases as well as in the reactivity of their resulting metabolites, with fenoterol being more susceptible to oxidation and affording more reactive intermediates. Because the therapeutic activity of these drugs depends on their binding to β2-adrenergic receptors on smooth muscle cells, structural modifications resulting from oxidation are likely to inhibit the interaction with their cognate receptor, resulting in diminished therapeutic efficacy. Peroxidase mediated oxidation and consequent drug inactivation, particular when airway inflammation is severe, may thus be a mechanism that renders patients refractory or resistant to the bronchodilatory effects of β2-agonists.This oxidative transformation, however, can be inhibited by physiological antioxidants/substrates (ascorbate, glutathione, thiocyanate) and pharmacological inhibitors of peroxidase (methimazole and dapsone), suggesting that such agents may offer means to prevent and/or enhance the therapeutic activity or other functions of the agonists in clinical setting.
Acknowledgements
This study was supported by grant from NIH (HL072068 to DWM).
Footnotes
Abbreviations: β2AR, β2-adrenergic receptors; ABTS, 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid); AscH–, ascorbate; Asc•–, ascorbate radical; BSA, bovine serum albumin; DMPO, 5,5′-dimethyl pyrroline N-oxide, spin trap; DTPA, diethylenetriamine pentaacetic acid; EPO, eosinophil peroxidase; EPR, electron paramagnetic resonance; GlOx, glucose oxidase; hfsc's, hyperfine splitting constants; LPO, lactoperoxidase; MNP, 2-methyl-2-nitrosopropane, spin trap; MPO, myeloperoxidase; PMN, neutrophils; TyrOH, tyrosine; TyrO•, tyrosyl radical.
We note that Br–, co-present with fenoterol, may participate in this reaction and contribute to the rapid enzyme turnover.
The multilane EPR spectrum detected during oxidation of metaproterenol was preceded by a singlet spectrum (not shown).
See footnote 2.
References
- 1.Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, Boivin JF, McNutt M, Buist AS, Rebuck AS. The use of beta-agonists and the risk of death and near death from asthma. N. Engl. J. Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, Blais L, McNutt M, Buist AS, Spitzer WO. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am. J. Respir. Cri.t Care Med. 1994;149(3 Pt 1):604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 3.Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am. J. Respir. Med. 2003;2(4):287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, Town GI, Kettle AJ. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic. Biol. Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
- 5.Duguet A, Iijima H, Eum SY, Hamid Q, Eidelman DH. Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2001;164:1119–1126. doi: 10.1164/ajrccm.164.7.2010085. [DOI] [PubMed] [Google Scholar]
- 6.Wu WJ, Samoszuk MK, Comhair SAA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreadis AA, Hazen SL, Comhair SAA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic. Biol. Med. 2003;35(3):213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage fluid in subjects with mild asthma. Am. Rev. Respir. Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Jeffery PK, Busse W, Johnson M, Vinola AM. Asthma: from bronchoconstriction to airway inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 10.Yasui K, Kobayashi N, Yamazaki T, Agematsu K, Matsuzaki S, Nakata S, Baba A. Differential effects of short-acting beta2-agonists on human granulocyte functions. Int. Arch. Allergy Immunol. 2006;139(1):1–8. doi: 10.1159/000089516. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana A, Kato M, Kimura H, Fujiu T, Suzuki M, Morikawa A. Inhibition by fenoterol of human eosinophil functions including beta2-adrenoceptor-independent actions. Clin. Exp.Immunol. 2002;130(3):415–23. doi: 10.1046/j.1365-2249.2002.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillissen A, Jaworska M, Scharling B, van Zwoll D, Schultze-Werninghaus G. Beta-2 agonists have antioxidant function in vitro. 1. Inhibition of superoxide anion, hydrogen peroxide, hypochlorous acid and hydroxyl radical. Respiration. 1997;64:16–22. doi: 10.1159/000196637. [DOI] [PubMed] [Google Scholar]
- 13.Gillissen A, Wickenburg D, van Zwoll D, Schultze-Werninghaus G. Beta-2-agonists have antioxidant function in vitro. 2. The effect of beta-2-agonists on oxidant mediated cytotoxicity and on superoxide anion generated by human polymorphonuclear leukocytes. Respiration. 1997;64:23–28. doi: 10.1159/000196638. [DOI] [PubMed] [Google Scholar]
- 14.Sawahata T, Neal RA. Horseradish peroxidase-mediated oxidation of phenol. Biochem Biophys Res Coomuns. 1982;109(3):988–994. doi: 10.1016/0006-291x(82)92037-x. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Taylor KE, Zou H, Biswas N, Bewtra JK. Phenol conversion and dimeric intermediates in horseradish peroxidase-catalyzed phenol removal from water. Environ Sci Technol. 1994;28:2154–2160. doi: 10.1021/es00061a025. [DOI] [PubMed] [Google Scholar]
- 16.Bayse GS, Michaelis AW, Morrison M. The peroxidase catalyzed oxidation of tyrosine. Biochim. Biophys. Acta. 1972;284:34–42. doi: 10.1016/0005-2744(72)90043-5. [DOI] [PubMed] [Google Scholar]
- 17.Marquez LA, Dunford HB. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. J. Biol. Chem. 1995;270(51):30434–30440. doi: 10.1074/jbc.270.51.30434. [DOI] [PubMed] [Google Scholar]
- 18.Heinecke JW, Li W, Daehnke HL, III, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloproxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993;268(6):4069–4077. [PubMed] [Google Scholar]
- 19.Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tien M. Myeloperoxidase-catalyzed oxidation of tyrosine. Arch. Biochem. Biophys. 1999;367(1):61–66. doi: 10.1006/abbi.1999.1226. [DOI] [PubMed] [Google Scholar]
- 21.Metodiewa D, Reszka K, Dunford HB. Oxidation of the substituted catechols dihydroxyphenylalanine methyl ester and trihydroxyphenylalanine by lactoperoxidase and its compounds. Arch. Biochem. Biophys. 1989;274(2):601–608. doi: 10.1016/0003-9861(89)90475-x. [DOI] [PubMed] [Google Scholar]
- 22.Metodiewa D, Reszka K, Dunford HB. Evidence for a peroxidatic oxidation of norepinephrine, a catecholamine, by lactoperoxidase. Biochem. Biophys. Res. Commun. 1989;160(3):1183–1188. doi: 10.1016/s0006-291x(89)80128-7. [DOI] [PubMed] [Google Scholar]
- 23.Monzani E, Gatti AL, Profumo A, Casella L, Gullotti M. Oxidation of phenolic compounds by lactoperoxidase. Evidence for the presence of a low-potential compound II during catalytic turnover. Biochemistry. 1997;36:1918–1926. doi: 10.1021/bi961868y. [DOI] [PubMed] [Google Scholar]
- 24.Jenzer H, Jones W, Kohler H. On the molecular mechanism of lactoperoxidase-catalyzed H2O2 metabolism and irreversible enzyme inactivation. J. Biol. Chem. 1986;261(33):15550–15556. [PubMed] [Google Scholar]
- 25.Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solution in the UV). Anal. Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 26.Kalyanaraman B, Felix CC, Sealy RC. Photoionization of melanin precursors: an electron spin resonance investigation using the spin trap 5,5-dimethyl-1-pyrroline-1-oxide (DMPO). Photochem. Photobiol. 1982;36(1):5–12. [Google Scholar]
- 27.Childs RE, Bardsley WG. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Divi RL, Doerge DR. Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry. 1994;33:9668–9674. doi: 10.1021/bi00198a036. [DOI] [PubMed] [Google Scholar]
- 29.Perbet G, Filiol C, Boule P, Lemaire J. Photolyse et photo-oxydation des diphénols en solution aqueous diluée. Journal de Chimie Physique. 1979;76(1):89–96. [Google Scholar]
- 30.Cross CE, van der Vliet A, O'Neill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994;102(suppl 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturgeon BE, Sipe HJ, Jr., Barr DP, Corbett JT, Martinez JG, Mason RP. The fate of the oxidizing tyrosyl radical in the presence of glutathione and ascorbate. Implications for the radical sink hypothesis. J. Biol. Chem. 1998;273(46):30116–30121. doi: 10.1074/jbc.273.46.30116. [DOI] [PubMed] [Google Scholar]
- 32.Harman LS, Carver DK, Schreiber J, Mason RP. One- and two-electron oxidation of reduced glutathione by peroxidases. J. Biol. Chem. 1986;261(4):1642–1648. [PubMed] [Google Scholar]
- 33.Reszka KJ, Matuszak Z, Chignell CF, Dillon J. Oxidation of biological electron donors and antioxidants by a reactive lactoperoxidase metabolite from nitrite (NO2−): an EPR and spin trapping study. Free Radic. Biol. Med. 1999;26(56):669–678. doi: 10.1016/s0891-5849(98)00244-5. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Schreiber J, Fischer V, Mason RP. Formation of glutathione-conjugated semiquinones by the reaction of quinines with glutathione: an ESR study. Arch. Biochem. Biophys. 1987;252(1):41–48. doi: 10.1016/0003-9861(87)90006-3. [DOI] [PubMed] [Google Scholar]
- 35.Rao DNR, Takahashi N, Mason RP. Characterization of a glutathione conjugate of 1,4benzosemiquinone-free radical formed in rat hepatocytes. J. Biol. Chem. 1988;263(34):17981–17986. [PubMed] [Google Scholar]
- 36.Yamazaki I, Tamura M, Nakajima R, Nakamura M. Physiological aspects of free-radical reactions. Environ. Health Perspectives. 1985;64:331–342. doi: 10.1289/ehp.8564331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohler H, Taurog A, Dunford HB. Spectral studies with lactoperoxidase and thyroid peroxidase: interconversions between native enzyme, compound II, and compound III. Arch. Biochem. Biophys. 1988;264(2):438–449. doi: 10.1016/0003-9861(88)90309-8. [DOI] [PubMed] [Google Scholar]
- 38.Metodiewa D, Marquez LA, Dunford HB. The activity of mammalian peroxidases (lactoperoxidase and myeloperoxidase) and their compounds III toward 2-t-butyl-4-methoxyphenol (butylated hydroxyanisole) and its dimer (2,2'-dihydroxy-3,3'-di-t-butyl-5,5'-dimethoxydiphenyl). Biochem. Int. 1991;23(2):281–290. [PubMed] [Google Scholar]
- 39.Davidson B, Soodak M, Neary JT, Strout HV, Kieffer JD, Mover H, Maloof F. The irreversible inactivation of thyroid peroxidase by methylmercaptoimidazole, thiouracil, and propylthiouracil in vitro and its relationship to in vivo findings. Endocrinology. 1978;103(3):871–882. doi: 10.1210/endo-103-3-871. [DOI] [PubMed] [Google Scholar]
- 40.Ohtaki S, Nakagawa H, Nakamura M, Yamazaki I. Reactions of purified hog thyroid peroxidase with H2O2, tyrosine, and methylmercaptoimidazole (goitrogen) in comparison with bovine lactoperoxidase. J. Biol. Chem. 1982;257(2):761–766. [PubMed] [Google Scholar]
- 41.Michot JL, Nunez J, Johnson ML, Irace G, Edelhoch H. Iodide binding and regulation of lactoperoxidase activity toward goitrogens. J. Biol. Chem. 1979;254:2205–2209. [PubMed] [Google Scholar]
- 42.Edelhoch H, Irace G, Johnson ML, Michot JL, Nunez J. The effects of thioureylene compounds (goitrogens) on lactoperoxidase activity. J. Biol. Chem. 1979;254:11822–11830. [PubMed] [Google Scholar]
- 43.van Zyl JM, Basson K, Kriegler A, van der Walt BJ. Mechanisms by which clofazimine and dapsone inhibit the myeloperoxidase system. A possible correlation with their anti-inflammatory properties. Biochem. Pharmacol. 1991;42(3):599–608. doi: 10.1016/0006-2952(91)90323-w. [DOI] [PubMed] [Google Scholar]
- 44.Kettle AJ, Winterbourn CC. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem. Pharmacol. 1991;41(10):1485–1492. doi: 10.1016/0006-2952(91)90565-m. [DOI] [PubMed] [Google Scholar]
- 45.Bozeman PM, Learn DB, Thomas EL. Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem. Pharmacol. 1992;44(2):553–563. doi: 10.1016/0006-2952(92)90449-s. [DOI] [PubMed] [Google Scholar]
- 46.Thomas EL, Jefferson MM, Joyner RE, Cook GS, King CC. Leukocyte myeloperoxidase and salivary lactoperoxidase: identification and quantitation in human mixed saliva. J. Dent. Res. 1994;73(2):544–55. doi: 10.1177/00220345940730021001. [DOI] [PubMed] [Google Scholar]
- 47.McCormick ML, Gaut JP, Lin TS, Britigan BE, Buettner GR, Heinecke JW. Electron paramagnetic resonance detection of free tyrosyl radical generated by myeloperoxidase, lactoperoxidase, and horseradish peroxidase. J. Biol. Chem. 1998;273(48):32030–32037. doi: 10.1074/jbc.273.48.32030. [DOI] [PubMed] [Google Scholar]
- 48.Gunther MR, Tschirret-Guth RA, Witkowska HE, Fann YC, Barr DP, Ortiz De Montellano PR, Mason RP. Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem. J. 1998;330(Pt 3):1293–1299. doi: 10.1042/bj3301293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone TJ, Waters WA. Aryloxy-radicals. Part III. Electron spin resonance spectra of radicals from some substituted resorcinols. J. Chem. Soc. 1964:4302–4307. [Google Scholar]
- 50.Valoti M, Sipe HJ, Jr., Sgaragli G, Mason RP. Free radical intermediates during peroxidase oxidation of 2-t-butyl-4-methoxyphenol, 2,6-di-t-butyl-4-methylphenol, and related phenol compounds. Arch. Biochem. Biophys. 1989;269(2):423–432. doi: 10.1016/0003-9861(89)90126-4. [DOI] [PubMed] [Google Scholar]
- 51.Roithova J, Schröder D, Schwartz H. Generation of the elusive meta-benzoquinone in gas phase. Angew. Chem. 2005;117:3152–3156. doi: 10.1002/anie.200461783. [DOI] [PubMed] [Google Scholar]
- 52.Philipp B, Schink B. Evidence of two oxidative reaction steps initiating anaerobic degradation of resorcinol (1,3-dihydroxybenzene) by the denitrifying bacterium Azoarcus anaerobius. J. Bacteriol. 1998;180(14):3644–3649. doi: 10.1128/jb.180.14.3644-3649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallus C, Schink B. Anaerobic degradation of a-resorcylate by Thaurea aromatica strain AR-1 prceeds via oxidation and decarboxylation to hydroxyhydroquinone. Arch Microbiol. 1998;169:333–338. doi: 10.1007/s002030050579. [DOI] [PubMed] [Google Scholar]
- 54.Kettle AJ, Winterbourn CC. Superoxide-dependent hydroxylation by myeloperoxidase. J. Biol. Chem. 1994;269(25):17146–17151. [PubMed] [Google Scholar]
- 55.Bandyopadhyay U, Bhattacharyya D. Kr., Chatterjee R, Banerjee RK. Irreversible inactivation of lactoperoxidase by mercaptomethylimidazole through generation of a thiyl radical: its use as a probe to study the active site. Biochem. J. 1995;306:751–757. doi: 10.1042/bj3060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGirr LG, Jatoe SD, O'Brien PJ. Myeloperoxidase catalysed cooxidative metabolism of methimazole: oxidation of glutathione and NADH by free radical intermediates. Chem. Biol. Interact. 1990;73(23):279–295. doi: 10.1016/0009-2797(90)90009-c. [DOI] [PubMed] [Google Scholar]
- 57.Dewey A, Bara A, Dean T, Walters H. Dapsone as an oral corticosteroid sparing agent for asthma. Cochrane Database Syst. Rev. 2002;4:1–8. doi: 10.1002/14651858.CD003268. CD003268. [DOI] [PubMed] [Google Scholar]