Abstract
The PhoBR regulatory system is required for the induction of multiple genes under conditions of phosphate limitation. Here we examine the role of PhoB in biofilm formation and environmental stress response in V. cholerae of the El Tor biotype. Deletion of phoB or hapR enhanced biofilm formation in phosphate-limited medium. Planktonic and re-dispersed biofilm cells of the ΔphoB mutant did not differ from wild type for the expression of HapR suggesting that PhoB negatively affects biofilm formation through a HapR-independent pathway. The ΔphoB mutant exhibited elevated expression of exopolysaccharide genes vpsA and vpsL compared to wild type. Deletion of hapR enhanced the expression of positive regulator vpsT but had no effect on the expression of vpsR. Contrastingly, deletion of phoB enhanced the expression of positive regulator vpsR but had no effect on the expression of hapR and vpsT. The ΔphoB mutant was more sensitive to hydrogen peroxide compared to wild type and to an isogenic ΔrpoS mutant. Conversely, the ΔphoB mutant was more resistant to acidic conditions and high osmolarity compared to wild type and to an isogenic ΔrpoS mutant. Taken together, our data suggests that phosphate-limitation induces V. cholerae to adopt a free swimming life style in which PhoB modulates environmental stress response in a manner that differs from the general stress response regulator RpoS.
Cholera is an acute water-borne diarrheal disease caused by Vibrio cholerae of serogroups O1 and O139 that continues to be a major public health concern in endemic areas of south Asia and Africa. Infecting Vibrios that overcome the gastric acid barrier swim toward and adhere to the intestinal mucosa and express cholera toxin, which is largely responsible for the profuse rice-watery diarrhea typical of this disease (Kaper et al., 1995). At a late stage of infection, V. cholerae down-regulates the expression of virulence factors and detaches to return to the environment (Zhu et al., 2002). The ability of V. cholerae to persist in the aquatic environment has become a major obstacle to the eradication of this disease. The formation of biofilm communities has been suggested to contribute to V. cholerae environmental fitness (Joelsson et al., 2007; Yildiz & Schoolnik, 1999). Cells within these biofilm communities have been reported to be more resistant to environmental stresses and protozoan grazing (Joelsson et al., 2007; Matz et al., 2005; Zhu & Mekalanos, 2003). Biofilm formation in V. cholerae is regulated by quorum sensing. Quorum sensing is a cell-to-cell communication process involving the production, secretion and detection of chemical signaling molecules known as autoinducers that allow individual bacterial cells to synchronize their behavior and respond as a population. Two autoinducer systems, cholera autoinducer 1 (CAI-1) and autoinducer 2 (AI-2), synergistically activate the expression of the master regulator HapR at high cell density (Miller et al., 2002). CAI-1 and AI-2 are recognized by their cognate receptor CqsS and LuxPQ, respectively (Miller et al., 2002). Sensory information is fed through a phosphorelay system to the τ54-dependent activator LuxO (Miller et al., 2002). At low cell density the autokinase domains of CqsS and LuxPQ become phosphorylated and phosphorus is transferred to LuxO (Miller et al., 2002). Phospho-LuxO then activates expression of multiple redundant small RNAs which in conjunction with the RNA binding protein Hfq, destabilize hapR mRNA (Lenz et al., 2004). When the concentration of autoinducer molecules produced by growing bacteria reaches a threshold, CqsS and LuxPQ switch from kinase to phosphatase. The flow of phosphorus is reversed and phospho-LuxO becomes de-phosphorylated and inactive allowing the expression of HapR (Lenz et al., 2004; Miller et al., 2002), which acts to inhibit biofilm formation (Hammer & Bassler, 2003; Zhu & Mekalanos, 2003). The formation of three-dimensional mature biofilms involve a complex genetic program that entails the expression of motility and mannose-sensitive hemagglutinin for surface attachment and monolayer formation, as well as the biosynthesis of an exopolysaccharide (vps) matrix (Watnick & Kolter, 1999). The genes responsible for vps biosynthesis are clustered in two operons in which vpsA and vpsL are the first genes of operon I and II, respectively. Expression of vps genes is regulated by a complex set of regulatory interactions that involve cyclic diguanylate (c-di-GMP) signaling (Beyhan et al., 2006; Beyhan et al., 2007; Lim et al., 2006; Lim et al., 2007; Tischler & Camilli, 2004), the positive regulators VpsT (Casper-Lindley & Yildiz, 2004) and VpsR (Yildiz et al., 2001) and the negative regulators CytR (Haugo & Watnick, 2002) and HapR (Jobling & Holmes, 1997; Yildiz et al., 2004). HapR has been reported to repress biofilm formation by lowering c-di-GMP and negatively affecting the expression of VpsT (Waters et al., 2008).
It has been shown that freshwater and estuarine ecosystems where Vibrios can survive and persist outside the human host are limited in phosphate content (Benitez-Nelson, 2000; Correl, 1999). In E. coli, phosphate starvation induces the general stress response regulator RpoS (Hengge-Aronis, 2002). V. cholerae has been shown to build very large intracellular polyphosphate (poly-P) stores (Ogawa et al., 2000). A V. cholerae poly-P deficient mutant exhibited reduced activity of the general stress response regulator RpoS which resulted in augmented sensitivity to low pH, high salinity and oxidative stress in low phosphate medium (Jahid et al., 2006). In Escherichia coli, deprivation of phosphate induces the expression of the PhoB regulon (Lamarche et al., 2008). PhoB is part of the PhoR/PhoB two component regulatory system. PhoR is an inner membrane histidine kinase that responds to periplasmic orthophosphate through its interaction with the phosphate transport system. Under conditions of phosphate limitation, phosphorus is transferred from phospho-PhoR to the response regulator PhoB. Phospho-PhoB then binds to DNA pho boxes to activate or repress the transcription of target genes (Lamarche et al., 2008). A proteomic comparison of wild type and phoB V. cholerae strain 569B revealed 140 differentially expressed proteins (von Kruger et al., 2006). Furthermore, it was shown that phosphate limitation induced the expression of genes belonging to both the PhoB and general stress response regulons suggesting a link between PhoB and RpoS (von Kruger et al., 2006). Furthermore, a V. cholerae phoB mutant colonized less in the rabbit ileal loop model suggesting a role for this regulator in intestinal colonization and pathogenesis (von Kruger et al.,1999). Recently, PhoB has been shown to modulate biofilm formation in a classical biotype V. cholerae strains that does not express HapR (Prat et al., 2009).
In E. coli and Pseudomonas aeruginosa expression of PhoB has been shown to affect surface adherence, biofilm formation, and stress response (Monds et al., 2001; Monds et al. 2007; Ferreira & Spira 2008; Ruiz & Silhavy, 2003). Since the expression of these phenotypes is crucial to the persistence of cholera, we decided to examine the role of PhoB in biofilm formation and stress response in an El Tor biotype strain representative of the current 7th pandemic. In the present study we show that under conditions of phosphate limitation PhoB acts to diminish biofilm formation by lowering the expression of the positive regulator VpsR and modulates resistance to environmental stresses in a manner independent of RpoS.
MATERIALS AND METHODS
Strains and media
Strains, plasmids and primers used in this study are shown in Table S1. All V. cholerae reporter strains and mutants were derived from C7258 (El Tor biotype, 1991 isolate from Peru). The E. coli strains TOP10 (Invitrogen) and SM10λpir (De Lorenzo et al., 1993) were used for cloning and plasmid propagation. For routine cultivation, strains were grown in LB medium (pH 7.4) supplemented with ampicillin (Amp, 100-μg/ml), polymixin B (PolB, 100-units/ml) or 5-bromo-4-chloro-3-indolyl-D-galactopyronoside (X-Gal, 20-μg/ml) as required. For the phosphate limitation studies V. cholerae strains were grown in EZ-rich defined medium (Teknova, Inc) consisting in MOPS minimal medium (pH 7.2) supplemented with D-glucose (0.2%), ACGU solution, supplement EZ (Teknova, Inc.) and different concentrations of inorganic phosphates (high phosphate, 1.32 mM K2HPO4; low phosphate 0.132 mM K2HPO4).
Strain construction
To construct a V. cholerae quorum sensing reporter strain we initially amplified 737-bp and 821-bp DNA fragments flanking the V. cholerae C7258 lacZ promoter using the primer pairs LacZ955/LacZ218 and LacZ63/LacZ758 and the Advantage 2 PCR kit (BD Biosciences Clontech). A 500-bp KpnI and HindIII fragment containing rrnB transcription terminator (Brosius et al., 1981) and the V. harveyi luxC promoter was extracted from plasmid pLuxLacZ described previously (Silva et al., 2008). The 737-bp fragment located upstream of the lacZ promoter, the rrnB-luxC promoter DNA and the 821-bp fragment lying downstream of the lacZ promoter were sequentially cloned in pUC19 and the entire cassette transferred to the suicide vector pCVD442 (Donnenberg & Kaper, 1991) to obtain pCVDLuxlacZ. The above suicide vector was transferred from SM10λpir to C7258 by conjugation and exconjugants selected on LB agar containing Amp and PolB. The segregant SZS007 in which the lacZ promoter region was replaced by the rrnBT1T2-luxC promoter fragment was obtained by sucrose selection as previously described (Silva et al., 2008) and confirmed by PCR and DNA sequencing. To construct a phoB deletion mutant, we amplified 758-bp and 760-bp chromosomal DNA fragments located upstream and downstream of phoB respectively using the primer pairs PhoB23/PhoB762 and phoB793/phoB1535. The fragments were sequentially cloned in pUC19, confirmed by DNA sequencing and the chromosomal fragment containing the phoB deletion transferred to pCVD442 (Donnenberg & Kaper, 1991). Similarly, 857-bp and 828-bp chromosomal DNA fragments flanking luxO were amplified using the primer pairs LuxO133/LuxO972 and LuxO1462/LuxO2272, the chromosomal deletion constructed in pUC19, confirmed by DNA sequencing and moved to pCVD442 (Donnenberg & Kaper, 1991). To construct a ΔhapR and ΔrpoS mutants we used similarly constructed suicide vector pCVDΔHapR and pCVDΔRpoS2 described previously (Liang et al., 2007). In all cases, mutation were created by conjugal transfer of the corresponding suicide vector to the quorum sensing reporter strain SZS007 to monitor HapR expression and biofilm formation in the same genetic background. The mutants were obtained by sucrose selection and confirmed by DNA sequencing across the deletion point. For genetic complementation, we amplified the entire phoBR operon using primers PhoCF and PhoCR. The amplicon was cloned into pUC19 to construct pPhoBR and confirmed by DNA sequencing.
Quantitative real-time reverse transcription PCR (qRT-PCR)
V. cholerae strains were grown in LB medium at 37 °C with agitation for 16 h, diluted 1:100 in EZ-rich defined medium with 0.132 mM phosphate and grown to OD600 of 0.3. Total RNA was isolated from the culture using the RNeasy kit (Qiagen Laboratories). The RNA samples were analyzed by quantitative real-time reverse transcription-PCR (qRT-PCR) using the iScript two-step RT-PCR kit with SYBR green (Bio-Rad Laboratories) as described previously (Liang et al., 2007; Silva et al., 2008). Relative expression values were calculated as 2-(Ct target-Ct reference) where Ct is the fractional threshold cycle. The amount of recA mRNA was used as a reference. We did not observe differences in recA expression between the different culture conditions and strains used in this study. The following primer combinations were used: CytR295 and CytR421 for cytR mRNA; HapR589 and HapR1046 for hapR mRNA; VpsA434 and VpsA676 for vpsA mRNA; VpsL607 and VpsL775 for vpsL mRNA; VpsR75 and VpsR206 for vpsR mRNA; and VpsT56 and VpsT252 for vpsT mRNA. A control mixture lacking reverse transcriptase was run for each reaction to exclude chromosomal DNA contamination.
Biofilm assay
Biofilm formation was measured by the crystal violet staining method and results normalized for growth and expressed as the OD570/OD600 ratio (Zhu et al., 2002). Strains were grown in 5 ml LB medium for 16 h, diluted 1:50 in fresh EZ rich defined medium with different phosphate concentrations and 100 μL of the inoculated medium was transferred to each well of 96-well flat-bottom polystyrene microtiter plates. The plates were incubated for 24 h at 30°C for biofilm development. For confocal microscopy, strains were grown in 5 ml LB medium for 16 h, diluted 1:50 in fresh EZ rich defined medium with 0.132 mM phosphate and 3 ml of the inoculated medium was transferred to 35 mm diameter glass-bottom dishes. The dishes were incubated for 24 h at 30°C for biofilm development. Following incubation, the planktonic cells were removed; the plates were washed 4 times with 4 ml normal saline each time and stained with 2.5 ml of 10μM Syto-9 (Invitrogen) for 30 min at 30°C. Stain solution was removed and the plates were washed once with 4 ml normal saline and then the biofilm on the glass-bottom was examined by laser confocal microscopy using 485- and 498-nm excitation and emission wavelength, respectively. To measure the expression of HapR in biofilm cells, strains were inoculated in 3 ml of EZ-rich defined medium with low phosphate in borosilicate tubes and incubated at 30°C for 24 h. The planktonic cells were removed and saved, the tubes were washed three times with normal saline and biofilm associated cells were brought into suspension in 0.5 ml normal saline by vortexing in the presence of 1 mm diameter borosilicate glass beads (Sigma).
Enzyme assay
β-Galactosidase activity was measured as described previously (Miller, 1971) using the substrate o-nitrophenyl-β-D-galctotopyranoside (ONPG). Specific activities are given in Miller units [1000 OD420/t. v. OD600)] where t is the reaction time and v is the volume of enzyme extract per reaction.
Stress response assays
V. cholerae strains were grown for 16 h in LB medium at 37°C. The culture was then diluted to 106 to 107 cells per ml in fresh low phosphate EZ-rich defined medium containing 1.2 M NaCl, 0.5 mM hydrogen peroxide, pH 4.5 or lacking carbon source. Cultures were incubated at 37°C with shaking (250 rpm), and samples were taken at different time points to determine viability by dilution plating on LB agar plates.
RESULTS
Phosphate limitation enhances HapR expression
Repression of HapR requires the regulator LuxO to be phosphorylated (Lenz et al., 2004). Therefore, we reasoned that phosphate limited conditions might increase the expression of HapR by diminishing the amount of high energy phosphate required to activate LuxO. To test this hypothesis we constructed the HapR reporter strain SZS007 to monitor the production of active HapR protein in high and low phosphate media. To this end, we replaced the V. cholerae native lacZ promoter in the C7258 chromosome by the HapR-regulated V. harveyi luxC promoter. Expression of β-galactosidase activity by the wild type strain containing the luxC-lacZ transcriptional fusion followed the typical U-shaped cell density dependent pattern (Fig. 1a). No β-galactosidase activity could be detected in the isogenic hapR deletion mutant SZS009 after growth to the highest cell density in LB medium (Fig. 1a). We next used this reporter strain to examine the effect of phosphate limitation on HapR expression. The reporter strain was grown to OD600 1 in high phosphate EZ Rich defined medium, the cells centrifuged and reconstituted in one volume of the same medium containing 0.132 mM and no phosphate. As shown in Fig. 1b, higher β-galactosidase activities were detected after incubation under phosphate limiting conditions. To further document the effect of phosphate limitation on HapR expression we took advantage of strain AJB26 derivative of V. cholerae C6709ΔlacZ that contains a chromosomally integrated hapR-lacZ transcriptional fusion previously shown to recapitulate the cell density dependent regulation of HapR (Silva & Benitez, 2004). This strain provided an opportunity to test the effect of phosphate limitation on HapR expression in a different strain with a different indicator system. Different from SZS007, strain AJB26 reports the production of a stable hapR leader mRNA driving LacZ expression. An experiment similar to Fig. 1b with AJB26 resulted in 11.9 ± 1.4 Miller units after 2 h incubation in high phosphate medium versus 20.0 ± 2.9 Miller units after incubation in low phosphate medium. These observations provide compelling evidence that phosphate limitation has a positive effect on the expression of the master quorum sensing regulator HapR. Since HapR represses biofilm formation, we hypothesized that elevated expression of HapR in the phosphate limited condition could act to diminish biofilm formation. However, the amount of biofilm formed in high and low phosphate EZ-rich defined medium (as measured by the crystal violet assay) was very low precluding the detection of significant differences (Fig. 2).
Fig. 1. Effect of phosphate limitation on HapR expression.
A. Strain SZS007 (WT) and SZS009 (ΔhapR) were diluted in LB and grown to high cell density. Samples were taken for OD600 readings and β-galactosidase activity (Miller units). B. Strain SZS007 was grown to OD600 1 in EZ-rich defined medium supplemented with 1.32 mM phosphate. Cells were collected by centrifugation and suspended in one volume of the same medium containing 1.32 mM, 0.132 mM and no phosphate. Cells were incubated at 30°C and β-galactosidase activity was measured as an indicator of HapR expression. Each value indicates the mean of three independent cultures. The standard deviation is indicated by error bars.
Fig. 2. Biofim formation by strain SZS007 (WT) and isogenic ΔphoB, ΔhapR, ΔluxO and ΔluxOΔphoB mutants.

Strains were allowed to form static biofilms for 24 h at 30°C in microtiter plates and adherent cells were stained using the crystal violet method. Panel A: Biofilm formation in EZ-rich defined medium with 1.32 mM phosphate. Panel B: Biofilm formation in EZ-rich defined medium with 0.132 mm phosphate. Panel C: Complementation analysis. Strain SZS007ΔphoB was transformed with the cloned phoBR operon (pPhoBR) and biofilm formation was measured in EZ-rich defined medium with 0.132 mM phosphate. Panel D. Comparison of HapR expression in planktonic and biofilm associated cells of strain SZS007 (WT) and its ΔphoB derivative. Strains were allowed to form biofilm in borosilicate tubes at 30°C for 24 h. The planktonic cells were removed and biofilm cells re-dispersed by vortexing with 1 mm diameter glass beads. Expression of HapR in the planktonic and biofilm sub-populations was determined by the expression of β-galactosidase activity (Miller units). In all panels, each value indicates the mean of three independent cultures. Standard deviations are indicated by error bars.
The global regulator PhoB, expressed under conditions of phosphate limitation, is responsible for activating numerous genes collectively known as the PhoB regulon (Lamarche et al., 2008) and has been shown to modulate biofilm formation in other Gram-negative bacteria (Monds et al., 2001; Monds et al., 2007). Therefore, we decided to investigate the role of this global regulator in HapR expression and biofilm formation by introducing a phoB deletion in strain SZS007.
The PhoB global regulator negatively affects biofilm formation under phosphate limiting conditions
A phoB deletion mutation was introduced in strain SZS007 as described in materials and methods. The resulting strain SZS011 showed similar growth rate and motility in LB and high phosphate EZ-rich defined medium but, as expected, reduced growth rate in phosphate-limited medium. We compared biofilm formation in high and low phosphate media between the wild type strain SZS007 and isogenic ΔphoB, ΔhapR, ΔluxO and ΔphoBΔluxO mutants. As shown in Fig. 2, in all cases the ΔhapR mutant displayed an enhanced biofilm forming phenotype while ΔluxO mutants (that make constitutive HapR) made negligible biofilm. These results demonstrate that under the experimental conditions used in this study including the phosphate-limited medium, biofilm formation is tightly regulated by LuxO/HapR. As expected, deletion of phoB had no effect on biofilm formation in high phosphate condition (Fig. 2a). However, deletion of phoB significantly enhanced biofilm formation under phosphate limitation (p < 0.01, t test) but to a lesser extent than deletion of hapR (Fig. 2b). Consistent with HapR being a much stronger repressor of biofilm formation, deletion of luxO (leading to constitutive hapR expression) completely abrogated the positive effect of phoB (Fig. 2b). In order to confirm that deletion of phoB enhances the formation of V. cholerae biofilms, we conducted a complementation assay. A DNA fragment encoding the complete phoBR operon was cloned in pUC19 to yield pPhoBR and introduced by electroporation in strain SZS011 (ΔphoB). As shown in Fig. 2c, restoring phoB in trans, but not the empty vector (pUC19), diminished biofim formation to the wild type level. These results clearly demonstrate that under conditions of phosphate limitation activation of PhoB acts to negatively modulate the formation of V. cholerae biofilms.
Since we showed that phosphate limitation enhanced the expression of HapR, we considered the possibility of PhoB negatively affecting biofilm formation by increasing the expression of HapR. To test this possibility, we allowed the wild type and ΔphoB mutant to form static biofilms and determined the expression of HapR in the planktonic and adherent sub-populations. As shown in Fig. 2d, no differences were found between the wild type and mutant strain which leads to the conclusion that expression of PhoB does not negatively affect biofilm formation by enhancing the expression of HapR. Given that HapR and PhoB appear to negatively affect biofilm formation in different ways, we used laser confocal microscopy to examine the three-dimensional architecture of wild type, ΔphoB and ΔhapR biofilms under phosphate limitation. As shown in Fig. 3, under these conditions the wild type strain adhered poorly while the ΔphoB mutant formed a more uniform monolayer. As expected the ΔhapR mutant displayed an enhanced biofilm forming phenotype. These findings suggest that PhoB might negatively affect biofilm formation by interfering with early events that mediate adherence and monolayer formation. It has been suggested that surface attachment can trigger the expression of additional genes and regulators required for exopolysaccharide matrix biosynthesis and development of a mature biofilm (Watnick & Kolter, 1999). Thus, we decided to examine the effect of PhoB on the expression of known regulators of exopolysaccharide biosynthesis and biofilm formation.
Fig. 3. Comparison of wild type, ΔphoB and ΔhapR biofilms by confocal microscopy.

Strain SZS007 (WT) and isogenic ΔphoB and ΔhapR mutants were allowed to form static biofilm in EZ-rich defined medium containing 0.132 mM phosphate in 35 mm glass-bottom dishes for 24 h at 30°C. The planktonic cells were removed and adherent Vibrios stained with Syto-9 for confocal microscopy.
PhoB negatively affects the expression of VpsR, a positive regulator of biofilm formation
Given the complex regulatory circuitry controlling biofilm formation and exopolysaccharide biosynthesis, we used qRT-PCR to compare the effects of PhoB and HapR on regulators of exopolysaccharide gene expression and biofilm formation. In agreement with our previous results (Liang et al., 2007a), deletion of hapR enhanced the expression of vpsA, vpsL and the positive regulator vpsT but had little effect on the expression of vpsR and cytR (Fig. 4). In concurrence with results presented in Fig. 2d, deletion of phoB did not affect the expression of hapR. Deletion of phoB also enhanced the expression of vpsA and vpsL (Fig. 4). However, contrary to the deletion of hapR, elimination of phoB had little effect on the expression of vpsT and cytR but significantly enhanced vpsR (Fig. 4). These results further support the conclusion that HapR and PhoB independently diminish biofilm formation through distinct pathways that converge to diminish expression of vpsA and vpsL.
Fig. 4. Comparison of wild type, ΔphoB, and ΔhapR strains for the expression of known regulators of biofilm formation using qRT-PCR.
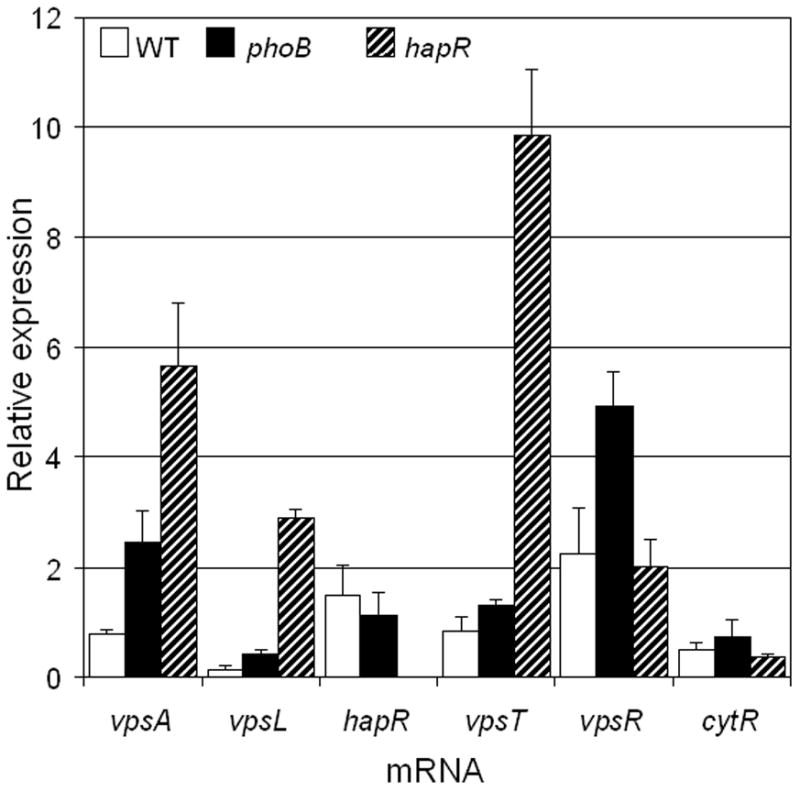
Strain SZS007 (WT) and isogenic mutants were grown in LB medium for 16 h, diluted 1:100 in EZ-rich defined medium with 0.132 mM phosphate and grown to OD600 0.3. Total RNA was isolated from each culture and RNA samples were analyzed by qRT-PCR. Each value indicates the mean of three independent cultures. Standard deviation are indicated by error bars.
PhoB modulates stress response
In addition to the formation of biofilm communities that provide protection to many environmental stressors at a population or social level, the general stress response contributes to environmental stress survival by armoring individual cells with the biochemical activities required to provide protection against many environmental stressors. It has been proposed that phosphate starvation and PhoB activate the general stress response (Ruiz & Silhavy, 2003). To determine the relationship between PhoB and the general stress response in V. cholerae we compared the ability of isogenic wild type, ΔphoB and ΔrpoS mutants to withstand environmental stresses. In Fig. 5 we show that, while strain SZS007 (wild type) and its isogenic ΔrpoS were similarly sensitive to 0.5 mM hydrogen peroxide, the ΔphoB was significantly more sensitive to this level of oxidative stress. The ΔrpoS mutant was more sensitive than wild type to higher hydrogen peroxide concentrations (data not shown). This result indicates that expression of PhoB provides protection to oxidative stress by a mechanism different from RpoS. As expected from previous studies (Jahid et al., 2006; Yildiz & Schoolnik, 1998), the ΔrpoS mutant was more sensitive than wild type to 1.2 M NaCl while the ΔphoB mutant was more resistant (Fig. 5). The ΔphoB mutant was also more resistant than the wild type strain and the ΔrpoS mutant to pH 4.5. Finally, the ΔrpoS mutant was more sensitive to carbon starvation compared to wild type and ΔphoB that behaved similarly with regard to this stress. Taken together, the above data is consistent with PhoB modulating environmental stress in a manner independent of the general stress response regulator RpoS.
Fig. 5. Comparison of wild type, ΔphoB, and ΔrpoS mutants for their ability to survive environmental stressors.

Strain SZS007 (WT) and isogenic ΔphoB and ΔrpoS mutants were grown in LB medium and the cultures were diluted in fresh EZ-rich defined medium with 0.132 mM phosphate and different stress conditions. Cultures were incubated at 37°C with shaking and samples were taken at different time points to determine viability.
DISCUSSION
The finding that V. cholerae builds large poly-P stores and our previous observation that a mutant impaired in poly-P biosynthesis was more sensitive to environmental stressors in low phosphate medium (Jahid et al., 2006; Ogawa et al., 2000) points to phosphate starvation as a critical environmental stress that could impact the survival of V. cholerae in its aquatic habitat. Unfortunately, very little is known about how phosphate starvation affects V. cholerae behavior and life style. Extracellular orthophosphate is the major source of high energy phosphate for biosynthesis and many signal transduction pathways such as quorum sensing. Therefore, it was not surprising that phosphate limitation enhanced HapR expression which is repressed when high energy phosphate is transferred to LuxO. Phosphate starvation is known to activate PhoB and induce the transcription of the PhoB regulon (Lamarche et al., 2008). The fact that HapR represses biofilm formation (Waters et al., 2008) and PhoB has been shown to negatively affect biofilm formation in other Gram-negative bacteria (Monds et al., 2001; Monds et al., 2007), prompted us to examine the relationship between HapR, PhoB and biofilm formation. To this end, we constructed a ΔphoB mutant of strain SZS007. We did not observe any phenotype for this mutant in LB or high phosphate medium (i.e. growth rate, motility, extracellular protease production). However, the mutant did exhibit reduced growth and alkaline phosphatase expression in low phosphate medium containing the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate (data not shown). Although we could not demonstrate a negative effect of phosphate starvation on biofilm formation in the wild type strain, deletion of phoB significantly enhanced the development of biofilms as measured by the crystal violet assay and confocal microscopy. Interestingly, in a ΔhapR genetic background phosphate limitation (a condition expected to induce PhoB) appeared to enhance rather than diminish biofilm formation. This result suggests the possibility of an unknown interaction between the quorum sensing and PhoB regulatory pathways. Analysis of HapR expression in the ΔphoB mutant indicated that PhoB does not negatively affect biofilm formation by enhancing HapR. Confocal microscopy suggested that deletion of phoB enhanced adherence and monolayer formation as reported in P. aeruginosa where PhoB acts by lowering c-di-GMP which in turn inhibits the secretion of the LapA adhesin (Monds et al., 2001; Monds et al., 2007). Surface attachment has been suggested to trigger the expression of additional genes involved in exopolysaccharide matrix biosynthesis in V. cholerae (Watnick & Kolter, 1999). Comparison of the expression of known regulators of biofilm formation in wild type, ΔphoB and ΔhapR mutants showed that HapR and PhoB negatively affect biofilm formation through distinct pathways with HapR repressing VpsT (Waters et al., 2008) and PhoB diminishing the expression of VpsR. We have previously shown that VpsR is modulated by the cAMP-cAMP receptor protein (CRP) complex (Liang et al., 2007a). Therefore, we propose that VpsR plays a critical role in biofilm formation by acting as a receiver of external carbon and phosphorus sensory information to modulate exopolysaccharide matrix biosynthesis. The regulation of vpsR resembles the E. coli ugp and psiE genes whose promoters are subject to dual regulation by CRP and PhoB (Kasahara et al., 1991; Kim et al. 2000). Analysis of the DNA region upstream the vpsR start codon using the Virtual footprint software (http://www.prodoric.de/vfp/index2.php) revealed a putative CRP binding site with a score (6.27) close to the average of a position weight matrix composed of 27 CRP binding sites. Interestingly, an overlapping string of bases resembling a pho box is located 13 nucleotides upstream of the putative CRP binding site. In this potential pho box, 8 bases out of the 12 most conserved positions were identical to the consensus sequence resulting in a positive hit score as reported by Yuan et al. (2006). These findings suggest the possibility of an antagonistic interaction between CRP and PhoB at the vpsR promoter. A recent study showed that deletion of phoB also enhanced biofilm formation in a V. cholerae strain of the classical biotype that does not express HapR-dependent quorum and modulated the expression of genes involved in c-di-GMP metabolism (Pratt et al., 2009). Therefore, PhoB-dependent modulation of V. cholerae behavior could represent a general regulatory pattern affecting the persistence of V. cholerae of both biotypes in the environment.
In E. coli, activation of the Pho regulon has been reported to upregulate translation of rpoS (Ruiz & Silhavy, 2003). Our data on the stress response behavior of a V. choleraeΔphoB mutant suggests that PhoB modulates stress response but differs from the pattern reported for rpoS and ppk mutants (Jahid et al., 2006; Yildiz & Schoolnik, 1998). For instance, global gene expression profiling of an rpoS mutant revealed that RpoS positively affects the expression of the catalase peroxidase PerA (VC1560) and cytochrome c551 peroxidase VC0089 (Silva et al., 2008). Contrastingly, deletion of phoB in V. cholerae was found to affect the expression the alkylhydroperoxidase VC0731 (von Kruger et al., 2006) reported to protect against oxidative stress under conditions of phosphate starvation (Moreau et al., 2001). These results are in agreement with our data suggesting that RpoS and PhoB activate different stress response mechanisms in V. cholerae.
Taken together, our results suggests that under conditions of phosphate limitation elevated expression of HapR and expression of PhoB act to diminish biofilm formation by diminishing VpsT and VpsR, respectively. In parallel, induction of PhoB under conditions of phosphate limitation modulates stress response in an RpoS-independent manner to provide planktonic cells with resistance mechanisms that could be specifically tailored to the phosphate-deprived environment. The finding that phosphate limitation and expression of PhoB appears to induce V. cholerae to switch to a planktonic life style poses an intriguing question. The planktonic life style could provide fitness when survival depends on inter-species competition for limiting amounts of soluble phosphate. A model for the integration of cell density and nutritional signals in the regulation of biofilm formation is shown in Fig. 6. According to this model, high cell density, carbon starvation and phosphate limitation promote a planktonic life style by enhancing the expression of the negative factor HapR and PhoB (in the case of phosphate limitation). Interestingly, the opposing effects of CRP and PhoB on VpsR expression suggest that VpsR might function to finely adjust the transition between life styles in response to the carbon-phosphate ratio in the environment. Clearly, more research is required to clarify how the complex interplay between cell density and nutritional signals in the aquatic environment coordinately affect biofilm formation, stress response and the persistence of V. cholerae.
Fig. 6.

Model for the integration of high cell density, carbon and phosphorus sensory information in the regulation of biofim formation.
Supplementary Material
Acknowledgments
The present study was supported by grant GM008248 from the National Institute of General Medical Sciences to A.J.S and PHS grant AI63187 from the National Institute of Allergy and Infectious Disease to J.A.B.
References
- Benitez-Nelson CR. The biogeochemical cycling of phosphorus in marine systems. Earth-Science Reviews. 2000;51:109–135. [Google Scholar]
- Beyhan S, Bilecen K, Salama SR, Casper-Lindley L, Yildiz FH. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of the VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR and hapR. J Bacteriol. 2007;189:388–402. doi: 10.1128/JB.00981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S, Tischler AD, Camilli A, Yildiz FH. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J Bacteriol. 2006;188:3600–3613. doi: 10.1128/JB.188.10.3600-3613.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J, Ullrich A, Raker MA, Gray A, Dull TJ, Gutell RR, Noller HF. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon in Escherichia coli. Plasmids. 1981;6:112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Casper-Lindley C, Yildiz FH. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J Bacteriol. 2004;186:1574–1578. doi: 10.1128/JB.186.5.1574-1578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll DL. Phosphorus: a rate limiting nutrient in surface waters. Poultry Sci. 1999;78:674–682. doi: 10.1093/ps/78.5.674. [DOI] [PubMed] [Google Scholar]
- De Lorenzo V, Eltis L, Kessler B, Timmis KN. Analysis of the Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–7. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira GM, Spira B. The pst operon of enteropathogenic Escherichia coli enhances bacterial adherence to epithelial cells. Microbiology. 2008;154:2025–2036. doi: 10.1099/mic.0.2008/016634-0. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50:101–114. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Haugo AJ, Watnick PI. Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol. 2002;45:471–483. doi: 10.1046/j.1365-2958.2002.03023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the Sigma S (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahid IK, Silva AJ, Benitez JA. Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low phosphate environment. Appl Environ Microbiol. 2006;72:7043–7049. doi: 10.1128/AEM.00924-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- Joelsson A, Kan B, Zhu J. Quorum Sensing Enhances Stress Response in Vibrio cholerae. Appl Environ Microbiol. 2007;73:3742–3746. doi: 10.1128/AEM.02804-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Morris G, Jr, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Makino K, Amemura M, Nakata A, Shinagawa H. Dual regulation of the ugp operon by phosphate and carbon starvation at two interspaced promoters. J Bacteriol. 1991;173:549–558. doi: 10.1128/jb.173.2.549-558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-K, Kimura S, Shinagawa A, Nakata A, Sung Lee K, Wanner BL, Makino K. Dual transcriptional regulation of the Escherichia coli phosphate-starvation-inducible psiE gene of the phosphate regulon by PhoB and the cyclic AMP receptor protein complex. J Bacteriol. 2000;182:5596–5599. doi: 10.1128/jb.182.19.5596-5599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche MG, Wanner BL, Crepin S, Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control QS in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Liang W, Silva AJ, Benitez JA. The cAMP Receptor Protein Modulates Colonial Morphology Phase in Vibrio cholerae. Appl Environ Microbiol. 2007a;73:7482–7487. doi: 10.1128/AEM.01564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Pascual-Montano A, Silva AJ, Benitez JA. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology. 2007;153:2964–2975. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- Lim B, Beyhan S, Yildiz FH. Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J Bacteriol. 2007;189:717–729. doi: 10.1128/JB.00834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006;60:331–348. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Matz C, McDougald D, Moreno AM, Yung PY, Yildiz FH, Kjelleberg S. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc Natl Acad Sci USA. 2005;102:16819–16824. doi: 10.1073/pnas.0505350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1971. [Google Scholar]
- Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- Monds RD, Newell PD, Gross RH, O’Toole GA. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf01biofilm formation by controlling secretion of the adhesin LapA. Mol Microbiol. 2007;63:656–679. doi: 10.1111/j.1365-2958.2006.05539.x. [DOI] [PubMed] [Google Scholar]
- Monds RD, Silby MW, Mahanty HK. Expression of the Pho regulon negatively regulates biofilm formation by Pseudomonas aeruginosa. Mol Microbiol. 2001;42:415–426. doi: 10.1046/j.1365-2958.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- Moreau PL, Gerard F, Lutz NW, Cozzone P. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol Microbiol. 2001;39:1048–1060. doi: 10.1046/j.1365-2958.2001.02303.x. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Tzseng C-M, Fraley CDE, Kornberg A. Inorganic polyphosphate in Vibrio cholerae: genetics, biochemical, and physiological features. J Bacteriol. 2000;182:6687–6693. doi: 10.1128/jb.182.23.6687-6693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JT, McDonough E, Camilli A. PhoB regulates motility, biofilm and c-diGMP in Vibrio cholerae. J Bacteriol. 2009 doi: 10.1128/JB.00708–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Silhavy T. Constitutive activation of the Escherichia coli Pho regulon upregulates rpoS translation in an Hfq-dependent fashion. J Bacteriol. 2003;185:5984–5992. doi: 10.1128/JB.185.20.5984-5992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Benitez JA. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J Bacteriol. 2004;186:6374–6382. doi: 10.1128/JB.186.19.6374-6382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Sultan SZ, Liang W, Benitez JA. Role of the histone-like nucleoid structuring protein (H-NS) in the regulation of RpoS and RpoS-dependent Genes in Vibrio cholerae. J Bacteriol. 2008;190:7335–7345. doi: 10.1128/JB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kruger WM, Humphreys S, Ketley JM. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate limitation response and intestinal colonization. Microbiology. 1999;145:2463–2475. doi: 10.1099/00221287-145-9-2463. [DOI] [PubMed] [Google Scholar]
- Von Kruger WM, Lery LM, Soares MR, de Neves-Manta FS, Batista e Silva CM, Neves-Ferreira AG, Perales J, Bisch PM. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics. 2006;6:1495–1511. doi: 10.1002/pmic.200500238. [DOI] [PubMed] [Google Scholar]
- Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Schoolnik GK. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;180:773–784. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Dolganov NA, Schoolnik GK. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS (ETr)-associated phenotypes in Vibrio cholerae O1 El Tor. J Bacteriol. 2001;183:1716–1726. doi: 10.1128/JB.183.5.1716-1726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol Microbiol. 2004;53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- Yuan Z-C, Zaheer R, Morton R, Finan TM. Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Research. 2006;34:2686–2697. doi: 10.1093/nar/gkl365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Developmental Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Miller BM, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



