Abstract
The self-renewing capacity of B1 cells infers homeostatic regulation; however, previous work suggests the low level of N-region addition characterizing B1 cells early in life increases with age, which implies that the B1-cell population is not a closed system. To explore this, we evaluated N-region addition in CD5+ B1 cells generated from adult BM. Adult BM cells were marked with GFP introduced by mouse stem cell virus transduction, and were then adoptively transferred into lethally irradiated recipients. Within 2–3 months, we found GFP-marked CD5+ B cells in the peritoneal cavities of recipients, which we demonstrate here meet a variety of criteria for B1-cell traits including Mac-1 surface expression; annexin, elfin, and Pax-5 gene expression; mitogenic responsiveness to phorbol ester; and spontaneous immunoglobulin secretion. Notably, we found by single-cell PCR that this population of BM-derived CD5+ B1 cells expressed immunoglobulin with abundant N-region addition (and little VH11/VH12 skewing), unlike CD5+ B1 cells obtained from unmanipulated animals but reminiscent of B2 cells. Further, we confirmed that native CD5+ B1 cells from older mice contain more N-region additions than native CD5+ B1 cells from younger mice. These results suggest that adult BM progenitors contribute to the peritoneal CD5+ B1-cell pool over time.
Keywords: Antibodies, B cells, Cell differentiation, Repertoire development
Introduction
B1 cells are distinguished from conventional B2 cells by phenotypic, transcriptomic and functional criteria (reviewed in [1-4]). B1 cells are defined by expression of the surface antigen, CD5, in the context of characteristic B-cell markers; such B cells are designated B1a or CD5+ B1 cells. CD5+ B1 cells typically express substantial levels of Mac-1, CD43, and CD80, in contrast to conventional B (B2) cells that fail to express Mac-1 or CD43 and express relatively meager amounts of CD80 [5]. CD5+ B1 cells are further distinguished from B2 cells by higher-level expression of IgM, lower level expression of IgD, lower level expression of B220, and failure to express CD23. A small subpopulation of B1 cells, designated B1b, shares the phenotype of B1a cells except for absent expression of the classical marker, CD5, but is separately regulated and distinguished functionally [6-10].
The composition of CD5+ B1 cells differs from that of B2 cells in a number of ways that can be detected transcriptomically and proteomically, prominent among which is elevated gene expression of annexin II and elfin by B1 but not by B2 cells, and diminished gene expression of Pax-5 by B1 in comparison to B2 cells [11-13]. Functionally, CD5+ B1 cells differ from B2 cells in the stimuli required to signal for initiation of proliferation. CD5+ B1 cells rapidly enter the S phase in response to treatment with phorbol ester (such as PMA) alone, whereas B2 cells respond to PMA only in conjunction with a calcium ionophore [14]. The initiation of proliferation in B1 cells by PMA is associated with specific and early induction of cyclin D2 and the generation of cyclin D3–cdk2 complexes [15, 16].
CD5+ B1 cells play an indispensable and unique role in generating protection against microbial infection. CD5+ B1 cells constitutively and spontaneously secrete immunoglobulin, termed natural antibody, unlike B2 cells, which require further differentiation for immunoglobulin secretion to occur [11, 17-20]. However, the immunoglobulin produced by CD5+ B1 cells differs from that produced by B2 cells. CD5+ B1-cell-derived immunoglobulin is repertoire skewed, and thus selected, as exemplified by overexpression (in comparison to B2 cells) of two phosphatidylcholine-binding VH gene segments, VH11 and VH12, which are protective against intestinal bacteria [21-25]. Other CD5+ B1-cell specificities protect against other microbial organisms [26, 27]. Notably, CD5+ B1 cells (B1a cells) uniquely produce natural antibody that provides early protection against Streptococcus pneumoniae, whereas CD5− B1 cells (B1b cells) do not, and thus from the standpoint of natural immunoglobulin CD5+ B1a cells, as opposed to CD5− B1b cells, are especially important [9]. CD5+ B1-cell repertoire skewing may relate to diminished variability in CD5+ B1-cell immunoglobulin, which results from reduced non-templated N-region addition and reduced somatic mutation [28-30]. Previous study of N-region addition suggested that the low level that characterizes CD5+ B1 cells early in life increases with age [30], but this has been disputed [31, 32]. Although CD5+ B1 cells display the capacity of self-renewal [33, 34], a time-dependent increase in N-addition, if true, would suggest that the CD5+ B1-cell population is not a closed system [30, 31], with important implications for the composition of natural antibody.
Two distinct paradigms have been proposed for the origin of B1 cells. The lineage model holds that B1 cells develop from a distinct, B1-cell-specific progenitor that exists early in ontogeny but is absent or rarely present in adult BM (reviewed in [1]). The differentiation model holds that B1 cells develop from the same progenitor as B2 cells, as a result of specific forms of BCR signaling, and that the common B-cell progenitor exists in adult BM (reviewed in [3]). Recently a distinct B220lo/−CD19+ progenitor for B1 cells has been identified by Dorshkind and colleagues, lending credence to the lineage model described above [35, 36], although it is not known whether all, or only some, B1 cells derive from this progenitor. The B1-cell progenitor was found to be present in both fetal liver and adult BM, but to a much lesser extent in the latter as opposed to the former. The bulk of B1 cells are produced during fetal and early neonatal life, and although it is accepted that adult BM can give rise to CD5− B1b cells, the capacity of adult BM to give rise to CD5+ B1 cells remains unclear. In fact, a majority of the B1 cells produced by the B220lo/−CD19+ Dorshkind progenitor in adoptive transfer experiments were CD5− B1 (B1b) cells [35]. Some of the confusion results from the use of phenotype as a marker for B1 cells, since activation can induce expression of CD5 on B2 cells [37].
In the present work, we sought to conclusively determine whether adult BM can give rise to authentic CD5+ B1 cells; positive results would support the idea that the B1 population in the adult is dynamic, with continual input from BM, rather than static as a result of self-renewal. To do so we carried out adoptive transfer of adult BM following retroviral transduction to mark progenitor cells with GFP, and then examined GFP-expressing peritoneal B1 cells that arose in recipient mice. We used a variety of assays beyond phenotype to verify that identified CD5+ B cells share common B1-cell traits. In this way we demonstrated convincingly that BM progenitors generate B1 cells. We then asked whether such adult BM-derived (BMD) CD5+ B1 cells contain few, or many, N-region additions. If few, the potential compositional change in the B1-cell population produced by the addition of CD5+ B1 cells derived from BM progenitors might make little functional difference in the generation of natural immunoglobulin. In contrast, if N-region addition is increased in adult BMD CD5+ B1 cells, as opposed to fetal liver-derived CD5+ B1 cells, then the composition of B1-cell-produced natural immunoglobulin could change over time, extrapolation from which would suggest that the anti-bacterial pool of pre-existing natural immunoglobulin might deteriorate. We found that adult BMD CD5+ B1 cells contain elevated levels of N-region addition, in comparison to native CD5+ B1 cells (and diminished levels of VH11 and VH12), and we further found that native CD5+ B1 cells from older mice express immunoglobulin with increased N-region addition in comparison to native CD5+ B1 cells from younger mice. Together, these results suggest a new paradigm in which the early B1-cell pool is altered over time by input of newly differentiated B1 cells derived from the BM.
Results
We evaluated BM production of CD5+ B1 cells in a global way, because it is not yet known whether all B1 cells, or only a portion of B1 cells, derive from B220lo/−CD19+ progenitors. To do this we utilized mouse stem cell virus (MSCV), which previous work has shown infects hematopoietic stem cells [38] and not mature lymphocytes. We transduced BM depleted of lin+ cells with an MSCV vector containing GFP, followed by adoptive transfer to lethally irradiated recipients. This provided the means (through GFP positivity) to track any and all mature cells that derived from transduced BM progenitors.
CD5+ B1 cells are found in the peritoneal cavities of BM recipients
We examined peritoneal washout cells obtained from adoptive hosts 8–12 wk following administration of MSCV-infected (lin−) BM. We counted these cells, which we then characterized phenotypically by immunofluorescent staining and flow cytometry. Results obtained with five reconstituted mice and with five normal adult BALB/c mice are presented in Table 1. The total number of cells recovered from peritoneal washout fluids differed little between BM chimera (adoptive host) mice and normal (non-irradiated) control mice (data not shown), suggesting that reconstitution was complete. Similarly, lymphocytes constituted about half of the peritoneal washout cells in both chimera and control mice. In chimeras, about 29% of lymphocytes overall expressed GFP, reflecting the efficiency of progenitor infection by MSCV. Similarly, 26% of B cells and 24% of T cells expressed GFP. We compared the relative abundance of various lymphocyte subsets contained within the GFP+ fraction of chimera peritoneal lymphocytes with the relative abundance of lymphocyte subsets contained within control peritoneal lymphocytes. We found that CD5+ B1 cells constituted 12% of the former and 35% of the latter (and, by way of comparison, 9%±2, mean±SEM, of the GFP− fraction of chimera peritoneal lymphocytes, n = 5). Thus, reconstitution of CD5+ B1 cells from BM stem cells was incomplete, as evidenced by analysis of T cells (B220−CD5+), which showed similar proportions among GFP+ chimera peritoneal lymphocytes and control peritoneal lymphocytes (9%±2 and 7%±2, n = 5). Although CD5+ B1-cell reconstitution from adult BM progenitors was incomplete, substantial CD5+ B1-cell development and expansion did occur, such that, on average, lin− adult BM produced about 90 000 BMD peritoneal CD5+ B1 cells per chimera mouse whereas each normal mouse contained about 600 000 recoverable peritoneal B1 cells.
Table 1.
Recovery of B1 cells from adoptive transfer hosts given MSCV.GFP-marked lineage-negative bone marrow
| Bone marrow chimera mice |
WT BALB/c Mice |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse | Lymphsa present in PWOb |
Lymphs that are GFP+c |
GFP+ lymphs that are B220+/ CD5+ |
GFP+ lymphs that are B220+/ CD5+ |
GFP+ cells among T Cells |
GFP+ cells among B Cells |
Mouse | Lymphs from present in PWOb |
Lymphs that are B220+/ CD5+ |
| #1 | 41.0d) | 42.0 | 8.2 | 10.0 | 30.0 | 30.0 | #1 | 41.0 | 34.0 |
| #2 | 42.0 | 19.0 | 10.4 | 9.8 | 12.0 | 20.0 | #2 | 43.0 | 34.5 |
| #3 | 32.0 | 17.0 | 13.6 | 14.2 | 20.0 | 19.0 | #3 | 58.0 | 32.1 |
| #4 | 48.0 | 40.0 | 20.8 | 8.9 | 40.0 | 40.0 | #4 | 50.0 | 40.0 |
| #5 | 60.0 | 26.0 | 9.3 | 3 | 25.0 | 25.0 | #5 | 44.0 | 36.1 |
| Mean | 45.5 | 25.5 | 12.5 | 9.0 | 24.3 | 26.0 | Mean | 47.2 | 35.3 |
| SEM | 4.5 | 5.4 | 2.2 | 2.1 | 5.4 | 4.5 | SEM | 3.1 | 1.3 |
Lymphs, lymphocytes
PWO, peritoneal washout gated for viable cells
Proportion of cells that fall within the lymphocyte gate
All figures are given as percentages
BMD CD5+ B1 cells and native CD5+ B1 cells are phenotypically similar
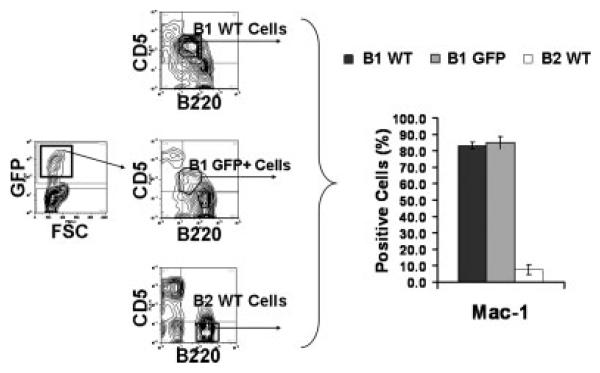
We analyzed BMD CD5+ B1 cells for expression of the surface marker Mac-1, which characterizes native B1 cells. To examine BMD CD5+ B1 cells we obtained peritoneal washout cells from BM chimeras and gated on GFP+ lymphocytes that expressed B220loCD5+. For comparison we examined native CD5+ B1 and B2 cells, which were isolated from adult WT BALB/c mice as B220loCD5+ peritoneal lymphocytes and B220+ splenocytes, respectively, and were analyzed concurrently with BMD CD5+ B1 cells. The gating of a representative experiment is shown in the left panel of Fig. 1, and results compiled from five independent experiments are shown in the right panel of Fig. 1. As expected, native CD5+ B1 and B2 cells differed markedly in phenotype with CD5+ B1 cells expressing much more Mac-1 (≥80% positive) than did B2 cells (≤8% positive). GFP+CD5+ B1 cells that developed in adoptive hosts from MSCV-infected BM stem cells expressed elevated levels of Mac-1 (≥80% positive) similar to native CD5+ B1 cells and unlike native B2 cells. Thus, the sorted BMD CD5+ B1-cell population expresses Mac-1 just like the sorted native CD5+ population.
Figure 1.
BMD CD5+ B1 cells and native CD5+ B1 cells are phenotypically similar. Peritoneal washout cells were obtained from chimeric adoptive hosts 12 wk following lethal irradiation and rescue by administration of MSCV.GFP-infected (lin−) adult BM. Peritoneal washout cells and spleen cells were obtained from control WT BALB/c mice. These groups were stained with anti-B220-APC, anti-CD5-PE-Cy5, and one of the following antibodies conjugated to PE: isotype control or anti-Mac-1. Peritoneal washout CD5+ B1 cells (B220loCD5+) and splenic B2 cells (B220+CD5−) were analyzed for PE staining; in the case of peritoneal washout cells from chimeric mice, GFP+CD5+ B1 cells were evaluated. A total of 104 cells were analyzed for each group. The gating of a representative experiment is shown in the panel on the left; mean results for PE-positive cells in five independent experiments are shown in the panel on the right, along with lines indicating the standard errors of the means.
BMD CD5+ B1 cells and native CD5+ B1 cells are transcriptionally similar
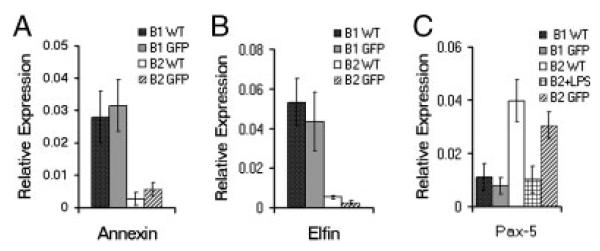
We analyzed BMD CD5+ B1 cells for expression of three genes whose transcription characterizes native B1 cells — annexin, elfin, and Pax-5. To examine BMD CD5+ B1 cells we obtained peritoneal washout cells from BM chimeras and gated on GFP+ lymphocytes that expressed B220loCD5+. For comparison we examined native CD5+ B1 cells from adult BALB/c mice, BMD (GFP+) B2 cells from chimeric mice, and native B2 cells from adult BALB/c mice (isolated as described in the Materials and methods). We prepared RNA from sorted cells and, following reverse transcription, we amplified annexin, elfin, and Pax-5 by real-time PCR, with normalization to β2 microglobulin. Results compiled from four independent experiments are shown in Fig. 2. As expected, native CD5+ B1 and B2 cells differed markedly in baseline transcription, with CD5+ B1 cells expressing much more annexin and elfin, and much less Pax-5, compared with B2 cells. As a control, we stimulated native B2 cells with LPS for 2 days and demonstrated a decline in Pax-5 as previously reported [11]. GFP+CD5+ B1 cells that developed in adoptive hosts from MSCV-infected BM stem cells expressed large amounts of annexin and elfin, like native CD5+ B1 cells; further, GFP+CD5+ B1 cells expressed little Pax-5, like native CD5+ B1 cells and LPS-stimulated B2 cells. In contrast, expression of annexin, elfin, and Pax-5 by BMD GFP+ B2 cells from chimeric mice recapitulated that of native B2 cells. Thus, BMD CD5+ B1 cells express typical transcriptomic features of normal adult CD5+ B1 cells.
Figure 2.
BMD CD5+ B1 cells and native CD5+ B1 cells are transcriptionally similar. Peritoneal washout cells and spleen cells were obtained from chimeric adoptive hosts 12 wk following lethal irradiation and rescue by administration of MSCV.GFP-infected (lin−) adult BM, immunofluorescently stained, and sorted for GFP+CD5+ peritoneal B1 (B220loCD5+) and GFP+ splenic B2 (B220+CD5−) cells. Peritoneal washout cells and spleen cells were obtained from control WT BALB/c mice, immunofluorescently stained, and sorted for CD5+ B1 and for B2 cells. RNA was prepared from CD5+ B1 and B2 cells and reverse transcribed. The levels of annexin, elfin, and Pax-5 relative to β2 microglobulin were determined by real-time PCR with the primers described in the Materials and methods. Mean results of four independent experiments are shown, along with lines indicating standard errors of the means.
BMD CD5+ B1 cells and native CD5+ B1 cells similarly proliferate to PMA
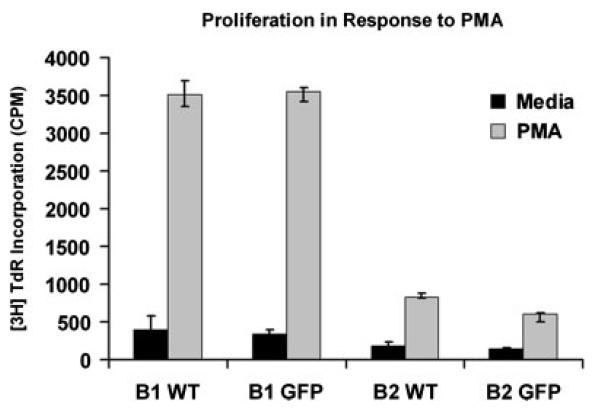
We analyzed BMD CD5+ B1 cells for the functional feature of rapid responsiveness to phorbol ester alone. We obtained peritoneal GFP+CD5+ B1 cells, and splenic GFP+ B2 cells, from BM chimeras, and peritoneal CD5+ B1 cells and splenic B2 cells from normal BALB/c mice, as described in Materials and methods. We stimulated B cells with PMA and added [3H]thymidine during the last 6 h of 24 h cultures. Results of a representative experiment are shown in Fig. 3. As expected, native CD5+ B1 and B2 cells differed markedly in the degree of S phase entry produced by PMA, with CD5+ B1 cells proliferating vigorously in contrast to B2 cells, which proliferated very little. GFP+CD5+ B1 cells that developed in adoptive hosts from MSCV-infected BM stem cells proliferated in response to PMA to the same extent as native CD5+ B1 cells, and to a much greater extent than native B2 cells. In contrast, proliferation in response to PMA by BMD GFP+ B2 cells from chimeric mice recapitulated the very low level characteristic of native B2 cells. Thus, BMD CD5+ B1 cells recapitulate the unique PMA-responsiveness of normal adult CD5+ B1 cells.
Figure 3.
BMD CD5+ B1 cells and native CD5+ B1 cells similarly proliferate to PMA. GFP+CD5+ B1 cells and GFP+ B2 cells from chimeric mice, and CD5+ B1 and B2 cells from WT mice, were sort-purified as described in the legend to Fig. 2. B cells were cultured in medium or stimulated with PMA at 300 ng/mL for 24h with 0.5 μCi [3H] thymidine added during the last 6 h of culture. Incorporation of thymidine was assessed by scintillation counting. Results for one of three comparable experiments are shown, representing the means of triplicate wells along with lines indicating standard errors of the means.
BMD CD5+ B1 cells and native CD5+ B1 cells similarly secrete immunoglobulin
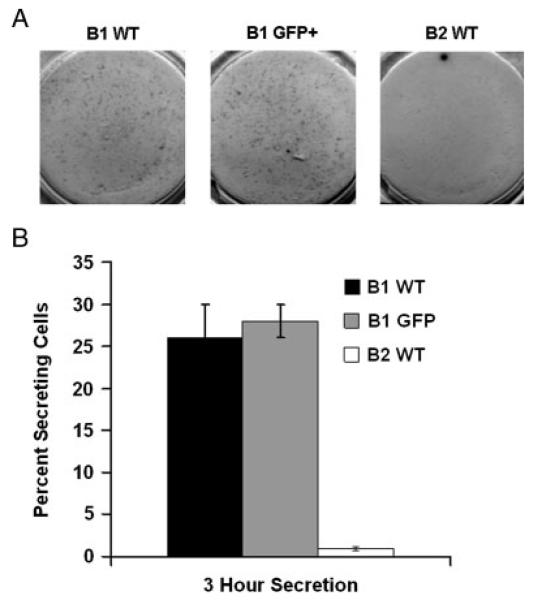
We analyzed BMD CD5+ B1 cells for the functional feature of spontaneous IgM secretion. We obtained peritoneal GFP+CD5+ B1 cells from BM chimeras, and peritoneal CD5+ B1 and splenic B2 cells from normal BALB/c mice, as described in Materials and methods. We placed B cells on coated ELISPOT plates immediately following sort purification and incubated B cells for 3 h. We developed spots with alkaline phosphatase-conjugated goat anti-mouse IgM. Results of a representative experiment are shown in Fig. 4A (and as a larger image in the Supporting Information Fig. 1), and results compiled from three independent experiments are shown in Fig. 4B. As expected, native CD5+ B1 and B2 cells differed markedly in spontaneous secretion of IgM, with a much larger fraction of CD5+ B1 cells registering as immunoglobulin-secreting cells, amounting to about 25%, in comparison to B2 cells, very few of which secreted immunoglobulin. The proportion of GFP+CD5+ B1 cells that secreted IgM in this short-term assay was essentially the same as the proportion of native CD5+ B1 cells that secreted IgM. Thus, BMD CD5+ B1 cells recapitulate the constitutive, spontaneous immunoglobulin secretion that typifies normal adult CD5+ B1 cells.
Figure 4.
BMD CD5+ B1 cells and native CD5+ B1 cells similarly secrete immunoglobulin. GFP+CD5+ B1 cells from chimeric mice, and CD5+ B1 and B2 cells from WT mice, were sort-purified as described in the legend to Fig. 2. B cells were immediately seeded onto plates coated with anti-mouse Ig, incubated for 3 h, and developed with alkaline phosphatase-conjugated goat anti-mouse IgM. Results of a representative experiment are shown in (A); mean numbers of anti-IgM-secreting cells in three independent experiments, as determined by Phoretix software, are shown in (B), along with lines indicating the standard errors of the means.
BMD CD5+ B1 cells express distinctly higher levels of N-region addition than native CD5+ B1 cells
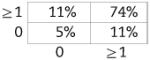
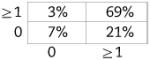
Now shown to be verifiably CD5+ B1 in nature, we examined BMD CD5+ B1 cells for N-region addition by single-cell PCR, as described in the Materials and methods. Cumulative results are presented in Table 2, displayed in the tabular form described by Kantor et al. [39] (sequences are provided in Supporting Information Fig. 2). We found, as expected, that native CD5+ B1-cell immunoglobulin sequences overall contained fewer N-region additions than did native, or chimeric GFP+, B2-cell immunoglobulin. Thus, native CD5+ B1 cells were devoid of all N-region addition at both V-D and D-J junctions in 55% of immunoglobulin sequences, whereas B2 cells lacked all N-region addition in only 5–7% of sequences, an order of magnitude less. Surprisingly, BMD CD5+ B1 cells were devoid of all N-region addition at both V-D and D-J junctions in only 13% of immunoglobulin sequences. In other words, BMD CD5+ B1-cell immunoglobulin sequences were reminiscent of native B2 cells in terms of N-region addition, and were not at all like native CD5+ B1 cells. The unexpected structure of BMD CD5+ B1-cell immunoglobulin sequences was statistically significant. At both the V-D and D-J junctions, the difference between BMD CD5+ B1 cells and native CD5+ B1 cells in sequences completely lacking N-region addition was significant for each (p<0.03, Student's t-test), whereas the difference between BMD CD5+ B1 cells and native B2 cells was not. Considered together (V-D+D-J), the difference between BMD CD5+ B1 cells and native CD5+ B1 cells in N-less sequences was even more highly significant (p<0.001), whereas the difference between BMD CD5+ B1 cells and native B2 cells was again not significant. These results were confirmed by Chi Square analysis, by which measure BMD CD5+ B1 cells were significantly different from native CD5+ B1 cells (p<0.001) and BMD CD5+ B1 cells were not significantly different from native B2 cells. Thus, BMD CD5+ B1 cells differ from native CD5+ B1 cells in immunoglobulin structure.
Table 2.
Proportion of immunoglobulin sequences containing N-region addition
| B1 WT (n=56) | B1 GFP+ (n=30) | B2 WT (n=38) | B2 GFP+ (n=38) | |
|---|---|---|---|---|
| N-region additions at D-J | 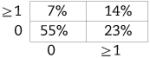 |
 |
 |
 |
| N-region additions at V-D | ||||
Native CD5+ B1 cells acquire N-region additions with age
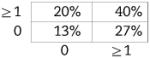
The B2-like level of N-region addition in BMD CD5+ B1 cells, combined with the previous report that the representation of N-additions within the CD5+ B1-cell population increases with advancing age [30], suggests that the former contributes to the latter. However, the conclusion that CD5+ B1-cell N-region additions increase with increasing age has been disputed [31, 32]. Because of the important implications for immune defense of an open, rather than a closed, B1-cell population, we examined N-region addition by single-cell PCR in peritoneal CD5+ B1 cells obtained from mice in our colony aged up to 6 months. Cumulative results are presented in Table 3. We found that native CD5+ B1 cells from 6-month-old animals were devoid of all N-region addition at both V-D and D-J junctions in only 30% of immunoglobulin sequences (sequences are provided in Supporting Information Fig. 2). This is quite different from native CD5+ B1 cells obtained from 2-month-old animals, wherein all N-region addition was absent from both V-D and D-J junctions in 55% of immunoglobulin sequences (Table 2). This difference is statistically significant. At both the V-D and D-J junctions, the difference between 6-month-old native CD5+ B1 cells and 2-month-old native CD5+ B1 cells in sequences completely lacking N-region addition is significant for each (p<0.04 and p<0.01, respectively). Considered together (V-D+D-J), the difference between 6-month-old native CD5+ B1 cells and 2-month-old native CD5+ B1 cells in N-less sequences is even more highly significant (p<0.002). The significant difference between 6-month-old native CD5+ B1 cells and 2-month-old native CD5+ B1 cells in N-region addition was confirmed by Chi Square analysis (which further showed no significant difference between BMD (GFP+) CD5+ B1 cells and 6-month-old native CD5+ B1 cells). Thus, the pool of native CD5+ B1-cell immunoglobulin sequences acquires N-region additions with age.
Table 3.
Proportion of Ig sequences from 6-month-old mice containing N-region addition
| B1 WT 6 month (n=54) | |
|---|---|
| N-region additions at D-J | 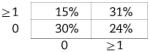 |
| N-region additions at V-D |
BMD CD5+ B1 cells and native CD5+ B1 cells differ with respect to expression of VH11 and VH12
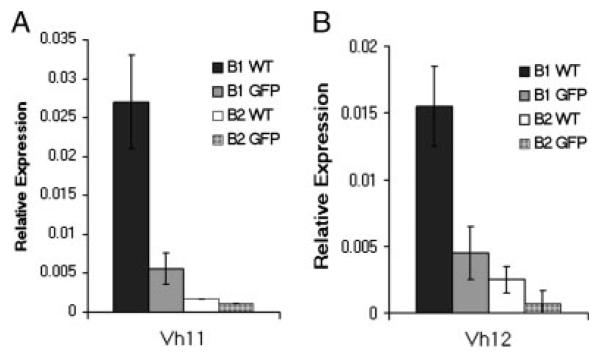
Having found that BMD CD5+ B1 cells differ from native CD5+ B1 cells in one aspect of immunoglobulin repertoire, specifically, N-region addition, we examined another. We analyzed BMD CD5+ B1 cells for expression of VH11 and VH12, which are typically overrepresented among native CD5+ B1-cell immunoglobulin sequences in comparison to B2-cell sequences. We obtained peritoneal GFP+ CD5+ B1 cells, and splenic GFP+ B2 cells, from BM chimeras, and peritoneal CD5+ B1 and splenic B2 cells from normal BALB/c mice, as described in Materials and methods. We prepared RNA from sorted cells and, following reverse transcription, amplified VH11 and VH12 by real-time PCR, with normalization to β2 microglobulin. Results compiled from six independent experiments are shown in Fig. 5. As expected, native CD5+ B1 and B2 cells differed markedly in VH11/VH12 expression, with CD5+ B1 cells expressing much higher levels of VH11/VH12 compared with B2 cells. VH11/VH12 expression by BMD GFP+ B2 cells from chimeric mice recapitulated the low level observed in native B2 cells. Surprisingly, GFP+ CD5+ B1 cells that developed in adoptive hosts from MSCV-infected adult BM progenitors expressed substantially less VH11/VH12 than native CD5+ B1 cells and approximated the low levels seen in native and BMD B2 cells. Thus, BMD CD5+ B1 cells differ from native CD5+ B1 cells in VH usage.
Figure 5.
BMD CD5+ B1 cells and native CD5+ B1 cells differ with respect to expression of VH11 and VH12. GFP+CD5+ B1 cells and GFP+ B2 cells from chimeric mice, and CD5+ B1 and B2 cells from WT mice, were sort-purified as described in the legend to Fig. 2. RNA was prepared from B cells and reverse transcribed. The levels of VH11 (A) and VH12 (B) relative to β2 microglobulin were determined by real-time PCR with the primers described in the Materials and methods. Mean results of six independent experiments are shown, along with lines indicating standard errors of the means.
BMD CD5+ B1 cells cannot be confused with CD5-expressing B2 cells
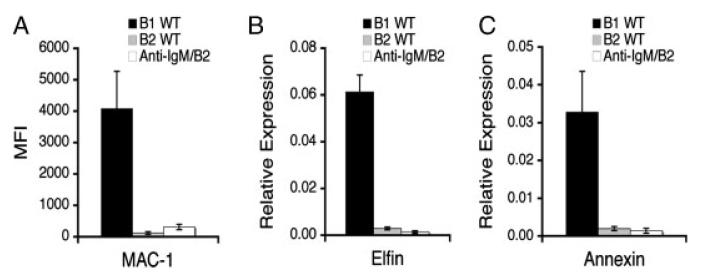
Our finding that BMD CD5+ B1 cells differ in N-region addition and VH11/VH12 expression in comparison to native B1 cells and in these parameters are not significantly different from native B2 cells raised the remote possibility that some of the BMD CD5+ B1 cells that we examined represent antigen-activated B2 cells that express CD5, despite the many similarities between BMD CD5+ B1 cells and native CD5+ B1 cells in terms of surface antigen phenotype, specific gene expression, unique mitogenic responsiveness, and spontaneous immunoglobulin secretion. This consideration relates to early work by Wortis and colleagues indicating that B2 cells stimulated by anti-Ig for 2 days acquire CD5 expression [37], and, in fact, some time ago we showed that anti-Ig activated B2 cells respond to PMA, suggesting at least a superficial similarity to native B1 cells [40]. To address this issue in more detail, we stimulated splenic B2 cells with anti-Ig for 2 days and then sort-purified and studied CD5+ B2 cells. Results are shown in Fig. 6. We found that isolated CD5+ B2 cells failed to express Mac-1, failed to express annexin, and failed to express elfin, to any great extent, in direct contrast to the properties of native and BMD CD5+ B1 cells. Because Mac-1, annexin, and elfin expression by BMD CD5+ B1 cells was virtually identical to native B1 cells (Figs. 1 and 2); these results indicate that putative activated B2 cells, if such were to express CD5, could not comprise a significant proportion of the BMD CD5+ B1-cell pool, inasmuch as this would of necessity lead to a substantial and readily detectable decline in Mac-1, annexin, and elfin expression in this population, which was not observed. We further tested the ability of MSCV to induce CD5 expression on B2 cells, and found that it did not (data not shown), ruling out the remote possibility that somehow a small amount of non-replicative MSCV might have carried over with adoptive cells and then weeks later influenced surface marker expression among B2 cells. All together this work confirms in a very definitive way the identity of BMD B1 cells, which had previously been extrapolated from simple phenotypic data, and excludes the possibility that activated B2 cells could be confused with BMD B1 cells.
Figure 6.
BMD CD5+ B1 cells cannot be confused with CD5-expressing B2 cells. (A) Peritoneal washout cells and spleen cells were obtained from control WT BALB/c mice and were immunofluorescently stained to detect B1 cells (B1 WT, B220+CD5+) and B2 cells (B2 WT, B220+CD5−). Once gated on B220+CD5+ or B220+CD5− each population was evaluated for expression of Mac-1. For anti-Ig-stimulated B2 cells (Anti-IgM/B2) spleen cells were obtained from control WT BALB/c mice and B2 cells were prepared by negative selection using Miltenyi beads. These B2 cells were then placed in culture for 48 h with anti-IgM (15 μg/mL) at the end of which time cells were immunofluorescently stained and B220+CD5+ cells were evaluated for expression of Mac-1. Mean Mac-1 expression (MFI) on WT B1 cells, WT B2 cells, and anti-Ig-stimulated CD5+ B2 cells is shown for three independent experiments, along with lines indicating standard errors of the means. (B/C) RNA was prepared after sort purification of the B-cell populations described above and was reverse transcribed. The levels of elfin (B) and annexin (C) relative to β2 microglobulin were determined by real-time PCR with the primers described in the Materials and methods. Mean results of three independent experiments are shown, along with lines indicating standard errors of the means.
Discussion
The results presented herein demonstrate that peritoneal CD5+ B1 cells produced by BM progenitors that are targets for MSCV infection bear a panoply of typical native CD5+ B1-cell characteristics, but, at the same time, differ with respect to immunoglobulin structure in terms of N-region addition and in terms of repertoire selection.
In previous studies establishing the lineage paradigm, it was found in adoptive transfer experiments that fetal liver produced CD5+ B1 cells whereas adult BM was relatively deficient in this respect [6, 41], although CD5− B1 cells were produced, leading to the suggestion that B1a, B1b, and B2 cells represent three distinct and separately regulated populations [6, 7]. Still, adult BM appears capable of generating small numbers of phenotypically CD5+ B cells, which have been observed at very late times after adoptive transfer [6, 41, 42]. However, in these studies B1 cells were identified primarily by cell surface phenotype, rather than the full range of B1-cell characteristics known today, so their status was determined on the basis of a relatively permissive threshold.
Recently, Dorshkind and colleagues reported identification of a specific progenitor, characterized as a lin−, B220lo/−CD19+ stem cell, that gave rise to B1 cells [35]. The identification of a B1-cell progenitor provides strong evidence that B1 cells constitute a distinct B-cell lineage [36], although it remains unclear whether B220lo/−CD19+ stem cells are the source of all, or only some, B1 cells. For this reason we did not restrict ourselves to working with a single progenitor in defining the capacity of adult BM to give rise to CD5+ B1 cells.
The B220lo/−CD19+ progenitor cell was found to be abundantly present in fetal liver and much less abundant, though present, in adult BM [35], which might be thought to explain the relative capacities of fetal liver and adult BM to reconstitute the B1-cell population. There are two issues with this provisional conclusion:
(i) B220lo/−CD19+ progenitor cells obtained from adult BM preferentially produced peritoneal CD5− B1 (B1b) cells as opposed to CD5+ B1 (B1a) cells, similar to that which had been seen in earlier adoptive transfer studies [6, 41].
(ii) B220lo/−CD19+ progeny were classified as B1 cells primarily on the basis of cell surface phenotype without testing for key B1-cell functions studied here such as specific gene expression, unique mitogenic responsiveness, and spontaneous immunoglobulin secretion [35].
As noted above, classification on the basis of phenotype alone is unacceptably permissive because it could encompass activated B2 cells that express CD5 [37] instead of, or in addition to, true B1 cells. Thus, our work authenticates the identity of BMD CD5+ B1 cells by a variety of static and functional criteria demonstrating that they not only appear, but also behave, as true CD5+ B1 cells, and at the same time our work clarifies the capacity of adult BM progenitors to produce such genuine CD5+ B1 cells. Although reconstitution was not complete – the proportion of B1 cells among GFP+ lymphocytes in adoptive hosts was about 1/3 the proportion of B1 cells among lymphocytes in normal control animals – the level found was substantial.
With the true B1-cell nature of BMD B1 cells established by multiple and varied criteria, we focused on the immunoglobulin produced, examining in particular N-region addition. Here we found that BMD CD5+ B1 cells differed markedly from native CD5+ B1 cells, in that the former contained relatively high levels of N-additions that were significantly different than the very low levels present in native CD5+ B1 cells. This cannot be attributed to contamination of sorted BMD B1 cells with putatively BCR-activated B2 cells expressing CD5 because we showed that such cells express very little Mac-1, annexin, or elfin. In fact, anti-Ig-stimulated B2 cells that were sort-purified for CD5 positivity expressed less than 1/20 the amount of surface Mac-1 and annexin/elfin transcripts as compared with native B1 cells. To produce the results presented in Table 2, wherein only 13% of BMD CD5+ B1-cell immunoglobulin lacked N-region addition, by dilution of true B1 cells (whose immunoglobulin was 55% N-addition-less) with B2 cells (whose immunoglobulin was 5–7% N-addition-less), B2 cells would have to comprise more than 80% of the studied BMD CD5+ B-cell pool. This is clearly ruled out because the levels of Mac-1, annexin, and elfin expressed by BMD CD5+ B1 cells were not substantially diminished as compared with native B1 cells – levels of Mac-1, annexin, and elfin expressed by BMD CD5+ B1 cells would be reduced by over 75% in comparison to native CD5+ B1 cells, if putatively BCR-activated B2 cells were responsible for the levels of N-region addition determined, and this was not observed (compare Figs. 1 and 2).
The recovery of N-addition containing B1 cells from adoptive recipients cannot be attributed to expansion of possibly contaminating B1 cells that may have been present in BM depleted of lin+ cells and then become MSCV-transduced for several reasons:
(i) BM inocula contained fewer than 0.4% B220loCD5+ cells (see the Materials and methods).
(ii) Native B1 cells from the peritoneal cavity were not susceptible to MSCV transduction under the growth factor conditions used for BM stem cell transduction (data not shown).
(iii) Early work indicated that the expansive capability of peritoneal B1 cells in adoptive hosts is severely limited, ranging from very little to “several fold” [34, 43], clearly insufficient to produce the numbers of GFP+ B1 cells that we recovered from adoptive hosts, were those cells to have been derived from very few mature B1-cell contaminants of lin-depleted BM that may have become transduced by MSCV.
(iv) The B1 cells we recovered from adoptive hosts contained abundant N-region additions, in contrast to native B1 cells; had the B1 cells we recovered actually derived from massive expansion of native B1 cells that accompanied BM stem cells, then these recovered B1 cells should have expressed minimal N-region addition.
Although it is theoretically possible that MSCV might have affected gene expression in early lymphoid progenitors so as to alter B-cell development with respect to V-D-J recombination, there is no evidence for this remote possibility and BMD B1 cells were normal in all other respects. It is also theoretically possible that MSCV might have placed CD5 on CD5 negative B1b cells, but the proportion of B1b cells within the GFP+ fraction of chimera peritoneal lymphocytes (7%±1) was the same as the proportion of B1b cells among native peritoneal lymphocytes (6%±1), strongly suggesting that MSCV transduction and adoptive transfer did not lead to an unnatural shift of B1b cells toward CD5 expression and confusion with CD5+ B1a cells. Rather, the unexpected finding of high N-region addition in BMD CD5+ B1 cells implies two things: (i) a different N-region-rich mechanism accounts for CD5+ B1-cell production by adult versus fetal/neonatal progenitors such that B1 cells are derived from distinct precursors at different ages, or from the same progenitor which, however, may acquire terminal deoxynucleotidyl transferase (TdT) in migrating to the BM (and, consistent with the latter possibility, we found in preliminary experiments that B220lo/−CD19+ progenitors from adult BM expressed TdT) and (ii) new, rather than self-replenishing, CD5+ B1 cells can be produced during adult life leading to continual seeding of the peritoneal cavity by BMD CD5+ B1 cells that contain substantial N-region additions, which may explain the increase in overall N-region segments of peritoneal CD5+ B1 cells over time [30], which, although disputed in the past [31, 32], we have here supported with concrete evidence. These results suggest that the CD5+ B1-cell population is not a closed system but receives contributions from BM progenitors during adult life. In this respect it is important to note that in our work BM donors were at least 8 wk old, beyond the time when, according to the conventional paradigm, peritoneal B1 cells have become self-replenishing and are devoid of exogenous input [31]. Moreover, in the present work we have examined adoptive hosts at early times (8–12 wk) after BM transfer, verified B1-cell status by multiple identifying characteristics, utilized promiscuous primers that amplify all VH families, and evaluated a large number of sequences from which the differences we found were statistically significant, all of which provides additional support for our conclusions.
Accompanying the change in immunoglobulin structure dictated by increased N-region addition, we found markedly decreased utilization of VH11 and VH12 by BMD B1 cells as compared with native B1 cells. Because the B1-cell repertoire is selected, it may be speculated that increased N-region addition altered the specificity of immunoglobulin sequences incorporating VH11/VH12 such that phosphatidylcholine binding was changed. However, it is at least theoretically possible that immunoglobulin and B1-cell selection were altered by a putative relative deficiency in the adult of antigens involved in B1-cell skewing to VH11 and VH12, or by radiation delivered to adoptive recipients that affected a selecting micro-environment, or through other, unknown mechanisms.
These results have potentially profound ramifications. To the extent that adult BM gives rise to CD5+ B1 cells that are integrated into the B1-cell pool, that pool will change because adult BMD CD5+ B1 cells differ in immunoglobulin structure as compared with native CD5+ B1 cells. Germ-line-like natural immunoglobulin produced by CD5+ B1 cells bind, at low affinity, numerous bacterial antigens. Thus, as adult BMD CD5+ B1 cells with N-region additions slowly replace CD5+ B1 cells without, germ-line-like immunoglobulin will be replaced by immunoglobulin that has diversified so that it is no longer germ-line. As a result, it may be speculated that the natural anti-bacterial specificities produced by CD5+ B1 cells might well decline as time goes on. Most importantly, our work suggests that the B1-cell population is not a closed, self-renewing system, and infers that the degree to which it is open to BM emigrants will influence the role and effectiveness of B1 cells in producing natural immunoglobulin capable of combating pathogenic microorganisms during the life of the organism.
Materials and methods
Mice
Male BALB/cByJ mice of 6–8 wk of age were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were cared for and handled in accordance with National Institutes of Health and institutional guidelines.
MSCV construct
The MSCV2.2 internal ribosomal entry site GFP vector was kindly provided by Dr. Luk Van Parijs [38]. The virus was grown using BOSC packaging cells grown in DMEM with 10% fetal bovine serum, 5 mM l-glutamine, and penicillin/streptomycin. The BOSC cells were transfected using FUGENE (Amersham), 1 μg MSCV2.2 vector, and 1 μg of the retroviral envelope plasmid pCL-eco [44]. One day after transfection the BOSC cells were fed with the medium described above. The following day the supernatant from the BOSC cells was removed, cleared of cells by centrifugation, and used for infection of BM cells.
Adoptive transfer
Five days before BM harvest, donor mice were injected (i.p.) with 5 mg 5-fluorouracil. BM was harvested from hind legs and depleted of lin+ cells using biotin-labeled antibodies and anti-biotin magnetic beads (Miltenyi). The BM depleted of lin+ cells was then placed in culture overnight with the following cytokine cocktail: m-FMS-like tyrosine kinase 3 ligand (100 ng/mL), mSCF (100 ng/mL), and m-thrombopoietin (50 ng/mL). BM cells were then transduced three times over the next 3 days by suspending the cells in viral supernatant containing the cytokine cocktail and polybrene (5 μg/mL) and placed on a plate coated with fibronectin (5 μg/cm2). One day before transplant, BM cells were washed and resuspended in medium containing only cytokine cocktail. On the day of transplant recipient BALB/c mice were lethally irradiated at 900 rad. BM cells (containing fewer than 0.4% B220loCD5+ cells) were washed twice in PBS, resuspended in PBS, and then injected (i.v.) into irradiated recipients at 0.5–1.5 × 106 cells per mouse in 0.25 mL.
Cell purification and flow cytometry
B1- and B2-cell populations were isolated as previously described [45]. Briefly, peritoneal washout cells and splenocytes were obtained from 8–14-wk-old WT mice or BM chimeras 8–12 wk post transplant and stained with fluorescence-labeled antibodies to B220 and CD5 (peritoneal washout cells) or B220 (splenocytes). B-cell populations (native peritoneal CD5+ B1 cells: B220loCD5+; BMD peritoneal CD5+ B1 cells: GFP+B220loCD5+; native splenic B2 cells: B220hiCD5−; BMD splenic B2 cells: GFP+B220hiCD5−) were then purified using a FACSAria cell sorter (BD Biosciences). Post-sort re-analysis of the B-cell populations showed them to be ≥98% pure. Peritoneal washouts from WT and BM chimera mice were blocked with rat anti-mouse CD16/CD32 antibody (clone 2.4G2), stained with immunofluorescent antibodies, and then analyzed on a FACSCalibur flow cytometer (BD Biosciences) with appropriate gating. In some experiments purified B2 cells were stimulated by anti-IgM (15 μg/mL for 2 days) and then sort purified for CD5 (and B220) expression. Images were constructed with FlowJo 6.0 software (Tree Star). The following antibodies were obtained from BD Pharmingen: Biotin-conjugated rat anti-mouse CD45R/B220 (clone RA3-6B2); PE-conjugated rat anti-mouse CD80 (clone 16-10A1), CD43 (clone S7), Mac-1 (clone M1/70), IgG1 isotype control, PE-Cy5-conjugated rat anti-mouse CD5 (clone 53-7.3); Streptavidinconjugated APC.
Gene expression
Gene expression was assayed by real-time PCR as previously described [11]. Briefly, RNA was prepared from B cells using Ultraspec (BiotecX) and chloroform extraction. Following isolation the RNA was treated with DNaseI (Ambion) to remove contaminating DNA. cDNA was prepared using avian myeloblastosis virus reverse transcriptase (Bio-Rad). Gene expression was then measured by real-time PCR using iTaq SYBR Green (Bio-Rad) and normalized with β2 microglobulin. The following primer sets were used: β2 microglobulin (F-CCCGCCTCACATTGAAATCC/R-GCGTATGTATCAGTCTCAGTGG); Pax-5a (F-GCTACTCTGCACCGACGCTG/R-GGGCTGCAGGGCTGTAATAGT); elfin (F-ATGTGTGCACCGACTGTGGC/R-GGTCCTCTCCATCACTCGCG); annexin II (F-CCAAGTGCCTACGGGTCAGT/R-TGTCCTGCCTCTGCACATTG); VH11 (F-GCAATAAACTACGCACCATCCA/R-TGTCCTCCGATCGCACATT); VH12 (F-TTTCTACAACCCATCCCTCCAG/R-TACATGGCTGTGTCCTCTGTGG). Single gene products from each primer set were confirmed by running the products on a 2% agarose gel (Supporting Information Fig. 3).
Proliferation
Proliferation was measured by thymidine incorporation as previously described [5]. Briefly, FACS-sorted B cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin in duplicate in 96-well U-bottom plates. The B cells were stimulated for 24 h with 300 ng/mL of PMA. Cultures were pulsed with 0.5 μCi of [3H]thymidine (Amersham) for 6 h prior to the end of the 24 h culture. Thymidine incorporation was measured by scintillation counting of beta particle emissions.
Immunoglobulin secretion
Immunoglobulin secretion was measured by ELISPOT assay as previously described [11]. Briefly, FACS-sorted, naive B cells were distributed at various dilutions onto MultiScreen*-IP Plates (Millipore) pre-coated with goat anti-mouse Ig (H+L) and then incubated for 3 h at 37°C and 5% CO2. Plates were treated with alkaline phosphatase-conjugated goat anti-mouse IgM (Southern Biotechnology Associates) and developed with 5-bromo-4-chloro-3-indolyl phosphate/p-NBT chloride substrate (KPL). Ig-secreting cells were enumerated using Phoretix Expression software (NonLinear Dynamics).
N-region addition PCR, sequencing, and analysis
Peritoneal washout cells and splenocytes were obtained from WT mice and from BM chimeras and stained with Hoechst 33342 (5 μg/mL) for 1 h at 37°C, then washed and stained with anti-CD5 and anti-B220 antibodies. WT peritoneal CD5+ B1 (B220+ CD5+), BMD peritoneal CD5+ B1 (GFP+B220+CD5+), and WT splenic B2 (B220+ CD5−) cells were sorted onto a 48-well AmpliGrid (Advalytix). Deposition of 1 cell per well was confirmed by fluorescence microscopy. Reverse transcription and PCR (Qiagen OneStep RT-PCR) were carried out in a total volume of 1 μL reaction with two primers [39], each at 0.6 μM (MsVHE = GGGAATTCGAGGTGCAGCTGCAGGAGTCTGG; MsCμE = ATGGCCACCGAATTCTTATCAGA) after overlaying with 5 μL mineral oil, as follows: 49°C for 30 min; 94°C for 15 min; 35 cycles at 93°C for 30 s, 49°C for 30s, 71°C for 30 s; and then a final extention at 71°C for 10 min (AmpliSpeed PCR cycler, Advalytix). Contents of the 1 μL reaction were then removed from the center of each well and diluted with 49 μL of dH2O.
A second semi-nested amplification was then performed using the same MsVHE primer and an internal constant region primer (MsCμN = TGTAAAACGACGGCCAGTCATTTGGGAAGGACTGA) plus 4 μL of the first round PCR product, in a total volume of 25 μL. The PCR reaction was first heated to 95°C for 15 min and then run for 40 cycles at 94°C for 30 s, 50°C for 30 s, 72°C for 30 s, and a final extention at 72°C for 10 min. The products were run on a 1% agarose gel and purified using the Qiagen gel purification kit. These products were then sequenced (Genewiz) using the MsVHE primer. Sequences were then analyzed using an online sequence analysis tool for VDJ sequences (IMGT, the international ImMunoGeneTics information system).
Supplementary Material
Acknowledgements
The authors thank Dr. Joseph R. Tumang for helpful discussion and critical review of the manuscript. This work was supported by Public Health Service grants AI29690 and AI60896 awarded by the National Institutes of Health.
Abbreviations
- BMD
BM-derived
- MSCV
mouse stem cell virus
Footnotes
Current address: Karen Repetny, Millennium Pharmaceuticals, Inc., Cambridge, MA 02139, USA
Supporting Information for this article is available at www.wiley-vch.de/contents/jc_2040/2009/38920_s.pdf
Conflict of interest: The authors have declared no conflict of interest.
References
- 1.Herzenberg LA. B-1 cells: the lineage question revisited. Immunol. Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. B cell development pathways. Annu. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 3.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 4.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J. Immunol. 2002;168:4257–4261. doi: 10.4049/jimmunol.168.9.4257. [DOI] [PubMed] [Google Scholar]
- 5.Tumang JR, Hastings WD, Bai C, Rothstein TL. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur. J. Immunol. 2004;34:2158–2167. doi: 10.1002/eji.200424819. [DOI] [PubMed] [Google Scholar]
- 6.Kantor AB, Stall AM, Adams S, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tung JW, Mrazek MD, Yang Y, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc. Natl. Acad. Sci. USA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top Microbiol. Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 11.Tumang JR, Frances R, Yeo SG, Rothstein TL. Cutting edge: Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 12.Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur. J. Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- 13.Frances R, Tumang JR, Rothstein TL. Extreme skewing of annexin II and S100A6 expression identified by proteomic analysis of peritoneal B-1 cells. Int. Immunol. 2007;19:59–65. doi: 10.1093/intimm/dxl122. [DOI] [PubMed] [Google Scholar]
- 14.Rothstein TL, Kolber DL. Peritoneal B cells respond to phorbol esters in the absence of co- mitogen. J. Immunol. 1988;140:2880–2885. [PubMed] [Google Scholar]
- 15.Tanguay DA, Colarusso TP, Pavlovic S, Irigoyen M, Howard RG, Bartek J, Chiles TC, Rothstein TL. Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J. Exp. Med. 1999;189:1685–1690. doi: 10.1084/jem.189.11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanguay DA, Colarusso TP, Doughty C, Pavlovic S, Rothstein TL, Chiles TC. Differences in the signaling requirements for activation of assembled cyclin D3-cdk4 complexes in B-1 and B-2 lymphocytes. J. Immunol. 2001;166:4273–4277. doi: 10.4049/jimmunol.166.7.4273. [DOI] [PubMed] [Google Scholar]
- 17.Klinman DM, Holmes KL. Differences in the repertoire expressed by peritoneal and splenic Ly-1 (CD5)1B cells. J. Immunol. 1990;144:4520–4525. [PubMed] [Google Scholar]
- 18.Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-11B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- 19.Forster I, Rajewsky K. Expansion and functional activity of Ly-11B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 20.Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J. Exp. Med. 1992;175:1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J. Exp. Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennell CA, Mercolino TJ, Grdina TA, Arnold LW, Haughton G, Clarke SH. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur. J. Immunol. 1989;19:1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- 23.Hardy RR, Carmack CE, Shinton SA, Riblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 24.Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol. Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- 25.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennell CA, Arnold LW, Haughton G, Clarke SH. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J. Immunol. 1988;141:2788–2796. [PubMed] [Google Scholar]
- 29.Forster I, Gu H, Rajewsky K. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 1988;7:3693–3703. doi: 10.1002/j.1460-2075.1988.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu H, Forster I, Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the 3joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990;9:2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantor AB. V-gene usage and N-region insertions in B-1a, B-1b and conventional B cells. Semin. Immunol. 1996;8:29–35. doi: 10.1006/smim.1996.0005. [DOI] [PubMed] [Google Scholar]
- 32.Tornberg UC, Holmberg D. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J. 1995;14:1680–1689. doi: 10.1002/j.1460-2075.1995.tb07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur. J. Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa K, Hardy RR, Stall AM, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly- 1 B cell lineage. Eur. J. Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- 35.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 36.Herzenberg LA, Tung JW. B cell lineages: documented at last! Nat. Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 37.Cong YZ, Rabin E, Wortis HH. Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int. Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 38.Cherry SR, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol. Cell. Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 40.Rothstein TL, Kolber DL, Murphy TP, Cohen DP. Induction of phorbol ester responsiveness in conventional B cells after activation via surface Ig. J. Immunol. 1991;147:3728–3735. [PubMed] [Google Scholar]
- 41.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc. Natl. Acad. Sci. USA. 1991;88:11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang CA, Henry C, Iacomini J, Imanishi-Kari T, Wortis HH. Adult bone marrow contains precursors for CD5+ B cells. Eur. J. Immunol. 1996;26:2537–2540. doi: 10.1002/eji.1830261039. [DOI] [PubMed] [Google Scholar]
- 43.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retro-viruses. J. Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frances R, Tumang JR, Rothstein TL. Cutting edge: B-1 cells are deficient in Lck: defective B cell receptor signal transduction in B-1 cells occurs in the absence of elevated Lck expression. J. Immunol. 2005;175:27–31. doi: 10.4049/jimmunol.175.1.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.