Introduction
Monoclonal antibody (mAb) medications offer promising treatment for autoimmune disease, cancer, and a host of other diseases including substance abuse [1–3]. These medications have several advantages over traditional small molecule medicines. For example, mAbs have high affinity and precise selectivity for their disease targets, act as pharmacokinetic antagonists to block or blunt the action of their target antigen, and have an extremely long biological half-life (e.g., ~21 days for human IgG1) [4]. The long half-life is a particularly valuable pharmacological property since it allows for very long intervals between dosing. This long mAb half-life is usually assumed to result in an equally prolonged binding function, but direct evidence for prolonged function is often difficult to obtain.
The use of antibodies for treatment of drug abuse and addiction provides a novel therapeutic opportunity that would greatly benefit from a prolonged pharmacological action and less need for daily patient compliance. Toward this goal, our group has produced a significant number of mouse mAbs for use in preclinical studies of the acute and chronic treatments of adverse health effects associated with (+)-methamphetamine (METH) abuse [5–7]. The METH-specific mAb were produced using several METH-like haptens, coupled to a carrier protein. These METH antigens are useful for active immunizations, in addition to generating mAb for passive immunization. Our METH-specific mAbs reverse or reduce the pharmacological actions of METH by pharmacokinetic antagonism, which includes high affinity mAb binding, significant reductions in METH brain concentrations, and substantial increases in METH blood concentrations.
METH’s primary sites of action are in the brain [8], and the drug has extensive extravascular distribution with an apparent volume of distribution (Vd) = 9 L/kg in rats [9]. In contrast, mAb medications are confined mostly to the plasma and extracellular fluid space, which has a Vd = 0.141 L/kg for mouse mAbs in rats [10]. This suggests the maximum theoretical reduction in the METH Vd in the presence of an anti-METH mAb would be about 64-fold. MAb high affinity binding is considered a critical mechanism for therapeutic action, and it is generally assumed that in vivo efficacy (and binding function) can be predicted by the in vitro mAb affinity (or KD value) for the target ligand. However, comparison of pharmacological data from acute and chronic in vivo studies with METH and various moderate-to-high affinity anti-METH mAbs has now lead us theorize that in vitro affinity measures are not always a good predictor of long-term efficacy.
To illustrate, METH-induced stimulant effects were acutely treated 30 min after METH dosing with equal amounts of a moderate (mAb6H8 KD = 250 nM) or a high (mAb6H4 KD = 11 nM) affinity anti-METH mAb [6]. MAb6H4 was significantly more effective than mAb6H8 at rapidly reversing the METH-induced locomotor activity. In a contrasting chronic study, rats were pretreated with a single dose of either mAb6H4 or mAb6H8 and then challenged with METH doses at various times points. One day after mAb treatment, the high affinity mAb6H4 was much more effective at antagonizing METH-induced locomotor effects than mAb6H8. However, neither mAb appeared to antagonize METH-induced effects during later METH challenges on experimental days 4 and 7. These short lived mAb effects are surprising since the reported terminal elimination half-life (t1/2λz) for mouse mAbs in rats is 8.4 days [10].
In stark contrast to the short duration of action of these anti-METH mAbs, an anti-phencyclidine mAb6B5 (PCP KD = 1.3 nM) produced in our laboratory alters in vivo PCP pharmacokinetics for up to 28 days in rats [11], and measurement of mAb-bound PCP concentrations indicates a “functional” PCP elimination half-life of 15.4 days. A single low dose of anti-PCP mAb can also antagonize adverse PCP-related health effects for at least two weeks, even at doses that are 1/100th the molar amount of the PCP body burden [12].
In the current studies, we examined in vitro immunochemical properties, in vivo pharmacokinetics, and the in vivo functional properties that contribute to the duration of action of five different anti-METH mAbs (Table 1). The results of the various short-term and chronic studies suggested that while the mAb clearance was not different among the mAbs, the binding function of most of the anti-METH mAbs was sustainably reduced over time in vivo, rendering them at least partially inactive. Nevertheless, one of the antibodies, mAb4G9, showed prolonged action and greater efficacy by all measures.
Table 1.
Immunochemical characteristics of anti-METH monoclonal antibodiesa
Methods
Drugs and Reagents
METH [(+)-N-methyl-1-phenylpropan-2-amine hydrochloride], AMP [(+)-1-phenylpropan-2-amine sulfuric acid], 4-OH METH [(+/−)-4-(2-methylaminopropyl)phenol hydrochloride], and 4-OH-(+)-AMP [(+/−)4-(2-aminopropyl)phenol hydrobromide] were obtained from the National Institute on Drug Abuse (Rockville, MD). [3H]-(+)-METH ((+)-[2′,6′-3H(n)]methamphetamine; 23.5 Ci/mmol) and (±)-[2,6-3H2(n)]-amphetamine ([3H]-(±)AMP; 45 Ci/mmol) were obtained from the Research Triangle Institute (Research Triangle Park, NC), as a gift from the National Institute on Drug Abuse. N-Succinimidyl[2,3-3H]propionate ([3H]NSP) was purchased from Amersham Biosciences (GE Healthcare, Piscataway, NJ). All other reagents were purchased from Sigma Aldrich (St. Louis, MO) unless noted otherwise.
Anti-METH mAb Production and Purification
Synthesis of METH-like haptens, hapten conjugation to carrier protein and immunization of mice, and production of hybridoma cell lines are described in previous studies [5, 13]. Methods for the large scale production of anti-METH mAb, purification and formulation were described previously [13]. Some of the mAbs were produced in a Wave bag bioreactor system (GE Healthcare, Piscataway, NJ). Briefly, DMEM media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) was used for hybridoma starter cells and bag growth conditions. Starter culture cells were grown to a cell density of 1.5 × 105 cells/ml and used to inoculate the wave bag at a 1:4 ratio of seed culture to media. Following inoculation, the mAbs were produced under the following conditions. The Wave bags were rocked at 10 rpm at an angle of 7 degrees and a temperature of 37° C. The culture media was sampled daily and analyzed for pH, pCO2, pO2, glucose and lactate on a Rapidlab 1265 blood gas analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Cell density was also analyzed daily by microscopy and hemocytometer. The cultures were grown until cell viability reduced to 1.5 × 105 cells/ml, typically about 7 to 10 days.
Immunological Characteristics of the mAbs
The cross-reactivity profile of mAbs generated against the various METH-like haptens and numerous structurally related and unrelated compounds was determined by radioimmunoassay (RIA) using the method of Peterson et al. [13], which was based in part on the method of Owens, et al. [14] for the study of anti-PCP mAbs. The isotype of the mAbs were determined using a mouse–hybridoma isotyping kit (Boehringer Mannheim Corporation, Indianapolis, IN). The isoelectric points (pI) of mAb6H8, and mab9B11 were estimated using the ProtParam program on the ExPASy proteomics server: http://expasy.org [15]. The pI of mAb6H4, mAb4G9 and mAb6H7 were determined by isoelectric focusing using Invitrogen Novex pH 3–10 gels according to the manufacturer s instructions.
Equilibrium Dialysis for Determination of mAb In Vitro Dissociation Constants (KD) in Rat Serum and Buffer
For determination of mAb KD values in serum, a saturation analysis was carried out using normal Sprague-Dawley rat serum after adding purified anti-METH mAb. We selected mAb6H4, mAb4G9 and mAb9B11for KD determination in serum. We previously determined these KD values in buffer by RIA [13]. Preliminary studies were conducted with each mAb to determine the linear range of mAb protein binding concentration and the amount of mAb protein needed for the analysis. The mAb concentrations for the studies were selected for each analysis based on the midpoint of the linear range of [3H]-METH binding and was then used for the final KD determination. This experiment was also used to determine the time needed to reach equilibrium.
Dialysis membranes (6,000 MWCO) were soaked in water for 1 hr and then in 20% ethanol for a minimum of 20 min to rehydrate the membranes. Prior to use, membranes were rinsed with water, followed by Sorensen s Buffer (0.13 M sodium phosphate, pH 7.4). This high salt buffer was chosen to prevent osmotic volume shifts from one side of the membrane to the other in the presence of serum proteins. The 96-well equilibrium dialysis apparatus was assembled according to manufacturer s instructions (HT Dialysis, Gales Ferry, CT). Radiolabeled METH in Sorenson s buffer (5 μl) was added to 50 μl of normal rat serum (Pel-Freeze, Rodgers, AR) containing the mAb to be tested. [3H]-METH concentrations included a full range of concentrations above and below the KD values previously determined by RIA. The samples were then placed in one side (mAb side) of the dialysis well. On the serum side of the well, 50 μl of normal rat serum (without mAb) was added so that nonspecific [3H]-METH binding was equal on both sides of the dialysis chamber. Because serum was on both sides of the membrane, it was necessary to buffer the serum pH value to the in vivo physiological value of 7.35 by adding 5 μl of 10X Sorensen s buffer to the 50 μl of serum on each side of the dialysis membranes. After sample loading, the tops of the wells were covered with an adhesive sealing film to prevent evaporation. The 96 well dialysis block was then incubated overnight (~18 hrs) at 37°C with gentle rocking. Aliquots (10 μl) from each side of the two-sided well were removed from the dialysis chambers and counted by liquid scintillation spectrometry. The amount of [3H]-METH decays per minute (DPM) bound to mAb was determined by subtracting the [3H]-METH on the serum only (“free”) side from the METH DPM on the serum plus mAb (total binding) side of the chamber. The DPM/μl was then converted to a nM concentration based on the specific activity of the METH (28.3 Ci/mmol). MAb KD values in pH 7.35 Sorensen s buffer were also determined as described for serum except Sorensen s buffer was used on both sides of the dialysis chambers instead of serum. All three mAbs were run in triplicate on three separate occasions in both buffer and serum.
Animals
Male Sprague-Dawley rats with indwelling jugular vein catheters (medical-grade polyurethane tubing; 0.025 in ID × 0.040 in OD) were obtained from Charles River Laboratories (Wilmington, MA). Animals were habituated to the environment for one week prior to the start of experiments. On the day before the studies began, the catheters were flushed with 0.2 ml sterile saline followed by 0.05 ml of saline containing 25 units of heparin. Catheters were maintained by flushing as described above, every 3–4 days. The rats were housed separately and fed each day with three food pellets (~20 g), which resulted in animal weights of 250–280 g throughout the experiment. All animal experiments were conducted with the prior approval of the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Tritium Labeling of anti-METH mAb
Each of the mAbs for the serum pharmacokinetic studies were labeled with [3H]-NSP as described in McClurkan et al. [16]. The resulting [3H]-mAb was used as a tracer dose to allow accurate determination of mAb concentration over time in the rats. Briefly, 250 μCi of [3H]-NSP was dried to remove solvents under a light stream of nitrogen in a siliconized tube. The mAb (1 mg in 250 μl of 0.1 M Sodium Borate, pH 8.5) was then added to the tube along with a stir bar. The reaction was stirred for 1 hr on ice. The reaction mixture was then passed over a G-25 gel filtration column (equilibrated with phosphate buffered saline, pH 7.4) to separate uncoupled [3H]-NSP from [3H]-mAb. Fractions containing [3H]-mAb were combined and dialyzed against antibody administration buffer (15 mM sodium phosphate, 150 mM sodium chloride, pH 6.5 or pH 7.3, depending on the isoelectric point of the mAb). These reactions yielded ~60% incorporation of [3H]-NSP.
MAb Pharmacokinetic Studies
For the studies of mAb pharmacokinetics, rats with dual jugular catheters were housed in metabolism cages. On day 0, rats (n = 4 per mAb group) were administered 100 mg/kg mAb along with a tracer dose of the [3H]-NSP labeled mAb (~30 μCi) via the left jugular vein catheter. Blood samples were collected at predetermined times over the next 31 days after mAb administration. For the 1, 2, 4, 8 hr and 1 day time points, 200 μl of blood was collected from the right jugular vein catheter. At later time points, larger blood volumes (250–500 μl) were collected. In some cases, the catheter would fail before the end of the 31 day study. In these cases, blood was collected from the tail vein. Preliminary validation studies showed no statistically significant differences in the amount of radioactivity in blood collected from the tail vein vs. the jugular catheter (data not shown). From the whole blood, 20 μl (in duplicate) was immediately placed in 5 ml liquid scintillation fluid (Ecoscint A, National Diagnostics, Atlanta, GA) and then counted by liquid scintillation spectrometry. The remaining blood was allowed to clot, centrifuged, and the serum fraction was then collected. Serum samples (10 μl in duplicate) were then counted for total radioactivity content by liquid scintillation spectrometry.
To confirm that the radioactivity in the serum samples was associated only with intact mAb, all serum samples were subjected to size exclusion chromatography on a TSKgel G2000SW XL high performance liquid chromatography (HPLC) column (7.3 mm ID × 30 cm; TOSOH Bioscience, Montgomeryville, PA) using a 50 mM NaPO4, 100 mM Na2SO4 (pH 6.7) buffer. The buffer flow rate was 1 ml/min, and the absorbance at 280 nm was monitored for serum protein peaks as an internal quality control parameter to ensure consistency of chromatography. The column was also calibrated under these conditions with protein standards, including mouse IgG mAb. Fractions (20 sec) were collected in Ecoscint A scintillation fluid. Each fraction was then vortexed and counted by liquid scintillation spectrometry for determination of tritium in the sample. In all cases, >90% of the radioactivity was associated with the mAb-containing fractions. We also performed 10% trichloroacetic acid precipitation on randomly selected samples and found that >95% of the radioactivity was associated with the precipitated pellet (data not shown), thus helping to validate the accuracy and reproducibility of the method. The concentration of [3H]-mAb from the HPLC quantitation was then used to calculate unlabeled mAb-equivalent concentrations in each serum sample. This unlabeled mAb concentration (in ng/ml) was determined by multiplying by the ratio of mAb dose/μCi labeled mAb.
Determination of In Vivo mAb Functional Half-lives
For these studies, rats (n = 4 per mAb group) with dual jugular vein catheters were housed individually in standard cages. Osmotic minipumps (2 week, Alzet, Durect Corp., Cupertino, CA) were prepared to deliver a METH (free base) dose of 5.6 mg/kg/day dissolved in sterile saline. The pumps were implanted subcutaneously between the scapulae under halothane anesthesia. METH levels reached steady state approximately 8–11 hrs after implantation.
About 16 hrs after pumps implantation, the rats were anesthetized with halothane. Blood samples (~250 μl) were immediately collected via the right jugular vein catheters for determination of pre-mAb METH serum steady state levels. The catheter was flushed with 200 μl sterile saline. A mAb dose (180 mg/kg, the same dose for all five mAb in this study) equimolar in binding sites to the body burden of METH was then administered via the left jugular vein catheter. Following the mAb dose, the catheter was flushed with 200 μl of saline. At 5 min after the mAb administration, a blood sample was collected via right jugular vein catheter. Blood samples (~350 μl) were collected via the right jugular catheter at 24 hrs, 4, 7, and 13 days after mAb administration. On day 13, rats were anesthetized with halothane and sacrificed by decapitation. Trunk blood and brain samples were immediately collected. After allowing blood to clot, serum was collected by centrifugation. Serum and brain samples were stored at −80°C until analysis. METH and AMP concentrations in serum and tissues were determined by LC-MS/MS analysis by a previously reported method from our laboratory [17].
Serum and Brain METH Disposition during the First 24 hrs after mAb Treatment
To better understand functional differences between mAb6H4 and mAb4G9, experiments were performed in groups of rats that were sacrificed at various time points up to 24 hrs after mAb treatment. METH and AMP concentrations were then determined in these serum and brain samples. These studies were performed essentially as described in the previous section except for the use of a 3 day osmotic pump (Alzet, Durect Corp., Cupertino, CA) to deliver the METH dose (5.6 mg/kg/day). Following mAb6H4 or mAb4G9 administration (same doses and time of administration as in the previous section), rats (n = 4–5 per time point) were sacrificed by decapitation at predetermined time points (1, 5, 30, 75, 120 min and 24 hr). Brains and serum samples were immediately collected and processed as described in the previous section.
In a parallel set of studies, the METH blood:serum ratio was determined over time. METH administration via sc 3-day osmotic pump and mAb doses were used as described in the previous section. Following mAb administration, a ~250 μl blood sample was taken at 1, 5, 30, 75,120 min and 24 hr. Of this sample, 100 μl of blood was transferred to a separate tube and 400 μl of 18 mΩ water was added, the sample was vortexed and then sonicated for 10 min. The remaining blood was centrifuged and serum was collected. The blood and serum samples were stored at −80°C until analysis.
Quantification of METH and AMP in Serum and Tissue
A 10–100 μl aliquot of each serum sample, along with internal LC-MS/MS standards for METH and metabolites (i.e., 4-OH-AMP, AMP, and 4-OH-METH) was treated with 100 μl of ice cold 20% (w/v) trichloroacetic acid (TCA). Prior to TCA treatment, all sample volumes were brought to 100 μl with normal rat serum. For the whole blood samples, 100 μl of the diluted blood was extracted as described for serum. Brains were homogenized with 4 volumes of ice cold water using a PowerGen 125 homogenizer (Fisher Scientific) for ~30–40 sec. A 100 μl aliquot of each tissue homogenate was treated as described for the serum analysis. Samples were vortex-mixed for 20 sec followed by 15 min on a rotating mixer at 4°C. After centrifugation at 22,200 x g for 5 min, the samples were filtered (0.45 μm nylon membrane) and transferred to vials for injection and analysis by LC-MS/MS. This LC-MS/MS procedure was previously validated and reported [17].
The METH concentrations in the brain samples (uncorrected for the blood remaining in brain vasculature) were then determined using equation 1: CMETH = (CH) * (VH)/(WT). CMETH is the concentration of METH (ng/g) in tissue (uncorrected for blood content), CH is the concentration of METH in the tissue homogenate in ng/ml, VH is the volume of homogenate, and WT is the weight of the tissue prior to homogenization. More precise METH and AMP concentrations were then calculated by correcting for the amount of METH and AMP present in the blood contained in the brain sample. For calculation of the drug concentrations in brains collected on day 13, blood drug concentrations were estimated using equation 2: Cb = C * (1 + H[fu* ρ − 1]) from Rowland and Tozer [18], where Cb = blood drug concentration, C= serum drug concentration, H = hematocrit, fu = fraction of unbound drug, and ρ = a measure of affinity of drug for red blood cells (determined from the blood to serum drug ratio in the absence of mAb). For the brain concentrations in the 24 hr tissue distribution study with mAb6H4 and mAb4G9, blood concentrations were known from the blood to serum ratio experiment. The corrected concentration of METH in the brains was calculated using equation 3: CMETH(cor) = [CMETH − (Cb) * (Vf)b]/[1 − (Vf)b] [adapted from 19]. CMETH(cor) and Cb are the corrected tissue (ng/g) and blood (ng/ml) METH concentrations, respectively and CMETH is from equation 1. The volume fraction of blood, (Vf)b, remaining in brain was obtained from Khor and Mayersohn [19].
The following calculations were used for determination of the percentage of the METH dose in the brain during METH infusion steady state conditions. The body burden of METH at steady state from a 5.6 mg/kg/day infusion dose was calculated to be 0.35 mg/kg by converting the infusion dose to mg/kg/min and dividing by the elimination rate constant for METH (λn = 0.011/min). We then multiplied the experimentally determined brain tissue METH concentration by the total rat brain weight, which averages 1.5 g in a 250 g male Sprague-Dawley rat.
Equilibrium Dialysis for Determination of Serum METH Binding
These studies utilized the equilibrium dialysis unit described in a previous section. Sorensen s buffer (55 μl) was added to one side of the dialysis well, which is divided by the dialysis membrane. [3H]-METH (~50,000 DPM in 5 μl) was added to the serum samples (50 μl), and the serum samples were then placed in the other side of the dialysis well. The fraction of unbound METH was determined by dividing the unbound radioactivity on the buffer side by the total radioactivity from the serum side. We also determined AMP serum protein binding in mAb4G9 samples. MAb4G9 was the only mAb in these studies with significant cross reactivity to AMP [13]. These studies were conducted as described for [3H]-METH, except [3H]-AMP was used.
Pharmacokinetic Analysis
All pharmacokinetic analyses were performed using WinNonlin version 5.0 (Pharsight Corporation, Mountain View, CA). To determine the serum pharmacokinetic parameters of individual anti-METH mAbs, the average concentration-versus-time curves for mAb were analyzed by model-dependent methods using nonlinear least-squares fitting. A curve was fit to the data points using a two- and three-compartment i.v. bolus model, with no weighting, 1/y, or 1/y2 weighting. The best-fit line was chosen by visual inspection and analysis of the residuals. For the METH serum data in the presence of various mAb, the area under the METH serum concentration time curve (AUC) from time of mAb administration until the end of the study (13 day; ) were determined using the linear trapezoidal rule and Origin Graphing and Analysis software version 7.0 (OriginLab Corp., Northampton, MA).
Statistical Analysis
Comparisons of in vitro mAb KD values determined in serum or buffer and mAb pharmacokinetic parameters determined in vivo were accomplished using a one-way analysis of variance. When significant differences were found, a Bonferroni s t-test for all pairwise comparisons was used. For comparisons of serum METH and AMP concentrations in the mAb functionality study between control (pre-mAb and no mAb) and mAb treated groups, a two-way repeated measures analysis of variance with the levels treatment and time was used. If significant differences were found, a Tukey s test was used to make all pairwise comparisons. In the study of mAb6H4 and mAb4G9 effects on brain and serum METH and AMP levels in the 24 hr serum and brain study, a two-way analysis of variance with a post-hoc Tukey s test was used to compare mAb6H4 and mAb4G9 groups at each time point. Separate one-way ANOVAs followed by a post-hoc Dunnett s test were conducted to compare METH and AMP brain and serum levels between mAb treatment groups with a no mAb control group. A significance level of p<0.05 was used for all studies.
Results
In vitro Determination of mAb KD Values in Serum and Buffer
Our previous behavioral studies suggested anti-METH mAb6H4 lost activity in vivo (see introduction) and led us to theorize that exposure to the serum matrix could affect mAb affinity. Therefore, we determined in vitro METH KD values of three representative anti-METH mAbs in buffer and serum by equilibrium dialysis. While determination of KD values by equilibrium dialysis yielded different values from the previously determined RIA values, we found no statistically significant differences in KD values in serum compared with buffer by equilibrium dialysis (Table 2). In another series of studies, the in vitro binding of several mAbs in serum and buffer was followed for three days at 37° C by equilibrium dialysis. No loss of binding capacity was found over time under these conditions (data not shown). Thus, in vitro KD and Bmax values were unaltered by in vitro incubation.
Table 2.
In vitro KD values of mAbs in serum and buffer at pH 7.35
| KD (nM) |
||
|---|---|---|
| mAb Name | Buffer1 | Serum |
| mAb6H4 | 5.7 ± 2.7 (11) | 3.3 ± 1.3 |
| mAb4G9 | 34.2 ± 10.3 (35) | 36.5 ± 8.8 |
| mAb9B11 | 15.0 ± 5.9 (41) | 19.9 ± 1.7 |
Pharmacokinetics of mAbs in Rats
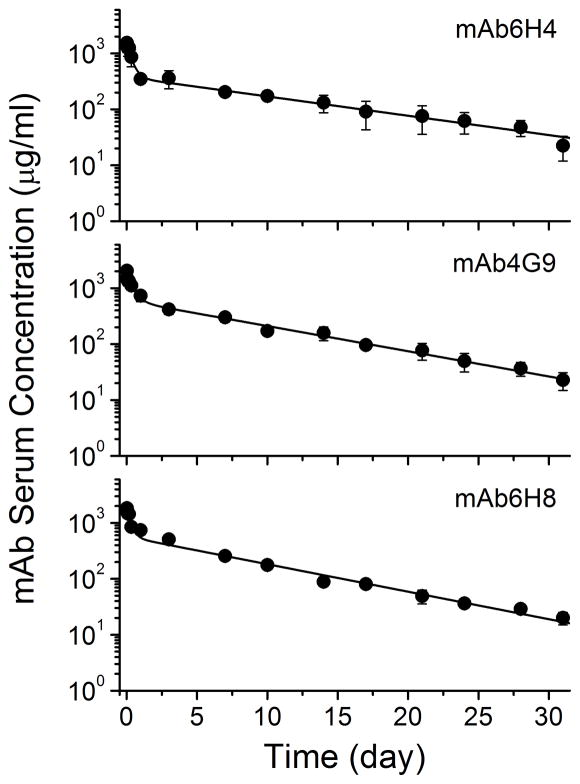
We next determined the pharmacokinetics of three representative anti-METH mAbs. Analysis of whole blood and serum at each time point revealed that mAb was not present in the red blood cells (data not shown). The average serum mAb concentration-time profiles are shown in Figure 1 and the pharmacokinetic parameters derived from these data are shown in Table 3. The pharmacokinetic parameters were remarkably similar among the different mAbs. There were no statistically significant differences between systemic clearance (Cls), t1/2λz, or values between the mAbs. The apparent volume of distribution (Vd) of mAb6H4 was significantly higher than mAb4G9 and mAb6H8 by about 60% and 40%, respectively. We did not consider this a substantive difference.
Figure 1. Serum pharmacokinetics of anti-METH mAb.
MAb (100 mg/kg) and a tracer dose of 3H-NSP labeled mAb (30–40 μCi) were administered to rats (n =4 per mAb group) and serum samples were collected over a 31 day time period. The fraction of 3H-NSP associated with intact IgG was determined after HPLC size exclusion chromatography. The total amount of IgG (μg/ml) in the serum was calculated based on the fraction of intact IgG-equivalents and the ratio of IgG dose (mg) to radiolabeled IgG dose (DPM). Values represent the mean ± SD.
Table 3.
Pharmacokinetic parameters determined for anti-METH mAbs in rats
| mAb Name | t1/2λz (day) | Cls (ml/kg/day) | Vd (ml/kg) | (mg/ml/day) |
|---|---|---|---|---|
| mAb6H4 | 6.9 ± 1.8 | 25.6 ± 4.5 | 226.9 ± 31.11 | 4.0 ± 0.6 |
| mAb4G9 | 6.9 ± 1.0 | 18.6 ± 1.0 | 141.5 ± 27.8 | 5.4 ± 0.3 |
| mAb6H8 | 6.1 ± 0.5 | 18.9 ± 2.2 | 152.5 ± 24.1 | 5.3 ± 0.6 |
Significantly different from mAb4G9 and mAb6H8.
We also followed excretion of radiolabeled mAb catabolism products in the urine over the 30 day study and found no significant differences among mAbs in the excretion of [3H]-labeled catabolism products. The total radioactivity in the urine (as catabolic products), expressed as percent of total dose was 58 ± 6%, 66 ± 8%, and 62 ± 11% for mAb6H4, mAb4G9 and mAb6H8, respectively. The radioactivity in the urine samples was not precipitated by 10% trichloroacetic acid, indicating excretion of mAb catabolism fragments. Consequently, none of the radioactivity in the urine was associated with intact mAb. This was not surprising since the IgG molecule is too large (~150 kDa) to be cleared by glomerular filtration.
Functional mAb Pharmacokinetics as Measured by Time-dependent In Vivo Changes in METH and AMP Binding in Rats
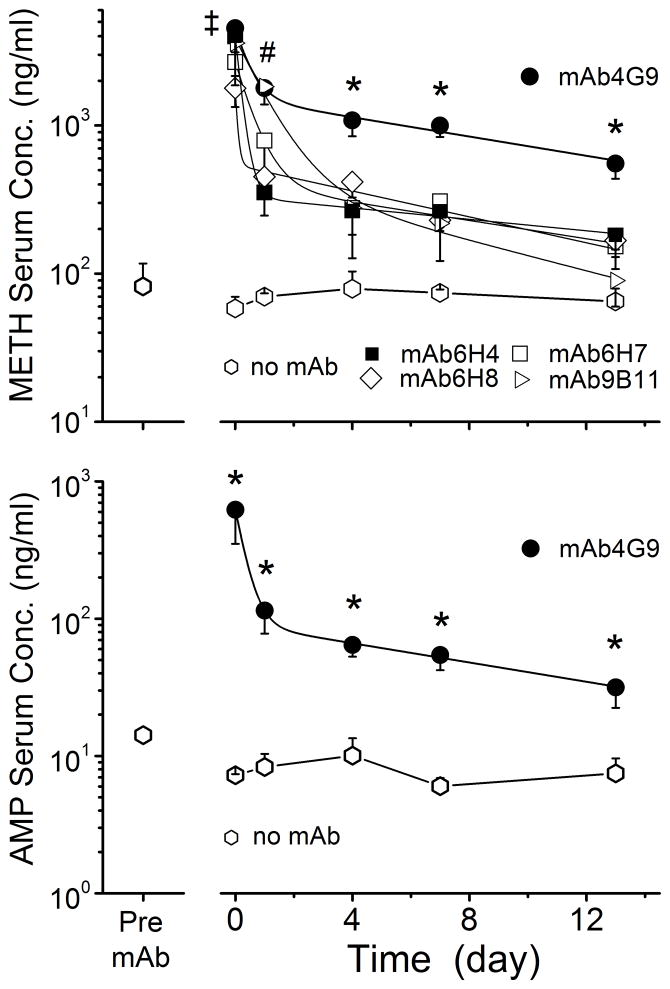
In the third series of studies, the in vivo binding function of five different anti-METH mAbs (Table 1) was assessed by comparing the effects of the mAb on the serum pharmacokinetics of METH and its metabolite, AMP. Prior to mAb treatment, the steady-state levels of METH and AMP in all animals were about 75 and 15 ng/ml, respectively. Following treatment with the various anti-METH mAbs, METH levels were significantly increased by all mAbs at the 5 min time point. However, the duration and magnitude of changes in METH levels were different for the various mAbs (Figure 2). Administration of mAb9B11 also resulted in increased serum METH concentrations over controls up to the 1 day time point, but not at any of the later time points. MAb4G9 significantly increased METH concentrations at all time points, up to the 13 day time point, compared to the control (no mAb) group and the pre-mAb levels. The METH values (i.e., AUC values from the time of dosing to the end of the 13 day study) are shown in Table 4. All mAbs significantly increased METH serum compared with the buffer-treated (no mAb) group; however, the effect of mAb4G9 on METH was significantly and substantially greater than all other mAb treatments.
Figure 2. METH serum levels before and after treatment with various anti-METH mAb.
Groups of rats (n = 4 per group) were infused with METH (5.6 mg/kg/day) via sc osmotic minipumps. Once METH achieved steady-state levels (pre-mAb), each rat group received one of five different anti-METH mAbs (180 mg/kg, equimolar in binding sites to the METH body burden). Serum samples were collected pre-mAb treatment and at various time points after mAb. METH (upper panel) and AMP (lower panel) concentration were determined by LC-MS/MS. Values represent the mean ± SD.
Only AMP concentrations in the presence of mAb4G9 are shown in the lower panel since it was the only mAb to substantially increase AMP concentrations throughout the 13 day experiment. The (‡) indicates all mAb treatments 5 min post-mAb treatment were significantly different from pre-mAb control. The (#) indicates mAb4G9 and mAb9B11 groups (at 1 day), and (*) indicates mAb4G9 treatment (at 4, 7 and 13 day) were significantly different from pre-mAb control.
Table 4.
METH and AMP serum area under the concentration-time curve values in rats receiving a 5.6 mg/kg/day METH infusion from the time of treatment with buffer or mAb (time 0) until day 13.
| Buffer or mAb Name | Serum
(ng/ml-day) |
|
|---|---|---|
| METH | AMP | |
| Buffer | 934 ± 81b | 170 ± 44 |
| mAb6H4 | 5,219 ± 1094c | 168 ± 12 |
| mAb4G9 | 15,029 ± 1435b | 1,072 ± 152b |
| mAb9B11 | 6,117 ± 2745c | 218 ± 60 |
| mAb6H7 | 5,591 ± 1380c | 152 ± 25 |
| mAb6H8 | 4,489 ± 757c | 174 ± 42 |
Determined by RIA, data from Peterson et al. [13].
Significantly different from all other groups.
Significantly different from buffer and mAb4G9 treated groups.
The effect of mAb4G9 on serum AMP levels is shown in Figure 2 (lower panel). It was the only mAb that had a significant effect on AMP levels throughout the 13 day study (see Table 4). MAb4G9 significantly increased AMP levels at all time points compared with the no mAb control group and the pre-mAb levels in the mAb4G9 treated group. We also examined brain concentrations at the final 13 day time point. At this time point, METH and AMP brain concentrations were not significantly reduced in any of the mAb-treated controls compared with the no mAb treatment group.
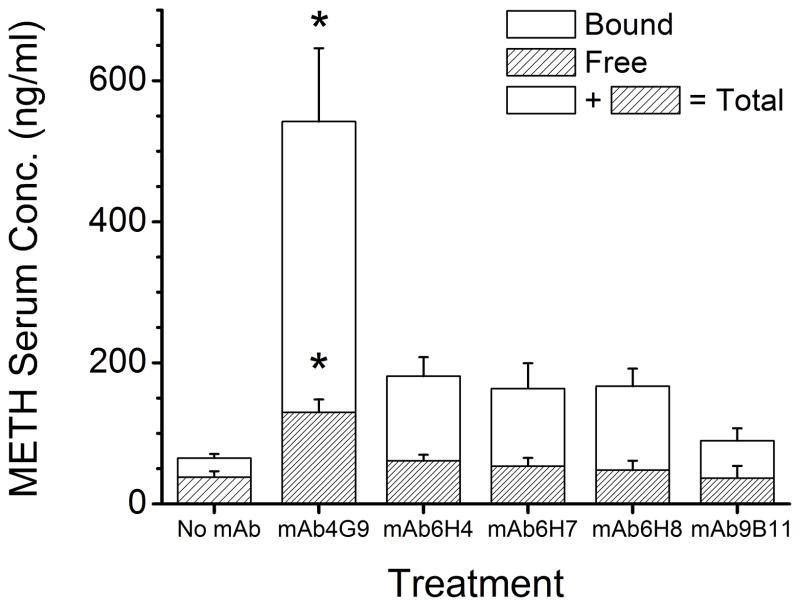
METH serum protein binding was determined in serum samples from the 13 day time point. METH serum protein binding was significantly increased in all mAb-treated groups compared to the no mAb control group. METH serum protein binding, free METH concentration, and total METH concentrations in the mAb4G9 treated group were significantly higher than all other mAb-treated groups (Figure 3).
Figure 3. Free and bound METH serum concentrations in rats receiving a 5.6 mg/kg/day METH infusion 13 days after treatment with anti-METH mAb.
Groups of rats (n = 4 per group) were infused with METH (5.6 mg/kg/day) via sc osmotic minipumps. Once METH achieved steady-state concentrations, each group received one of five different anti-METH mAb (180 mg/kg, equimolar in binding sites to the METH body burden). METH percent binding was determined by equilibrium dialysis. The hatched and open bars indicate the separate bound and free serum concentrations. The total concentrations are indicted by the combined height the bound and free concentrations. The (*) indicate bound, free and total METH concentrations significantly different from all other treatment groups.
Effect of mAb6H4 and mAb4G9 on METH and AMP Serum Concentrations over the First 24 hr Period after mAb Dosing
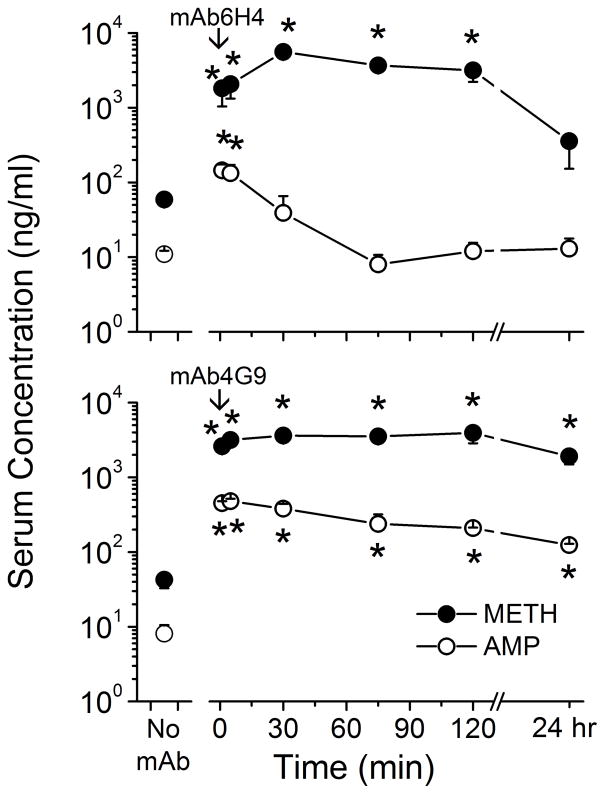
MAb6H4 and mAb4G9 were the highest affinity mAbs, but had dramatically different in vivo binding function over time (Figure 2 and Table 4). Thus, these mAbs were selected for more detailed studies to characterize binding function changes at earlier time points. The effect of these mAbs on serum and brain METH and AMP concentrations during a 5.6 mg/kg/day METH infusion was determined from 1 min to 24 hr after mAb dosing. MAb6H4 administration resulted in a significant increase in METH serum concentrations at the 1, 5, 30, 75 and 120 min time points compared with pre-mAb serum METH concentrations (Figure 4, upper panel). However, at 24 hr after mAb6H4, METH serum concentrations had returned to pre-mAb levels. MAb6H4 resulted in increased AMP serum concentrations at the 2 and 20 min time points, but AMP concentrations were not affected at any other time points.
Figure 4. Effect of mAb6H4 (top panel) and mAb4G9 (lower panel) on METH (closed circles) and AMP (open circles) concentrations in rat serum.
Rats (n =4 per group) received METH (5.6 mg/kg/day) via sc osmotic minipumps. Once steady-state concentrations were reached, anti-METH mAb6H4 or mAb4G9 (180 mg/kg, equimolar in binding sites to the METH body burden) or buffer only (no mAb) was administered. Rats were sacrificed at various time points for determination of serum METH and AMP concentrations by LC-MS/MS. Values represent the mean ± SD. The (*) indicate significant differences from buffer control (no-mAb) concentrations.
MAb4G9 mediated significantly increased METH and AMP serum concentrations in all of the time points compared with pre-mAb steady state METH and AMP serum levels (Figure 4, lower panel). Interestingly, METH serum levels were significantly higher at 5 min and 24 hr in mAb4G9 treated rats than mAb6H4 treated rats, but METH serum levels were significantly higher at 30 min in mAb6H4 treated rats compared with mAb4G9 treated rats. AMP serum levels were significantly higher in mAb4G9 treated rats than mAb6H4 treated rats at all time points examined.
Effect of mAb6H4 and mAb4G9 on METH and AMP Brain Concentrations over the First 24 hr Period after mAb Dosing
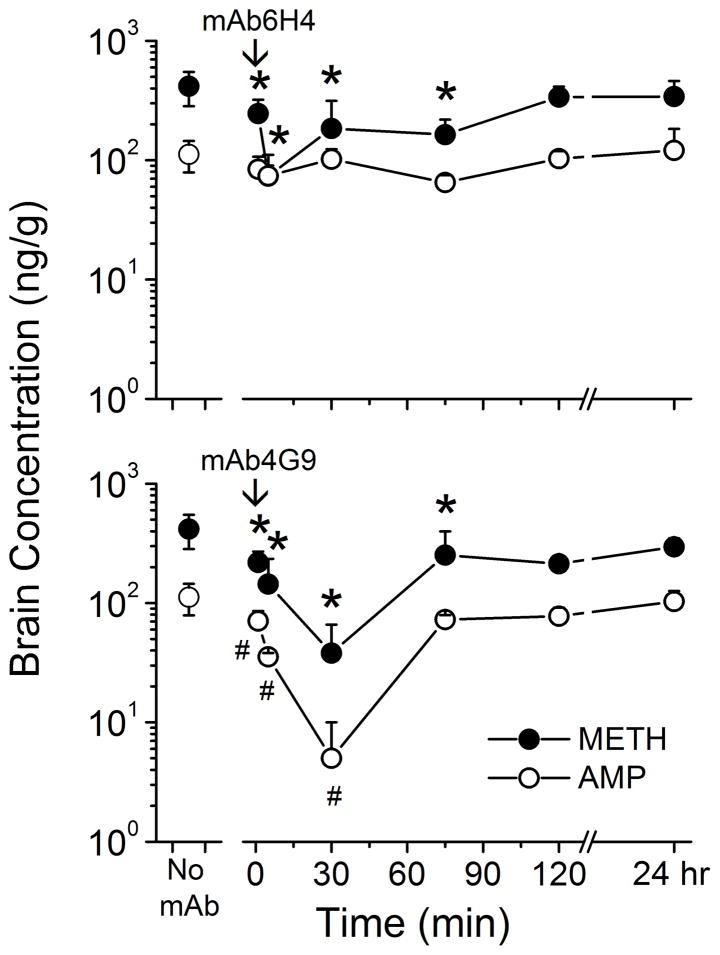
Figure 5 shows the effect of mAb6H4 and mAb4G9 on brain METH and AMP concentrations from the same animals as in the previous section. MAb6H4 significantly lowered METH brain concentrations at the 1, 5, 30 and 75 min time points compared to the pre-mAb controls (Figure 5, upper panel). MAb6H4 had no significant effect on AMP brain concentrations.
Figure 5. Effect of mAb6H4 (top panel) and mAb4G9 (lower panel) on METH (closed circles) and AMP (open circles) concentrations in the rat brains.
Rats (n = 4 per group) received METH (5.6 mg/kg/day) via sc osmotic minipumps. Once steady-state concentrations were reached, anti-METH mAb6H4 or mAb4G9 (180 mg/kg, equimolar in binding sites to the METH body burden) or buffer only (no mAb) was administered. Rats were sacrificed at various time points for determination of brain METH and AMP concentrations by LC-MS/MS. METH brain concentrations were decreased only at the 5 min time point after mAb6H4 treatment, compared with pre-mAb levels. Values represent the mean ± SD. The (* and #) indicate significant differences from buffer control (no-mAb) METH and AMP concentrations, respectively.
MAb4G9 significantly lowered METH brain concentrations at the 1, 5, 30, 75 and 120 min time point post-mAb administration, compared with pre-mAb levels (Figure 5, lower panel). AMP brain levels were decreased at the 1, 5, and 30 min time points by mAb4G9, compared with pre-mAb controls. The mAb-induced decrease in METH brain concentrations at the 30 min time point was significantly greater in mAb4G9 treated rats than in mAb6H4 treated rats. Brain AMP concentrations were significantly decreased at all time points, up to 75 min, in mAb4G9 treated rats compared with mAb6H4 treated rats. This finding is not noted in Figure 5.
METH and AMP Serum Protein Binding and the Blood to Serum Ratio over the First 24 hrs after mAb Dosing
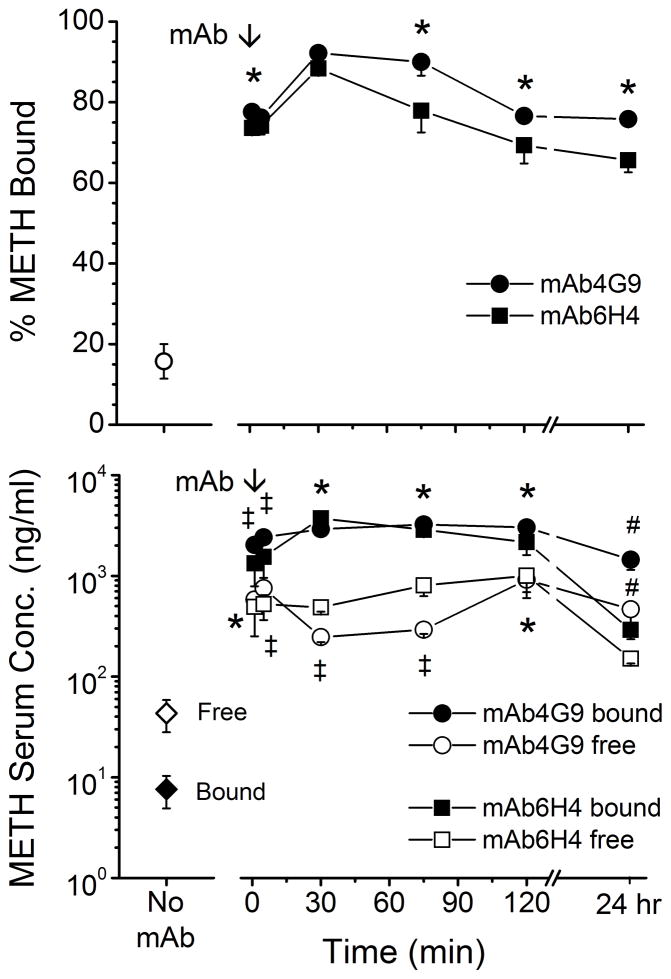
Because the functional activity of mAb6H4 and mAb4G9 is dependent on the extent of METH binding in serum, we examined METH protein binding in the serum from the previous two experiments. The percentage of METH bound in serum from animals treated with both mAbs was significantly increased compared with pre-mAb controls at all time points (Figure 6, upper panel). For both mAb groups, the percent bound peaked at the 30 min time point and then declined about 10% (mAb4G9) to 20% (mAb6H4). The percentage of METH bound in the mAb4G9 rats was significantly higher than the mAb6H4 group at the 1 min, 75 min, 120 min, and 24 hr time points. We used the percentage of METH bound data along with METH total serum concentrations (from Figure 4) to calculate free and bound METH serum concentrations (Figure 6, lower panel). Both mAbs significantly increased METH bound and free serum concentrations at 1, 5, 30, 75, and 120 min compared with pre-mAb concentrations, but at the 24 hr time point only mAb4G9 significantly increased METH bound and free serum concentrations.
Figure 6. METH serum protein binding in the 24 hr period following mAb administration.
Serum samples from rats receiving a sc infusion of METH (5.6 mg/kg/day). Pre- and post-mAb treatments were subjected to equilibrium dialysis for determination of METH serum protein binding. Values represent the mean ± SD. Top panel shows the percent METH bound in the serum samples. The (*) indicate significant differences between the percentage METH bound in mAb6H4 samples vs. mAb4G9 samples. Lower panel shows free and bound METH serum concentrations based on the percentage of METH bound. The (*) indicates mAb6H4 and mAb4G9 groups significantly different from control, the (‡) indicates mAb6H4 and mAb4G9 values significantly different from control and each other, the (#) indicates mAb4G9 values significantly different from mAb6H4 and control values.
At the 1 min and 5 min time points, mAb4G9 bound serum concentrations were significantly higher than in mAb6H4 treated rats, while at the 30 and 75 min time points mAb4G9 significantly decreased free METH serum concentrations compared with mAb6H4. Finally, at the 5 min time point, mAb6H4 free serum METH concentrations were lower than in mAb4G9 treated rats. AMP protein binding was also determined in mAb4G9 samples. AMP was 95–99% bound at all time points (data not shown).
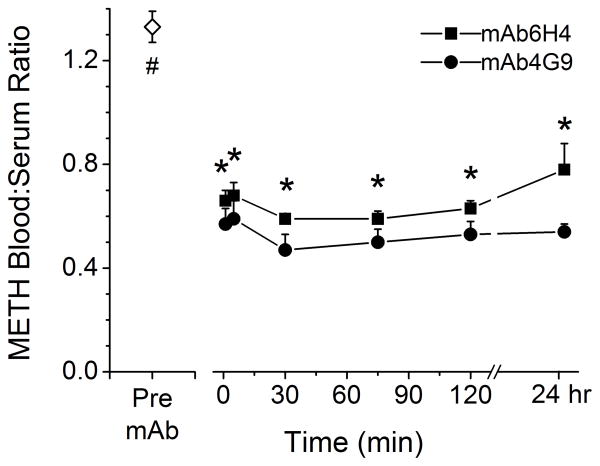
Because the METH free serum concentrations were higher in mAb-treated rats than in rats that did not receive mAb, we studied the blood to serum ratio of METH in a separate set of studies (Figure 7). In the pre-mAb samples, METH equally distributed between the red blood cells and serum, yielding a METH blood to serum ratio of ~1.3. This indicated nearly equal distribution of METH between red blood cells and serum in the absence of mAb. For mAb4G9 treated rats, the blood to serum ratio was ≤0.5 at all time points examined. This suggested that the METH was highly bound to mAb4G9, which is only in the serum fraction of whole blood. In contrast, the blood to serum ratio in mAb6H4-treated rats was ~0.5 at early time points, but was ~0.8 at 24 hrs indicating a significantly lowered ability of the mAb to keep the METH partitioned into the serum fraction of the whole blood. The blood to serum ratio in mAb4G9 treated rats was significantly lower than in mAb6H4 treated rats at all time points except at 1 min after completion of mAb dosing.
Figure 7. METH blood to serum ratio in the absence and presence of mAb.
Rats (n = 4 per group) received METH (5.6 mg/kg/day) via sc osmotic minipump. Once steady-state concentrations were reached anti-METH mAb6H4 or mAb4G9 (180 mg/kg, equimolar in binding sites to the METH body burden) was administered. Blood samples were collected pre- and post mAb treatment for determination of blood and serum METH concentrations by LC-MS/MS. Blood to serum ratio was determined by dividing blood concentration by the serum concentration. Values represent the mean ± SD. The (#) indicates the pre-mAb value is significantly different from all post-mAb values. The (*) indicate mAb6H4 and mAb 4G9 values significantly different from one another.
Discussion
We studied potential anti-METH mAb medications in experiments aimed at understanding the pharmacokinetic and functional determinates of mAb efficacy and duration of action. We found differences in METH and AMP in vivo binding activity, and it appeared that some mAbs lost significant binding function over the two week study. We first suspected non-specific serum matrix effects were altering mAb function; however, in vitro studies of mAb affinity over a 24 hr period did not reveal any alterations in in vitro mAb function (Table 2). Furthermore, pharmacokinetic studies of three representative anti-METH mAbs revealed no substantive differences in the pharmacokinetic parameters among the mAbs (Figure 1 and Table 3). This finding refutes our hypothesis that time-dependent reductions in mAb in vivo function and efficacy are caused by substantial changes in mAb clearance. Indeed, the mAb pharmacokinetic values were similar to those of Bazin-Redureau et al. [10], who used mouse mAbs that do not bind METH. It is worth noting that mAb6H4 and mAb6H8 were both of the IgG1κ subclass while mAb4G9 was an IgG2bκ. Previous studies also show murine IgG1 and IgG2 subclasses with κ light chains have similar pharmacokinetic parameters [20]. It is known that different mAb isotypes have different affinities for Fcγ [21], which potentially could affect in vivo efficacy of the mAbs. Nevertheless, mAb isotype did not appear to affect the outcome of mAb serum pharmacokinetics or in vivo inactivation, based on our studies with both IgG1 and IgG2b antibodies. Also, the mechanism of anti-METH mAb therapeutic benefit results from a pharmacokinetic antagonism of METH effects, and thus does not involve activation of in vivo immune cascades like some other mAb medications.
When we studied the time course of anti-METH mAb binding using a novel in vivo functional binding assay (Table 1), we found mAb-dependent differences in the longevity of METH binding. Keep in mind that these studies of mAb binding were conducted during a constant METH infusion that replaced 50% of the drug every hour (METH t1/2λz = 1 hr). Importantly, functional longevity did not correlate with mAb affinity for METH for four of the five mAbs (Figure 2). Indeed, some of the mAbs appeared to lose METH binding capacity at a more rapid rate than others, as seen by comparing the initial decreases in METH serum concentrations within 1–3 days after mAb administration. In most cases, these METH concentration (bound to mAb) decreases were greater than the mAb concentration decreases that occur during the initial mAb pharmacokinetic distribution phase (see Figure 1). Overall differences were also readily apparent from the METH serum values among the different mAb treatment groups compared to controls (Table 4). MAb4G9 (KD=35 nM) appeared to be the most effective and long-lasting mAb. It caused a 16-fold and 6-fold increase in METH and AMP serum values (respectively, compared to controls), while all other mAbs (KD values = 11–250 nM) only increased METH serum values 4.8–5.6-fold over buffer controls (i.e., no mAb), with no substantive effect on AMP .
While lower affinity for METH likely contributed to a lower METH serum binding in some cases (e.g., mAb6H8), our data indicate that most of the mAbs were at least partially inhibited or inactivated in vivo in a time-dependent manner. This finding supports the hypotheses that time-dependent in vivo decreases in function are responsible for decreased effectiveness. We think this decreased in vivo function is likely due to an increased KD value (lower affinity) and/or decreased Bmax value.
MAb6H4 (the mAb with the lowest KD value in this study) appeared to lose substantial in vivo METH binding activity within 24 hr (Figures 2, 3 and 4). These findings are in agreement with previous rat behavioral studies using mAb6H4 [6] in which MAb6H4 significantly reduced METH-induced locomotor activity immediately after the first METH challenge, but not on subsequent METH doses on days 4 and 7.
It is possible that an endogenous ligand structurally related to METH might be responsible for inhibiting METH binding. However, all of our mAbs are routinely screened for cross-reactivity with a wide range of structurally related and unrelated ligands (see Peterson et al. [13]). None of these ligands cause inhibition of METH binding. It is also possible that high concentrations of structurally similar amino acids in serum [22, 23] could inhibit METH binding. However, testing of the high concentrations of aromatic amino acids (phenylalanine, tryptophan, histidine and tyrosine) showed no inhibition of METH binding to mAb (unpublished observations).
A METH metabolite (besides AMP) might also inhibit or inactivate METH binding in vivo. For example, we routinely analyze for 4-OH METH in rat serum samples, but the concentration is usually below levels of accurate quantitation (2 nM). MAb6H4 shows the highest affinity for 4-OH METH (METH Ki =19 nM), and in vivo 4-OH METH concentrations range from 93–537 nM at the early time points after mAb6H4 administration (1–30 min). At these same time points, METH concentrations range from 5.9–37 μM. Therefore, it is unlikely that 4-OH METH significantly inhibits METH binding to mAb6H4.
MAb half-life (Table 3) is highly dependent on the neonatal Fc receptor (FcRn) [24]. IgG is repeatedly internalized to vascular endothelial cells via pinocytosis, and bound to FcRn in acidified endosomes in a pH-dependent manner (pH <6.5) [25]. FcRn then recycles the IgG back to the blood, where IgG is released at neutral pH. This change in pH during recycling could cause irreversible changes in protein structure and function, particularly in mAbs with a pI value close to the pH of the acidified endosomes (e.g., mAb6H4 and mAb9B11 pI values are around 6; Table 1). Several studies report pH- and ionic strength-induced changes in mAb conformation and stability, and in some cases the changes are irreversible [26, 27].
MAb inactivation could occur through binding to METH-like metabolic transition state analogs, or non-enzymatic deamidation of glutamine and asparagine residues. This last modification, in particular, has been shown to result in alterations in the functional activity of mAbs [28]. Examination of the primary sequences (see [13]) of all five anti-METH mAbs revealed they all have possible deamidation sites. Importantly, mAb6H4, mAb6H7 and mAb6H8 show potential deamidation sites in their complementarity determining regions (Figure 2).
Brain concentrations of METH were significantly decreased by mAb4G9 (Figure 5) despite apparent mAb-induced increases in free serum METH concentrations (Figure 6). This appears to contradict a basic principle of pharmacology that drug tissue levels are dependent on free drug serum concentrations. We have repeatedly observed similar results with both anti-METH mAbs and anti-phencyclidine mAb6B5 [11, 12]. We hypothesize that 1) the extremely small volume of the vascular cerebral space relative to the general circulation, 2) the blood-brain barrier which restricts mAb but not METH to the vasculature, and 3) the extremely small fraction of the total administered METH dose localizing to the brain [0.1–0.2% for METH and PCP, 7]) combine to allow temporary greater drug-mAb occupancy with each pass through the brain. This scenario in the brain vs. the systemic circulation (respectively) can be envisioned by considering the mAb Vd of 0.142 L/kg (mAb4G9, Table 3) vs. the METH Vd of 9 L/kg [9].
A complementary functional explanation involves the dynamic in vivo antibody-ligand interactions. This continuous process of METH s on and off interactions with the mAbs could keep a larger fraction of the free drug near the mAb-METH binding site; analogous to the way a juggler uses his hand to keep several balls engaged while giving each ball only limited time in his hands. This juggling of several METH molecules could keep more METH confined to the small volume of distribution of the mAb and prevent it from crossing into tissue compartments like the brain.
MAb4G9 was generated from the MO10 hapten covalently bound to ovalbumin (see Table 1 and Peterson et al. [13]). This mAb appeared to be significantly less affected by time-dependent changes in METH binding. We hypothesize that the immunizing hapten played a significant role in this extended period of function. The longer MO10 spacer arm likely improved immune presentation over other haptens with shorter linkers in these studies, and allowed a greater degree of flexibility for immune presentation of the METH-like hapten. Indeed, these factors likely contributed to the ability of mAb4G9 to recognize METH and AMP, with relatively high affinity. Our previous studies suggest the METH binding pocket of mAb4G9 is wider and shallower than the mAb6H4 binding pocket [13], which was generated from a P6 METH hapten with a shorter spacer arm. We also hypothesize that since mAb4G9 recognizes METH and AMP with high affinity, the charged site of the secondary amine of METH and AMP was less exposed to interactions with the mAb binding site. This suggests the interactions of the METH charged site with specific amino acids in the other mAbs in this study played a role in time-dependent reductions in binding function. These interactions could occur through non-specific binding with oppositely charged endogenous ligands or through covalent modifications. It is also possible that the longer linker of MO10 could expose a broader surface of the METH molecule, which allowed for increased molecular interactions between the METH molecule and the antigen binding pocket. In ongoing studies in our laboratory, we have recently tested three additional prototype mAbs resulting from immunization with the MO10 hapten covalently bound to ovalbumin or bovine albumin. Two of the three resulting mAbs have similar in vivo longevity to mAb4G9 (unpublished observation). These data suggest the METH-like MO10 hapten is a major innovation for generating anti-METH active and passive vaccination for the treatment of METH addiction.
Previous preclinical studies in rats using anti-METH mAbs show even very high iv doses (e.g., 1,000 mg/kg) given over several minutes do not do produce any observable behavioral or cardiovascular effects [29]. In addition, we have never observed adverse effects resulting from mice or rats immunized with METH-like haptens. These important safety data suggest there is no significant mAb in vivo cross reactivity with endogenous ligands or adverse effects on behavioral or physiological functions.
These detailed preclinical studies indicate that utilization of anti-METH mAb4G9, or another recently discovered MO10-generated antibody designated mAb7F9 that has even higher affinity for METH, could be very effective for treating METH-induced medical problems in humans. Indeed a major goal for these preclinical studies was to develop predictive in vivo testing models to aid in choosing a mAb with optimal in vivo efficacy and long-term function before proceeding to human testing. We think the data from the current studies substantially improve the potential for a successful therapeutic outcome. Especially since we plan to use a chimeric mAb with the Fc region of human origin and a proven long-acting Fv binding site from the mouse. These data also suggest the MO10 hapten would be an excellent choice for use in an active vaccine.
In summary, these studies demonstrate the importance of both in vitro and in vivo characterization of the function of therapeutic mAbs. Our studies show mAb in vivo function is not always predicted from in vitro immunochemical characterization. Pharmacokinetic studies of mAb protein are also important, but may lead to inaccurate assumptions regarding longer-term in vivo efficacy. Studies are underway to address complex mechanistic questions such as Is METH required for inactivation? and Are specific amino acids being modified? Nevertheless, the studies presented here underscore the need for extensive preclinical characterization of mAb therapeutics, and report important results showing the novel prolonged function of anti-METH mAb4G9. It is also clear that careful structure-activity studies with mAbs generated from a range of haptens (Table 1) can provide better choices for optimal vaccines.
Acknowledgments
This work was supported by National Institute on Drug Abuse grants R01 DA11560 and P01 DA14361 to SMO, F32 DA018039 to ECP, and R01 DA05477 to FIC. Additional support was provided by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds act of 2000.
S. Michael Owens and W. Brooks Gentry have financial interests in and serve as Chief Scientific Officer and Chief Medical Officer, respectively, of InterveXion Therapeutics, LLC, a pharmaceutical biotechnology company, whose main interest is in developing new monoclonal antibodies for treatment of human diseases, including drug abuse.
The authors thank Melinda Gunnell for production and formulation of the mAbs, and Sherri Wood for assistance with animal studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century.[erratum appears in Nat Rev Drug Discov. 2003 Mar;2(3):240] Nature Reviews Drug Discovery. 2003;2(1):52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. American Journal of the Medical Sciences. 2002;324(1):14–30. doi: 10.1097/00000441-200207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacology & Therapeutics. 2005 Oct;108(1):76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Knapp MJ, Colburn PA. Clinical uses of intravenous immune globulin. Clinical Pharmacy. 1990;9(7):509–29. [PubMed] [Google Scholar]
- 5.Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, et al. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. European Journal of Pharmacology. 2003;461(2–3):119–28. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. European Journal of Pharmacology. 2005;521(1–3):86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Laurenzana EM, Byrnes-Blake KA, Milesi-Halle A, Gentry WB, Williams DK, Owens SM. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metabolism & Disposition. 2003;31(11):1320–6. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- 8.Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse (New York, NY) 2001 Feb;39(2):161–6. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Riviere GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. The Journal of pharmacology and experimental therapeutics. 1999 Dec;291(3):1220–6. [PubMed] [Google Scholar]
- 10.Bazin-Redureau MI, Renard CB, Scherrmann JM. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. Journal of Pharmacy & Pharmacology. 1997;49(3):277–81. doi: 10.1111/j.2042-7158.1997.tb06795.x. [DOI] [PubMed] [Google Scholar]
- 11.Proksch JW, Gentry WB, Owens SM. Anti-phencyclidine monoclonal antibodies provide long-term reductions in brain phencyclidine concentrations during chronic phencyclidine administration in rats. Journal of Pharmacology & Experimental Therapeutics. 2000;292(3):831–7. [PubMed] [Google Scholar]
- 12.Laurenzana EM, Gunnell MG, Gentry WB, Owens SM. Treatment of adverse effects of excessive phencyclidine exposure in rats with a minimal dose of monoclonal antibody. Journal of Pharmacology & Experimental Therapeutics. 2003;306(3):1092–8. doi: 10.1124/jpet.103.053140. [DOI] [PubMed] [Google Scholar]
- 13.Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, et al. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. Journal of Pharmacology & Experimental Therapeutics. 2007;322(1):30–9. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- 14.Owens SM, Zorbas M, Lattin DL, Gunnell M, Polk M. Antibodies against arylcyclohexylamines and their similarities in binding specificity with the phencyclidine receptor. Journal of Pharmacology & Experimental Therapeutics. 1988;246(2):472–8. [PubMed] [Google Scholar]
- 15.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic acids research. 2003 Jul 1;31(13):3784–8. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClurkan MB, Valentine JL, Arnold L, Owens SM. Disposition of a monoclonal anti-phencyclidine Fab fragment of immunoglobulin G in rats. The Journal of pharmacology and experimental therapeutics. 1993 Sep;266(3):1439–45. [PubMed] [Google Scholar]
- 17.Hendrickson H, Laurenzana E, Owens SM. Quantitative determination of total methamphetamine and active metabolites in rat tissue by liquid chromatography with tandem mass spectrometric detection. AAPS Journal. 2006;8(4):E709–17. doi: 10.1208/aapsj080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland M, Tozer TN. Clinical Pharamacokinetics Concepts and Applications. 3. Philadelphia: Lippincott Willams and Wilkins; 1995. [Google Scholar]
- 19.Khor SP, Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. I. Theoretical considerations. Drug Metabolism & Disposition. 1991;19(2):478–85. [PubMed] [Google Scholar]
- 20.Montano RF, Morrison SL. Influence of the isotype of the light chain on the properties of IgG. Journal of Immunology. 2002;168(1):224–31. doi: 10.4049/jimmunol.168.1.224. [DOI] [PubMed] [Google Scholar]
- 21.Roskos LK, Davis CGM, Schwab G. The clinical pharmacology of therapeutic monoclonal antibodies. Drug Development Research. 2004;61(3):108–20. [Google Scholar]
- 22.Gustafson JM, Dodds SJ, Burgus RC, Mercer LP. Prediction of brain and serum free amino acid profiles in rats fed graded levels of protein. Journal of Nutrition. 1986;116(9):1667–81. doi: 10.1093/jn/116.9.1667. [DOI] [PubMed] [Google Scholar]
- 23.Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathe V, Huneau JF, et al. Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;292(4):R1400–7. doi: 10.1152/ajpregu.00566.2006. [DOI] [PubMed] [Google Scholar]
- 24.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Bronson CL, Wani MA, Oberyszyn TM, Mohanty S, Chaudhury C, et al. Beta 2-microglobulin deficient mice catabolize IgG more rapidly than FcRn- alpha-chain deficient mice. Exp Biol Med (Maywood) 2008 May;233(5):603–9. doi: 10.3181/0710-RM-270. [DOI] [PubMed] [Google Scholar]
- 26.Dejaegere A, Choulier L, Lafont V, De Genst E, Altschuh D. Variations in antigen-antibody association kinetics as a function of pH and salt concentration: a QSAR and molecular modeling study. Biochemistry. 2005;44(44):14409–18. doi: 10.1021/bi050986v. [DOI] [PubMed] [Google Scholar]
- 27.Jiskoot W, Beuvery EC, de Koning AA, Herron JN, Crommelin DJ. Analytical approaches to the study of monoclonal antibody stability. Pharmaceutical Research. 1990;7(12):1234–41. doi: 10.1023/a:1015925519154. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Analytical Chemistry. 2005;77(5):1432–9. doi: 10.1021/ac0494174. [DOI] [PubMed] [Google Scholar]
- 29.Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006 Jun;6(6):968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]