Abstract
Coronary computed tomographic angiography (CCTA) is a direct but minimally invasive method of visualizing coronary arteries. Acceptable indications for this technique include the assessment of suspected or known coronary artery anomalies, the evaluation of chest pain syndromes in patients with non diagnostic stress tests or who are unable to exercise, and exclusion of an ischemic etiology in patients with unexplained left ventricular dysfunction. Assessment of coronary stents with a diameter of <3.0 mm and imaging of asymptomatic patients with a goal of establishing prognosis are currently not accepted indications for CCTA.
Keywords: Angiography, computed tomography, coronary artery disease, diagnosis
Introduction
Coronary computed tomographic angiography (CCTA) is creating great excitement among health care professionals concerned with the imaging of coronary artery disease (CAD). Before the advent of computed tomography (CT) scanners with temporal and spatial resolution high enough to generate reliable diagnostic images of the lumen of major epicardial coronary artery branches, direct visualization of the coronary arteries required invasive, selective coronary artery catheterization. Conversely, all non invasive cardiovascular tests for CAD rely on indirect indicators of luminal obstruction such as reversible perfusion abnormalities on stress myocardial perfusion imaging, or inducible regional wall motion abnormalities on stress echocardiography. This reliance on indirect indicators limits the diagnostic accuracy of conventional stress imaging.
Technical issues
Coronary computed tomographic angiography and cardiac magnetic resonance are direct but non invasive or minimally invasive methods of visualizing coronary arteries. CCTA is currently easier, quicker, and more robust to perform than cardiac magnetic resonance, and is therefore more widely offered. The type of CT scanner most commonly used for CCTA is multidetector CT, also referred to as multislice CT. State-of-the-art multidetector CT scanners acquire 64 slices or more with each gantry rotation, at a gantry rotation speed of 333 ms/360° or less. The physics principles of image formation in CT are discussed elsewhere in this issue of Heart and Metabolism.
Practical issues
From a practical perspective, performing CCTA requires intravenous injection of contrast material and exposes the patient to ionizing radiation [1]; the contrast volume and dose of radiation required for the technique can exceed those required for coronary catheterization [2]. Beat-to-beat variations in the duration of the cardiac cycle lead to variation in the end-diastolic left ventricular dimensions, and increases in heart rate shorten the diastolic resting period of the heart. For these reasons, the image quality of CCTA in patients with an irregular or fast heart rate can be reduced or non diagnostic because of misregistration between adjacent image slices or blurring of the coronary arteries by motion artifact [3].
Clinical features of coronary computed tomographic angiography
Unlike non invasive stress imaging, CCTA can detect coronary plaques that are not large enough to cause ischemia but may be prone to rupture [4]. Therefore, CCTA may eventually prove to have not only diagnostic but also prognostic value. Unlike selective coronary angiography, CCTA allows visualization of both the coronary lumen and the coronary artery wall and may therefore be superior to selective coronary angiography for the assessment of coronary plaque burden, because so-called ‘vascular remodeling’ [5] can create a normal appearance of the lumen in coronary artery segments that have enlarged in diameter to accommodate plaque.
Current indications
Table I lists the uses of cardiovascular CT that an intersocietal working group and technical panel consisting of cardiologists and radiologists recently deemed appropriate, given the current body of evidence [6].
Table I.
Appropriate indications for cardiovacular computed tomography [6].
| Indication | Median scorea |
|---|---|
| 9 | Evaluation of suspected coronary anomaliesb |
| Evaluation of suspected aortic dissection or thoracic aortic aneurysm | |
| Evaluation of suspected pulmonary embolism | |
| 8 | Evaluation of chest pain syndrome (in patients with uninterpretable or equivocal stress test)b |
| Evaluation of suspected cardiac tumor or thrombus (in patients with technically limited images from echocardiogram, MRI, or TEE) | |
| Evaluation of pericardial conditions (in patients with technically limited images from echocardiogram, MRI, or TEE) | |
| Evaluation of pulmonary vein anatomy before invasive radiofrequency ablation for atrial fibrillation | |
| Non invasive coronary vein mapping before placement of biventricular pacemaker | |
| Non invasive coronary arterial mapping, including internal mammary artery, before repeat cardiac surgical revascularization | |
| 7 | Evaluation of chest pain syndrome (in patients with intermediate pre-test probability of CAD whose ECG is uninterpretable or who are unable to exercise)b |
| Acute chest pain (in patients with intermediate pre-test probability of CAD who have no ECG changes and negative serial enzymes)b | |
| Assessment of complex congenital heart disease, including anomalies of coronary circulation, great vessels, and cardiac chambers and valvesb | |
| Evaluation of coronary arteries in patients with new-onset heart failure, to assess etiologyb |
Scores assigned by panel members ranged from 1 (test is not generally acceptable and is not a reasonable approach for the indication) to 9 (test is generally acceptable and is a reasonable approach for the indication). Indications that received a median score of 7 or higher were considered appropriate.
Indication requires coronary computed tomography angiography. CAD, coronary artery disease; ECG, electrocardiogram; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography.
Assessment of coronary artery anomalies
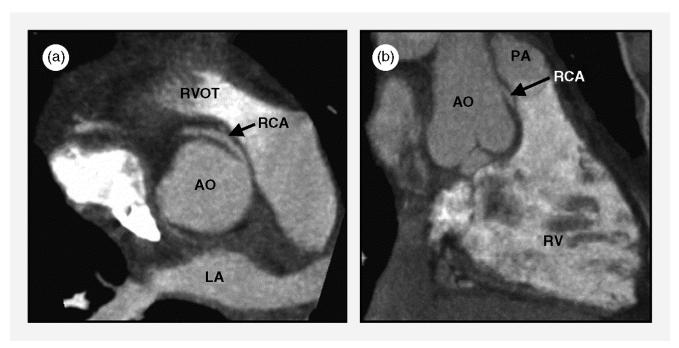
Coronary computed tomographic angiography is ideally suited for the assessment of patients with known or suspected coronary anomalies, because the primary diagnostic task is qualitative [7]: does the anomalous coronary artery course between the aorta and the pulmonary artery or does it not? This distinction is important, because the risk of sudden cardiac death is increased in patients who have a coronary artery that originates abnormally from the contralateral coronary sinus of Valsalva and courses, on its way to its usual perfusion territory, between the aorta and the pulmonary artery (Figure 1). The presumed mechanism for sudden cardiac death in this type of coronary anomaly is compression of the anomalous coronary artery between the aorta and the pulmonary artery, which causes ischemia, particularly during strenuous exercise.
Figure 1.
Right coronary artery (RCA) with abnormal origin from the left coronary sinus of Valsalva and a proximal course between the right ventricular outflow tract (RVOT) and the aorta (AO), which creates potential for compression between the RVOT and pulmonary artery (PA) (anterior) and the aorta (posterior). (a) Maximum-intensity projection that approximates the horizontal long-axis orientation. (b) Maximum-intensity projection that approximates the vertical long-axis orientation. LA, left atrium; RV, right ventricle. (From Deibler et al [7], with permission.)
Numerous studies have unanimously concluded that CCTA with contemporary scanners is superior to selective coronary angiography for the assessment of the proximal course of congenitally abnormal coronary arteries.
Detection of coronary artery stenoses
Quantitative CCTA is currently limited by spatial resolution. With an in-plane resolution of approximately 0.3 mm and a slice thickness of approximately 0.5 mm, it is not always possible to make a confident distinction between a 50% and a 70% stenosis in a coronary artery with a caliber of 3 mm. Semiquantitative reporting that distinguishes between mild (1–30%), moderate (31–70%), and severe (>70%) luminal narrowing reflects image quality with current scanners most adequately. However, most studies that have examined the diagnostic accuracy of CCTA for identifying high-grade coronary artery stenoses have used luminal narrowing of >50% as the definition of ‘significant’ coronary artery disease likely to be associated with ischemia. Used in this way, the sensitivity of CCTA with state-of-the-art scanners at experienced centers is 85–95%, and the specificity is 93–97% [8].
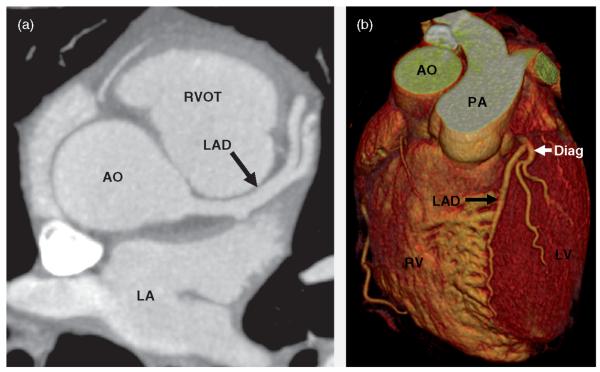
CCTA is useful in patients with chest pain syndromes, to tip the balance toward selective coronary angiography if at least moderate coronary artery stenoses are present on CCTA, or toward recommending non cardiac evaluation if the quality of the CCTA study is good and coronary artery stenoses are clearly absent [9] (Figure 2).
Figure 2.
Coronary computed tomographic angiogram in a patient without coronary calcifications or coronary artery stenoses. (a) Contrast-enhanced transaxial view. (b) Volume rendering. AO, aorta; Diag, diagonal branch; LA, left atrium; LAD, left anterior descending artery; PA, pulmonary artery; RV, right ventricle; RVOT, right ventricular outflow tract. (From Gerber & Walser [9], with permission.)
In all studies comparing CCTA and selective coronary angiography, the negative predictive value was universally very high (98–99%) [8]. Hence, CCTA may in the future prove useful as an alternative to selective coronary angiography in situations in which the pre-test likelihood is low but a need exists to confirm the absence of high-grade coronary artery stenoses, such as in patients with left ventricular dysfunction in whom an ischemic etiology must be excluded or in patients about to undergo non coronary cardiac surgery, such as valve replacement or repair, in whom the need for concurrent coronary artery bypass grafting must be excluded.
The diagnostic value of CCTA is reduced if image quality is limited. The factor most likely to reduce image quality with current CT scanners is severe coronary calcification, which is most prevalent in older patients or in patients with known, advanced coronary artery disease [3]. Therefore, patients older than 75–80 years of age or those with a history of adverse cardiac events or percutaneous coronary intervention are typically not good candidates for CCTA.
Other frequent uses of coronary computed tomographic angiography
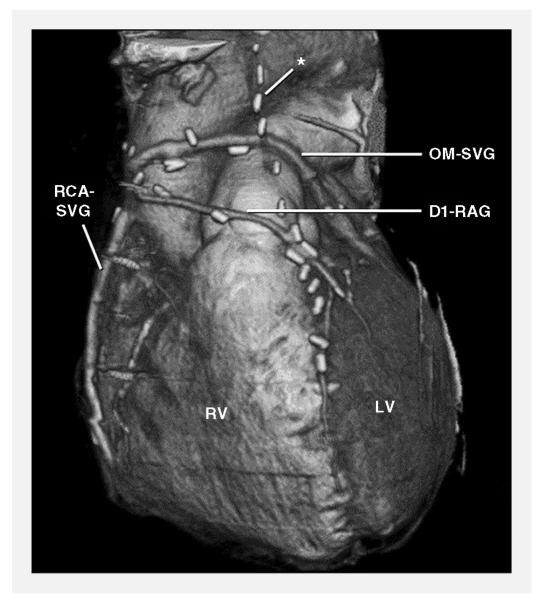
Computed tomographic angiography of coronary artery bypass grafts almost always results in excellent image quality [10] (Figure 3), but its clinical value is limited by difficulty in assessing the native coronary arteries downstream of the graft anastomosis as a result of the severe coronary calcification that is typically present in patients with CAD severe enough to have required coronary artery bypass grafting. Nevertheless, coronary artery bypass graft computed tomographic angiography may very occasionally help establish the patency of a clinically important graft that could not be selectively engaged during cardiac catheterization.
Figure 3.
Coronary artery bypass graft computed tomographic angiogram. *Occluded left internal mammary artery graft to the left anterior descending artery. D1-RAG, radial artery graft to first diagonal branch; LV, left ventricle; OM-SVG, saphenous vein graft to obtuse marginal branch; RCA-SVG, saphenous vein graft to right coronary artery; RV, right ventricle. (From Gerber et al [2], with permission.)
The assessment of coronary artery stents is not routinely possible, because of image artifacts resulting from the radio-dense metal of the stent struts [11]. Therefore, exclusion of in-stent restenosis in symptomatic patients is not an acceptable indication for CCTA except in patients with a stent diameter of >3.0 mm [12].
Coronary computed tomographic angiography for prognostication
The ability of CCTA to identify the presence of non calcified coronary plaque allows the detection of subclinical CAD. Detection, quantification, and characterization of subclinical CAD may eventually allow identification of patients who would benefit from aggressive, targeted risk-factor modification to avoid the occurrence of future adverse cardiac events. However, at the current stage of technical development, quantification of non calcified coronary plaque burden by CCTA is not reproducibly possible [4], the relationship between non calcified plaque and biologically ‘vulnerable’ plaque likely to rupture is not well established, and no outcome studies to date have addressed whether choosing management strategies based on the findings on CCTA will improve the prognosis of patients perceived to be at high risk. Therefore, currently, performing CCTA in asymptomatic patients with a goal of establishing prognosis and modifying lifestyle and pharmacologic management can not be recommended.
REFERENCES
- 1.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation. 2003;107:917–922. doi: 10.1161/01.cir.0000048965.56529.c2. [DOI] [PubMed] [Google Scholar]
- 2.Gerber TC, Breen JF, Kuzo RS, et al. Computed tomographic angiography of the coronary arteries: techniques and applications. Semin Ultrasound CT MR. 2006;27:42–55. doi: 10.1053/j.sult.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Gerber TC, Kuzo RS, Lane GE, et al. Image quality in a standardized algorithm for minimally invasive coronary angiography with multislice spiral computed tomography. J Comput Assist Tomogr. 2003;27:62–69. doi: 10.1097/00004728-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound [see comment] J Am Coll Cardiol. 2006;47:672–677. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 6.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/ SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Deibler AR, Kuzo RS, Vohringer M, et al. Imaging of congenital coronary anomalies with multislice computed tomography. Mayo Clini Proc. 2004;79:1017–1023. doi: 10.4065/79.8.1017. [DOI] [PubMed] [Google Scholar]
- 8.Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919–1928. doi: 10.1016/j.jacc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Gerber TC, Walser EM. Cardiovascular computed tomography and magnetic resonance imaging. In: Murphy JG, Lloyd MA, editors. Mayo Clinic cardiology: concise textbook. 3rd ed Mayo Clinic Scientific Press and Informa Healthcare USA, Inc.; Rochester (MN): 2007. pp. 185–204. [Google Scholar]
- 10.Ropers D, Pohle FK, Kuettner A, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006;114:2334–2341. doi: 10.1161/CIRCULATIONAHA.106.631051. [DOI] [PubMed] [Google Scholar]
- 11.Maintz D, Seifarth H, Raupach R, et al. 64-Slice multidetector coronary CT angiography: in vitro evaluation of 68 different stents. Eur Radiol. 2006;16:818–826. doi: 10.1007/s00330-005-0062-8. [DOI] [PubMed] [Google Scholar]
- 12.Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94:427–430. doi: 10.1016/j.amjcard.2004.04.057. [DOI] [PubMed] [Google Scholar]