Abstract
Schuknecht proposed a discrete form of presbycusis in which hearing loss results principally from degeneration of cochlear stria vascularis and decline of the endocochlear potential (EP). This form was asserted to be genetically linked, and to arise independently from age-related pathology of either the organ of Corti or cochlear neurons. Although extensive strial degeneration in humans coincides with hearing loss, EPs have never been measured in humans, and age-related EP reduction has never been verified. No human genes that promote strial presbycusis have been identified, nor is its pathophysiology well understood. Effective application of animal models to this issue requires models demonstrating EP decline, and preferably, genetically distinct strains that vary in patterns of EP decline and its cellular correlates. Until recently, only two models, Mongolian gerbils and Tyrp1B-lt mice, were known to undergo age-associated EP reduction. Detailed studies of seven inbred mouse strains have now revealed three strains (C57BL/6J, B6.CAST-Cdh23CAST, CBA/J) showing essentially no EP decline with age, and four strains ranging from modest to severe EP reduction (C57BL/6-Tyrc-2J, BALB/cJ, CBA/CaJ, NOD.NON-H2nbl/LtJ). Collectively, animal models support five basic principles regarding a strial form of presbycusis: 1) Progressive EP decline from initially normal levels as a defining characteristic; 2) Non-universality, not all age-associated hearing loss involves EP decline; 3) A clear genetic basis; 4) Modulation by environment or stochastic events; and 5) Independent strial, organ of Corti, and neural pathology. Shared features between human strial presbycusis, gerbils, and BALB/cJ and C57BL/6-Tyrc-2J mice further suggest this condition frequently begins with strial marginal cell dysfunction and loss. By contrast, NOD.NON-H2nbl mice may model a sequence more closely associated with strial microvascular disease. Additional studies of these and other inbred mouse and rat models should reveal candidate processes and genes that promote EP decline in humans.
Keywords: Cochlea; Aging; Stria vascularis; Spiral ligament; Mouse; Gerbil, Temporal Bone, Endocochlear Potential; Marginal Cells; Melanin, Intermediate Cells
Introduction
Age-related strial degeneration and subsequent endocochlear potential (EP) decline are taken to be the hallmarks of a form of presbycusis Schuknecht termed metabolic or strial (Schuknecht, 1993; Schuknecht and Gacek, 1993; Schuknecht et al., 1974). Compared to other posited forms, strial presbycusis was asserted to show the earliest onset (as early as the third decade of life), and to show the clearest genetic influence. Causally distinct pathology of the stria vascularis certainly seems plausible. The cellular processes that give rise to progressive hair cell and neural loss are often spatially and biochemically distinct from those in the stria, and examples of ‘pure’ and delimited strial pathology have been demonstrated in temporal bones. Yet EP decline has never been demonstrated in humans. While the incidence of strial presbycusis has been estimated to range from ~30% (Schuknecht and Gacek, 1993) of cases to 100% of ‘true’ presbycusis cases (Gates and Mills, 2005; Schmiedt et al., 2002), there are no universally accepted diagnostic criteria, so it is hard to be sure how frequently it occurs. Diagnosis is difficult both in living subjects, in whom the shape of the audiogram is of questionable value (Nelson and Hinojosa, 2003), and post-mortem, when the extent of strial dysfunction is difficult to infer from tissue samples. Presently, there are no verified mechanisms and no known predisposing genes.
Strial presbycusis as a distinct aging pathology
From the foregoing, the first question one might formulate about strial presbycusis is ‘Does it exist?’ Is it more nearly universal or mythical unicorn? And if there are no specific treatments, do we care which? Underneath some spoken or written discourses on the importance of presbycusis research, there is latent cynicism about the value of dissecting out its causes and forms. Most temporal bones studied—and indeed most animal models—indicate multiform aging cochlear pathology and multiple contributors to hearing loss. This is not surprising, given that all major cochlear cell types decrease in number with age (Ohlemiller and Frisina, 2008). The mechanisms driving this cell loss are probably those underlying most cellular aging, and may include oxidative stress, mitochondrial damage, calcium dysregulation, and loss of progenitor cells. If most aging cochleas are subject to loss of both sensory and strial cells, are potentially minor differences in why they die really important enough to study? We (Ohlemiller and Frisina, 2008) recently distinguished two general classes of age-related cochlear pathology, somewhat ponderously termed ‘biological-age synchronous’ (BAS) and ‘biological-age accelerated’ (BAA). Given a hypothetical set of biomarkers to establish the ‘true biological age’ of an individual, BAS aging of the cochlea will mirror the biological age. The quality of life will be impinged by multiple ailments, one of which ultimately becomes fatal. In such an individual, there will be hearing loss, but it will not stand out among other limitations, and any biologic fixes will probably overlap those for treating other tissues and organs. By contrast, BAA aging of the cochlea is taken to far outpace overall biological age, and to singularly interfere with quality of life. Rather than reflect broad cellular aging mechanisms, it will reflect processes and vulnerabilities specific to the cochlea. It may also primarily impact a specific cochlear cell population and give rise to a distinct form of presbycusis. Regardless of how it arises, presbycusis merits study. But it is the latter scenario that generates the most urgency. If strial presbycusis can be established, and its dissimilarity to noise injury is confirmed, it may emerge as the clearest example of biological-age accelerated cochlear aging. That said, we do not subscribe to the view that strial presbycusis is the only true form. This view originates from evidence that sensory and neural presbycusis often have an injury component, and the propensity for these will reflect gene/environment interactions (Ohlemiller and Frisina, 2008). Evidence presented here argues that strial presbycusis is no different in this regard.
Animal models offer the best strategy for linking specific patterns of strial pathology to EP reduction and uncovering related genes. Yet despite the fact that the EP is not difficult to measure, few animal studies have included this fundamental metric. Until recently, all direct evidence for age-related EP decline came from a single model, the Mongolian gerbil. Now accumulating data from inbred mouse and rat strains are revealing patterns in how the EP declines, and the nature of attendant cellular pathology. Supported principles include the existence of strial presbycusis as a causally and genetically distinct form, its strong modulation by environmental or probabilistic events, and a frequent role for marginal cell pathology in its appearance.
Requirements for a normal EP
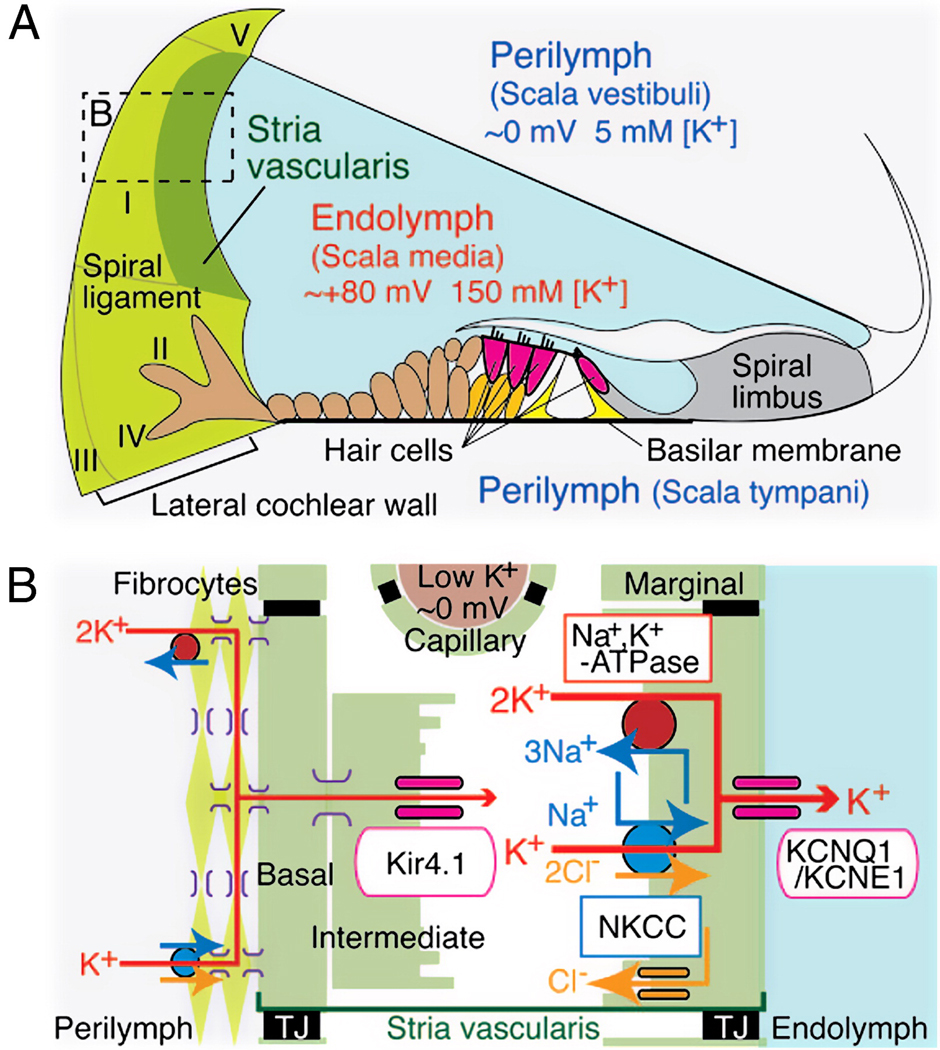
Proper strial function and a normal EP require that a host of ion channels, exchangers, and pumps within the stria and spiral ligament are present and functional (Fig. 1) (Hibino and Kurachi, 2006; Wangemann, 2006). The first requirement is an adequate K+ supply, since K+ is the dominant cation in endolymph, and the primary current carrier in transduction. Ideally this K+ could be reused, and moreover appears toxic if allowed to build up around hair cells (Bohne and Rabbit, 1983). In fact, evolution has solved this problem by efficiently ‘recycling’ K+ back to the stria via a nearly continuous cellular network that carries it through the lateral organ of Corti, through fibrocytes of the spiral ligament, and into the intrastrial space. Normally, the only points within this loop where K+ is extracellular are prior to its removal from the organ of Corti and after its arrival in the ligament, where it is released by the finger-like root cells and taken up by type II fibrocytes (Fig. 1). All other flow occurs within cells connected by gap junctions composed primarily of connexins 26 and 31. The principal generators of the EP within the stria are 1) Kir4.1 K+ channel expressed by intermediate cells, and 2) Na+/K+-ATPase, ion exchanger NKCC, and KCNQ1/KCNE1 K+ channels expressed by marginal cells. A large EP also requires that highly selective boundaries be established surrounding scala media, surrounding the intrastrial space, and around strial capillaries. Normally, claudin-based tight junctions (TJ in Fig. 1) seal off intercellular gaps, ensuring that most K+ passes from strial intermediate cells to marginal cells, then back into scala media.
Figure 1.
Schematic structure of the cochlear duct with the lateral wall. A. Appropriate ionic composition of the endolymph and the EP require that an ion barrier line scala media, the intrastrial space, and strial capillaries. A nearly continuous cellular network guides K+ from the organ of Corti through finger-like root cells into (primarily) type II and I fibrocytes. Fibrocytes must take up K+ from the exracellular space around root cells. Locations of five major types of fibrocytes are indicated by roman numerals. B. Schematic enlargement of the boxed area in A depicts the cells and components required to generate the EP. Type I fibrocytes, strial basal cells, intermediate cells, as well as capillary pericytes (not shown) are joined by gap junctions composed mostly of connexins 26 and 30. K+ enters the intrastrial space through Kir4.1, then through marginal cells via Na+/K+-ATPase, the NKCC ion exchanger, and KCNQ1/KCNE1 channel complexes. TJ, tight junction. (Reprinted with permission from (Nin et al., 2008))
The elaborate machinery and delicate boundaries needed to maintain the EP suggest many ways the process could be undermined by aging. Capillary loss is a common finding, and may play a primary role in some cases. Disruption of ion boundaries appears not to be a frequent factor. Age-related cell losses within the stria (Spicer and Schulte, 2005), within strial capillaries (Ohlemiller et al., 2009; Thomopoulos et al., 1997), and within Reissner’s membrane (De Fraissonette et al., 1993) all seem to occur in a way that maintains these boundaries. Moreover, we will see that even wholesale degeneration of the organ of Corti, as in some mouse models, must leave intact the barrier at the surface of the organ of Corti. Degeneration of the spiral ligament need not promote strial degeneration (Ohlemiller et al., 2006), but may reduce the EP by cutting off its K+ supply. The strial epithelium appears redundant, both in terms of overall volume and density of major cell types. Yet, loss of either intermediate or marginal cells could alter stoichiometric relations, such that the activities of critical channels, pumps and exchangers are no longer balanced (Diaz et al., 2007). The same effect could, of course, be achieved without cell loss, since dysfunctional cells could simply stop expressing adequate levels needed proteins. Age-related loss of Na+/K+-ATPase has been demonstrated in gerbils, and to correlate with EP decline (Schulte and Schmiedt, 1992). Whether marginal cell loss could account for this was not tested, however.
Insights from key animal models
Schuknecht developed the notion of strial presbycusis in papers published over 40 years beginning in 1953 (Schuknecht, 1953, 1964, 1993; Schuknecht and Gacek, 1993; Schuknecht et al., 1974). Despite modest sample size, limited quality of samples, and lack of direct physiological data, several of his assertions appear to be on target. Early studies in several species (Bohne et al., 1990; Covell and Rogers, 1957; Keithley et al., 1992) characterized the general features of age-related strial degeneration, including thinning, disorganization, and capillary loss. But these did not make clear when strial presbycusis could be asserted to be present. Concurrent studies in mice lacking strial intermediate cells (Steel, 1995; Steel and Barkway, 1989; Steel et al., 1987) clarified the importance of these cells for strial function, but the resulting pathology and EP reduction were early and profound, and not ‘aging-like.’ Real progress toward a conception awaited the discovery of animals with delayed, progressive EP decline.
Of men and gerbils
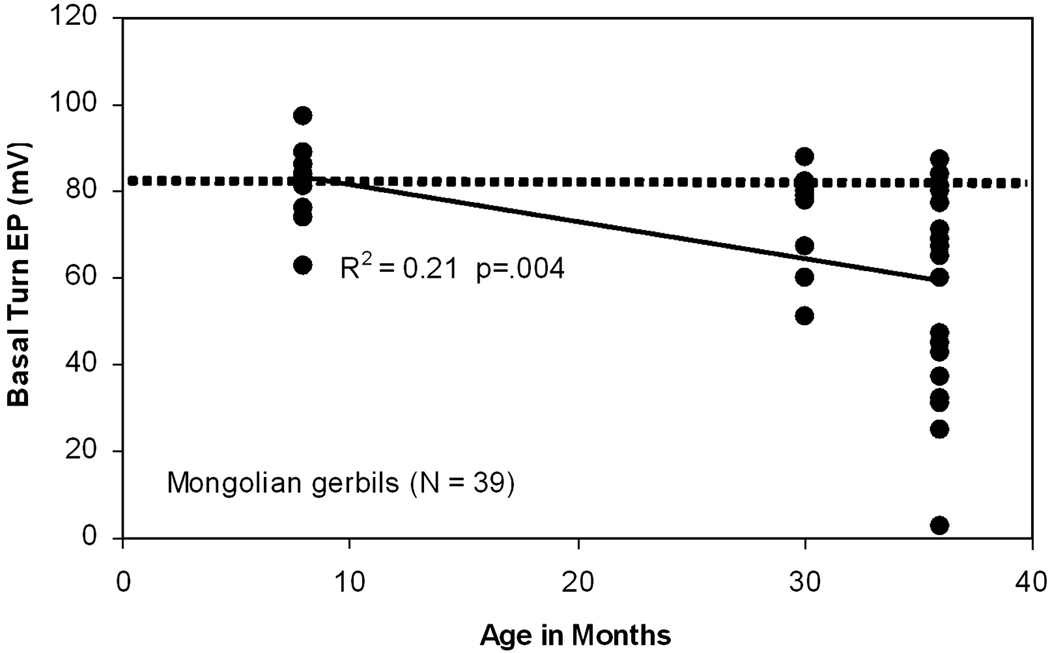
Many of the initial basic science insights came from work in gerbils (Gratton and Schulte, 1995; Gratton et al., 1996; Gratton et al., 1997a; Gratton et al., 1997b; Schmiedt, 1993; Schmiedt et al., 2002; Schulte and Schmiedt, 1992; Spicer and Schulte, 1998, 2002). First was the essential fact of EP decline. Figure 2 shows average EPs across the gerbil lifespan (Schmiedt, 1993). From an early average near 81 mV (dashed line), basal turn EPs decline by an average 20 mV by age 36 months. Given the generally modest hair cell and neural loss occurring by this age (Tarnowski et al., 1991), strial dysfunction appears to account for much of hearing loss (see also Fig. 7), and gerbils have been reasonably asserted to model relatively ‘pure’ strial presbycusis (Schmiedt et al., 2002). Although the variety of gerbils used in most hearing research are moderately inbred, the aging pattern of EP change does not affect all animals, suggesting that the link between predisposing genes in gerbils and EP decline is not deterministic. The very uniqueness of the gerbil EP data, however, may have led to a tendency to see all presbycusis through a gerbil-shaped lens. With detailed data from only one species, how could we be sure that EP decline isn’t a feature of all animals and humans? Recent mouse data have proven particularly significant on this point.
Figure 2.
Scatter plot of EP versus age in Mongolian gerbils. Horizontal dotted line indicates the average basal turn EP in young animals (81 mV). (Re-plotted from (Schmiedt, 1993))
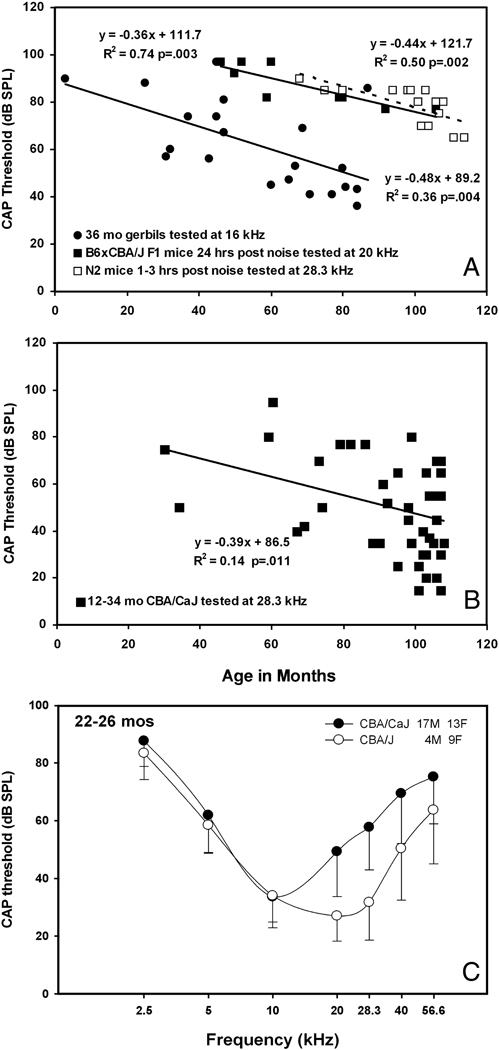
Figure 7.
A. Basal turn EP versus compound action potential (CAP) thresholds in gerbils and mice. Old gerbils are compared with young mice within 24 hrs of severe noise exposure (4–45 kHz, 110 dB SPL, 2 hrs). Both relations show slopes near −0.4 to −0.5 dB/mV. B. Old CBA/CaJ mice also show a significant correlation. This may indicate that age-related hearing loss in both gerbils and CBA/CaJ primarily reflects EP decline. C. Because the required gain of the cochlear amplifier is greater at higher frequencies, EP reduction is expected to affect high frequency thresholds more than low. Comparison of CAP thresholds in old CBA/J and CBA/CaJ mice suggest this pattern applies to CBA/CaJ. Data in A are re-plotted from (Ohlemiller and Gagnon, 2007a; Schmiedt, 1993).
With the gerbil as a plausible animal model of strial presbycusis, new questions could be asked, and important comparisons could be made with human temporal bones. It was possible, for example, to correlate gerbil EPs with functional strial volume and the expression of Na+/K+-ATPase, a critical marginal cell pump (Gratton et al., 1997b; Schulte and Schmiedt, 1992). These data supported less directly derived assertions in humans that strial epithelium is redundant (Pauler et al., 1988). It appears that up to 40% of strial functional volume or capacity may be lost without EP reduction. Through detailed histological comparisons of affected human temporal bones and gerbils with measured EP reduction, it was also possible to infer which cellular changes were most closely tied. Schuknecht felt that the initial changes were manifested in strial marginal cells. Initial gerbil reports focused on microvascular changes, noting that EP decline could be correlated with strial capillary loss (Gratton and Schulte, 1995; Gratton et al., 1996; Gratton et al., 1997a), and appeared to follow changes in the composition of capillaries (Thomopoulos et al., 1997). More recently, however, ultrastructural studies in gerbils (Spicer and Schulte, 2005) have favored Schuknecht’s suggestion, leading to the possibility that the same pathophysiological—and even genetic—processes may be at work in gerbils and some humans.
As we proceed to consideration of mouse models, the reader may note an awkwardness of comparing ‘the gerbil’ with multiple mouse models, all having different aging characteristics. Because inbred mouse strains age quite differently, and may model different forms of presbycusis, we do not attempt to say how ‘the mouse’ ages, and probably should avoid similar language for gerbils (or chinchillas, cats, guinea pigs). Each of these species comprises many genetically distinct populations, only some of which are recognized and have made it into the laboratory. Presumably the Mongolian gerbil line used primarily for research in the U.S. just happens to carry one or more genes that promote strial degeneration and EP decline. These may be homologous to genes found to promote EP decline in mice, and ultimately humans.
Tyrp1B-lt mice
Mice carrying at least one copy of the Tyrp1B-lt allele (Chr. 4) show an initially normal EP, followed by highly variable EP reduction after 2–3 months (Fig. 3A) (Cable et al., 1993). Their aging-like characteristics were revealed at about the same time as seminal gerbil studies, but similarities in the overall pattern with age were missed by the field. Like gerbils, only some Tyrp1B-lt mutants show EP reduction. Unlike the gerbil, however, average EP may not decline further after ~3 months of age. A hugely important aspect of this model is that the gene defect giving rise to EP reduction is identified. Tyrp1 codes for tyrosinase-related protein 1, a melanocyte protein involved melanin synthesis that operates downstream of tyrosinase (Sarangarajan and Boissy, 2001). Tyrp1 and tyrosinase share both similarity of protein structure and gene ancestry. While not all the functions of Tyrp1 are known, it is suspected to stabilize tyrosinase conformation, and possibly overall melanosome structure. Mice carrying Tyrp1B-lt show progressive loss of coat pigmentation, as hair follicle melanocytes gradually disappear. Thus there is a link in these mice between melanocyte dysfunction and EP decline. Intermediate cells, the stria’s melanocytes, are critical to strial function, and early loss of these cells, as in the case of cochleo-saccular mutations (Steel, 1995; Steel and Barkway, 1989) disrupts strial function and promotes rapid degeneration. Moreover, as we will see, even normal survival and function of intermediate cells lacking the capacity to produce melanin (as in albinos) has long term implications for the EP. A mutation that impairs melanocyte survival by interfering with melanin synthesis may therefore impact the EP in multiple ways. Death of hair follicle melanocytes in Tyrp1B-lt mice has been proposed to result from generation of a toxic intermediate by the aberrant protein. The dominant nature of the mutation thus may reflect a harmful gain-of-function. The anticipated progressive loss of strial intermediate cells has not been confirmed in Tyrp1B-lt mice, nor has any other degeneration been found to correlate with the EP. These mice merit more study, both for cellular mechanisms and genetics of EP reduction.
Figure 3.
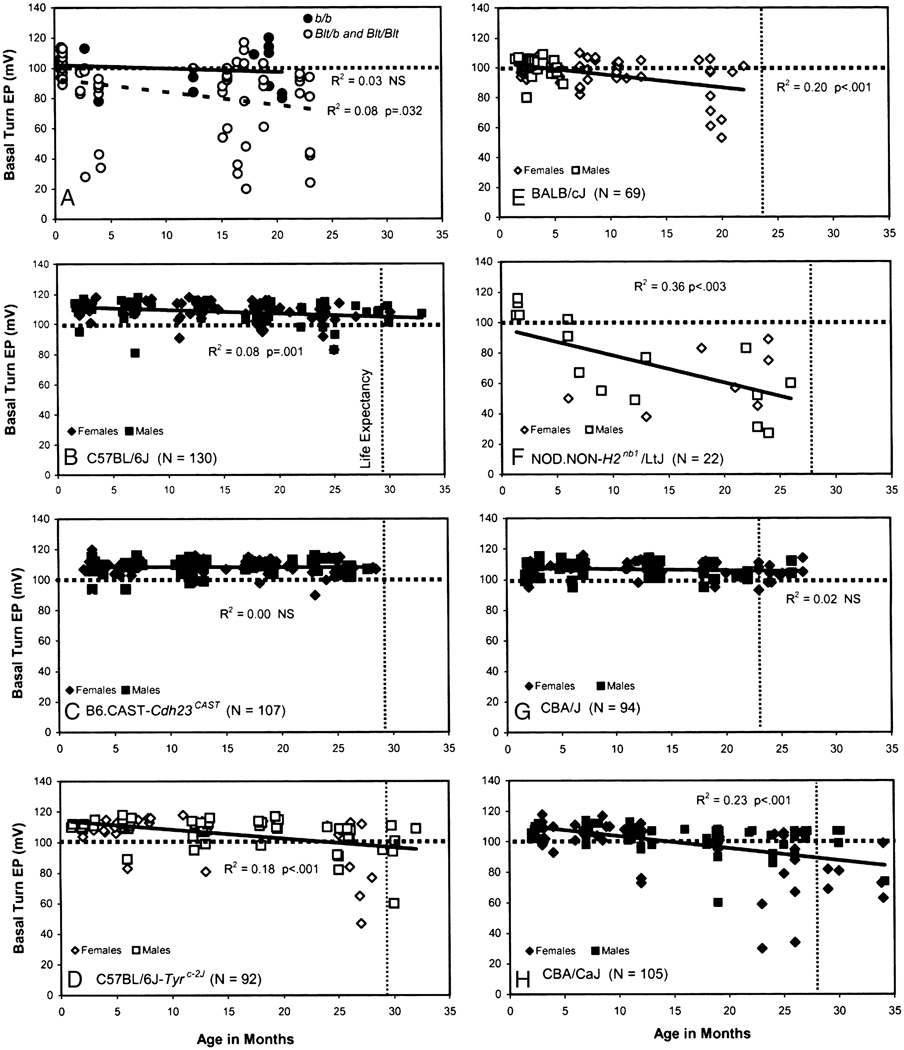
A–H. Scatter plots of age versus EP in eight different mouse models. Horizontal dotted line in each graph indicates 100 mV for reference. Vertical dotted lines indicate life expectancy (where known) averaged for males and females of each strain (Fox et al., 1997). Genders (where known) are also indicated. Carriers of Tyrp1B-lt in panel A were generated on a mixed background and compared with Tyrp1b/Tyrp1b littermates. A: Re-plotted from (Cable et al., 1993); B: Re-plotted from (Ohlemiller et al., 2009); D: Re-plotted from (Ohlemiller et al., 2009); E: Re-plotted from (Ohlemiller et al., 2006); F: Re-plotted from (Ohlemiller et al., 2008).
Age-invariant EP mouse strains
The implications of findings in gerbils for strial presbycusis depend in part on how common EP decline is. Finding animal models that do not show age-related EP decline is just as valuable as identifying those that do. We must be careful, however, regarding when in the lifespan the EP is asserted to be age-invariant. The wild type controls to Tyrp1B-lt mutants showed no average EP reduction by 22 months of age (Fig. 3A). But what if additional recordings were made at 25 months? Any claim of EP stability throughout life should be withheld until the entire ‘healthy lifespan’ has been tested. Failure to adhere to this maxim led us to initially miss valuable characteristics of BALB/c mice (Ohlemiller, 2002). It has also led us and others, perhaps prematurely, to judge the EP stable with age in 129S6/SvEvTac mice at 15 months of age (Ohlemiller and Gagnon, 2004b), and in CD-1 mice at 9 months of age (Wu and Marcus, 2003). A scan of Figure 3 reveals several different patterns of delayed EP reduction, some late in life (Fig. 3D) and some early (Fig. 3A,F). Does it matter if the trajectory of the EP does not map onto the posited trajectory in humans of EP decline, starting around middle age? We would argue it does not, at least until the range of mechanisms these models represent can be evaluated.
Life expectancies, where they are known, are indicated by vertical dashed lines in Figure 3 (averaged for both genders). We can therefore guess that the average EP shows little or no decline by the end of the expected lifespan in three mouse strains: C57BL/6J (Fig. 3B), B6.CAST-Cdh23CAST, (Fig. 3C), and CBA/J (Fig. 3G). One important implication is that EP decline is not ubiquitous, but instead must depend upon genetic factors. Another implication becomes clear when it is remembered that C57BL/6J (B6) mice are homozygous for two alleles (Cdh23ahl and Ahl3) that promote progressive hearing loss (Johnson et al., 1997; Nemoto et al., 2004). The first of these, Cdh23ahl, encodes a hair cell stereociliary protein, and is associated with hair cell loss. Also noted in this strain, with an unclear relation to Cdh23ahl, are progressive primary neural (Ohlemiller and Gagnon, 2004a), spiral ligament (Hequembourg and Liberman, 2001; Ichimiya et al., 2000), and strial degeneration (Mikaelian, 1979). It is not yet clear whether Cdh23ahl has any role in these. Elimination of the influence of this allele, as has been accomplished in B6.CAST-Cdh23CAST congenic mice, preserves hair cells (Keithley et al., 2004), but whether the lateral wall fares better has not been determined. Age related changes in the lateral wall of B6 have led them to be considered a strial presbycusis model, with confusing implications. The interpretation seemed to be that organ of Corti, neural, and lateral wall degeneration all progress together, and can be brought about by a hair cell mutation. If so, then B6 mice represent a mixed presbycusis model, and much apparent presbycusis in humans and animals is likely to be mixed. Vital clarification came when the EP was shown to be stable with age (Lang et al., 2002). Failing to meet the essential criterion, B6 apparently do not represent a strial presbycusis model. More importantly, moderate strial thinning and capillary loss, plus marked loss of spiral ligament fibrocytes in these mice need not lead to EP reduction. This was an important development, as it causally separates strial presbycusis from other forms, and indicates that its genetic underpinnings may be distinct from those in other degenerations. A minor caveat becomes necessary when lifelong EP trends in B6 (Fig. 3B) are compared with those in B6.CAST-Cdh23CAST, shown here for the first time in Figure 3C. Although the slope of a linear regression in B6 is slight, it is significantly different from zero. By contrast, the regression for B6.CAST-Cdh23CAST is virtually flat. Thus some interaction between organ of Corti pathology and the EP cannot be completely ruled out.
The third model presently known to show a normal EP at the end of its expected lifespan is CBA/J (Fig. 3G). As in B6.CAST-Cdh23CAST mice, the slope of a linear regression on EP in these mice is zero. CBA/J mice are commonly studied as a ‘good hearing’ and ‘good aging’ model, particularly as a counter model to B6. They have been used nearly interchangeably with another good hearing model, CBA/CaJ, and some mouse hearing papers have not bothered to state which of these was used. These strains, however, diverged genetically over 70 years ago (Fox et al., 1997), and are already suspected to possess different susceptibilities to noise (Gagnon et al., 2007). We will see that their aging characteristics differ, also.
BALB/cJ mice
BALB/cJ (BALB) mice share with B6 multiple genes that promote progressive sensory cell and hearing loss (Ohlemiller, 2006; Ohlemiller et al., 2006). We initially screened aging BALBs expecting to observe age-related EP decline both in these and in B6, as part of a broad degenerative pattern. Instead, intriguing differences emerged. First, the early ‘normal’ EP is slightly (~10 mV) but significantly lower in BALBs than in B6 (compare Fig. 3B,E). Second, unlike B6, the average EP clearly undergoes further decline in BALBs by 19 months of age. Two anatomical features of the cochlear lateral wall were found both to distinguish BALB from B6, and within BALBs, to correlate with EP. The first of these was strial marginal cell density, which is initially lower in BALBs than in B6, and then undergoes further decreases with age. The second was the thickness of the spiral ligament, which is narrower in BALBs (Fig 4) and becomes narrower still with age. Of a host of strial, ligament, and capillary metrics, no metrics other than marginal cell density and ligament thickness were found to favor B6, or to predict the EP. Notably, ligament cell density appeared more reduced by aging in B6 than in BALB. While the ligament results are novel and their meaning is not clear, the marginal cell results prominently echo earlier findings in humans and gerbils.
Figure 4.
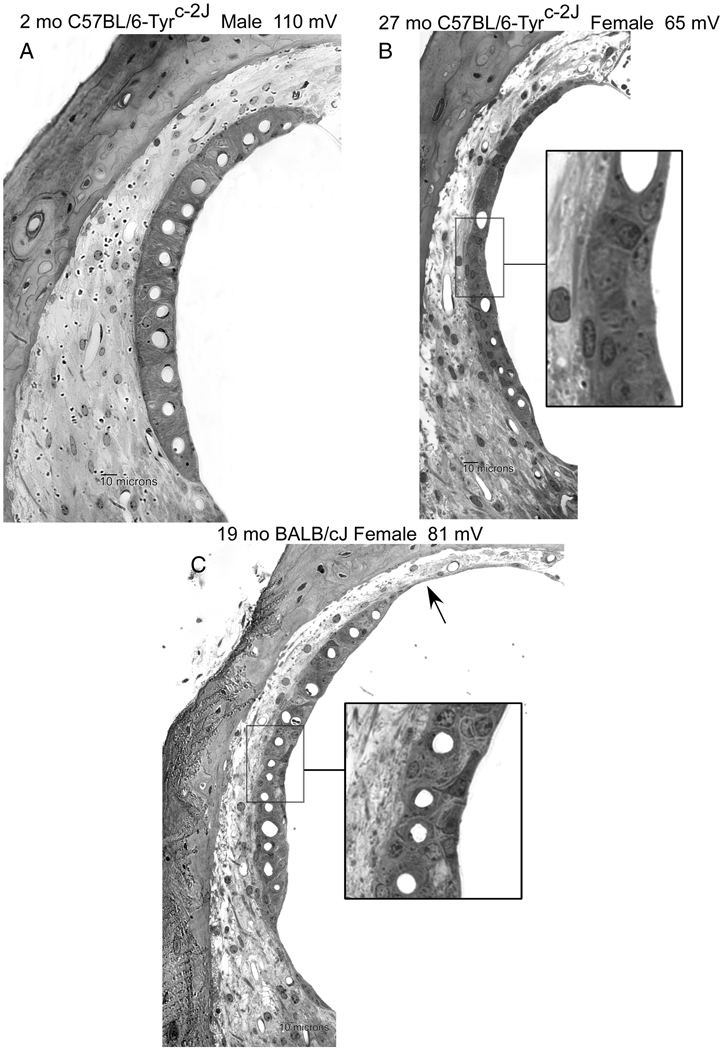
Examples of lateral wall in the lower cochlear base of young B6 albino (A), old B6 albino (B), and old BALB/c (C). Age, gender, and basal turn EP are indicated. Aging was associated with strial thinning and marginal cell loss in both strains. Severity of capillary loss and ligament thinning in B are atypical for this strain. BALB (C) features greater loss of marginal cells along the luminal surface. Marginal cells in C inset show dense staining and retraction of processes. Arrow in C denotes somewhat unusual strial atrophy.
The fact that BALBs begin life with fewer marginal cells than B6, and then lose these at a faster rate, points either to developmental processes that establish marginal cell numbers, to deficient cell replacement mechanisms, or to defective repair processes. Although strial intermediate cells, and possibly basal cells, are replaced (Conlee et al., 1994; Hirose et al., 2005), it is not clear whether marginal cells are. BALB and B6 mice differ genetically at many loci, so that few specific genes are suggested. Since they both carry Cdh23ahl (Johnson et al., 2000), this cannot explain their EP-aging profiles. Unlike B6, BALBs carry the dominant A (agouti) allele (Chr. 2), which may alter the type of strial melanin produced. Yet CBA/J mice, in which the EP is completely stable throughout life, also carry this allele. BALBs are also albino, which may play some role, as we will see next.
C57BL/6-Tyrc-2J (B6 albino)
The biochemical actions of eumelanin, the prevalent form of melanin, include binding of metals and ions such as Ca++ and inactivation of oxidative radicals. Given that melanin is protective in the skin and eye against injury and some apparent effects of aging (Riley, 1997; Schraermeyer and Heimann, 1999; Trachimowicz et al., 1981), it has long been supposed that it is also important for preservation of the cochlea against aging. Evidence for the physiological effects of melanin have nearly universally been sought by comparing pigmented and albino strains of animals differing at a host of unknown loci. This can complicate the interpretation of findings. Some evidence indicates that intermediate cells are replaced more slowly in albinos (Conlee et al., 1994), and that strial marginal cells in albinos may be more vulnerable to ototoxins (Taylor et al., 2008). Beyond that, little evidence has been brought to bear on the issue using optimal models. We exploited the fact of essential EP stability throughout life in B6 mice in a comparison of these with aging C57BL/6-Tyrc-2J albino mice (Ohlemiller et al., 2009). These mice carry a naturally occurring inactivating mutation of the tyrosinase (Tyr) locus on chromosome 7. Because the mutation arose on the B6 line, they are coisogenic with B6. They produce no melanin, but in all tissues retain a normal complement of melanocytes.
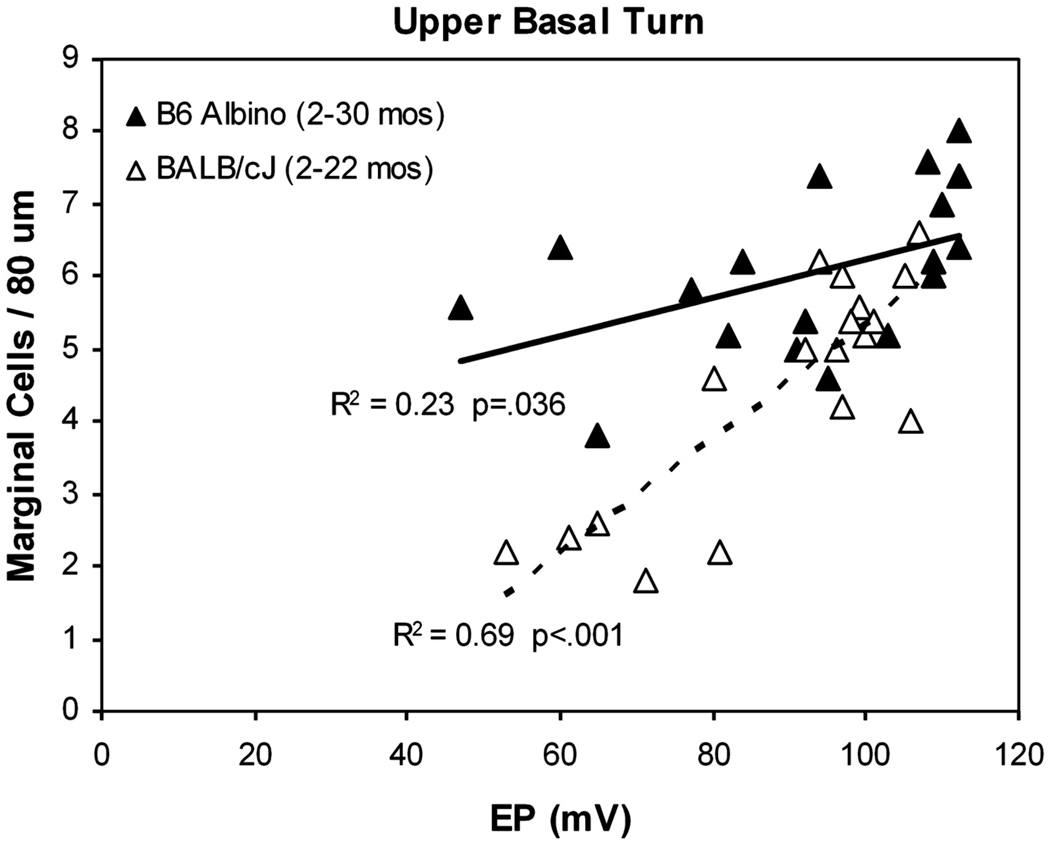
The progression of hearing loss in B6 and B6 albinos (not shown) was found to be identical, as was the distribution of EPs for ages out to ~2 years (Fig. 3B,D). After this, EPs in the two strains significantly diverge. From a wide array of histologic metrics, strial thickness most clearly differed between the two strains, with albinos exhibiting ~20 percent greater thinning than pigmented B6 by 24 months of age. Of major strial cell types, reduced strial thickness in albinos correlated uniquely with marginal cell loss. As in BALBs, marginal cell density within the cochlear upper basal turn predicted the EP (Fig. 5), although the slope of the relation was less steep than in BALBs. Like BALBs also, strial degeneration in B6 albinos was generally modest; Atrophic regions were rare, and cells clearly in the process of dying were not commonly observed (see Fig. 4).
Figure 5.
Scatter plot and linear regression on basal turn EP versus strial marginal cell density in the cochlear upper basal turn in B6 albino and BALB/c mice. Data for each strain include young and old animals fitted to a single line. BALB data are replotted from a previous paper (Ohlemiller et al., 2006). Both strains show significant correlation, and a similar degree of EP reduction in older animals. However, BALBs undergo greater net cell loss with age and exhibit a steeper relation.
The fact of similar EP decline in BALBs and B6 albinos suggests that albinism may represent a predisposing factor. Supporting this are recent data from aging Fischer 344/NHsd albino rat, in which a tendency toward EP reduction was also noted (Bielefeld et al., 2008). Prominence of marginal cell loss in BALBs and B6 albinos suggests that a major function of melanin may be to promote marginal cell survival. This might seem paradoxical, since it is intermediate cells that produce melanin. However, melanosome secretion into the extracellular space and uptake by surrounding cells is a ubiquitous process. In the stria, melanosomes are taken up by marginal and basal cells and fuse with lysosomes, where melanin deposits can remain indefinitely (Riley, 1997). In old mice, marginal cells are nearly outlined by dense melanin content (Hayashi et al., 2007; Ohlemiller et al., 2009), potentially reflecting an adaptive process. Among strial cells, marginal cells express an especially wide array of ion channels, transporters, and ATP-consuming pumps. These may place a premium on marginal cell operation for overall strial function, and place marginal cells at increased risk for oxidative stress (Spicer and Schulte, 2005).
Clearly, albinism is not a suggested requirement for strial presbycusis, nor do we interpret our findings in albinos as evidence that the Tyr locus is a candidate predisposing locus for this condition in humans. Instead, albino models point to a limiting fragility of strial marginal cells on strial function. Lack of melanin may represent just one of many factors that can bias the net rate of marginal cell loss with age. Accordingly, plausible candidate genes include more than 30 known to affect the type, density, or distribution of melanin (Eppig et al., 2007). Yet they also include genes involved in protective and repair processes, ion homeostasis, and blood flow. The very different slopes evident in the EP-versus-marginal cell relation in BALBs and B6 albinos (Fig. 5) seem likely to reflect additional factors operating in BALBs beyond any effect of pigmentation.
Strial epithelium is redundant (Pauler et al., 1988; Schulte and Schmiedt, 1992), and the EP actually appears amazingly resilient. Permanent EP reduction in any individual probably requires that a limiting resource such as marginal cell function fall below some critical threshold (Ohlemiller et al., 2009). The idea that strial functional reserve is reduced in aging is supported by recent experiments in which potassium cyanide was administered to young and old Fischer 344/NHsd albino rats (Bielefeld et al., 2008). While the EP reduction in young animals reversed within 10 minutes, the reduction in old animals persisted for at least an hour.
NOD.NON-H2nbl/LtJ
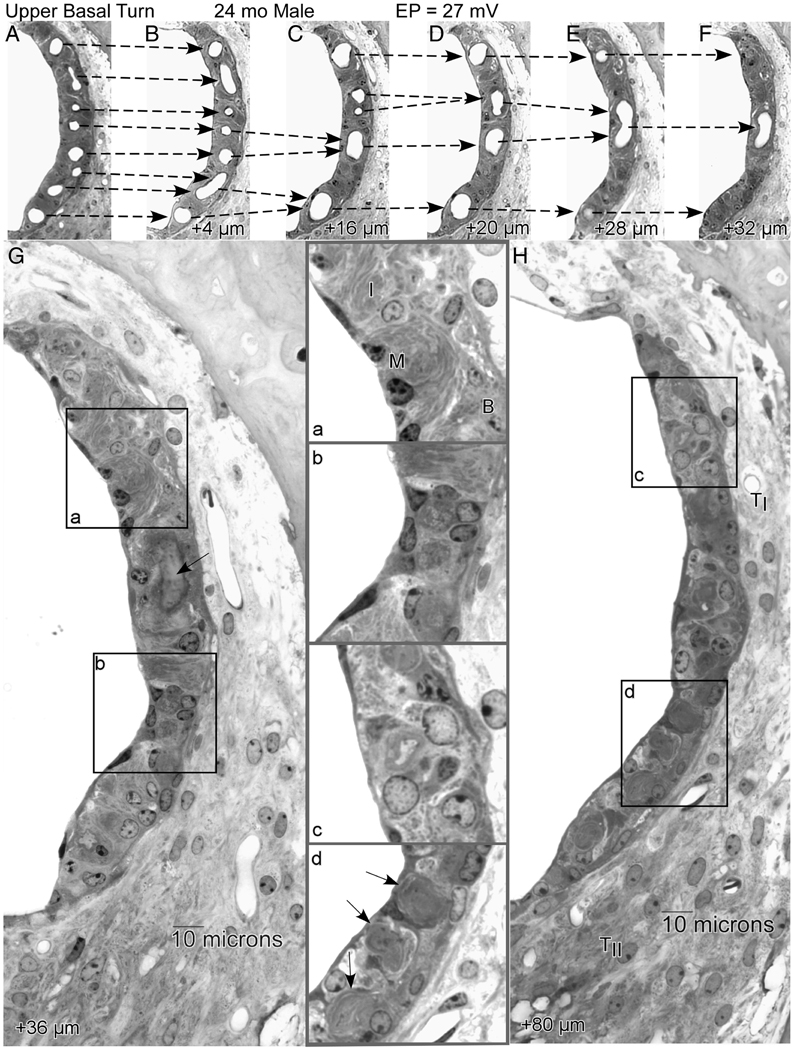
We examined NOD.NON-H2nb1/LtJ (NOD.NON) mice (Ohlemiller et al., 2008) as part of an aging screen of ‘poor hearing’ strains, again anticipating that wholesale pathology would include strial pathology and EP reduction. These mice undergo early and rapidly progressive hearing loss due to the presence of at least two alleles, Cdh23Ahl and Ahl2 (Johnson and Zheng, 2002). Unlike the original NOD/ShiLtJ inbred line to which they are congenic, NOD.NON lack H2g7 histocompatibility alleles that promote autoimmune disease, including Type I diabetes and Sjogren’s-like pathology. Although they carry pro-inflammatory alleles at other loci (see below), they do not show clear autoimmune disease. The NOD.NONs in our sample exhibited rapid hair cell and neural loss. In addition, they showed consistent and often severe EP reduction after 6 months of age (Fig. 3F). Unique among characterized models, this reduction features two components: Decline from typical normal levels after 6 months in all subjects, followed by more variable reduction that affects only some animals. The clear anatomic correlate of this reduction appeared to be strial atrophy secondary to microvascular degeneration (Fig. 6). Active degeneration of strial capillaries was frequently apparent, beginning in the deep base and apex and progressing toward the upper base, so that the cochlear upper base often contained the last normal appearing segment of stria. Microvascular pathology was often the only abnormality apparent by light microscope, and manifested in the form of darkly staining pericytes surrounding patent vessels that terminated in subsequent sections.
Figure 6.
A.–H. Sequential 4 µm sections proceeding apically from the upper base of a 24 month old NOD.NON-H2nbl/LtJ male showing progressive merging of strial capillaries that renders the stria completely avascular over a span of 80 µm. Dashed arrows in A.–F. show how capillaries progressively merge, ultimately forming a blind loop. The first completely avascular segment (G) features well-organized regions (inset a), as well as regions with increased numbers of poorly differentiated cells (inset b). The arrow in G shows the wall of the last capillary loop. By 80 µm (H), the stria is still present but thin, and shows hyperplasia of undefined cell types over most of its length (inset c). Traces of degenerated capillaries can still be seen (inset d, arrows). The ligament at this location appears normal. TI: Type I fibrocytes; TII: Type II fibrocytes; B: Basal cells; I: Intermediate cells; M: Marginal cells. (From (Ohlemiller et al., 2008))
NOD.NON-H2nb1/LtJ mice retain diabetogenic alleles from the NOD/ShiLtJ parent strain. Some of these may promote immune dysfunction and strial pathology. Autoimmune disease often has a hearing loss component, for which strial thinning and a low EP are the only clear correlates (Ruckenstein et al., 1999a; Ruckenstein et al., 1999b; Trune, 2002; Trune et al., 1991). Yet irreversible strial degeneration has not been shown in mouse autoimmune models, so that anatomic similarities to NOD.NON are limited. Loci other than H2 where NODs are believed to carry disease-promoting alleles include Idd10, Idd17, Idd18, Idd3 (for which interleukins 2 and 21 are candidates), and Idd16 (for which TNFα is a candidate) (Ikegami et al., 2003). These may represent candidate genes for the strial degeneration observed, and for strial presbycusis of microvascular origin. Like BALB and C57BL/6-Tyrc-2J mice, NOD.NONs are albino, so that a lack of melanin may also influence disease progression.
The literature includes many mouse models with early strial dysfunction that does not resemble aging. Likewise, it is not clear that the strial pathology in NOD.NON necessarily has mechanistic similarities to aging processes. However, comparison of the pattern of EP decline in these mice (Fig. 3F) with that in other models reveals a common probabilistic character: The extent of EP reduction varies widely, suggesting an interaction between genetic and other factors. This character matches conceptions of aging processes, which integrate multiple factors. Regarding our initial assumption that broad cochlear pathology will often include a strial component, the time course of hair cell/neural loss and strial degeneration in NOD.NON are quite different (Ohlemiller et al., 2008) and seem likely to represent independent processes. Creation and testing of additional lines that separate the multiple hearing loss alleles in NOD.NON will be required to resolve this issue.
Irrespective of how it arises, dynamic capillary loss in NOD.NON mice offers an opportunity to track changes in strial function and appearance as blood vessels withdraw, as well as how the vessels withdraw. Figure 6 shows by example how the stria of the upper basal turn changes in composition over a progressively apical 80 µm span in which a normal complement of capillaries disappears, leaving the stria completely avascular. Because the stria in the lower apex was completely absent in this animal, the sequence probably captures the end of vascularized stria, proceeding apically. The sequence begins with a normal appearing stria, possessing all cell layers and characteristic types (Fig. 6A). Over the next 32 µm, capillaries are seen to merge and end in blind loops, leaving the epithelium progressively less well supplied. At 36 µm (Fig. 6G) the imprint of the last capillary loop can be seen (arrow). At this point, the stria is composed both of regions still retaining three cell layers (inset a) and regions showing hyperplasia of poorly specified cells (inset b). At 80 µm distance (Fig. 6H), only abnormal cells are found (inset c), yet surprisingly the area of the epithelium is only modestly reduced. Note that the tracks of degenerated blood vessels can still be seen (inset d, arrows), suggesting a rapid withdrawal of the supporting vasculature. The ligament in this region appears normal, with several patent capillaries. Clearly, blood must exit the stria somehow, and normally follows capillaries that pass from the stria into spiral ligament in the region of Type II fibrocytes (Axelsson, 1988). The blind loops that appear in the NOD.NON stria suggest that blood flow reverses course within the stria as blood seeks an outlet. This probably results in highly turbulent flow, plus stagnation of poorly oxygenated red blood cells.
CBA/CaJ
As pointed out, two inbred mouse strains with similar names, CBA/J and CBA/CaJ, are often used as ‘good hearing’ and ‘good aging’ models. The nearly interchangeable use of CBA/J and CBA/CaJ may simply reflect the similarity of their names, when these strains are probably quite different. In a detailed comparison covering the expected lifespan of each (Ohlemiller and Gagnon, 2007b), we were surprised to find that CBA/CaJ undergo more rapid hearing loss than CBA/J after one year of age. By contrast, it is CBA/CaJ that may be more resistant to noise (Gagnon et al., 2007), so that these two characteristics need not always be closely tied. The divergence of hearing thresholds in CBA/J and CBA/CaJ was associated with very different EP-versus-aging characteristics (compare Fig. 3G,H), with significant EP decline apparent only in CBA/CaJs. Most of this occurred in females, potentially matching a pattern in humans that may reflect either an influence of sex hormones or autoimmune phenomena (Guimaraes et al., 2004; Ohlemiller and Frisina, 2008).
The anatomic correlates of EP decline in CBA/CaJ mice are still under study. Nevertheless, the fact that these mice retain hair cells and neurons and maintain reasonably good hearing as they age makes them the currently best mouse candidate of a ‘pure’ strial presbycusis model. This argument is supported by the relation between EP and hearing thresholds, as we consider next.
Diagnosing cases and models from EP-versus-threshold relations
The EP provides part of the electrochemical gradient that drives K+ currents through hair cells (Wangemann, 2006). A decrease in the EP is thus predicted to increase hearing threshold, and has been found experimentally to do so with a slope close to −1.0 dB/mV (Schmiedt et al., 2002; Sewell, 1984). This value applies to acute EP reduction using furosemide. In cases of relatively delimited strial presbycusis—that is, where little other pathology obscures the relation, the slope appears closer to −0.5 dB/mV (Fig. 7A), as estimated using data from gerbils (Schmiedt, 1993). This may reflect how the latter was measured, comparing across rather than within animals, and the need to rely on absolute thresholds rather than threshold shifts in aging. It is also possible that the shallower relation for aging somehow reflects differences between short and long term EP reduction. However, our own estimates for this relation immediately following noise exposure in mice are also well below unity, at about −0.4 dB/mV (Fig. 7A). We interpret this to reflect the simultaneous influence on thresholds of many factors, including synaptic injury, stereociliary injury, and altered mechanics, in addition to EP reduction. When multiple factors contribute to threshold elevation in differing amounts across subjects, there will be limits to how much increasing the EP appears to improve sensitivity. Aging may similarly be expected to inflict multiple impairments, even in cases where a single type of degeneration accounts for most hearing loss. Since the gerbil is taken to represent relatively isolated strial pathology, the very existence of a clear correlation between pooled EPs and thresholds in old gerbils (as in Fig. 7A) provides supportive evidence of this claim. A slope much shallower than −1.0 dB/mV for gerbils may nevertheless indicate the contributions of other factors. We asserted that CBA/CaJ mice may also represent a relatively isolated model of age-related strial pathology. If so, a clear EP-versus-threshold correlation should be observed in these mice. In fact, an estimate of −0.39 dB/mV emerges from pooled thresholds at 28.3 kHz in CBA/CaJs older than 12 months (Fig. 7B). In models wherein multiple kinds of age-associated degeneration strongly influence thresholds, no significant EP-versus-threshold correlation may be expected, and indeed we find no such correlations for other models such as BALB/cJ, NOD.NON-H2nbl/LtJ, or C57BL/6-Tyrc-2J (not shown). However, to claim that this could be used to diagnose these latter models as having multiple pathologies sets up a ‘straw man’ argument: At ages when these mice show EP decline, they are nearly deaf. As Schmiedt has pointed out (Schmiedt et al., 2002), the degree of hearing loss that EP reduction can impart is limited by the total gain of the cochlear amplifier. This may be limited to ~60 dB at high frequencies, and as little as 20 dB at low frequencies, depending on species. Thus, in cases where threshold elevation is greater than about 60 dB, EP decline alone cannot explain the shift. It follows that the shape of the audiogram should often provide clues to the presence of uncomplicated strial presbycusis. ‘Pure’ strial presbycusis might be revealed by modest hearing loss at low frequencies, combined with no more than ~60 dB loss at higher frequencies. Gerbils appear to follow this pattern (Schmiedt et al., 2002). As shown in Figure 7C, CAP thresholds in old CBA/CaJ and CBA/J differ exclusively at high frequencies, just as might be predicted if the extra hearing loss in CBA/CaJ results from EP decline. Unfortunately, a pattern of primarily high frequency hearing loss is a common clinical finding that can have many causes (Nelson and Hinojosa, 2003). Although EP-versus-threshold relations and the shape of audiograms may be useful for classifying models of strial presbycusis, this may not translate to the clinic.
Conclusions
Eight mouse lines in which EP-versus-age characteristics are well described show two general patterns. Five lines (Tyrp1B-lt, BALB/cJ, NOD.NON-H2nbl/LtJ, C57BL/6-Tyrc-2J, and CBA/CaJ) show highly variable EP decline that suggests combined influences of genetic, environmental, and stochastic factors. Four of these are albinos, or carry a known mutation of a gene involved in melanin synthesis (Tyrp1B-lt). In two of the albino strains (BALB/cJ, C57BL/6-Tyrc-2J), strial pathology and EP decline correlate best with strial marginal cell loss. These strains support a conception of the aging stria wherein marginal cells are the primary limiting resource for EP maintenance. Genetic and environmental factors, as well as chance events, may jointly bias the net rate of marginal cell loss with age. The idea that marginal cell pathology is central to many cases of strial presbycusis is supported by work in gerbils, as well as in human temporal bones. A fifth mouse strain showing EP reduction (NOD.NON) exhibits rapid strial degeneration subsequent to capillary degeneration. These mice may model forms of strial presbycusis having a microvascular origin, and offer a unique vantage on the dynamic process of strial capillary loss and local cellular response.
Mouse lines showing age-associated EP reduction do not necessarily represent mechanistic or genetic models of human strial presbycusis. Most of these lines feature multifaceted degeneration that complicates their interpretation. Nevertheless, they echo particular patterns and origins suggested to underlie the human disease. Moreover, specific genes (Tyrp1) and genetic pathways (melanin synthesis) emerge from these models as candidate genes—or gene families—whose mutations may promote the human disease. The posited fragility of marginal cells, and limiting nature of marginal cell function, also implicate genes that impact ion homeostasis, antioxidant protections, as well as cell repair and replacement. Untangling the complex degeneration in NOD.NON mice may reveal roles for immune-related genes in causing strial capillary loss. Finally, while it is not clear just how often presbycusis in humans is likely to reflect strial pathology alone, the identification of two potential animal models of relatively ‘pure’ strial presbycusis (gerbils and CBA/CaJ mice) argue that it is possible.
Providing useful counter-examples to the first group are three strains exhibiting little or no EP decline with age (C57BL/6J, B6.CAST-Cdh23CAST, CBA/J). The single major implication of these strains is that EP decline is not inevitable. These models support strial presbycusis as just one of several distinct forms. Findings in B6 further indicate that EP reduction does not necessarily accompany broad cochlear degeneration or marked cell loss within spiral ligament. Thus, strial presbycusis appears distinct from age-associated ligament degeneration.
Acknowledgements
Thanks to Drs. Rick Schmiedt and Karen Steel for comments and for sharing their data, and to Dr. H. Hibino for the use of Figure 1. Thanks also to Mary Rybak Rice, Allyson Rosen and Patty M. Gagnon for assistance with figures. Funded by NIH R01 DC08321 (KKO), R01 DC03454 (KKO), P30 DC04665 (R. Chole) and Washington University Med. School Department of Otolaryngology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axelsson A. Comparative anatomy of cochlear blood vessels. Am. J. Otolaryngol. 1988;9:278–290. doi: 10.1016/s0196-0709(88)80036-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Bielefeld E, Coling DE, Chen G-D, Li M, Tanaka C, Hu BH, Henderson D. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hearing Res. 2008;241:26–33. doi: 10.1016/j.heares.2008.04.006. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Rabbit KD. Holes in the reticular lamina after noise exposure: Implications for continued damage in the organ of Corti. Hearing Res. 1983;11:41–53. doi: 10.1016/0378-5955(83)90044-8. Scopus. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Gruner MM, Harding GW. Morphological correlates of aging in the chinchilla cochlea. Hearing Res. 1990;48:79–91. doi: 10.1016/0378-5955(90)90200-9. Scopus. [DOI] [PubMed] [Google Scholar]
- Cable J, Jackson IJ, Steel KP. Light (Blt), a mutation that causes melanocyte death, affects stria vascularis function in the mouse inner ear. Pigment Cell Res. 1993;6:215–225. doi: 10.1111/j.1600-0749.1993.tb00605.x. Scopus. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Gerrity LC, Bennett ML. Ongoing proliferation of melanocytes in the stria vascularis of adult guinea pigs. Hearing Res. 1994;79:115–122. doi: 10.1016/0378-5955(94)90133-3. Scopus. [DOI] [PubMed] [Google Scholar]
- Covell WP, Rogers JB. Pathologic changes in the inner ear of senile guinea pigs. Laryngoscope. 1957;67:118–129. doi: 10.1288/00005537-195702000-00002. [DOI] [PubMed] [Google Scholar]
- De Fraissonette A, Felix H, Hoffmann V, Johnsson L-G, Gleeson MJ. Human Reissner's membrane in patients with age-related normal hearing and with sensorineural hearing loss. Oto rhino laryngology. 1993;55:68–72. doi: 10.1159/000276381. Scopus. [DOI] [PubMed] [Google Scholar]
- Diaz RC, Vazquez AE, Dou H, Wei D, Cardell EL, Lingrel J, Shull GE, Doyle KJ, Yamoah EN. Conservation of hearing by simultaneous mutation of Na,K-ATPase and NKCCl. J. Assoc. Res. Otolaryngol. 2007;8:422–434. doi: 10.1007/s10162-007-0089-4. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. The Mouse Genome Database (MGD): new features facilitating a model system. Nucleic Acids Research. 2007;35(Database issue):D630–D637. doi: 10.1093/nar/gkl940. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RR, Witham BA, Neleski LA, editors. Handbook of genetically standardized JAX mice. Bar Harbor ME: The Jackson Laboratory; 1997. [Google Scholar]
- Gagnon PM, Simmons DD, Bao J, Lei D, Ortmann AJ, Ohlemiller KK. Temporal and genetic influences on protection against noise-induced hearing loss by hypoxic preconditioning in mice. Hearing Res. 2007;226:79–91. doi: 10.1016/j.heares.2006.09.006. Scopus. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schulte BA. Alterations in microvasculature are associated with atrophy of the sria vascularis in quiet-aged gerbils. Hearing Res. 1995;82:44–52. doi: 10.1016/0378-5955(94)00161-i. Scopus. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hearing Res. 1996;102:181–190. doi: 10.1016/s0378-5955(96)90017-9. Scopus. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schulte BA, Smythe NM. Quantification of the stria vascularis and strial capillary areas in quiet-reared young and aged gerbils. Hearing Res. 1997a;114:1–9. doi: 10.1016/s0378-5955(97)00025-7. Scopus. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Smyth BJ, Lam CF, Boettcher FA, Schmiedt RA. Decline in the endocochlear potential corresponds to decreased Na,K-ATPase activity in the lateral wall of quiet-aged gerbils. Hearing Res. 1997b;108:9–16. doi: 10.1016/s0378-5955(97)00034-8. Scopus. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hearing Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. Scopus. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sone M, Schachern PA, Wakamatsu K, Paparella MM, Nakashima T. Comparison of the quantity of cochlear melanin in young and old C57BL/6 mice. Archives of Otolaryngology Head and Neck Surgery. 2007;133:151–154. doi: 10.1001/archotol.133.2.151. Scopus. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: A major aspect of age-related cochlear degeneration in C57BL/6 mice. J. Assoc. Res. Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology. 2006;21:336–344. doi: 10.1152/physiol.00023.2006. Scopus. [DOI] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. Scopus. [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Suzuki M, Goro M. Age-related changes in the murine cochlear lateral wall. Hearing Res. 2000;139:116–122. doi: 10.1016/s0378-5955(99)00170-7. Scopus. [DOI] [PubMed] [Google Scholar]
- Ikegami H, Fujisawa T, Makino S, Ogihara T. Congenic mapping and candidate sequencing of susceptibility genes for type 1 diabetes in the NOD mouse. Ann. NY Acad. Sci. 2003;1005:196–204. doi: 10.1196/annals.1288.026. Scopus. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY. Ahl2, a second locus affecting age-related hearing loss in mice. Genomics. 2002;80:461–464. Scopus. [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least 10 inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. Scopus. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hearing Res. 1992;59:171–178. doi: 10.1016/0378-5955(92)90113-2. Scopus. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hearing Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Endocochlear potentials and compound action potential recovery: functions in the C57BL/6J mouse. Hearing Res. 2002;172:118–126. doi: 10.1016/s0378-5955(02)00552-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Mikaelian DO. The development and degeneration of hearing in the C57/bl6 mouse: Relation of the electrophysiologic responses from the round window to cochlear anatomy and behavioral responses. Laryngoscope. 1979;89:1–15. doi: 10.1288/00005537-197901000-00001. Scopus. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with flat audiometric patterns of hearing loss using a new method to quantify stria vascularis volume. Laryngoscope. 2003;113:1672–1686. doi: 10.1097/00005537-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Nemoto M, Morita Y, Mishima Y, Takahashi S, Nomura T, Ushiki T, Shiroishi T, Kikkawa Y, Yonekawa H, Kominami R. Ahl3, a third locus on mouse chromosome 17 affecting age-related hearing loss. Biochemical and Biophysical Research Communications. 2004;324:1283–1288. doi: 10.1016/j.bbrc.2004.09.186. Scopus. [DOI] [PubMed] [Google Scholar]
- Nin F, Hibino H, Doi KS, Suzuki T, Hisa Y, Kurachi Y. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Nat. Acad. Sci. USA. 2008;105:1751–1756. doi: 10.1073/pnas.0711463105. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Reduction in sharpness of frequency tuning but not endocochlear potential in aging and noise-exposed BALB/cJ mice. J. Assoc. Res. Otolaryngol. 2002;3:444–456. doi: 10.1007/s10162-002-2041-y. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. Scopus. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Apical-to-basal gradients in age-related cochlear degeneration and their relationship to 'primary' loss of cochlear neurons. J. Comp. Neurol. 2004a;479:103–116. doi: 10.1002/cne.20326. Scopus. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Cellular correlates of progressive hearing loss in 129S6/SvEv mice. J. Comp. Neurol. 2004b;469:377–390. doi: 10.1002/cne.11011. Scopus. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hearing Res. 2007a;224:34–50. doi: 10.1016/j.heares.2006.11.005. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Divergence of hearing sensitivity and endocochlear potential (EP) in CBA/J and CBA/CaJ mice after 12 months of age. Abstr., Assn. Res. Otolaryngol. 2007b;30:257. [Google Scholar]
- Ohlemiller KK, Frisina RD. Age-related hearing loss and its cellular and molecular bases. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection, and Repair. New York: Springer; 2008. pp. 145–194. [Google Scholar]
- Ohlemiller KK, Lett JM, Gagnon PM. Cellular correlates of age-related endocochlear potential reduction in a mouse model. Hearing Res. 2006;220:10–26. doi: 10.1016/j.heares.2006.06.012. Scopus. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Rybak Rice ME, Gagnon PM. Strial microvascular pathology and age-associated endocochlear potential decline in NOD congenic mice. Hearing Res. 2008;244:85–97. doi: 10.1016/j.heares.2008.08.001. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rice MR, Lett JM, Gagnon PM. Absence of strial melanin coincides with age associated marginal cell loss and endocochlear potential decline. Hearing Res. 2009 doi: 10.1016/j.heares.2008.12.005. (in press) [DOI] [PubMed] [Google Scholar]
- Pauler M, Schuknecht HF, White JA. Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope. 1988;98:754–759. doi: 10.1288/00005537-198807000-00014. [DOI] [PubMed] [Google Scholar]
- Riley PA. Molecules in focus: Melanin. Int. J. Biochem. Cell Biol. 1997;11:1235–1239. doi: 10.1016/s1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Faslpr mouse. Otolaryngology-Head and Neck Surgery. 1999a;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. Scopus. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Keithley EM, Bennett T, Powell HC, Baird S, Harris JP. Ultrastructural pathology in the stria vascularis of the MRL-Faslpr mouse. Hearing Res. 1999b;131:22–28. doi: 10.1016/s0378-5955(99)00018-0. Scopus. [DOI] [PubMed] [Google Scholar]
- Sarangarajan R, Boissy RE. Tyrp1 and oculocutaneous albinism Type 3. Pigment Cell Res. 2001;14:437–444. doi: 10.1034/j.1600-0749.2001.140603.x. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Cochlear potentials in quiet-aged gerbils: Does the aging cochlea need a jump start? In: Verillo RT, editor. Sensory Research: Multimodal Perspectives. Hillsdale NJ: Lawerence Erlbaum Assoc.; 1993. pp. 91–103. [Google Scholar]
- Schmiedt RA, Lang H, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: A model of metabolic presbycusis. J. Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. Scopus. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Lesions of the organ of Corti. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1953;57:366–383. [PubMed] [Google Scholar]
- Schuknecht HF. Further observations on the pathology of presbycusis. Arch.Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Pathology of the Ear. Second ed. Philadelphia: Lea and Febiger; 1993. [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann. Otol. Rhinol. Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. Scopus. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Watanuki K, Takahashi T, Belal AA, Kimura RS, Jones DD. Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope. 1974;84:1777–1821. doi: 10.1288/00005537-197410000-00012. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na,K-ATPase and endodochlear potentials decline with age in quiet-reared gerbils. Hearing Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Sewell W. The effects of furosemide on the endocochlear potential and auditory nerve fiber tuning curves in cats. Hearing Res. 1984;14:305–314. doi: 10.1016/0378-5955(84)90057-1. Scopus. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Evidence for a medial K+ recycling pathway from inner hair cells. Hearing Res. 1998;118:1–12. doi: 10.1016/s0378-5955(98)00006-9. Scopus. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Spiral ligament pathology in quiet-aged gerbils. Hearing Res. 2002;172:172–185. doi: 10.1016/s0378-5955(02)00581-6. Scopus. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Pathologic changes of presbycusis begin in secondary processes and spread to primary processes of strial marginal cells. Hearing Res. 2005;205:225–240. doi: 10.1016/j.heares.2005.03.022. Scopus. [DOI] [PubMed] [Google Scholar]
- Steel KP. Inherited Hearing Defects in Mice. Annual Review of Genetics. 1995;29:675–701. doi: 10.1146/annurev.ge.29.120195.003331. Scopus. [DOI] [PubMed] [Google Scholar]
- Steel KP, Barkway C. Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–463. doi: 10.1242/dev.107.3.453. Scopus. [DOI] [PubMed] [Google Scholar]
- Steel KP, Barkway C, Bock GR. Strial dysfunction in mice with cochleo-saccular abnormalities. Hearing Res. 1987;27:11–26. doi: 10.1016/0378-5955(87)90022-0. Scopus. [DOI] [PubMed] [Google Scholar]
- Tarnowski BI, Schmiedt RA, Hellstrom LI, Lee FS, Adams JC. Age-related changes in cochleas of mongolian gerbils. Hearing Res. 1991;54:123–134. doi: 10.1016/0378-5955(91)90142-v. Scopus. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A. Rapid hair cell loss: A mouse model for cochlear lesions. J. Assoc. Res. Otolaryngol. 2008;9:44–64. doi: 10.1007/s10162-007-0105-8. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hearing Res. 1997;111:31–41. doi: 10.1016/s0378-5955(97)00080-4. Scopus. [DOI] [PubMed] [Google Scholar]
- Trachimowicz RA, Fisher LJ, Hinds JW. Preservation of retinal structure in aged pigmented mice. Neurobiology of Aging. 1981;2:133–141. doi: 10.1016/0197-4580(81)90011-7. Scopus. [DOI] [PubMed] [Google Scholar]
- Trune DR. Mouse models for immunologic diseases of the auditory system. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. New York: CRC Press; 2002. pp. 505–531. [Google Scholar]
- Trune DR, Morton JI, Craven JP, Traynor SJ, Mitchell CR. Inner ear pathology in the Palmerston North autoimmune strain mouse. Am. J. Otolaryngol. 1991;12:259–266. doi: 10.1016/0196-0709(91)90003-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006;576.1:11–21. doi: 10.1113/jphysiol.2006.112888. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Marcus DC. Age-related changes in cochlear endolymphatic potassium and potential in CD-1 and CBA/CaJ mice. J. Assoc. Res. Otolaryngol. 2003;4:353–362. doi: 10.1007/s10162-002-3026-6. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]