Abstract
Purpose
There is no standard treatment for patients with advanced urothelial cancer who are ineligible (“unfit”) for cisplatin-based chemotherapy (CHT). To compare the activity and safety of two CHT combinations in this patient group, a randomized phase II/III trial was conducted by the EORTC (European Organisation for Research and Treatment of Cancer). We report here the phase II results of the study.
Patients and Methods
CHT-naïve patients with measurable disease and impaired renal function (30 mL/min < glomerular filtration rate [GFR] < 60 mL/min) and/or performance status (PS) 2 were randomly assigned to receive either GC (gemcitabine 1,000 mg/m2 on days 1 and 8 and carboplatin area under the serum concentration-time curve [AUC] 4.5) for 21 days or M-CAVI (methotrexate 30 mg/m2 on days 1, 15, and 22; carboplatin AUC 4.5 on day 1; and vinblastine 3 mg/m2 on days 1, 15, and 22) for 28 days. End points of response and severe acute toxicity (SAT) were evaluated with respect to treatment group, renal function, PS, and Bajorin risk groups.
Results
Three of 178 patients who were ineligible or did not start treatment were excluded. SAT was reported in 13.6% of patients on GC and in 23% on M-CAVI. Overall response rates were 42% (37 of 88) for GC and 30% (26 of 87) for M-CAVI. Patients with PS 2 and GFR less than 60 mL/min and patients in Bajorin risk group 2 showed a response rate of only 26% and 20% and an SAT rate of 26% and 25%, respectively.
Conclusion
Both combinations are active in this group of unfit patients. However, patients with PS 2 and GFR less than 60 mL/min do not benefit from combination CHT. Alternative treatment modalities should be sought in this subgroup of poor-risk patients.
INTRODUCTION
Up to 50% of patients with urothelial cancer are not eligible (“unfit”) for cisplatin-based standard chemotherapy because of impaired renal function, performance status, or comorbidity.1–4 So far, no standard chemotherapy has been established for this patient group.5
Carboplatin-based regimens are widely used as an alternative to cisplatin combination chemotherapy in unfit patients. Carboplatin is a platinum analog that is less nephrotoxic than cisplatin, but it appears to be slightly inferior.6–9 Methotrexate/carboplatin/vinblastine (M-CAVI) is a well-tolerated palliative combination chemotherapy regimen with a response rate (RR) of 30% to 57% and a median survival of about 9 months.9–14 A number of new agents and combinations have been explored to reduce toxicity and improve efficacy in the treatment of urothelial cancer. Among them is gemcitabine, a pyrimidine antimetabolite, that provides an RR of approximately 25% when used as a monotherapy.15–19 Gemcitabine is well tolerated and can be safely used in patients with impaired renal function (glomerular filtration rate [GFR] ≥ 30 mL/min).20
The European Organisation for Research and Treatment of Cancer-Genitourinary Tract Cancer (EORTC GU) group has categorized patients with urothelial cancer to be “fit” or “unfit” for cisplatin-containing chemotherapy in order to develop separate investigational strategies in these patients.12,21 Patients unfit for cisplatin therapy were defined by either performance status (PS) 2 and/or impaired renal function (GFR < 60 mL/min).
For this randomized phase II/III trial, a feasibility study was conducted to define a recommended dose of gemcitabine/carboplatin in the group of patients unfit for cisplatin. Fixed-dose gemcitabine 1,000 mg/m2 on days 1 and 8 and carboplatin area under the serum concentration-time curve (AUC) 5 on day 1 at dose level 1 showed dose-limiting myelotoxicity. At one dose level lower, with carboplatin AUC 4.5, the regimen was well tolerated and recommended for further investigation.22
This phase II/III study aimed to assess the activity and toxicity of two carboplatin combinations—one with methotrexate and vinblastine and the other with gemcitabine—in patients with advanced urothelial transitional-cell carcinoma ineligible for cisplatin-based chemotherapy. The phase II results are reported here.
PATIENTS AND METHODS
Patients
Patients with histologically proven transitional-cell carcinoma of the urinary tract (including renal pelvis, ureters, urinary bladder), unresected lymph node(s) (N+), distant metastases (M1, stage IV) or unresectable primary bladder cancer (T3-4), and with measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST)23 were included in this trial. Lesions occurring in tissues that had been previously irradiated were to be assessed only if irradiation treatment had been completed at least 3 months earlier and if the lesions had since progressed or were new. No previous systemic treatment, either cytotoxic or biologic, was allowed. All patients had to be ineligible (unfit) for cisplatin-based chemotherapy, defined by either a WHO PS 2 and/or an impaired renal function (GFR > 30 but < 60 mL/min). GFR could be assessed by direct measurement (ethylenediaminetetra-acetate or creatinine clearance) or, if not available, by calculation from serum/plasma creatinine.24 Corrected serum calcium was to be within the normal limits.
Absence of any psychological, familial, sociological, or geographical condition potentially hampering compliance with the study protocol and follow-up schedule was required. Fertile men and potentially childbearing women were required to use an appropriate contraceptive method during and for 6 months after completion of chemotherapy. Patients with previous systemic chemotherapy (including adjuvant and neoadjuvant chemotherapy); inadequate bone marrow function (WBC < 4,000/μL or platelets < 125,000/μL); liver function impairment (bilirubin > 1.25× upper limit of normal [ULN] and/or AST/ALT > 3× ULN; in the case of known liver metastases AST/ALT > 5× ULN); presence of brain metastases or other CNS lesions; a concomitant, second, or previous malignancy except for cured basal-cell skin cancer; carcinoma in situ of the cervix; and pregnant or lactating women were all ineligible.
The protocol was approved by the ethics review board of the participating institutions. Before randomization, written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, applicable guidelines for good clinical practice, and applicable laws and regulations of the countries where the study was conducted, whichever represented the greater protection of the individual.
Treatment Schedule
Patients were centrally randomly assigned by the EORTC Headquarters to receive either gemcitabine/carboplatin (GC) or M-CAVI, using the minimization technique with stratification for PS, renal function (GFR), and institution. Patients on M-CAVI received methotrexate 30 mg/m2 IV on days 1, 15, and 22. However, it was omitted in patients presenting pleural effusions or ascites until complete resolution. Vinblastine 3 mg/m2 IV was given on days 1, 15, and 22. Carboplatin doses in milligrams were calculated as 4.5 × (GFR + 25) given over 1 hour IV on day 1 in both treatment arms and given every 4 weeks on the M-CAVI treatment arm. Patients allocated to GC received gemcitabine 1,000 mg/m2 over 30 minutes IV on days 1 and 8, followed by carboplatin on day 1, every 3 weeks.
Treatment was continued until disease progression or intolerable toxicity. In case of complete response, two more cycles were to be given. Granulocyte colony-stimulating factor (GCSF) was allowed and documented but reserved for those patients in whom the recommended dose modifications were insufficient.
On both treatment arms, cycles were not started unless WBC was ≥ 3,000/μg, ANC ≥ 1,500/μg, and platelets were ≥ 100,000/μg. On the M-CAVI arm, treatment was withheld on days 15 and 22 if these values were not reached. Gemcitabine was given with 50% dose reduction if WBC was 1,000 to 1,900/g or ANC was 500 to 1,000/g or platelets were 50,000 to 99,000/μg, or withheld when any value was below these limits. If patients required more than 2 weeks for hematologic recovery, or if there was grade 4 neutropenia with fever, or grade 4 thrombocytopenia for more than 3 days, or thrombocytopenia with active bleeding during the nadir, treatment was continued with 75% of all drugs in both treatment arms.
On both arms, dose was adjusted for nonhematologic toxicity, including mucositis. Although prophylactic leucovorin was not allowed, leucovorin was permitted 24 hours after methotrexate administration in patients experiencing grade 3 or 4 mucositis. Grade 3 and 4 nonhematologic toxicities required 25% dose reduction and withdrawal from the study or continuation with 50% dose (at the discretion of the investigator), respectively.
Because of toxicity concerns on the M-CAVI arm, an amendment to the protocol was implemented in February 2002, specifying that “methotrexate should be omitted when the GFR is less than 30 mL/min or the serum creatinine level is more than 2 mg/dL. The dose of methotrexate is to be reduced by 50% if the serum creatinine level is between 1.5 and 2.0 mg/dL.”
Treatment Evaluation
The primary objective of the phase II part of this study was to evaluate the antitumor activity (objective tumor response) and toxicity of the two treatment arms. Toxicity was evaluated using Common Toxicity Criteria, version 2.0. During treatment, blood counts and serum creatinine were determined weekly. Before each chemotherapy cycle, history, physical examination, blood count, blood chemistries (serum creatinine, bilirubin, alkaline phosphatase, AST, ALT, lactate dehydrogenase, GFR, and calcium) and measured or calculated creatinine clearance were required. In addition, before start of treatment, height, ECG, and cystoscopy (if the primary tumor was to be evaluated) were performed.
Tumor measurements were assessed radiologically (computed tomography scans, chest x-ray) before start of treatment and after every two cycles, with response assessed according to the RECIST.23 It was strongly recommended to confirm the responses to fulfill RECIST requirements. However, they could not always be confirmed after a minimum of 4 weeks ( Discussion).
Statistical Considerations: Study Objectives
Because of limited experience with GC in unfit patients, the study started as a randomized phase II trial to simultaneously assess activity and toxicity of the two regimens. If the RR (complete response plus partial response) was sufficiently high and the severe acute toxicity (SAT) rate was acceptably low, the two treatment regimens would be further studied in a phase III setting. The two-stage Bryant and Day design25 was used, which takes into account both RR and toxicity.
SAT was defined as the occurrence of any of the following events, either directly or at least possibly related to treatment administration: mucositis grade 3 or 4, thrombocytopenia grade 4 associated with bleeding, neutropenic fever grade 3 or 4, renal toxicity grade 3 or 4, and death. An RR of 45% and an SAT rate of 15% were considered as acceptable for continuation in phase III. Response and SAT rates of 30% were considered to be unacceptable.
With α = .20 and β =.05, each arm of the study was conducted in two steps. In the first step, 45 patients would be registered on each treatment. If 13 or fewer responses were observed on any arm, that arm would be stopped because of an inadequate RR. If 14 or more patients with SAT were observed on any arm, that arm would be stopped because of excessive toxicity. Otherwise the trial would be kept open until a total of 78 patients had been entered on each arm. In the second step, if 26 or fewer responses were observed among these 78 patients, it would be concluded that the regimen was not sufficiently active to warrant further testing. If 20 or more patients with SAT were observed, it would be concluded that the regimen should not be studied further because of excessive toxicity. If 27 or more responses and 19 patients or fewer with SAT were observed on each arm, then the trial would be continued as a randomized phase III study.
Patients were centrally randomly assigned by the EORTC Headquarters to receive either GC or M-CAVI, using the minimization technique with stratification for PS, renal function (GFR), and institution.
RESULTS
The phase II part of the study was open for enrollment between January 2001 and June 2005. There was one preplanned stop in recruitment between June 2003 and March 2004 after 112 patients had been accrued to determine whether the criteria for proceeding to the second step had been met.
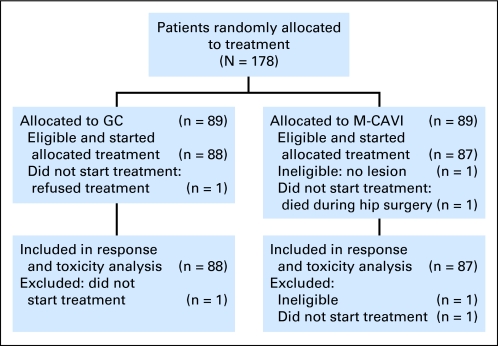
A total of 178 patients from 28 institutions in 12 countries were randomly assigned, 89 on each treatment arm. The sample size was extended slightly because it was unknown how many patients would ultimately be eligible and start treatment. Three patients were excluded: one patient on M-CAVI who was ineligible (no lesion), and one patient on each arm who did not start treatment (one refused, one died after hip surgery). Therefore, 88 patients on GC and 87 on M-CAVI fulfilled the criteria for toxicity and activity evaluation (Fig 1).
Fig 1.
CONSORT diagram. GC, gemcitabine plus carboplatin; M-CAVI, methotrexate plus carboplatin plus vinblastine.
Patient characteristics (Table 1) were generally well balanced between the treatment arms, as were the stratification factors (Table 2). There was only a slight imbalance in the distribution of liver and visceral metastases (Table 1).
Table 1.
Patient Characteristics
| Characteristic | GC(n = 88) |
M-CAVI(n = 87) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 69 | 78.4 | 68 | 78.2 |
| Female | 19 | 21.6 | 19 | 21.8 |
| Age, years | ||||
| Median | 71 | 72 | ||
| Range | 36-85 | 34-86 | ||
| Performance status | ||||
| 0 | 15 | 17.0 | 12 | 13.8 |
| 1 | 37 | 42.0 | 39 | 44.8 |
| 2 | 36 | 40.9 | 36 | 41.4 |
| Associated chronic disease (eg, hypertension, diabetes mellitus, cardiovascular disorders, depression, peptic ulcers, emphysema) | 41 | 47 | 39 | 45 |
| GFR, mL/min | ||||
| Median | 50 | 47 | ||
| Range | 30-125 | 30-115 | ||
| Primary tumor only target | 13 | 14.8 | 12 | 13.8 |
| TNM classification of metastases | ||||
| M0 | 18 | 20.5 | 23 | 26.4 |
| M1 | 68 | 77.3 | 61 | 70.1 |
| MX | 2 | 2.3 | 3 | 3.4 |
| Visceral metastases | ||||
| No | 50 | 56.8 | 41 | 47.1 |
| Yes | 38 | 43.2 | 46 | 52.9 |
| Liver involved | ||||
| No | 70 | 79.5 | 60 | 69.0 |
| Yes | 14 | 15.9 | 22 | 25.3 |
| Unknown | 4 | 4.5 | 5 | 5.7 |
| Bajorin risk group | ||||
| 0 | 37 | 42.0 | 31 | 35.6 |
| 1 | 28 | 31.8 | 30 | 34.5 |
| 2 | 23 | 26.1 | 26 | 29.9 |
Abbreviations: GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vincristine; GFR, glomerular filtration rate.
Table 2.
Stratification Factors
| Factor | GC(n = 88) |
M-CAVI(n = 87) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| PS 2 only | 12 | 13.6 | 14 | 16.1 |
| GFR, < 60 mL/min only | 52 | 59.1 | 51 | 58.6 |
| PS 2 and GFR < 60 mL/min | 24 | 27.3 | 22 | 25.3 |
Abbreviations: GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vincristine; PS, performance status; GFR, glomerular filtration rate.
Toxicity
The median number of chemotherapy cycles was 4.5 on GC and 3 on M-CAVI (Table 3), with 31 patients receiving only one chemotherapy cycle (12 on GC and 19 on M-CAVI). Dose reductions and delays as well as the need for growth factors are detailed in Table 3. SAT, at least possibly treatment related, was reported in 12 patients (13.6%) on GC and in 20 patients (23.0%) on M-CAVI (Table 4). Mucositis grade 3 occurred in one patient (1.1%) on GC and five patients (5.7%) on M-CAVI, thrombocytopenia grade 4 with bleeding in three patients (3.4%) on GC and zero patients on M-CAVI, neutropenic fever grade 3/4 in five patients (5.7%) on GC and 12 patients (13.8%) on M-CAVI, renal toxicity grade 3/4 in three patients (3.4%) on GC and two patients (2.3%) on M-CAVI, and death due to treatment in two patients (2.3%) on GC and four patients (4.6%) on M-CAVI.
Table 3.
Amount of Chemotherapy, Dose Reductions and Delays, Renal Function Assessment, and Growth Factor Use
| Variable | GC(n = 88) |
M-CAVI(n = 87) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Chemotherapy cycles | ||||
| Median | 4.5 | 3 | ||
| Range | 1-10 | 1-23 | ||
| > Six cycles | 11 | 12.4 | 5 | 5.7 |
| Reason for dose reductions | ||||
| Any | 63 | 71.6 | 73 | 83.9 |
| Hematologic | 43 | 48.9 | 48 | 55.2 |
| Renal | 7 | 8.0 | 13 | 14.9 |
| Reason for dose delay | ||||
| Any | 67 | 76.1 | 53 | 60.9 |
| Hematologic | 32 | 36.4 | 33 | 37.9 |
| Renal | 4 | 4.5 | 1 | 1.1 |
| GFR, calculated at least once | ||||
| Calculated | 78 | 88.6 | 78 | 89.7 |
| Measured | 3 | 3.4 | 5 | 5.7 |
| EDTA | 6 | 6.8 | 3 | 3.4 |
| GCSF, secondary prophylaxis | 5 | 5.6 | 10 | 11.4 |
Abbreviations: GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vincristine; GFR, glomerular filtration rate; EDTA, ethylenediaminetetra-acetate; GCSF, granulocyte colony-stimulating factor.
Table 4.
Results According to Treatment Arm
| Treatment | Confirmed ORR |
Confirmed and Unconfirmed ORR |
Confirmed and Unconfirmed CR |
Severe Acute Toxicity |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| GC (n = 88) | 33 | 38 | 37 | 42 | 3 | 3.4 | 12 | 14 |
| M-CAVI (n = 87) | 17 | 20 | 26 | 30 | 4 | 4.6 | 20 | 23 |
Abbreviations: ORR, overall response rate; CR, complete response; GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vincristine.
Death related to toxicity occurred after one cycle in four patients (two on GC and two on M-CAVI) and in two patients after two and three cycles on M-CAVI. Reasons for treatment-associated deaths were thrombocytopenia and hemorrhage in one patient on GC and neutropenia and/or infections in all the other cases. The use of secondary GCSF was documented according to the protocol in 10 M-CAVI and five GC patients. In 13 of 15 patients, GCSF was used in only one cycle. Three and four cycles with GCSF were reported in one patient each on GC and M-CAVI, respectively.
Activity
Best confirmed overall response rates (ORRs), complete response plus partial response, were 38% (33 of 88) on GC and 20% (17 of 87) on M-CAVI (Table 4). Complete remissions were rare, with three (3.4%) on each treatment. Thirteen additional patients had unconfirmed responses, 10.3% (nine patients) on the M-CAVI arm versus 4.5% (four patients) on the GC arm.
An analysis of patients according to the number of poor stratification factors is given in Table 5. In a post hoc attempt to evaluate outcome measures in this unfit patient population by using the Bajorin risk groups based on PS and visceral metastases, PS 0 and 1 were transformed into Karnofsky performance status (KPS) ≥ 80% and PS 2 was transformed into KPS less than 80%. When adding the presence or absence of visceral metastases, patients were regrouped into three prognostic groups, depending on their number of adverse prognostic factors (Bajorin risk groups 0, 1, or 2).26 RRs and the percentages of SAT as well as the chance of receiving only one chemotherapy cycle differed substantially between these three groups (Table 6) with patients in risk group 2 receiving less treatment, experiencing more SAT, and having a lower RR. These results confirm the validity of the Bajorin prognostic groups in this patient population.
Table 5.
Results According to Stratification Parameters
| Stratification | Only One Cycle of Therapy*(n = 16/175) |
ORR |
Severe Acute Toxicity |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| PS 2 or GFR < 60 mL/min | 7/129 | 5 | 51/129 | 39.5 | 20/129 | 15.5 |
| PS 2 and GFR < 60 mL/min | 9/46 | 20 | 12/46 | 26.1 | 12/46 | 26.1 |
Abbreviations: ORR, overall response rate (confirmed and unconfirmed); PS, performance status; GFR, glomerular filtration rate.
Excluding patients with progression or toxicity as reasons for stopping therapy.
Table 6.
Results According to Bajorin Risk Groups*
| Risk Group | Only One Cycle of Therapy†(n = 16/175) |
ORR |
Severe Acute Toxicity |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| 0 (n = 68) | 4 | 6 | 32 | 47 | 11 | 16 |
| 1 (n = 58) | 2 | 3 | 21 | 39 | 9 | 16 |
| 2 (n = 49) | 10 | 20 | 10 | 20 | 12 | 25 |
Abbreviation: ORR, confirmed and unconfirmed overall response rate.
Predicting outcome of therapy: Karnofsky performance status, < 80%, visceral metastases.26
Excluding patients with progression or toxicity as reasons for stopping therapy.
DISCUSSION
To the best of our knowledge, this is the first randomized phase II/III trial evaluating two chemotherapy regimens in purely unfit urothelial cancer patients. The categories “fit” and “unfit” as used here, were first defined by the EORTC GU Group for investigating new treatment strategies. The unfit patient groups as well as the elderly and multimorbid groups have been highly underrepresented in clinical trials, not only in urothelial cancer.27–32 In our study, there was no age restriction. The median age was 71 to 72 years, which is about 8 to 10 years older than patients in other trials that study cisplatin-based chemotherapy.33,34 Renal function impairment increases with age35 and is a well-known comorbid condition in urothelial cancer patients.1,36 The concept of our study as well as the entry criteria reflect a clinical need that has been poorly addressed so far.
Only recently has more attention been paid to chemotherapy in the elderly and in patients with comorbidities.37,38 Age alone, however, is not necessarily a predictor of physiologic fitness.37 The definition of unfit bladder cancer patients here follows this concept.
In general, chemotherapy dosages are derived from studies with fit patients. This might be the cause for increased toxicity in the elderly and unfit.39,40 Carboplatin-based regimens have been tested extensively in those ineligible for cisplatin therapy.12,13 A 57% RR, comparable to that for standard MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), has been reported for the carboplatin combination with methotrexate and vinblastine in patients with a median age of 70 years, a KPS of 70%, and a lowered creatinine clearance.14 In that study, Small et al did not observe an appreciably different toxicity rate in patients older than 70 years.14 Of note, a dose-finding study with only unfit patients was performed for the investigational arm of our trial.22 The carboplatin dose of AUC 4.5 was recommended for further investigation and used in both treatment arms in this study. Toxicity of the two regimens under investigation differed in several points. Because of toxicity concerns on M-CAVI, a study protocol amendment was implemented to lower the methotrexate toxicity. Regarding death related to toxicity (2.3% for GC and 4.6% for M-CAVI), a case-by-case review was performed by the study's principal investigators, and a literature search revealed that in chemotherapy studies of different solid tumors and lymphomas with only elderly and unfit patients, death related to toxicity rates between 2.3% and 13.1% have been published.41–52 We did not observe a substantial difference in toxicity rates when comparing our data with those of recent MVAC trials without GCSF in fit patients, where approximately 14% neutropenic fever and a 3% to 4% treatment-related mortality were reported.33,53 A direct comparison of the toxicities per treatment arm will be possible in the phase III part of the study. The 14% and 23% SAT rates observed for GC and M-CAVI, respectively, fulfilled the statistical criteria for continuing to phase III. The ORRs of the two regimens were found to be within the expected range (42% for GC and 30% for M-CAVI).
Treatment of this unfit patient group with urothelial cancer turned out to be a challenge. In this regard, this randomized trial reflects daily clinical practice and its difficulties.30 First, a large number of patients who had only one chemotherapy cycle was observed, even when excluding those with progression and toxicity. The reasons were manifold and included patients' refusal of further treatment despite a lack of measurable SAT. Interestingly, the frequency of receiving only one chemotherapy cycle was highest in patients with two poor stratification factors and in those with two Bajorin poor prognostic factors (both 20%). Second, there was an imbalance of unconfirmed responses (four for GC v nine for M-CAVI), the reasons for which were multifactorial, including toxicity, progression, protocol violation, and the decisions of patients and investigators to stop protocol treatment. Third, and most importantly, in those patients (approximately 25%) with two poor stratification or two Bajorin poor risk factors, the ORRs with both GC and M-CAVI were low and toxicity was high. Alternative treatment modalities, other than combination chemotherapy, should be considered in this subgroup of poor-risk patients, as long as a survival benefit from combination chemotherapy that does not contain cisplatin is unproven. Mono-chemotherapy54,55 with the primary goal of palliation, the use of new drugs with alternative mechanisms of action, investigational therapy within clinical trials, or best supportive care might be more reasonable ways to proceed.
In conclusion, as far as we know, this is the first randomized trial evaluating two chemotherapy regimens in unfit urothelial cancer patients. Our results reveal that on GC there is more thrombocytopenia grade 4 with hemorrhage, and on M-CAVI there is more neutropenic fever, mucositis grade 3, and deaths related to toxicity. Both combinations are active in these unfit patients and fulfilled the criteria to continue the phase III part of the study. However, patients with two poor stratification factors did not benefit from combination chemotherapy, and alternative treatment regimens should be investigated.
Acknowledgment
We appreciate the temporary study coordination by G. Kaiser and the contributions of J.J. Croles, T. Gil, J.B. Vermorken, P. Carpentier, I. Billiet, M. Nogue-Aliguer, J.H. Schornagel, W. Kirkels, Th. M. De Reijke, B. Mellado, G. Daugaard, A. Horwich, P. Harper, J. Graham, V. Serretta, A. Bono, I. Bodrogi, A. Sella, and O. Koriakine.
Footnotes
Supported by Grants No. 2U10 CA11488-28 through 5U10 CA011488-38 from the National Cancer Institute, Bethesda, MD, and by Eli Lilly Study Code B9E-MC-S018.
Presented in part at the Genitourinary Cancers Symposium of the American Society of Clinical Oncology, February 14-16, 2008, San Francisco, CA, and at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Joaquim Bellmunt, Eli Lilly (C); Ronald de Wit, Eli Lilly (C) Stock Ownership: None Honoraria: Maria De Santis, Eli Lilly; Joaquim Bellmunt, Eli Lilly; Iwona Skoneczna, Eli Lilly; Ronald de Wit, Eli Lilly Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joaquim Bellmunt, Ronald de Wit, Richard Sylvester
Administrative support: Sandrine Marreaud, Richard Sylvester
Provision of study materials or patients: Maria De Santis, Joaquim Bellmunt, Graham Mead, J. Martijn Kerst, Michael Leahy, Pablo Maroto, Iwona Skoneczna
Collection and assembly of data: Richard Sylvester
Data analysis and interpretation: Maria De Santis, Richard Sylvester
Manuscript writing: Maria De Santis
Final approval of manuscript: Maria De Santis, Joaquim Bellmunt, Graham Mead, J. Martijn Kerst, Michael Leahy, Pablo Maroto, Iwona Skoneczna, Sandrine Marreaud, Ronald de Wit, Richard Sylvester
REFERENCES
- 1.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 2.Balducci L. Evidence-based management of cancer in the elderly. Cancer Control. 2000;7:368–376. doi: 10.1177/107327480000700412. [DOI] [PubMed] [Google Scholar]
- 3.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 4.Balducci L, Yates J. General guidelines for the management of older patients with cancer. Oncology (Williston Park) 2000;14:221–227. [PubMed] [Google Scholar]
- 5.De Santis M, Bachner M. New developments in first- and second-line chemotherapy for transitional cell, squamous cell and adenocarcinoma of the bladder. Curr Opin Urol. 2007;17:363–368. doi: 10.1097/MOU.0b013e3282c4b0cb. [DOI] [PubMed] [Google Scholar]
- 6.Petrioli R, Frediani B, Manganelli A, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer. 1996;77:344–351. doi: 10.1002/(SICI)1097-0142(19960115)77:2<344::AID-CNCR18>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Dogliotti L, Carteni G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Dreicer R, Manola J, Roth BJ, et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer. 2004;100:1639–1645. doi: 10.1002/cncr.20123. [DOI] [PubMed] [Google Scholar]
- 9.Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–1972. doi: 10.1002/(sici)1097-0142(19971115)80:10<1966::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.de Wit R, Tesselaar M, Kok TC, et al. Randomised phase II trial of carboplatin and iproplatin in advanced urothelial cancer. Eur J Cancer. 1991;27:1383–1385. doi: 10.1016/0277-5379(91)90015-6. [DOI] [PubMed] [Google Scholar]
- 11.Mottet-Auselo N, Bons-Rosset F, Costa P, et al. Carboplatin and urothelial tumors. Oncology. 1993;50:28–36. doi: 10.1159/000227258. [DOI] [PubMed] [Google Scholar]
- 12.Bellmunt J, Albanell J, Gallego OS, et al. Carboplatin, methotrexate, and vinblastine in patients with bladder cancer who were ineligible for cisplatin-based chemotherapy. Cancer. 1992;70:1974–1979. doi: 10.1002/1097-0142(19921001)70:7<1974::aid-cncr2820700727>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Klocker J, Pont J, Schumer J, et al. Carboplatin, methotrexate and vinblastin (Carbo-MV) for advanced urothelial cancer. A phase II trial. Am J Clin Oncol. 1991;14:328–330. doi: 10.1097/00000421-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Fippin LJ, Ernest ML, et al. A carboplatin-based regimen for the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer. 1996;78:1775–1780. doi: 10.1002/(sici)1097-0142(19961015)78:8<1775::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer. 1998;34:1208–1212. doi: 10.1016/s0959-8049(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 16.Pollera CF, Ceribelli A, Crecco M, et al. Weekly gemcitabine in advanced bladder cancer: A preliminary report from a phase I study. Ann Oncol. 1994;5:182–184. doi: 10.1093/oxfordjournals.annonc.a058775. [DOI] [PubMed] [Google Scholar]
- 17.Stadler WM, Kuzel T, Roth B, et al. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol. 1997;15:3394–3398. doi: 10.1200/JCO.1997.15.11.3394. [DOI] [PubMed] [Google Scholar]
- 18.Moore MJ, Tannock IF, Ernst DS, et al. Gemcitabine: A promising new agent in the treatment of advanced urothelial cancer. J Clin Oncol. 1997;15:3441–3445. doi: 10.1200/JCO.1997.15.12.3441. [DOI] [PubMed] [Google Scholar]
- 19.Albers P, Siener R, Härtlein M, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma - prognostic factors for response and improvement of quality of life. Onkologie. 2002;25:47–52. doi: 10.1159/000055202. [DOI] [PubMed] [Google Scholar]
- 20.von der Maase H. Gemcitabine in transitional cell carcinoma of the urothelium. Expert Rev Anticancer Ther. 2003;3:11–19. doi: 10.1586/14737140.3.1.11. [DOI] [PubMed] [Google Scholar]
- 21.de Wit R. Overview of bladder cancer trials in the European Organization for Research and Treatment. Cancer. 2003;97:2120–2126. doi: 10.1002/cncr.11288. [DOI] [PubMed] [Google Scholar]
- 22.Bellmunt J, de Wit R, Albanell J, et al. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur J Cancer. 2001;37:2212–2215. doi: 10.1016/s0959-8049(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51:1372–1383. [PubMed] [Google Scholar]
- 26.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 27.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 30.Extermann M, Albrand G, Chen H, et al. Are older French patients as willing as older American patients to undertake chemotherapy? J Clin Oncol. 2003;21:3214–3219. doi: 10.1200/JCO.2003.08.091. [DOI] [PubMed] [Google Scholar]
- 31.Ries LAG, Melbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975-2005. [Google Scholar]
- 32.Yee KW, Pater JL, Pho L, et al. Enrollment of older patients in cancer treatment trials in Canada: Why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 33.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 34.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial cancer without prior systemic therapy; EORTC30987/Intergroup Study. J Clin Oncol. 2007;25(suppl):242s. doi: 10.1200/JCO.2011.38.6979. abstr LBA5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 36.Nogué-Aliguer M, Carles J, Arrivi A, et al. Gemcitabine and carboplatin in advanced transitional cell carcinoma of the urinary tract: An alternative therapy. Cancer. 2003;97:2180–2186. doi: 10.1002/cncr.10990. [DOI] [PubMed] [Google Scholar]
- 37.Launay-Vacher V, Izzedine H, Rey JB, et al. Incidence of renal insufficiency in cancer patients and evaluation of information available on the use of anticancer drugs in renally impaired patients. Med Sci Monit. 2004;10:CR209–212. [PubMed] [Google Scholar]
- 38.Lichtman SM, Wildiers H, Launay-Vacher V, et al. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14–34. doi: 10.1016/j.ejca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1:18–24. [PubMed] [Google Scholar]
- 40.Wedding U, Honecker F, Bokemeyer C, et al. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control. 2007;14:44–56. doi: 10.1177/107327480701400106. [DOI] [PubMed] [Google Scholar]
- 41.Dimopoulos MA, Anagnostopoulos A, Pantazopoulos D, et al. Primary treatment of muscle-invasive bladder cancer in elderly patients and in patients with impaired heart or lung function with gemcitabine and docetaxel. Proc Am Soc Clin Oncol. 1999;18 abstr 1297. [Google Scholar]
- 42.Linardou H, Aravantinos G, Efstathiou E, et al. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: Phase II study of the Hellenic Co-operative Oncology Group. Urology. 2004;64:479–484. doi: 10.1016/j.urology.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 43.Constenla M, Lorenzo I, Carrete N, et al. Docetaxel (TXT) monotherapy & G-CSF for advanced breast cancer (ABC) in elderly patients (EP) Proc Am Soc Clin Oncol. 2000;19 abstr 425. [Google Scholar]
- 44.Tirelli U, Balzarotti M, Uziel L, et al. Comprehensive geriatric assessment (CGA)-adapted chemotherapy (CT) in 100 elderly patients (pts) (> 70 years) with diffuse large B-cell non-Hodgkin's lymphoma (DLBCL) J Clin Oncol, 2007;25(suppl):708s. abstr 19515. [Google Scholar]
- 45.Argiris A, Li Y, Murphy BA, et al. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol. 2004;22:262–268. doi: 10.1200/JCO.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Macpherson N, O'Reilly SE, Connors JM, et al. ODBEP chemotherapy for elderly patients with advanced stage Hodgkin's disease. Proc Am Soc Clin Oncol. 1996;15 abstr 1323. [Google Scholar]
- 47.Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: A phase II trial. Oncology. 2005;69:384–390. doi: 10.1159/000089992. [DOI] [PubMed] [Google Scholar]
- 48.Gómez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol. 1998;16:2065–2069. doi: 10.1200/JCO.1998.16.6.2065. [DOI] [PubMed] [Google Scholar]
- 49.Tirelli U, Zagonel V, Serraino D, et al. Non-Hodgkin's lymphomas in 137 patients aged 70 years or older: A retrospective European Organization for Research and Treatment of Cancer Lymphoma Group Study. J Clin Oncol. 1988;6:1708–1713. doi: 10.1200/JCO.1988.6.11.1708. [DOI] [PubMed] [Google Scholar]
- 50.Buffoni L, Dongiovanni D, Barone C, et al. Fractionated dose of cisplatin (CDDP) and vinorelbine (VNB) chemotherapy for elderly patients with advanced non-small cell lung cancer: Phase II trial. Lung Cancer. 2006;54:353–357. doi: 10.1016/j.lungcan.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Italiano A, Ortholan C, Oudard S, et al. First-line docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-refractory prostate cancer: A French national study. Eur Urol. 2008;55:1368–1376. doi: 10.1016/j.eururo.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 52.Kasahara K, Shibata K, Shirasaki H, et al. A phase II trial of carboplatin, oral etoposide, and vincristine in elderly or unfit patients with small cell lung cancer (SCLC) Proc Am Soc Clin Oncol. 2002;21:332a. abstr 1326. [Google Scholar]
- 53.Fosså SD, Sternberg C, Scher HI, et al. Survival of patients with advanced urothelial cancer treated with cisplatin-based chemotherapy. Br J Cancer. 1996;74:1655–1659. doi: 10.1038/bjc.1996.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagneto B, Zai S, Marenco D, et al. Single-agent gemcitabine in previously untreated elderly patients with advanced bladder carcinoma: Response to treatment and correlation with the comprehensive geriatric assessment. Oncology. 2004;67:27–32. doi: 10.1159/000080282. [DOI] [PubMed] [Google Scholar]
- 55.Chester JD, Hall GD, Forster M, et al. Systemic chemotherapy for patients with bladder cancer–current controversies and future directions. Cancer Treat Rev. 2004;30:343–358. doi: 10.1016/j.ctrv.2003.12.005. [DOI] [PubMed] [Google Scholar]