Abstract
Understanding early immunological events during HIV-1 infection that may set the course of disease progression is important for identifying correlates of viral control. This study explores the association of differentiation profiles of HIV-specific and total memory CD8+ T cells with viral set point. A cohort of 47 HIV-1-infected individuals, with differing viral set points at 12 mo, were recruited during acute infection. We identified that the magnitude of IFN-γ+ T cell responses at 6 mo postinfection did not associate with viral set point at 12 mo. A subset of 16 individuals was further studied to characterize CD8+ T cells for expression patterns of markers for memory differentiation, survival (CD127), senescence (CD57), and negative regulation (programmed death-1). We show that viral control and the predicted tempo of HIV disease progression in the first year of infection was associated with a synchronous differentiation of HIV-specific and total CD8+ memory subpopulations. At 6–9 mo postinfection, those with low viral set points had a significantly higher proportion of early differentiated HIV-specific and total memory CD8+ cells of a central memory (CD45RO+CD27+CCR7+) and intermediate memory (CD45RO−CD27+CCR7−) phenotype. Those with high viral set points possessed significantly larger frequencies of effector memory (CD45RO+CD27−CCR7−) cells. The proportions of memory subsets significantly correlated with CD38+CD8+ T cells. Thus, it is likely that a high Ag burden resulting in generalized immune activation may drive differentiation of HIV-specific and total memory CD8+ T cells.
Human immunodeficiency virus 1 infection generally leads to a decline of immune function, which in the absence of successful antiretroviral therapy results in progression to AIDS. However, a small group of individuals is able to naturally control viral replication and maintain high levels of CD4+ cells, known as long-term nonprogressors or elite controllers (1). It is now well established that virus fitness and/or host genetic background can contribute to the delay of HIV disease progression (2–5). More controversial is the role of HIV-specific CD4+ and CD8+ responses in natural viral control. Divergent data show the impact of HIV-specific CD4+ T cell responses on disease progression (6, 7) and, in simian models, there is evidence that CD8+ T cells may play an important role in the control of viremia (8, 9). These data strengthen the hypothesis that the development of HIV-specific CD8+ T cell responses contributes to the delay of disease progression in humans and coincides with a reduction in initial viremia during primary infection (2). Although an inverse relationship has been described between the proportion of HIV-specific CD8+ T cells and viral load (10), more recent studies have raised questions about whether the frequency of HIV-specific CD8+ T cells is associated with viral control (11–13). Furthermore, neither the breadth nor the magnitude of HIV-specific IFN-γ+ CD8+ T cell responses in chronically infected patients has been shown to be a marker of viral control (12–15). These latter observations collectively infer that the quality, more than the quantity, of CD8+ T cell responses might play a role in viral control.
The hypothesis that the quality of CD8+ T cells is important in controlling disease, and would be important to elicit in vaccine-induced immunity, is supported by data showing that proliferation and polyfunctional cytokine responses associate with control of HIV (16–18). Phenotype and function of T cells are integrally linked, and studies have shown that stages of HIV-specific CD8+ T cell differentiation may be an important qualitative assessment. Functionally suboptimal HIV-specific cells accumulate in a pre-terminally differentiated stage (19), and viral control in early and chronic infection is associated with terminally differentiated HIV-specific effector memory CD8+ cells (14, 20). The differentiation status of total CD8+ memory cells may also be important during HIV infection, where recent data have shown that a late differentiated and aged total CD8+ memory compartment is associated with faster disease progression (21).
Few studies have investigated the differentiation profiles of CD8+ T cell responses during acute and early infection, with most data having been collected during chronic infection in cross-sectional studies. It is well established that plasma viral load at approximately 1 year after infection, known as viral set point, is a strong predictor of subsequent CD4+ decline rates and progression to AIDS (22), implying that early events in HIV infection may set the course of viremia and hence for subsequent disease progression. In SIV infection, massive destruction of the CD4+ memory T cell compartment occurs in acute infection at mucosal surfaces, particularly in the gut (23). Additionally, phenotypic defects and increased apoptosis manifest in the first few weeks after SIV infection (24). As the global vaccine community reflects on the unsuccessful Ad5 HIV vaccine trial (25), identity of T cell quality and the factors that influence it during the early stages of infection may be important for vaccine development.
In this study, we examined the association of T cell responses and memory differentiation with viral set point at 1 year in a subtype C HIV-1 acute infection cohort. We show that total HIV-1-specific IFN-γ responses are unrelated to viral control, but a more refined analysis of CD8+ T cell memory differentiation revealed associations with viral set point.
Materials and Methods
Study subjects
Acute HIV-infected cohort
A longitudinal cohort of acutely HIV-1-infected women were enrolled as part of the Centre for AIDS Programme of Research in South Africa (CAPRISA) acute infection study in Durban, South Africa. This study cohort has been described previously (26, 27). The time postinfection was estimated either by a prospective RNA-positive/Ab-negative measurement or taken as the midpoint between the last Ab-negative test and first Ab-positive ELISA test. Study participants were followed for 12 mo, and follow-up is ongoing. Data from events within the first 3–12 mo are reported herein. None of the study individuals was on antiretroviral therapy during the first year of infection. The University of KwaZulu-Natal, University of Witwatersrand, and University of Cape Town Research Ethics Committees approved this study, and all the subjects provided written informed consent for participation in this study. A total of 47 study participants were analyzed by ELISPOT, and a subset of 16 individuals was analyzed in more depth for HIV-specific memory phenotypes (Table I).
TABLE I.
Characteristics of study participants
| CD4 Count | % CD8+IFN-γ+ Cells (ICS)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Viral | at Time | ||||||||
| Postinfection at | Set Point | of Analysis | ||||||||
| Patient ID | Analysis (wk) | (copies/ml)a | (cells/mm3) | HLA-A | HLA-B | HLA-C | Nef | Gag | Pol | Env |
| CAP045 | 41 | 472 | 904 | 2301/2902 | 1510/4501 | 602/1602 | 0.19 | |||
| CAP228 | 37 | 974 | 1,411 | 2301/2601 | 4403/5101 | 3c/7 | 0.97 | 0.39 | 0.16 | 0.07 |
| CAP084 | 19 | 4,565 | 623 | 2902/7401 | 1503/4407 | 2c/7 | 2.87 | 0.27 | ||
| CAP257 | 30 | 9,859 | 489 | 2301/2902 | 4202/4403 | 1701/— | 0.55 | |||
| CAP271 | 22 | 12,732 | 628 | 205/2301 | 1401/4403 | 303/804 | 0.04 | 0.06 | ||
| CAP217 | 31 | 24,836 | 529 | 202/2901 | 1503/5801 | 210/602 | 1.15 | |||
| CAP040 | 26 | 26,325 | 417 | 30c/— | 1510/4201 | 304/1701 | 0.3 | |||
| CAP229 | 38 | 37,686 | 690 | 101/— | 5801/— | 602/— | 0.67 | |||
| CAP088 | 26 | 46,304 | 864 | 29c/66 | 4501/5802 | 602/— | 1.26 | 0.5 | 3.5 | 3.65 |
| CAP030 | 42 | 63,338 | 537 | 2c/34 | 4403/4501 | 4c/16 | 0.88 | |||
| CAP129 | 32 | 84,959 | 676 | 26c/8001 | 18c/8101 | 202/4c | 0.09 | 0.08 | 0.54 | |
| CAP174 | 24 | 117,279 | 404 | 301/7401 | 4901/5802 | 6c/7 | 0.24 | |||
| CAP239 | 32 | 160,922 | 1,092 | 101/2902 | 4201/5801 | 602/7101 | 0.41 | 1.55 | ||
| CAP256 | 23 | 199,234 | 456 | 2902/6601 | 1503/5802 | 4c/602 | 1.29 | 0.7 | 0.4 | |
| CAP206 | 36 | 257,196 | 329 | 3204/74c | 702/4403 | 210/702 | 0.13 | |||
| CAP210 | 31 | 289,662 | 398 | 6802/— | 1510/— | 304/— | 9.44 | |||
| Median (n = 16) (47)d | 31 (24) | 41,995 (36,400) | 580 (464) | 0.69 | 0.55 | 0.54 | 0.335 | |||
| Range (n = 16) | 19–42 | 471–289,662 | 329–1,411 | |||||||
Measured by taking the mean of three time points around 52 ± 6 wk.
Peptide pools found to stimulate a positive IFN-γ response by intracellular cytokine staining (ICS).
Low-resolution HLA typing only.
Median time postinfection at recruitment, viral set point, and CD4 count did not differ significantly between the subgroup studied in detail (n = 16) and the larger group (n = 47).
HIV-uninfected cohort
We recruited 15 HIV-uninfected individuals from the Perinatal HIV Research Unit, Soweto, South Africa, who were the HIV-uninfected partner in an HIV serodiscordant relationship. Participation in this study was approved by the Human Research Ethics Committee of the University of the Witwatersrand. After providing informed consent, participants were tested for HIV with dual HIV rapid tests performed in parallel. All participants were HIV uninfected by all assays at the enrollment and follow-up visits.
Calculation of viral set point
Viral set point was calculated as the geometric mean of viral load measurements at three time points at week 52 ± 6 wk postinfection (between 46 and 58 wk) to minimize possible spurious viral load measurement. In the case where there was an atypical viral load occurring within the 52 ± 6 wk window, defined as a viral load >1 log different from the other measurements within that period, the datum point was removed and replaced with the next measurement. CAP84 was the only example where a viral load blip at 52 wk was replaced with a measurement beyond the 6 wk period.
Plasma viral load determination, CD4 T cell counts, and HLA typing
Plasma HIV-1 RNA levels were quantified using the COBAS AMPLICOR HIV-1 monitor test version 1.5 (Roche Diagnostics). Absolute blood CD4+ and CD8+ T cell counts were measured using a FACSCalibur flow cytometer and expressed as cells/mm3. HLA typing was performed as described in Chopera et al. (28).
Synthetic subtype C peptides
A set of 432 synthetic overlapping peptides spanning the entire expressed HIV-1 clade C proteome corresponding to gene products from the HIV-1 consensus C (Gag, Vif, Vpr, and Vpu), isolate Du151 (Pol, Nef, Tat, and Rev), and isolate Du179 (gp160 Env) were synthesized using 9-fluorenyl-methoxycarbonyl chemistry and standard based solid phase techniques (Natural and Medical Sciences Institute, University of Tubingen, Tubingen, Germany). The nonconsensus synthesized peptides were based on sequences from isolates used for manufacture of a clade C vaccine (29). The estimated purity of peptides was >80% as measured by HPLC and mass spectrometry. Individual peptides were diluted in DMSO (Sigma-Aldrich) and prepared as previously described (13).
Cell preparation
PBMC were isolated by standard Ficoll-Hypaque density gradient centrifugation (Amershan Pharmacia) and cryopreserved in 90% heat-activated FBS (Invitrogen) plus 10% DMSO and stored in liquid nitrogen until needed. Frozen PBMC were thawed and rested in RPMI 1640 (Invitrogen) containing 10% heat-inactivated FBS and 50 U gentamicin (Invitrogen) at 37°C and 5% CO2 for 18 h before use in ELISPOT and intracellular cytokine staining assays.
IFN-γ ELISPOT assay
HIV-1-specific T cell responses were quantified by the IFN-γ ELISPOT assay using the set of overlapping peptides arranged in a pool-matrix format as previously described (13). Briefly, PBMC were plated at 1 × 105 cells/ml with peptides at a concentration of 2 µg/ml in 96-well polyvinylidene difluoride plates (Millipore) that had been coated with 5 µg/ml anti-IFN-γ mAb, 1-DIK (Mabtech) overnight at 4°C. PHA (Calbiochem) stimulation at 4 µg/ml was used as positive control, and no peptide stimulation (medium alone) was used as negative control. As part of an ongoing quality assurance program, thawed PBMC that had been tested previously for responses to a pool of optimal peptides corresponding to CMV, EBV, and flu viruses were included on the same plate as a positive quality control sample for assay consistency. Plates were incubated overnight at 37°C, 5% CO2 and developed as previously described (13). Individual spots were counted with an automated ImmunoSpot plate reader (Cellular Technology) and expressed as spot-forming cells per million PBMC. Responses were initially evaluated by reacting PBMC with peptides arranged in a pool-matrix format and then followed with a second ELISPOT assay to confirm positive responses at the single-peptide level in triplicate. The following criteria were used to define positive responses: 1) reactivity to peptide pools were ≥67 spot-forming cells per 106 PBMC after background subtraction and at least three times greater than the mean background activity, and 2) a matching peptide in the matrix pool array.
Surface phenotypic and intracellular cytokine staining using flow cytometry
Flow cytometric detection of phenotypic and functional markers was performed essentially as described in (30). The following Abs and fluorophores were used: CD3-Cy7-allophycocyanin, CD3-Cy7-PE, IFN-γ-FITC, CD38-allophycocyanin (all BD Pharmingen), CD4-Cy5.5-PE (Caltag Laboratories), CD27-Cy5-PE, CD45RO-Texas Red-PE, CD127-PE (all Beckman Coulter), programmed death-1 (PD-1)4-biotin (R&D Systems), CD57-quantum dot (QD) 545, CD8-QD705, streptavidin-QD655, CD14-Pacific Blue, and CD19-Pacific Blue, conjugated according to standard protocols. Detailed dot plots of PD-1 labeling are provided in supplemental Fig. S2.5 Unconjugated Abs were from BD Pharmingen. All Abs were pretitered to optimal concentrations. Briefly, PBMC were stimulated with anti-CD28 and anti-CD49d (1 µg/ml) either alone or together with one to four peptide pools (Gag, Pol, Nef, and Env) at 1 µg/ml for 6 h in the presence of brefeldin A (10 µg/ml; Sigma-Aldrich). Table I shows the selection of peptide pools used to stimulate cells from a subset of 16 participants of the study. Cells were then stained with CCR7 for 10 min at 37°C, followed by surface staining with CD4, CD8, CD45RO, CD27, CD127, CD57, PD-1, CD14, and CD19 Abs, as well as violet amine reactive dye (Vivid; Molecular Probes). This was followed by incubation with streptavidin-Qdot655 for 10 min, and cells were then fixed and permeabilized using Cytofix/Cytoperm buffer (BD Biosciences) and stained intracellularly with CD3 and IFN-γ. For examining immune activation, unstimulated PBMC were surface stained with a separate panel that included CD3, CD8, CD27, CD45RO, CD38, CD19, and the viability dye Aqua Blue (Molecular Probes). After washing, cells were resuspended in 1% paraformaldeyde (Electron Microscopy Solutions). Approximately 500,000 events were acquired per sample on an LSRII flow cytometer (BD Biosciences), and analysis was performed using FlowJo v8.5.3 (Tree Star). Dead cells (Vivid+ or Aqua Blue+), monocytes (CD14+), and B cells (CD19+) were excluded from the analysis. A time gate was first evaluated, and then cells were gated on singlets, live CD3+, CD8+, CD8+ memory, and then either IFN-γ and combinations of markers, or CD38+ cells for measuring immune activation. The gating strategy is provided in supplemental Fig. S3. Memory subsets and CD38+ cells are expressed as a percentage of total CD8+ memory cells (i.e., excluding naive CD45RO−CCR7+CD27+CD8+ cells, or in the case of the activation panel, excluding CD45RO−CD27+ CD8+ cells). A positive cytokine response was defined as at least twice background (no Ag, only costimulatory Abs), >0.05% after subtraction of background, and at least 50 events. The latter criterion was introduced so as to minimize the possibility of error due to a low number of events when further subdividing these cells into the five memory subsets. All data points for IFN-γ-specific responses after Fig. 1 represent positive peptide pool responses; that is, negative responses were excluded from subsequent analysis in the IFN-γ+ compartment.
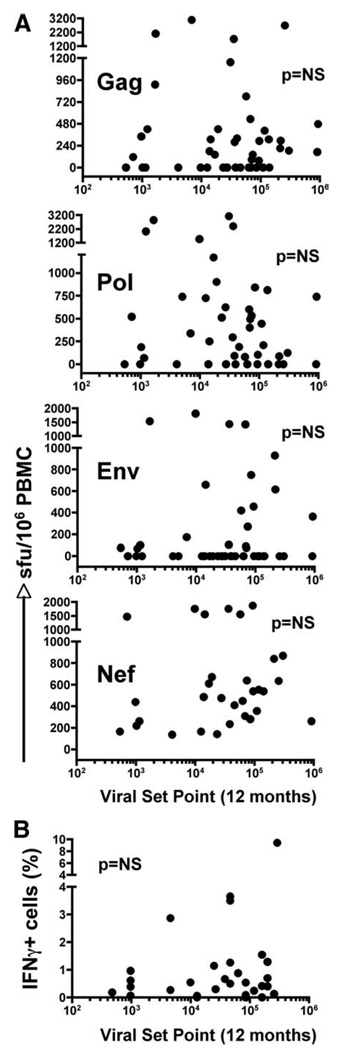
FIGURE 1.
Correlation of the magnitude of HIV-specific IFN-γ+ CD8+ T cell responses at 6 mo postinfection measured by ELISPOT or intracellular cytokine staining flow cytometry with viral set point at 12 mo postinfection. A, IFN-γ+ T cell responses were determined using the ELISPOT assay against pools of peptides corresponding to regions of the HIV-1 subtype C proteome in 47 individuals recruited during acute HIV infection. Each dot represents the cumulative sum of positive responses within each protein region (Gag, Pol, Env, and Nef) per individual at 6 mo postinfection. Magnitudes of responses were correlated with viral set point at 12 mo postinfection. B, IFN-γ+ CD8+ T cell responses at 6 mo postinfection were assessed in a subgroup of 16 study subjects by intracellular cytokine staining to detect responses toward Gag, Pol, Env, and Nef peptide pools. The percentage of IFN-γ+CD8+ T cells per individual was correlated with the viral set point at 12 mo postinfection. Statistical associations were determined using two-tailed nonparametric Spearman rank correlation.
Statistical analysis
Statistical analysis and graphical presentation were performed using InStat and GraphPad Prism version 3.0 software. Data are expressed as median values with interquartile ranges and analyzed by the use of nonparametric statistics. Statistical analysis of significance was calculated using either Mann-Whitney or Kruskal-Wallis ANOVA using Dunn’s test for multiple comparisons. All tests were two-tailed, and a value of p < 0.05 was considered statistically significant. The relationship between proportions of memory subpopulations and plasma viral load set point (in RNA copies/ml) was analyzed using Spearman rank correlations.
Results
HIV-specific IFN-γ+ T cell responses at 6 mo postinfection do not associate with viral set point at 12 mo
We first sought to investigate whether IFN-γ-specific T cell responses measured at 6 mo postinfection, using the ELISPOT assay, associated with viral set point at 12 mo after subtype C infection. HIV-specific IFN-γ+ T cell responses were measured in 47 individuals with a range of viral set point levels at 12 mo, from 400 to 1,750,000 RNA copies/ml. Fig. 1A shows no significant association between the magnitude of HIV-specific T cell responses to Gag, Pol, Nef, or Env at 6 mo postinfection and viral set point at 12 mo. Interestingly, the lack of association of responses with set point underlie the notion that possible important T cell responses, especially to Gag, may only emerge later during infection (31). We have also shown recently that the total magnitude of the IFN-γ+ response made earlier at 3 mo postinfection had very poor predictive power with viral set point (32). Similarly, when measuring total IFN-γ+ responses using intracellular cytokine staining in a subgroup of 16 individuals, shown in Table I, we identified that the major IFN-γ+ responses were from CD8+ T cells and that the proportion of these cells poorly associated with viral set point (Fig. 1B). These data demonstrate that early HIV-specific IFN-γ+ T cell responses can be of considerable magnitude but appear to have no bearing on viremia during the first year of infection.
Defining CD8+ T cell differentiation profiles
To refine the analysis further, we used polychromatic flow cytometry to investigate in greater detail the level of total and IFN-γ+ HIV-specific CD8+ T cell memory differentiation in the 16 individuals at a median time postinfection of 31 wk. The subgroup of 16 individuals did not differ significantly from the larger group of 47 individuals with respect to time from infection at recruitment, viral load, or CD4 count at 12 mo (Table I). Furthermore, there was no evidence of particular HLA allele frequencies being over- or under-represented in the subgroup compared with the larger group of individuals (data not shown). In this study we present our findings on the CD8+ memory compartment, and focus elsewhere on CD4+ memory in early infection (W. A. Burgers et al., manuscript in preparation). Fig. 2A shows representative dot plots of total CD8+ T cell subpopulations based on differential expression of CD45RO, CD27 and CCR7, where naive T cells (TNaive) along with five distinct memory populations can be distinguished, namely central memory cells (TCM), transitional memory cells (TTM), effector memory cells (TEM), cells that we have termed intermediate memory (TInt), and effector (TEff) cells. When we evaluated the proportion of these subsets in HIV-uninfected and HIV-infected individuals, no statistically significant differences were observed, although there was a trend toward a greater proportion of TTM and fewer TEff in HIV-infected individuals (Fig. 2B). Further characterization of the differentiation status of these memory subsets in HIV-infected individuals was made by assessing expression of CD127, the IL-7 receptor associated with long-term survival of cells (33); CD57, a marker of replicative senescence (34); and PD-1, a negative regulator of activation that has recently been linked with immune dysfunction (35–37). Most TNaive and TCM cells expressed CD127 (~90% and 77% respectively, Fig. 2C), while very few of these cells expressed CD57 (<3%). At the other extreme, TEff were characterized by the lowest expression of CD127 (by ~10% of these cells, Fig. 2C) and the highest expression of CD57 (by ~50%). Interestingly, PD-1 displayed an unexpected expression pattern, with more TCM and TTM expressing PD-1 compared with TEff. This has also been found by others (38), although we observed a higher proportion of TCM that expressed PD-1. Note that frequencies of CD127, CD57, and PD-1 expression on TEM were highly heterogeneous between individuals. Since CD127 characterizes long-lived cells and CD57 expression defines replicative senescence, we ranked the CD8+ T cell memory subpopulations based on the predicted ability to survive and proliferate from highest to lowest: TNaive→TCM→TTM→TInt→TEM→TEff (Fig. 2C, right panel, and D).
FIGURE 2.
Representation and definition of CD8+ T cell memory subpopulations. Expression of differentiation markers including CD45RO, CD27, CCR7, CD57, PD-1, and CD127 were analyzed on total CD8+ T cells at 6–9 mo postinfection using flow cytometry. Using these markers, we were able to discriminate six different subsets: central memory (TCM, CD45RO+CD27+CCR7+), transitional memory (TTM, CD45RO+ CD27+CCR7−), effector memory (TEM, CD45RO+CD27−CCR7−), naive (TNaive, CD45RO−CD27+CCR7+), intermediate (TInt, CD45RO−CD27dim CCR7−), and effector (TEff, CD45RO−CD27−CCR7−). A, Representative flow plots of CD45RO, CD27, and CCR7 profiles of total CD8+ T cells and the gating strategy for detection of TCM, TEM, TTM, TNaive, TInt, and TEff. B, Comparison of memory subset distribution in HIV-uninfected (n = 15) and HIV-infected individuals (n = 16). C, Percentage expression of CD127, CD57, and PD-1 in TCM, TEM, TTM, TNaive, TInt, and TEff. Each datum point represents an HIV-positive individual. Proportional differences in expression of CD27, CCR7, CD45RO, CD127, CD57, and PD-1 are shown as heat maps, based on the mean percentage expression within the different CD8+ T cell subpopulations. D, Representative flow plots for CD57/CD127 and CD127/PD-1 staining in TNaive, TCM, TEM, TTM, TInt, and TEff CD8+ T cells subsets.
Distribution of HIV-1-specific and total CD8+ memory cell populations
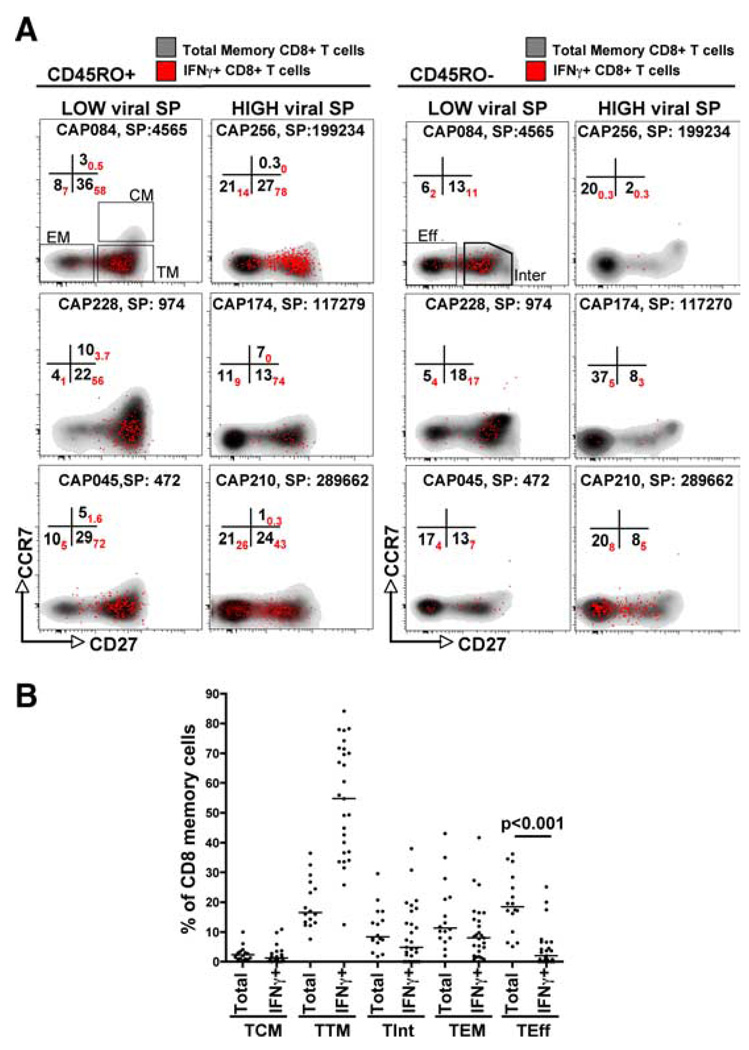
We next assessed the differentiation phenotypes of both HIV-specific and total CD8+ T cells, with the former consisting of discernible IFN-γ responses to Gag, Pol, Nef, or Env peptide pools (Table I). Representative density and overlay dot plots, showing the phenotype of total and IFN-γ+CD8+ T cells, at 6–9 mo postinfection in individuals with low and high viral set points are shown in Fig. 3A. Within the CD45RO+ compartment (allowing us to detect TCM, TTM, and TEM), total and HIV-specific CD8+ TCM were more abundant in individuals with low viral set point. Conversely, individuals with high viral set point were characterized by a higher frequency of TEM in both total and HIV-specific CD8+ T cells. Within the CD45RO− compartment (allowing us to detect TInt and TEff), the IFN-γ+CD8+ TInt memory subset was more abundant in individuals with low viral set point. There was variability in TEff frequencies in individuals with high viral set point, and these cells were not always observed in this compartment. These plots represent our main finding that lower set point at 12 mo postinfection was associated with a pool of less differentiated CD8+ memory T cells in both total and HIV-specific cells at 6–9 mo postinfection. Although there was a large level of heterogeneity between total CD8+ memory cells and IFN-γ-specific cells, the two populations tended to mirror each other, with the exception that there were significantly fewer HIV-specific TEff (p < 0.001), and a there was a trend toward a greater number of HIV-specific TTM compared with total memory cells (Fig. 3B).
FIGURE 3.
Distribution of CD8+ memory subpopulations between total memory cells and HIV-specific cells. PBMC from HIV-infected individuals (n = 16) at 6–9 mo postinfection were stimulated with Gag, Nef, Pol, and Env peptide pools and the frequency of CD8+ memory subsets was quantified from IFN-γ-producing T cells (IFN-γ+) and total memory CD8+ T cells (total memory). A, Representative density and overlay dot plots of memory subsets in total CD8+ T cells (density) and Gag-specific IFN-γ+ CD8+ T cells (red dots) defined according to CD45RO, CD27, and CCR7 expression levels. Left panels, CD45RO+ memory CD8+ T cells at 6–9 mo postinfection in three individuals (CAP084, CAP228, and CAP045) having a low viral set point (SP) at 12 mo postinfection and three individuals (CAP256, CAP174, and CAP210) with high viral set point at 12 mo postinfection. Right panels, CD45RO− memory CD8+ T cells in low and high viral set point individuals. Numbers in the quadrants correspond to the proportion of each memory subset in total memory CD8+ T cells (in black), expressed as a percentage of total memory cells, while the proportion of IFN-γ+CD8+ T cells are in red. Patient identification numbers and viral set point (SP) at 12 mo are shown. B, TCM, TTM, TInt, TEM, and TEff memory subsets expressed as a percentage of total memory cells. For total memory, each datum point represents a study individual, and for IFN-γ+ cells, each datum point represents a positive HIV-specific response. The median is shown as a horizontal bar. Statistical comparisons were performed using Krukal-Wallis ANOVA and multiple comparisons using Dunn’s test.
Differentiation status of HIV-specific and total CD8+ T cells associates with viral set point
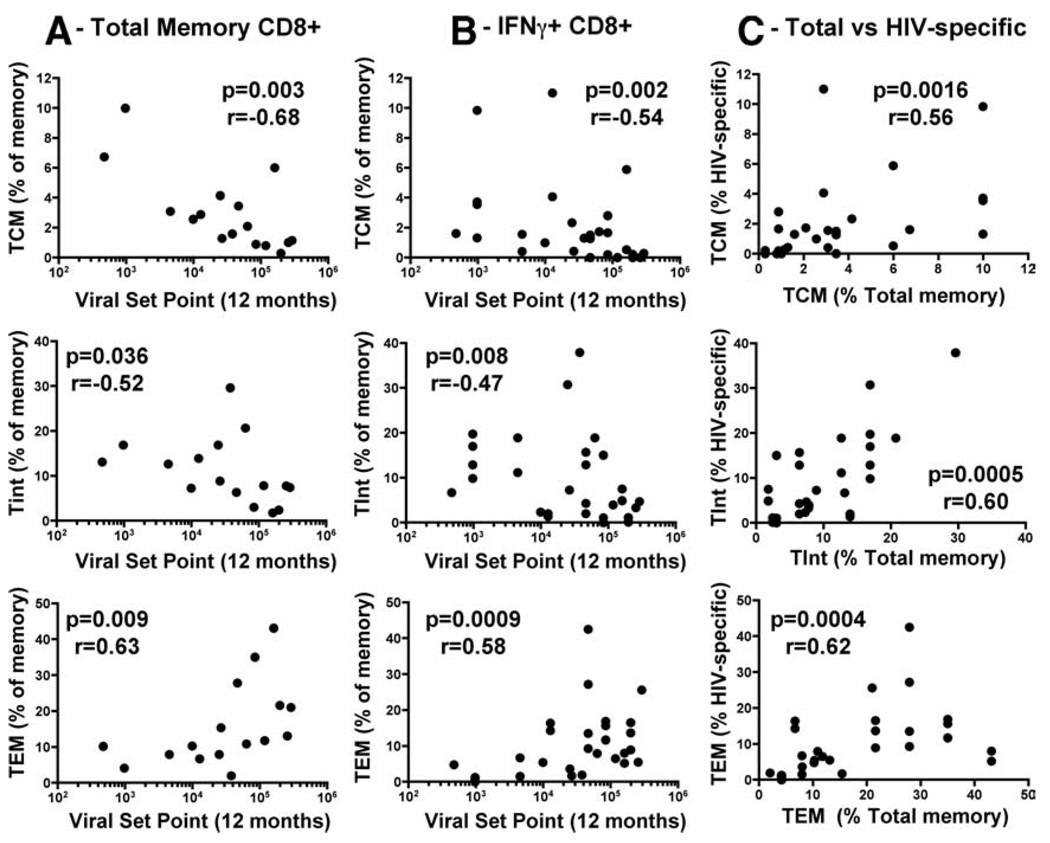
To further investigate the relationship between CD8+ memory subsets and viral set point, we correlated TCM, TTM, TInt, TEM, and TEff memory populations at 6–9 mo with viral set point at 12 mo. Fig. 4A shows that the frequencies of total TCM and TInt inversely correlated with viral set point (r = −0.68, p = 0.003 and r = −0.52, p = 0.036, respectively), while the frequency of total CD8+ TEM positively associated with viral set point (r = 0.63, p = 0.009). No significant relationships were found between viral set point and TTM and TEff cell populations (data not shown). We also examined the frequencies of TNaive cells in the total CD8+ T cell population and found that there was a significant negative association with viral set point (p = 0.01, r = −0.59, data not shown). To take into account variation in CD8+ counts among study subjects, we also performed correlations between the absolute number of cells in each memory subpopulation (based on absolute CD8+ cells/µl) and viral set point. The absolute number of TCM, TInt, and TEM cells reflected the relationships found for percentage of memory cells (r = −0.6, p = 0.014; r = −0.52, p = 0.03; and r = 0.49, p = 0.05, respectively; supplemental Fig. S1). We next assessed the relationship between HIV-1-specific IFN-γ+CD8+ TCM, TTM, TInt, TEM, and TEff profiles with viral set point and found that there was a significant negative association between TCM and TInt with set point (r = −0.54, p = 0.002 and r = −0.47, p = 0.008, respectively; Fig. 4B). Additionally, the proportion of IFN-γ+CD8+ T cells that possessed a TEM phenotype positively correlated with viral set point (r = 0.58, p = 0.0009; Fig. 4B). As in total CD8+ T cells, no correlations were observed between the proportion of TTM and TEff in HIV-specific cells and viral set point (data not shown). Further analyses showed significant positive correlations between total and HIV-specific TCM, TInt, and TEM (r = 0.56, p = 0.0016; r = 0.6, p = 0.0005; and r = 0.62, p = 0.0004, respectively; Fig. 4C), revealing that there was a synchronous differentiation profile of HIV-specific and total CD8+ T cells.
FIGURE 4.
Correlations of the percentage of TCM, TInt, and TEM at 6–9 mo postinfection with viral set point at 12 mo postinfection: (A) in total CD8+ memory T cells, (B) in HIV-specific IFN-γ+CD8+ memory T cells, and (C) between total and HIV-specific CD8+ memory T cells. For total memory, each datum point represents a study individual, and for IFN-γ+ cells, each datum point represents a positive HIV-specific response. Statistical associations were determined using two-tailed nonparametric Spearman rank correlation.
CD8+ T cell memory differentiation associates with immune activation
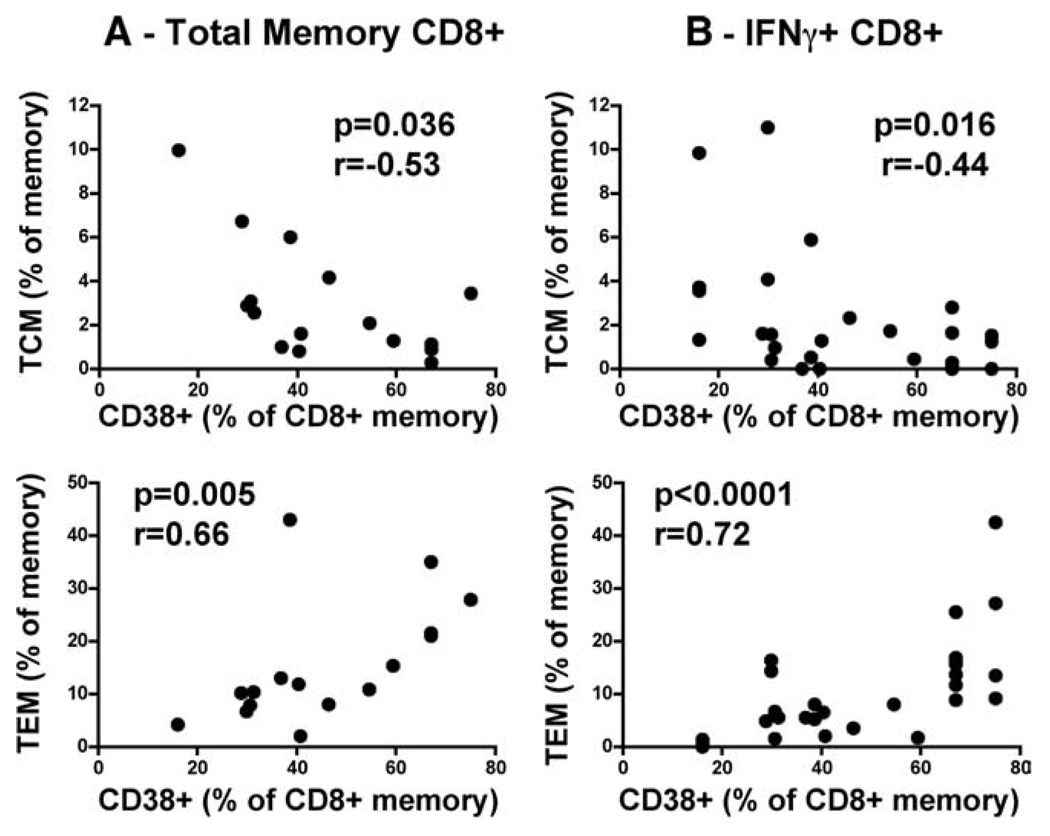
When we examined the relationship of differentiation profiles with concurrent viral load (at the time of analysis), we found a significant inverse correlation between HIV-specific TCM and a positive correlation of TEM with viral load (r = −0.41, p = 0.02 and r = 0.47, p = 0.009, respectively). The strength of these correlations did not hold when associating total memory CD8+ phenotypes with concurrent viral load (TCM, r = −0.47, p = 0.067; TEM, r = 0.39, p = 0.134). This would suggest that viremia exclusively drives HIV-specific CD8+ T cell differentiation, but not necessarily total memory differentiation. To examine whether the overall level of T cell activation was possibly related to memory differentiation, we examined CD38 surface expression on total CD8+ memory T cells using a separate activation phenotype Ab staining panel. It is well known that CD38 is a marker of generalized immune activation, and expression on CD8+ T cells is a strong indicator of disease progression (39). We found an inverse correlation between proportions of total CD8+ TCM and a positive correlation for total CD8+ TEM with CD38+CD8+ total memory cells (r = −0.53, p = 0.036 and r = 0.66, p = 0.005; Fig. 5A). This relationship also held true for HIV-specific cells (TCM, r = −0.44, p = 0.016; TEM, r = 0.72, p < 0.0001; Fig. 5B), and the level of immune activation correlated significantly with viral set point (r = 0.63, p = 0.008). These data infer that immune activation is possibly a strong driver of memory differentiation. Thus far, we have shown that the predominance of a particular CD8+ memory subset significantly associates with viral set point. High set point at 12 mo is preceded by an imbalance of the TNaive→TCM→TTM→TInt→TEM→TEff profile within the memory CD8+ T cell compartment, with a shift toward a more differentiated profile in both total and HIV-specific IFN-γ+CD8+ T cells. These imbalances are strongly associated with varying levels of immune activation as measured by CD38 surface expression.
FIGURE 5.
Relationship between immune activation and memory differentiation. Correlation of the percentage of CD38+ memory CD8+ T cells with TCM and TEM at 6–9 mo postinfection: in (A) total CD8+ memory T cells and in (B) HIV-specific IFN-γ+CD8+ memory T cells. Statistical associations were determined using two-tailed nonparametric Spearman rank correlation.
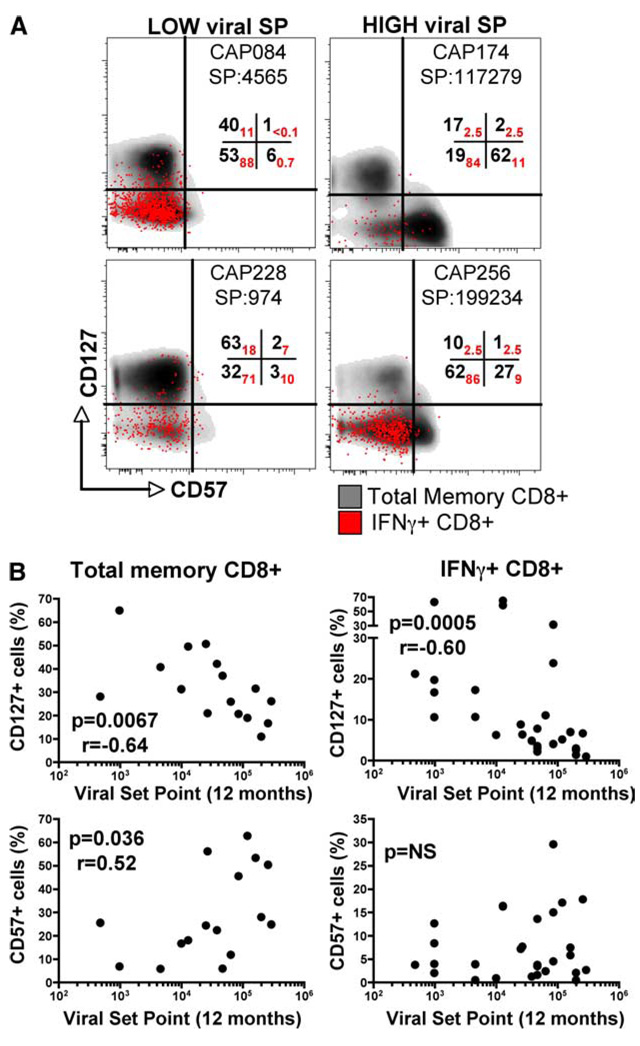
Survival and differentiation phenotypes correlate with viral set point
To investigate the predicted survival capacity of HIV-specific CD8+ T cells, we examined the expression of CD127 and CD57 on IFN-γ+CD8+ T cells. Representative CD127 and CD57 staining profiles for individuals with low or high viral set points are shown in Fig. 6A, where density plots represent total memory subsets overlayed with HIV-specific responses. From these examples it was apparent that there were fewer CD127+ and more CD57+ cells within the total memory population found in individuals with a high viral set point (Fig. 6A), while individuals with a low viral set point had greater frequencies of CD127+ HIV-specific IFN-γ-producing cells. There was a significant negative association between CD127 expression in total memory and HIV-specific CD8+ T cells with viral set point (r = −0.64, p = 0.0067 and r = −0.60, p = 0.0005, respectively; Fig. 6B), suggesting that HIV-specific cells from individuals able to control viremia have a better ability to be homeostatically maintained through the IL-7 survival pathway. The expression frequencies of CD57 showed a significant positive correlation with viral set point in the total CD8+ memory compartment (r = 0.52, p = 0.036), but this was not mirrored in HIV-specific IFN-γ+ cells. For PD-1 expression, neither the percentage of cells nor the levels of PD-1 (as measured by median fluorescent intensity) were significantly associated with viral set point (data not shown). Note that expression levels of CD57 on HIV-specific cells were marginal compared with CD57 expression on total memory CD8+ T cells (median, ~5% and 25%, respectively). Low expression of CD57 on HIV-specific cells has previously been reported (40).
FIGURE 6.
Phenotypic expression of CD57 and CD127 on total memory and HIV-specific CD8+ memory subpopulations. A, Representative CD127 vs CD57 density plots depicting total memory (gray) with overlayed IFN-γ+CD8+ T cells (red dots) in two study individuals with a low 12 mo viral set point (left panels) and two individuals with a high viral set point (right panels). Numbers in the quadrants represent the percentage of CD127+CD57−, CD127+CD57+, CD127+ CD57−, and CD127−CD57+ cells in total memory CD8+ T cells (in black) and in IFN-γ+CD8+ T cells (in red). Patient identification numbers and viral set point (SP) at 12 mo are shown. B, Correlation of the percentage of CD127+ and CD57+ cells in total memory (left panel) and IFN-γ+CD8+ T cells (right panel) at 6–9 mo postinfection with viral set point at 12 mo postinfection. Statistical associations were determined using two-tailed nonparametric Spearman rank correlation.
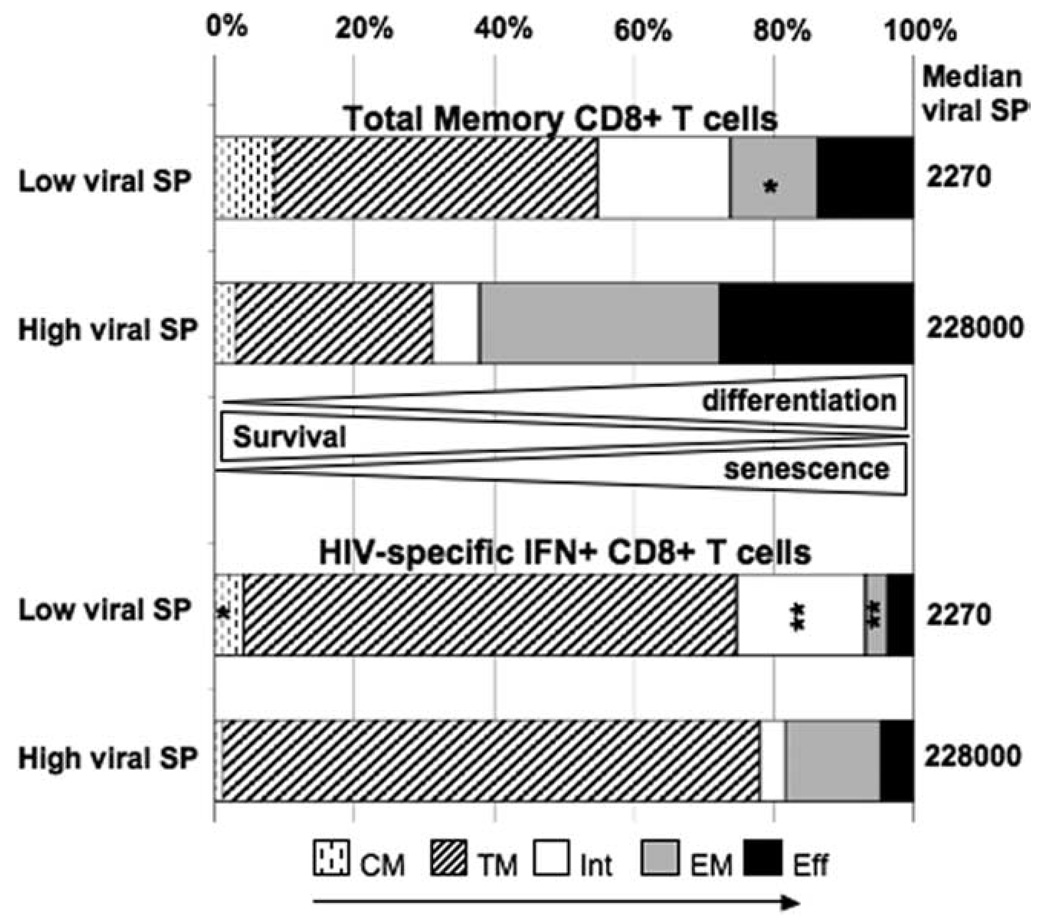
To summarize, when we compared the distribution of TNaive→TCM→TTM→TInt→TEM→TEff subsets in HIV-specific and total memory CD8+ T cells in individuals with low vs high viral set points, we observed that a high set point was preceded by a higher frequency of effector memory cells and lower frequencies of central and intermediate memory cells (Fig. 7). Conversely, a low set point at 12 mo was preceded by a larger pool of less differentiated central and intermediate CD8+ T cell memory phenotypes (Fig. 7). These data suggest that pools of central and intermediate memory CD8+ T cells accumulate in the peripheral blood of individuals who are able to later control viral replication at 12 mo.
FIGURE 7.
Balance of memory CD8+ T cell populations in HIV-positive individuals with low or high viral set point at 12 mo postinfection. Representation of the proportion of CD8+ T cells memory subpopulations (TCM, TEM, TTM, TInt, and TEff) in total memory and HIV-specific CD8+ T cells at 6–9 mo postinfection in HIV-infected individuals with low (n = 4) or high (n = 4) viral set point. Asterisks indicate significant differences between low and high viral set point individuals (*, p < 0.05; **, p < 0.005).
Discussion
In this study we have investigated features of the early CD8+ T cell response that mediate viral control within the first year of infection in a subtype C HIV-1 acute infection cohort (26). We have shown that the magnitude of HIV-1-specific IFN-γ+ T cell responses to Gag, Pol, Env, and Nef during early infection were not associated with viral set point at 12 mo. When we examined differentiation phenotypes of both total and HIV-specific CD8+ T cells, we found that a less differentiated profile correlated with a lower viral set point, while a more differentiated profile correlated with a higher viral set point. Unraveling relationships in early HIV infection between T cell differentiation, immune activation, viral control, and disease progression that may influence this skewing is important to advance our understanding of HIV pathogenesis.
T cells are composed of different memory subsets, or maturational stages, which are characterized by differential homing capacities, survival times, and functional abilities (41, 42). According to our present understanding, TNaive and TCM preferentially home to lymph nodes, while TEM and TEff subsets, lacking CCR7 expression, are localized in tissues. Phenotype and function are linked, where TCM are endowed with a better proliferation potential, being able to self-renew, compared with TEM and TEff, which are considered terminally differentiated cells and die upon restimulation (43, 44). TEM are endowed with ready effector functions, and ex vivo human TEM are characterized by higher levels of perforin and granzyme B expression and display greater cytotoxic abilities compared with TCM (45). In mice, however, Barber et al. showed that upon reactivation, TCM and TEM were equally efficient at cytotoxic killing in vivo (46). While TCM cells express little perforin and granzyme, they can acquire these functions rapidly upon secondary encounter with Ag. Thus, TCM may be more effective at mediating protective immunity by virtue of their ability to proliferate, which may be particularly important for persisting infections. Thus, according to the functional characteristics of TCM, an early accumulation of these cells preceding low viral set point suggests a role for these cells in viral control. We could hypothesize therefore that long-lived TCM and TInt found in individuals with low set points may reflect a greater ability to proliferate and subsequently control viremia over time. The alternative possibility is that differentiation status may simply reflect antigenic burden and levels of immune activation.
One of the most intriguing findings in our study was the synchronous differentiation profile of HIV-specific and total CD8+ memory cells. This begs the question: What factors may shape the immune system in the first year of infection? The most obvious candidate is HIV itself, providing persistent Ag stimulation for T cells. Reactivation of latent CMV, EBV, and other co-viral infections may also provide a plethora of specific antigenic stimuli within the CD8+ memory compartment (47). Additionally, indirect stimulation of memory cells by a highly activated immune system may also explain the effects on the total CD8+ memory compartment. Immune activation can be caused by TLR ligands within HIV RNA (48) or by microbial translocation from the gut (49). The degree to which all of these factors contribute to the early skewing of the total CD8+ memory compartment is not known; however, the link between CD8+ memory differentiation and immune activation has previously been proposed (40). Results from this study suggest that the highly differentiated status of CD8+ T cells is a consequence of persistent immune activation, which affects not only HIV-specific cells but all memory cells, and could therefore lead to extensive cell division and differentiation, resulting in premature exhaustion of cell functions. Our data support this, where we show that higher immune activation levels in bulk CD8+ memory cells, as measured by CD38+ expression, are associated with fewer TCM and more TEM. There is also the possibility that early skewing of HIV-specific CD8+ memory cells is related to the differentiation status of the memory compartment before HIV infection. Nonetheless, our data suggest that the establishment of a “protective” immune response could, in part, rely on the generation and maintenance of early differentiated cells endowed with greater self-renewal and survival abilities.
The use of CD27 staining in combination with CCR7 allowed us to define an intermediate population of CD8+ T memory cells (CD45RO−CD27+CCR7−), which we termed “TInt”. These cells have been previously noted in studies examining CD8+ T cell responses to vaccinia virus and CMV (50, 51). Our data show that, in addition to TCM, the proportions of TInt in both total and HIV-specific memory CD8+ T cells were inversely associated with viral set point. We also identified the TInt population in total CD8+ memory cells in HIV-uninfected individuals at similar proportions, where they constitute a sizeable proportion of the total CD8+ memory compartment. They appear distinct from TEff, with few TInt cells expressing CD57, and the proportion of cells expressing CD127 is intermediate between TCM and TEM. This suggests that these cells represent a memory subset in early differentiation, and that they may be endowed with proliferation abilities and be sensitive to IL-7. Indeed, Wills et al. (51) showed that these cells can proliferate strongly. Further investigations are needed to define the functional capacities of this subset and to establish its relationship to other memory subsets in a maturational pathway.
Our data are in apparent contrast to two recent studies that show that late differentiated HIV-specific CD8+ T cells (CD45RA+ CCR7− cells, termed terminally differentiated effector cells or TEMRA in these studies) were associated with viral control in HIV infection (14, 20). Chronically infected individuals controlling viral load had a greater number of these cells than progressors (14), and in early infection, their presence was associated with lower viral set points (20). In these studies memory subsets were discriminated on the basis of CD45RA and CCR7 expression, so terminally differentiated cells were equivalent to our combined TInt and TEff subsets, since we used CD27 to further define these subpopulations. Indeed, if we group these subsets together, we reach a similar conclusion in our study, with HIV-specific CD45RO− CCR7−CD8+ cells correlating inversely with viral set point (data not shown). However, it is clear that the CD45RO−(RA+)CCR7− subset is highly heterogeneous in terms of CD127 and CD57 expression, and additional markers such as CD27 aid in distinguishing between cells that may be functionally diverse.
We further characterized memory populations on the basis of CD127, CD57, and PD-1 expression. A paucity of CD127-expressing cells has been observed in chronic HIV infection and is related to persistent viremia (52, 53). Indeed, it has been shown that the accumulation of CD8+CD127− cells correlates with higher HIV viral load and lower CD4 counts (54). In line with these findings, we show in early HIV infection that CD127 expression was higher on HIV-specific IFN-γ+ cells in individuals naturally controlling viral replication compared with individuals possessing high viral set points, consistent with our observations of preserved early memory subsets. With regard to CD57 expression, although there was a trend toward higher CD57 expression on CD8+ T cells in those with high set points, the overall expression of CD57 was very low (<10%). The modest expression of CD57 on these cells, despite their late differentiated phenotype, could be due to the rapid disappearance of the HIV-specific CD57+ cells, in keeping with their high sensitivity to activation-induced cell death (34). We also did not find any significant association between PD-1 expression on total and HIV-specific IFN-γ+CD8+ T cells and viral control. Surprisingly, when analyzing total memory subsets, we observed high frequencies of PD-1+ cells in early and intermediate differentiated subsets (i.e., TCM and TInt), cells that were associated with better viral control at 1 year. This is in apparent conflict with evidence linking increased PD-1 expression to immune dysfunction, higher viral loads, and lower CD4 counts in chronic HIV infection (36, 37, 55). Our observations are in agreement with Sauce et al. (38), who found that PD-1 expression is higher on less differentiated than terminally differentiated memory subsets, where these PD-1+ cells remain fully functional, suggesting that PD-1 does not solely play a role in T cell exhaustion but could be implicated in T cell differentiation and/or activation. Further investigations are required to establish the precise role played by PD-1 in balancing stimulatory signals with inhibitory signals that maintain homeostasis of differentiated cells. Moreover, PD-1 expression is up-regulated in concert with markers of immune activation, so high levels of PD-1 in chronic HIV infection may be linked to the generalized immune activation, which is a hallmark of progressive disease (56). Other recent studies also support a regulatory role for PD-1 in memory cell differentiation (57, 58).
Overall, we have shown that viral control and the tempo of HIV disease progression in the first year of infection are linked to the presence of particular CD8+ memory subpopulations. Phenotypes associated with more differentiated memory cells accumulate by 6–9 mo in individuals with higher viral set points at 12 mo and are associated with a higher CD8+ T cell activation status. Conversely, the presence of less differentiated and longer lived CD8+ cells correlates with lower viral set point at 1 year after infection and is associated with a lower cell activation status. In conclusion, the level of immune activation may be a central driver underlying memory cell differentiation, where we hypothesize that persistently elevated activation signals push memory cell differentiation to TEff and senescence. What is cause and effect between viral load, memory differentiation, and immune activation remains the vexing issue.
Supplementary Material
Acknowledgments
We thank the participants and clinical and laboratory staff at CAPRISA for the specimens. We thank Laurie Lamoreaux and Jennifer Fischer for technical help, and Steve Perfetto for LSRII training.
Footnotes
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services Grant U19 A151794; the Canada-Africa Prevention Trials Network; and the Bill and Melinda Gates Comprehensive T Cell Vaccine Immune Monitoring Consortium award. W.B. was supported by a Columbia University-Southern Africa Fogarty AIDS International Training Fellowship.
Abbreviations used in this paper: PD-1, programmed death 1; TCM, central memory T cell; TEff, effector T cell; TEM, effector memory T cell; TInt, intermediate memory T cell; TNaive, naive T cell; TTM, transitional memory T cell.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao YZ, Andrews CA, Mcleod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, Obrien SJ, Rinaldo C, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MBA. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type-1 infection. J. Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, et al. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS. 2003;17:2581–2591. doi: 10.1097/00002030-200312050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 gag-specific IFN-γ+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 2002;169:6376–6385. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 7.Jansen CA, De Cuyper IM, Hooibrink B, van der Bij AK, van Baarle D, Miedema F. Prognostic value of HIV-1 Gag-specific CD4+ T-cell responses for progression to AIDS analyzed in a prospective cohort study. Blood. 2006;107:1427–1433. doi: 10.1182/blood-2005-07-2907. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virusinfected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 10.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao YZ, Rowland-Jones SL, Cerundolo V, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 11.Gea-Banacloche JC, Migueles SA, Martino L, Shupert WL, McNeil AC, Sabbaghian MS, Ehler L, Prussin C, Stevens R, Lambert L, et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 12.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, et al. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 2004;78:3233–3243. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addo MM, Draenert R, Rathod A, Verrill CL, Davis BT, Gandhi RT, Robbins GK, Basgoz NO, Stone DR, Cohen DE, et al. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS ONE. 2007;2:e321. doi: 10.1371/journal.pone.0000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betts MR, Casazza JP, Koup RA. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol. Lett. 2001;79:117. doi: 10.1016/s0165-2478(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 16.Day CL, Kiepiela P, Leslie AJ, van der Stok M, Nair K, Ismail N, Honeyborne I, Crawford H, Coovadia HM, Goulder PJ, et al. Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J. Virol. 2007;81:434–438. doi: 10.1128/JVI.01754-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 20.Northfield JW, Loo CP, Barbour JD, Spotts G, Hecht FM, Klenerman P, Nixon DF, Michaëlsson J. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ TEMRA cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J. Virol. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J. Acquired Immune Defic. Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Mattapalli JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 24.Mueller YM, Petrovas C, Do DH, Altork SR, Fischer-Smith T, Rappaport J, Altman JD, Lewis MG, Katsikis PD. Early establishment and antigen dependence of simian immunodeficiency virus-specific CD8+ T-cell defects. J. Virol. 2007;81:10861–10868. doi: 10.1128/JVI.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? . Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, Abdool Karim Q, Grobler A, Barnabas N, Iriogbe I, Abdool Karim SS CAPRISA 002 Acute Infection Study Team. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS ONE. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mlisana K, Auld SC, Grobler A, van Loggerenberg F, Williamson C, Iriogbe I, Sobieszczyk ME, Abdool Karim SS CAPRISA Acute Infection Study Team. Anaemia in acute HIV-1 subtype C infection. PLoS ONE. 2008;3:e1626. doi: 10.1371/journal.pone.0001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopera DR, Woodman Z, Mlisana K, Mlotshwa M, Martin DP, Seoighe C, Treurnicht F, de Rosa DA, Hide W, Karim SA, et al. Transmission of HIV-1CTL escape variants provides HLA-mismatched recipients with a survival advantage. PLoS Pathog. 2008;4:e1000033. doi: 10.1371/journal.ppat.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson C, Morris L, Maughan MF, Ping LH, Dryga SA, Thomas R, Reap EA, Cilliers T, van Harmelen J, Pascual A, et al. Characterization and selection of HIV-1 subtype C isolates for use in vaccine development. AIDS Res. Hum. Retroviruses. 2003;19:133–144. doi: 10.1089/088922203762688649. [DOI] [PubMed] [Google Scholar]
- 30.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat. Prot. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 31.Goulder PJR, Altfeld MA, Rosenberg ES, Nguyen T, Tang YH, Eldridge RL, Addo MM, He SQ, Mukherjee JS, Phillips MN, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray CM, Mlotshwa M, Riou C, Mathebula T, de Assis Rosa D, Mashishi T, Seoighe C, Ngandu N, van Loggerenberg F, Morris L, et al. on behalf of the CAPRISA 002 Acute Infection Study Team. HIV-specific IFNγ-ELISPOT responses targeting specific regions of the proteome during primary subtype C infection are poor predictors of the course of viraemia and set point. J. Virol. 2008 doi: 10.1128/JVI.01678-08. [Epub ahead of print on Oct. 22, 2008; doi 10.1128] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 34.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 35.Barber DL, Wherry EJ, Masopust D, Zhu BG, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 36.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 37.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 38.Sauce D, Almeida JR, Larsen M, Haro L, Autran B, Freeman GJ, Appay V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T-cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 40.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:173–185. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 42.Fritsch RD, Shen XL, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 43.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 44.Riou C, Yassine-Diab B, van Grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J. Exp. Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 46.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 47.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, Venet A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 48.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bayhi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 50.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 52.Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, Woodberry T, Brander C, Addo M, Klenerman P, Ahmed R, Walker BD. HIV-pecific CD8 T cells express low levels of IL-7Rα: implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercier F, Boulassel MR, Yassine-Diab B, Tremblay C, Bernard NF, Sekaly RP, Routy JP, et al. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Rα on central and effector memory CD4+ and CD8+ T cell subsets. Clin. Exp. Immunol. 2008;152:72–80. doi: 10.1111/j.1365-2249.2008.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 2005;174:2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 55.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazenberg MD, Otto SA, van Benthem BHB, Roos MTL, Coutinho RA, Lange JMA, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 57.Bowen DG, Shoukry NH, Grakoui A, Fuller MJ, Cawthon AG, Dong C, Hasselschwert DL, Brasky KM, Freeman GJ, Seth NP, et al. Variable patterns of programmed death-1 expression on fully functional memory T cells after spontaneous resolution of hepatitis C virus infection. J. Virol. 2008;82:5109–5114. doi: 10.1128/JVI.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He XH, Jia QT, Li FY, Saltis M, Liu Y, Xu LH, Zha QB. CD8+ T cells specific for both persistent and non-persistent viruses display distinct differentiation phenotypes but have similar level of PD-1 expression in healthy Chinese individuals. Clin. Immunol. 2008;126:222–234. doi: 10.1016/j.clim.2007.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.