Abstract
Purpose
Tumor growth requires the development of independent vascular networks that are often primitive in morphology and function. We examined whether microvessel morphology contributes to the considerable biologic heterogeneity of prostate cancer.
Methods
We evaluated microvessel morphology as a predictor of prostate cancer mortality among 572 men in the Health Professionals Follow-Up Study diagnosed with cancer during 1986 to 2000. We immunostained prostatectomy tumor block sections for endothelial marker CD34 and assessed microvessel density, vessel size (area and diameter), and irregularity of vessel lumen using image analysis. Proportional hazards models were used to assess microvessel density and morphology in relation to lethal prostate cancer.
Results
Poorly differentiated tumors exhibited greater microvessel density, greater irregularity of the vessel lumen, and smaller vessels. During 20 years of follow-up, 44 men developed bone metastases or died of cancer. Men with tumors exhibiting the smallest vessel diameter, based on quartiles, were 6.0 times more likely (95% CI, 1.8 to 20.0) to develop lethal prostate cancer. Men with the most irregularly shaped vessels were 17.1 times more likely (95% CI, 2.3 to 128) to develop lethal disease. Adjusting for Gleason grade and prostate-specific antigen levels did not qualitatively change the results. Microvessel density was not linked to cancer-specific mortality after adjusting for clinical factors.
Conclusion
Aggressive tumors form vessels that are primitive in morphology and function, with consequences for metastases. Vascular size and irregularity reflect the angiogenic potential of prostate cancer and may serve as biomarkers to predict prostate cancer mortality several years after diagnosis.
INTRODUCTION
Prostate cancer is biologically heterogeneous. Many localized cancers are slow-growing, even in the absence of therapy,1,2 whereas some men with apparently organ-confined disease develop metastases despite local therapy.3,4 An understanding of the key molecular events associated with prostate cancer progression remains to be elucidated.
Angiogenesis is one potential pathway that contributes to prostate cancer heterogeneity. The establishment of a vascular supply plays a pivotal role in the development of cancer by providing for the distribution of growth factors, nutrients, and oxygen critical for growth of solid tumors.5 In addition, angiogenesis can facilitate metastases by supplying tumor cells direct access to vasculature. Although angiogenesis begins with integration of existing host vasculature, continued tumor growth requires new vessel networks that are often primitive in organization, function, and morphology.6
The extent of neoangiogenesis, as estimated by microvessel density, is related to the tumor's aggressive potential7,8 and is inversely related to cancer survival for several malignancies.9 In prostate cancer, increased tumor microvessel density predicts poorly differentiated tumors10 and biochemical failure after treatment.11,12 Prospective studies of angiogenesis and prostate cancer death, a more relevant clinical outcome, are limited. Moreover, no study to date has comprehensively assessed morphologic aspects of angiogenesis, such as vessel size and shape, in relation to prostate cancer outcomes. Quantitative assessment of angiogenesis could have potential for the classification of prostate cancer into biologic subtypes.
We investigated microvessel density and morphologic measurements of angiogenesis in relation to tumor characteristics and lethal prostate cancer among 572 men treated with prostatectomy in the Health Professionals Follow-Up Study (HPFS). The research was approved by the institutional review boards at the Harvard School of Public Health and Partners Healthcare.
METHODS
Study Population
The HPFS prostatectomy cohort is a community sample of men with clinically localized prostate cancer (T1/T2) diagnosed from 1986 to 2000 and who elected prostatectomy as curative therapy. The men were participants in the ongoing prospective HPFS study of 51,529 male dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians in the United States. They completed biennial questionnaires to collect lifestyle and medical information. Incident prostate cancer was initially identified through self-report or, rarely, when prostate cancer was mentioned on death certificates and was confirmed by review of medical records, pathology reports and death certificates.
Hospital pathology departments where cohort members underwent surgery were contacted to retrieve archival formalin-fixed paraffin-embedded prostatectomy specimens. Of 1,593 men who underwent prostatectomy, we retrieved blocks for 64% (n = 1,023). Most hospitals sent blocks for the entire case, although some selected blocks containing tumor. The current analysis is based among 572 men for whom the first batch of morphologic assessment of angiogenesis was completed. The clinical characteristics for these men are similar to the entire HPFS prostatectomy cohort.
Clinical and Follow-Up Data
Information on clinical and pathologic stage, age, and prostate-specific antigen (PSA) at diagnosis was abstracted from medical records. Study pathologists reviewed hematoxylin and eosin slides to provide uniform Gleason grading.13 Information on race/ethnicity was collected on the baseline HPFS questionnaire in 1986, and body mass index was calculated using height and weight reported on questionnaires immediately preceding the cancer diagnosis.
Men were observed prospectively for clinical course through March 2008. Death certificates and relevant medical records were reviewed to confirm the cause; follow-up is more than 98% complete. Beginning in 2000, we also collected information on development of distant metastases abstracted from medical records.
Biomarkers of Angiogenesis
Morphologic assessment of angiogenesis was determined on serial sections of the prostatectomy blocks. The study pathologist identified all blocks containing cancer and evaluated between one and nine blocks with cancer per case. Protein expression for endothelial cell marker CD34 was ascertained by immunohistochemistry on 5-μm sections using primary mouse monoclonal antibody anti-CD34 (QBEND10; Biogenex, San Ramon, CA) at 1:200 dilution for 30 minutes after incubation with peroxidase-blocking reagent (Dual Endogenous Enzyme Block, Dako, Carpinteria, CA). Detection was accomplished by a peroxidase-labeled polymer conjugated to antimouse immunoglobulin G, liquid 3,3′-diaminobenzidine+Substrate Chromogen System (K3468; Dako), and EnVision+Dual Link System (Dako). Immunohistochemistry was performed in an OptiMax Automated Cell-Staining System (BioGenex). Slides were counterstained with hematoxylin (Sigma-Aldrich, St Louis, MO).
Semiautomated image analysis was accomplished under supervision of the pathologist using Image ProPlus 4.5 software (Media Cybernetics, Bethesda, MD). Slide images under high-powered fields (×200) were captured using a Spot RT Slider Camera (Diagnostic Instruments, Sterling Heights, MI) mounted on a Nikon Eclipse E800 microscope (Nikon Instruments, Melville, NY). The pathologist guided the imaging and directed the system to disregard vessels that were transected multiple times. Images were obtained from each focus of cancer. For blocks with large cancer volume, multiple nonoverlapping images from the focus were obtained. Microvessel density was calculated as the number of vascular structures in a high-powered field. Vessel size was determined as the average vessel diameter (in micrometers) and area comprised by a vessel (in square micrometers). Irregularity of the vessel lumen was calculated as perimeter2/4 · Π × area, with a value of 1.0 indicating a perfect circle and values greater than 1.0 indicating increasing irregularity. Vessels that were incompletely captured on the section were included in the calculation of microvessel density, but not size or shape. Greater microvessel density, smaller vessel size, and more irregularly shaped vessels are hypothesized to be characteristic of cancers with greater angiogenic potential14,15 (Table 1). The laboratory remained blinded to lethal outcome status during the immunohistochemistry and morphologic evaluation.
Table 1.
Histopathologic Measures of Microvessel Density and Morphology: HPFS Prostatectomy Cohort, 1986 to 2000
| Measure | Microvessel Density* | Vessel Area | Diameter of Blood Vessels | Irregularity of Vessel Lumen† |
|---|---|---|---|---|
| What is more angiogenic? | Greater No. | Smaller size | Smaller diameter | Irregular shape |
| Mean | 76 vessels | 542 μm2 | 25.2 μm | 4.0 |
| Range | 13-491 | 104-2795 | 12.9-55.6 | 1.6-7.9 |
| Correlations‡ | ||||
| Microvessel density | 1 | |||
| Vessel area | −0.15 | 1 | ||
| P | .0003 | |||
| Diameter | −0.10 | +0.91 | 1 | |
| P | .02 | < .0001 | ||
| Irregularity | −0.07 | −0.31 | −0.26 | 1 |
| P | .08 | < .0001 | < .0001 |
Abbreviation: HPFS, Health Professionals Follow-Up Study.
Microvessel density is the number of vessels per high-powered field.
A score of 1.0 indicates a perfect circle.
Age-adjusted Pearson correlation coefficients.
Statistical Analysis
For each individual, there were up to nine measurements, and minimum, mean, and maximum values were calculated across each angiogenesis measure. Because the findings were qualitatively similar, we present only the results using the mean values.
We compared angiogenesis measures by age, PSA, and Gleason grade. We used unconditional logistic regression models to calculate odds ratios and 95% CIs to evaluate the associations of the vascular parameters (quartiles) to predict extraprostatic disease (T3/T4/N1) at prostatectomy. We used Cox proportional hazards models to evaluate the angiogenesis biomarkers and time to development of lethal prostate cancer. Person-time was calculated from date of diagnosis to development of metastases, cancer death, or censored at time of death from other causes or end of follow-up (January 1, 2007). Hazard ratios (95% CI) were used as effect measures. We adjusted for age at diagnosis (years, continuous), Gleason grade (≤ 6, 7, 8 to 10), and PSA at diagnosis (< 4.1, 4.1 to 9.9, ≥ 10 ng/mL) and further adjusted for pathologic stage. We calculated C statistics to assess the ability of the angiogenesis measures to predict lethal prostate cancer, comparing the clinical variables (age, Gleason, PSA at diagnosis, stage) to that including clinical variables and the angiogenesis measures (quartiles). Analyses were undertaken using SAS Statistical Analysis (version 9.1; SAS Institute, Cary, NC).
RESULTS
The HPFS prostatectomy cohort included 572 men with incident prostate cancer diagnosed from 1986 to 2000, with the majority diagnosed in the era since introduction of PSA screening (Table 2). The median age at diagnosis was 66.3 years, and 95% of men were white.
Table 2.
Clinical and Demographic Characteristics of the HPFS Prostatectomy Cohort (N = 572), 1986 to 2000*
| Characteristic | No. | % |
|---|---|---|
| Total, N | 572 | |
| Age at diagnosis, years | ||
| Median | 66.3 | |
| Range | 47.0-79.8 | |
| Race | ||
| White | 519 | 95.6 |
| African American | 9 | 1.7 |
| Asian | 9 | 1.7 |
| Other | 6 | 1.1 |
| Pathologic tumor stage | ||
| T2 | 398 | 70.2 |
| T3a† | 98 | 17.3 |
| T3b‡ | 41 | 7.2 |
| T4/N1 | 30 | 5.3 |
| Gleason grade | ||
| 2-5 | 27 | 4.7 |
| 6 | 128 | 22.4 |
| 7 | 333 | 58.2 |
| 3 + 4 | 199 | 34.7 |
| 4 + 3 | 134 | 23.5 |
| 8-10 | 84 | 14.7 |
| Year of diagnosis | ||
| 1986-1989, pre-PSA era | 42 | 7.3 |
| 1990-1993, peri-PSA era | 174 | 30.4 |
| 1994-2000, PSA era | 356 | 62.2 |
| PSA at diagnosis, ng/mL | ||
| Median | 7.0 | |
| Range | 0.4-77 | |
| Follow-up time, years | ||
| Median | 9.9 | |
| Range | 0.8-19.9 | |
| Body mass index | ||
| Median | 25.1 | |
| Range | 17.8-38.3 | |
Abbreviations: HPFS, Health Professionals Follow-Up Study; PSA, prostate-specific antigen.
Numbers do not add up to 572 if data are missing.
T3a indicates penetration through capsule wall.
T3b indicates involvement of the seminal vesicles.
We calculated age-adjusted Pearson coefficients for within-tumor correlations between the parameters defining vascular density and structure (Table 1). Men whose tumors had a greater microvessel density also tended to have smaller vessel diameter. There was a weak correlation between microvessel density and irregularity of vessel lumen. In contrast, vessel area and diameter were strongly correlated. There was a strong inverse correlation between vessel size and shape, such that tumors with smaller vessel diameter and smaller vessel area exhibited greater irregularity of the vessel lumen, although this correlation would be expected based on the method for calculating vessel irregularity.
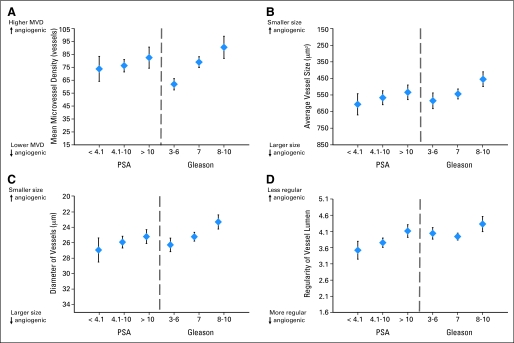
PSA levels at diagnosis were unrelated to microvessel density, but were significantly related to vessel diameter and irregularity (Fig 1). Compared with men with low PSA (< 4.0 ng/mL), those with PSA greater than 10.0 had microvessels that were on average smaller diameter (P = .07) and more irregularly shaped (P = .001). PSA was less correlated with microvessel area (P = .18). Gleason grade was strongly related to microvessel density, with Gleason 7 (P < .0001) and Gleason 8 to 10 (P < .0001) tumors exhibiting increased microvessel density compared with low-grade tumors. Compared with low-grade tumors, Gleason 8 to 10 tumors had vessels that were smaller in diameter (P < .0001) and area (P = .0007) and displayed greater irregularity in the vessel lumen (P = .052). Among Gleason 7 tumors, those with a dominant 4 (4 + 3) tended to also exhibit greater microvessel density and have vessels that comprised smaller area compared with dominant pattern 3 (3 + 4) tumors, although the differences were not statistically significant. When we focused specifically on the pattern in the high-powered field, the associations were considerably stronger. Compared with fields of Gleason pattern 3, those with pattern 4 had vessels that were smaller diameter (20.3 v 23.7 μm; P < .001), smaller area (323 v 488 μm2), were more irregularly shaped (4.5 v 4.0; P < .001), and greater density (82 v 61 vessels; P < .001).
Fig 1.
Microvessel density (MVD) and architecture in prostate tumors according to prostate-specific antigen (PSA) at diagnosis and Gleason grade, Health Professionals Follow-Up Study prostatectomy cohort, 1986 to 2000. Mean and 95% CIs comparing MVD, vessel area and diameter, and irregularity, stratified by PSA levels at diagnosis (< 4.1, 4.1 to 10, ≥ 10 ng/mL) and pathologic Gleason grade (2 to 6, 7, 8 to 10), are shown.
On prostatectomy, 29.8% of men had extraprostatic disease (Table 2). Seven percent had tumors involving the seminal vesicles, whereas 5% involved the bladder wall or extended to the regional lymph nodes. In Table 3, we examined the angiogenesis biomarkers to predict presence of extraprostatic disease. Among men with tumors with the greatest microvessel density based on quartiles, 39% had extraprostatic disease, compared with 21% of those with the smallest microvessel density. The odds of having extraprostatic disease was 2.5 (95% CI, 1.5 to 4.2) times higher comparing extreme quartiles based on microvessel density. Adjusting for Gleason and PSA somewhat attenuated the relation, although microvessel density remained a significant predictor. Vessel size was also associated with extraprostatic extension. Both smaller vessel area (odds ratio comparing extreme quartiles, 1.9; 95% CI, 1.1 to 3.2) and smaller diameter (odds ratio, 2.0; 95% CI, 1.2 to 3.5) were associated with a greater likelihood of extension outside the prostate. The relation between irregularity of the vessel lumen and extraprostatic extension was more modest and was not significant after adjusting for clinical variables. Associations between the angiogenesis measures and extraprostatic disease were similar when we excluded T3a tumors.
Table 3.
ORs and 95% CIs of Angiogenesis Markers to Predict Extraprostatic Disease: HPFS Prostatectomy Cohort, 1986 to 2000
| Biomarker and Quartile | What Is More Angiogenic? | Median of Quartile | Extraprostatic |
OR* | 95% CI | OR† | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Microvessel density, vessels | ||||||||
| Q4 greater | Greater No. | 115.8 | 56 | 39.4 | 2.5 | 1.5 to 4.2 | 1.7 | 1.0 to 3.0 |
| Q3 | 75.3 | 49 | 34.3 | 2.0 | 1.2 to 3.4 | 1.6 | 0.9 to 2.9 | |
| Q2 | 58.8 | 34 | 24.1 | 1.2 | 0.7 to 2.1 | 0.9 | 0.5 to 1.7 | |
| Q1-fewer | 41.4 | 30 | 21.3 | REF | REF | |||
| P, test for trend | .0002 | .021 | ||||||
| Vessel area, μm2 | ||||||||
| Q1 smaller area | Smaller area | 284 | 63 | 44.1 | 2.3 | 1.4 to 3.9 | 1.9 | 1.1 to 3.2 |
| Q2 | 416 | 39 | 27.3 | 1.1 | 0.7 to 1.9 | 1.0 | 0.6 to 1.7 | |
| Q3 | 552 | 32 | 22.9 | 0.9 | 0.5 to 1.6 | 0.8 | 0.5 to 1.4 | |
| Q4-larger area | 832 | 35 | 24.8 | REF | REF | |||
| P, test for trend | .0025 | .038 | ||||||
| Vessel diameter, μm | ||||||||
| Q1 smaller diameter | Smaller diameter | 19.7 | 63 | 44.1 | 2.5 | 1.5 to 4.2 | 2.0 | 1.2 to 3.5 |
| Q2 | 23.0 | 34 | 23.9 | 1.0 | 0.6 to 1.8 | 0.9 | 0.5 to 1.5 | |
| Q3 | 26.1 | 39 | 27.7 | 1.2 | 0.7 to 2.1 | 1.0 | 0.6 to 1.8 | |
| Q4 larger diameter | 31.4 | 33 | 23.4 | REF | REF | |||
| P, test for trend | .0017 | .027 | ||||||
| Regularity of vessels, units | ||||||||
| Q4 less regular | Irregular shape | 5.4 | 51 | 35.7 | 2.0 | 1.2 to 3.4 | 1.7 | 1.0 to 3.1 |
| Q3 | 4.4 | 40 | 28.2 | 1.4 | 0.8 to 2.4 | 1.3 | 0.7 to 2.3 | |
| Q2 | 3.6 | 48 | 34.0 | 1.9 | 1.1 to 3.3 | 1.8 | 1.0 to 3.1 | |
| Q1 more regular | 2.7 | 30 | 21.3 | REF | REF | |||
| P, test for trend | .036 | .14 | ||||||
Abbreviations: OR, odds ratio; HPFS, Health Professionals Follow-Up Study; REF, reference.
Odds ratios adjusted for age at diagnosis.
Odds ratios adjusted for age at diagnosis (continuous), Gleason grade (categorically, 2 to 6, 7, 8 to 10), and prostate-specific antigen at diagnosis (categorically, < 4, 4.1 to 10, > 10, not measured).
During up to 20 years of follow-up (median, 9.9 years), 35 men died of prostate cancer, nine men were alive with bone metastases, and 94 men died of other causes (and never developed metastases). In contrast to its relation with extraprostatic extension, microvessel density was not a significant predictor of lethal disease (Table 4). Vessel size and shape, however, provided good discrimination of lethal prostate cancer. Comparing extreme quartiles, 23 of 143 cases were lethal among men with the smallest vessel area, compared with three among 143 cases with the largest vessel area. Adjusting for age, the hazard ratio for lethal disease in relation to vessel area was 6.6 (95% CI, 2.0 to 21.9) comparing the smallest and largest area. Smaller vessel area and smaller diameter remained independent predictors after adjusting for Gleason grade, PSA, and age at diagnosis. The strongest association was noted for irregularity of the vessel lumen. Among men with the greatest lumen irregularity, 22 died of prostate cancer or developed metastases, compared with only one man in the quartile with the most regularly shaped lumens. The age-adjusted hazard ratio comparing extreme quartiles was 17.1 (95% CI, 2.3 to 128). Additionally adjusting for clinical characteristics, tumors with greater vessel irregularity experienced a 10-fold greater risk of developing lethal disease compared with those with the most regularly shaped vessels. In secondary analyses, adjusting for pathologic stage, vessel irregularity remained a strong predictor of outcome, although the effect was somewhat attenuated (P for trend approximately .013). Vessel area and diameter were no longer statistically significant.
Table 4.
HRs and 95% CIs of Angiogenesis Biomarkers to Predict Time to Lethal Prostate Cancer: HPFS Prostatectomy Cohort, 1986 to 2007
| Biomarker and Quartile | What Is More Angiogenic? | No. of Deaths/Metastases | HR* | 95% CI | HR† | 95% CI | HR‡ | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Microvessel density | ||||||||
| Q4 greater | Greater No. | 10 | 1.7 | 0.7 to 4.4 | 0.9 | 0.3 to 2.3 | 1.0 | 0.4 to 2.6 |
| Q3 | 16 | 2.4 | 1.0 to 5.6 | 1.5 | 0.6 to 3.5 | 1.5 | 0.6 to 3.7 | |
| Q2 | 10 | 1.4 | 0.5 to 3.5 | 0.8 | 0.3 to 2.1 | 0.9 | 0.3 to 2.3 | |
| Q1-fewer | 8 | REF | REF | REF | ||||
| P, test for trend | .25 | .94 | .92 | |||||
| Vessel area | ||||||||
| Q1 smaller area | Smaller area | 23 | 6.6 | 2.0 to 21.9 | 4.3 | 1.3 to 14.7 | 4.0 | 1.2 to 13.3 |
| Q2 | 13 | 3.5 | 1.0 to 12.4 | 3.0 | 0.9 to 10.8 | 3.2 | 0.9 to 11.4 | |
| Q3 | 5 | 1.6 | 0.4 to 6.7 | 1.3 | 0.3 to 5.6 | 1.5 | 0.4 to 6.4 | |
| Q4-larger area | 3 | REF | REF | REF | ||||
| P, test for trend | .0003 | .0035 | .0083 | |||||
| Vessel diameter | ||||||||
| Q1 smaller diameter | Smaller diameter | 22 | 6.0 | 1.8 to 20.0 | 3.9 | 1.1 to 13.2 | 3.6 | 1.1 to 12.1 |
| Q2 | 11 | 2.9 | 0.8 to 10.3 | 2.1 | 0.6 to 7.7 | 2.3 | 0.6 to 8.2 | |
| Q3 | 8 | 2.6 | 0.7 to 9.7 | 1.9 | 0.5 to 7.4 | 2.1 | 0.6 to 8.0 | |
| Q4-larger diameter | 3 | REF | REF | REF | ||||
| P, test for trend | .0011 | .013 | .024 | |||||
| Regularity of vessels | ||||||||
| Q4 less regular | Irregular shape | 22 | 17.1 | 2.3 to 128 | 13.1 | 1.8 to 97.4 | 10.9 | 1.5 to 81.4 |
| Q3 | 14 | 11.4 | 1.5 to 87.5 | 10.2 | 1.3 to 77.6 | 10.1 | 1.3 to 77.6 | |
| Q2 | 7 | 6.5 | 0.8 to 52.5 | 5.4 | 0.7 to 43.9 | 5.6 | 0.7 to 45.4 | |
| Q1 more regular | 1 | REF | REF | REF | ||||
| P, test for trend | .0001 | .0007 | .0038 |
Abbreviations: HR, hazard ratio; HPFS, Health Professionals Follow-Up Study; REF, reference.
Hazard ratios adjusted for age at diagnosis.
Hazard ratios adjusted for age at diagnosis (continuous) and Gleason grade (categorically, 2 to 6, 7, 8 to 10).
Hazard ratios adjusted for age at diagnosis (continuous), Gleason grade (categorically, 2 to 6, 7, 8 to 10), and prostate-specific antigen at diagnosis (categorically, < 4, 4.1 to 10, > 10).
In secondary analyses, we considered whether the angiogenesis measures predicted lethal outcomes among men with low to moderate risk prostate cancer, defined by Gleason grade ≤ 7. Although the number of outcomes was small (n = 19), the data suggested that vessel irregularity and smaller vessel size predict outcome even among men with low to moderate grade tumors.
The discrimination of models that included the clinical factors was compared with the model also including the angiogenesis biomarkers using lethal prostate cancer as an end point. The addition of angiogenesis markers led to marked improvement in prediction of lethal prostate cancer. The C statistics were 0.82 (95% CI, 0.76 to 0.87) for the model including age, Gleason grade (re-review), and PSA at diagnosis and 0.87 (95% CI, 0.82 to 0.92) for the model also including vessel area, diameter, and vessel irregularity. When pathologic stage was also included in the model, the C statistics were 0.88 (95% CI, 0.84 to 0.93) for the clinical model and 0.92 (95% CI, 0.89 to 0.96) for the model also including vessel size and shape.
DISCUSSION
In this large United States prostatectomy cohort, we demonstrate a strong link between quantitative morphologic measures of angiogenesis in relation to prostate cancer progression. Compared with low Gleason grade tumors, poorly differentiated tumors exhibited significantly greater microvessel density and smaller vessel size on the basis of both diameter of the vessel and vessel area. The association between Gleason grade and vessel regularity was weaker.
The HPFS prostatectomy cohort included almost one third whose tumors showed extraprostatic extension, nodal extension, or spread to the seminal vesicles or bladder on the basis of pathologic findings. Tumor vessel morphology and density were predictive of extraprostatic extension at time of prostatectomy. Pathologically advanced stage tumors exhibited greater microvessel density as well as smaller and less regularly shaped vessel lumen as compared with tumors that were localized.
Vessel size and shape were most strongly associated with development of bone metastases or cancer death several years after diagnosis. Men whose tumors where infiltrated by vessels that were of smaller size and were more irregular in shape were at increased risk of developing lethal disease. These histopathologic angiogenesis measures were predictive beyond their correlations with Gleason grade, PSA levels, and stage, suggesting that biomarkers of prostate cancer angiogenesis at diagnosis can predict outcomes several years hence and may help to stratify a patient's risk of progression and perhaps suggest the need for additional therapy in the adjuvant setting.
Our data on vessel morphology and architecture further support the importance of angiogenesis in prostate cancer progression and the hypothesis that disease progression is marked by a rapid formation of neovasculature in tumors that are of smaller size and more poorly formed.14,15 The leaky and disordered function of these vessels has important implications for progression and prognosis.16 Because tumor angiogenesis predicts future outcomes, the biology of the prostate cancer, as reflected in tumor angiogenesis, seems to be determined early in its development. The overall number of vessels may not be as strong of an indication of neoangiogenesis, because microvessel density reflects both new and existing vasculature.
Our data suggest that microvessel morphology and architecture are linked to prostate cancer progression apart from age, Gleason grade, and PSA at diagnosis. The relation between microvessel density, vessel size, and shape as a predictor of extraprostatic disease suggests that angiogenesis biomarkers may be useful in guiding tumor staging at diagnosis. Moreover, the finding that vessel size and irregularity were strong predictors of fatal disease many years in the future may have clinical utility and guide therapeutic choices, in combination with other tumor biomarkers or clinical information.
Several studies have shown that greater microvessel density is associated with worse tumor grade10,11 and more advanced stage.11,17 As a predictor of prognosis, greater microvessel density has been associated with an increased likelihood of biochemical failure after prostatectomy18–20 and worse cancer-specific survival.11,21 However, other studies have found that microvessel density is not an independent predictor of prostate cancer outcomes after adjusting for Gleason grade.22–24 In our own data, microvessel density was a predictor of pathologic evidence of extraprostatic disease and high Gleason. On a univariate level, the data suggested a positive association with cancer death, albeit not significant, which may reflect the relatively small number of deaths in the cohort, with limited statistical power to detect modest effects. Given the strong correlation with Gleason grade, microvessel density did not independently predict lethal prostate cancer. In part, this may be due to the fact that microvessel density is reflecting both new and mature vasculature. Moreover, our findings may be attenuated, as they do not account for the amount of stromal tissue relative to tumor.
There are no published studies to date on the relation between morphologic aspects of vessels, such as size and shape, in relation to prostate cancer. Irregularly shaped vessels have been associated with worse disease progression in pancreatic cancer,25 Hodgkin's lymphoma,26 laryngeal cancer,27 and malignant melanoma.28 Interestingly, smaller vessel diameter was associated with more advanced disease for bladder cancer,29 whereas larger vessels were predictive of advanced melanoma.28 Taken together with our results, these data suggest that the morphologic characteristics that reflect the pattern and maturity of the growing vascular network may be better indicators of neoangiogenesis, cancer aggressiveness, and metastatic potential.
Our study is one of the largest to evaluate biomarkers of angiogenesis and prostate cancer death. For validation of prognostic biomarkers, prostate cancer–specific death is a critical outcome. Although PSA recurrence is associated with an increased risk of prostate cancer death, most men with recurrences do not die of cancer,30,31 so studies based on intermediary measures may not provide the complete picture. The HPFS cohort is carefully monitored with prospective and complete follow-up, which is critical, because deaths can occur decades after diagnosis.1,2 The cohort has been carefully annotated with respect to important clinical characteristics. More than half of the participants were diagnosed in the PSA era, which allows an extrapolation of our findings to the recent clinical picture of prostate cancer. The cohort includes men in the United States, primarily white, and generally of a high socioeconomic status. Although these characteristics do not impact the study validity, they may influence the generalizability of the findings. Our study included prostatectomy specimens; evaluation of prostate biopsy specimens is an important next step. Concato et al21 directly assessed prostate cancer biopsies and found a small positive association (hazard ratio, 1.8; 95% CI, 1.2 to 2.6) between microvessel density and total mortality, independent of clinical characteristics.
Experimental studies suggest that antiangiogenic therapies may normalize tumor vasculature and microenvironment, reduce vascular permeability, and restore the balance of pro- and antiangiogenic factors, at least transiently.15 Data from our study reflect that antiangiogenic therapy may represent an important drug target.
In conclusion, a quantitative assessment of microvessel architecture is informative in assessing the extent of prostate cancer neoangiogenesis. Microvessel size and shape may be valuable markers to distinguish cancers with indolent biology from those with an aggressive potential.
Acknowledgment
We thank the participants of the Health Professionals Follow-Up Study. We thank Walter Willett, MD, PhD, for guidance on this project; Luba Bondarenko for block collection; Mira Kaufman and Alvin Wing for database support; Kimberly Carter for laboratory support; and Orly Stampfer for editorial assistance.
Footnotes
Supported by Grant No. Number P01CA055075 (principal investigator Walter Willett, MD, PhD) from the National Cancer Institute. L.A.M. is funded by a Young Investigators Award (Michael Milken Scholar) from the Prostate Cancer Foundation.
The content of this article is solely the responsibility of the authors, and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lorelei A. Mucci, Edward Giovannucci, Steven K. Clinton
Financial support: Edward Giovannucci, Steven K. Clinton
Provision of study materials or patients: Lorelei A. Mucci, Steven K. Clinton
Collection and assembly of data: Lorelei A. Mucci, Anna Powolny, Edward Giovannucci, Zhiming Liao, Stacey A. Kenfield, Rulong Shen, Meir J. Stampfer, Steven K. Clinton
Data analysis and interpretation: Lorelei A. Mucci, Stacey A. Kenfield, Rulong Shen, Meir J. Stampfer, Steven K. Clinton
Manuscript writing: Lorelei A. Mucci, Anna Powolny, Steven K. Clinton
Final approval of manuscript: Lorelei A. Mucci, Anna Powolny, Edward Giovannucci, Zhiming Liao, Stacey A. Kenfield, Rulong Shen, Meir J. Stampfer, Steven K. Clinton
REFERENCES
- 1.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 3.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 4.Porter CR, Kodama K, Gibbons RP, et al. 25-year prostate cancer control and survival outcomes: A 40-year radical prostatectomy single institution series. J Urol. 2006;176:569–574. doi: 10.1016/j.juro.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Philadelphia, PA: Lippincott; 1997. Angiogenesis. [Google Scholar]
- 6.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 9.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis: Correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 10.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 11.Borre M, Offersen BV, Nerstrom B, et al. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940–944. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberman MA, Partin AW, Veltri RW, et al. Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer. 1997;79:772–779. doi: 10.1002/(sici)1097-0142(19970215)79:4<772::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 15.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 16.Shchors K, Evan G. Tumor angiogenesis: Cause or consequence of cancer? Cancer Res. 2007;67:7059–7061. doi: 10.1158/0008-5472.CAN-07-2053. [DOI] [PubMed] [Google Scholar]
- 17.Rogatsch H, Hittmair A, Reissigl A, et al. Microvessel density in core biopsies of prostatic adenocarcinoma: A stage predictor? J Pathol. 1997;182:205–210. doi: 10.1002/(SICI)1096-9896(199706)182:2<205::AID-PATH846>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Hall MC, Troncoso P, Pollack A, et al. Significance of tumor angiogenesis in clinically localized prostate carcinoma treated with external beam radiotherapy. Urology. 1994;44:869–875. doi: 10.1016/s0090-4295(94)80173-8. [DOI] [PubMed] [Google Scholar]
- 19.Halvorsen OJ, Haukaas S, Hoisaeter PA, et al. Independent prognostic importance of microvessel density in clinically localized prostate cancer. Anticancer Res. 2000;20:3791–3799. [PubMed] [Google Scholar]
- 20.Revelos K, Petraki C, Scorilas A, et al. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2007;27:3651–3660. [PubMed] [Google Scholar]
- 21.Concato J, Jain D, Li WW, et al. Molecular markers and mortality in prostate cancer. BJU Int. 2007;100:1259–1263. doi: 10.1111/j.1464-410X.2007.07136.x. [DOI] [PubMed] [Google Scholar]
- 22.Bettencourt MC, Bauer JJ, Sesterhenn IA, et al. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol. 1998;160:459–465. [PubMed] [Google Scholar]
- 23.Krupski T, Petroni GR, Frierson HF, Jr, et al. Microvessel density, p53, retinoblastoma, and chromogranin A immunohistochemistry as predictors of disease-specific survival following radical prostatectomy for carcinoma of the prostate. Urology. 2000;55:743–749. doi: 10.1016/s0090-4295(99)00598-1. [DOI] [PubMed] [Google Scholar]
- 24.Rubin MA, Buyyounouski M, Bagiella E, et al. Microvessel density in prostate cancer: Lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology. 1999;53:542–547. doi: 10.1016/s0090-4295(98)00561-5. [DOI] [PubMed] [Google Scholar]
- 25.Giannopoulos G, Kavantzas N, Parasi A, et al. Morphometric microvascular characteristics in the prognosis of pancreatic and ampullary carcinoma. Pancreas. 2007;35:47–52. doi: 10.1097/mpa.0b013e31804bfbab. [DOI] [PubMed] [Google Scholar]
- 26.Korkolopoulou P, Thymara I, Kavantzas N, et al. Angiogenesis in Hodgkin's lymphoma: A morphometric approach in 286 patients with prognostic implications. Leukemia. 2005;19:894–900. doi: 10.1038/sj.leu.2403690. [DOI] [PubMed] [Google Scholar]
- 27.Laitakari J, Nayha V, Stenback F. Size, shape, structure, and direction of angiogenesis in laryngeal tumour development. J Clin Pathol. 2004;57:394–401. doi: 10.1136/jcp.2002.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massi D, Franchi A, Borgognoni L, et al. Tumor angiogenesis as a prognostic factor in thick cutaneous malignant melanoma: A quantitative morphologic analysis. Virchows Arch. 2002;440:22–28. doi: 10.1007/s004280100480. [DOI] [PubMed] [Google Scholar]
- 29.Korkolopoulou P, Konstantinidou AE, Kavantzas N, et al. Morphometric microvascular characteristics predict prognosis in superficial and invasive bladder cancer. Virchows Arch. 2001;438:603–611. doi: 10.1007/s004280100400. [DOI] [PubMed] [Google Scholar]
- 30.D'Amico AV, Cote K, Loffredo M, et al. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003;169:1320–1324. doi: 10.1097/01.ju.0000049200.30192.d1. [DOI] [PubMed] [Google Scholar]
- 31.D'Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer specific survival following radiation therapy during the prostate specific antigen era. J Urol. 2003;170:S42–S46. doi: 10.1097/01.ju.0000094800.63501.15. discussion S46-S47. [DOI] [PubMed] [Google Scholar]