Abstract
Study Objectives:
Overweight and obesity are thought to increase the risk of obstructive sleep apnea syndrome (OSAS) among children. However, previous results have been inconsistent and appear to be confounded by both ethnicity and the different ages of children studied. To determine whether the association between excess weight and OSAS varies with age across childhood, we assessed polysomnographic data from a series of Caucasian children and adolescents referred for clinical evaluation of snoring.
Methods:
Sleep and OSAS severity were assessed using polysomnography in 234 children aged 2.0 to 18.0 years. All children were referred for overnight evaluation of suspected OSAS. Severity of OSAS as a function of body mass and age were then evaluated.
Results:
Risk of OSAS among adolescents (age ≥ 12 years) was increased 3.5 fold with each standard-deviation increase in body mass index z-score. Risk of OSAS was not significantly increased with increasing body mass among younger children.
Conclusions:
Similar to adults, adolescent children show an increased risk for having OSAS in association with overweight and obesity. For Caucasian children, overweight and obesity should be considered a significant risk for OSAS among adolescents or from age 12 years, especially when in combination with other established risk factors, including snoring and adenotonsillar hypertrophy.
Citation:
Kohler MJ; Thormaehlen S; Kennedy JD; Pamula Y; van den Heuvel CJ; Lushington K; Martin AJ. Differences in the association between obesity and obstructive sleep apnea among children and adolescents. J Clin Sleep Med 2009;5(6):506-511.
Keywords: Obesity, OSAS, children, adolescents
Over the last decade, the prevalence of obesity in children and adolescents has risen dramatically in many countries, leading some researchers to speak of an “international epidemic of childhood obesity.”1 This rise in obesity has caused concern as to its impact on both rates and severity of sleep-related breathing disorders, most notably obstructive sleep apnea syndrome (OSAS). OSAS is characterized by intermittent complete and partial obstruction of the upper airway during sleep, resulting in hypoxia and cortical arousal, and is currently reported to occur in 2% to 3% of children. Milder primary snoring is reported in 5% to 10%.2 As in the case of obesity, OSAS also is thought to independently impair cardiovascular and metabolic function in children,3 and it is associated with a range of cognitive, behavior, and social problems.4 The reported association between OSAS and body mass among children appears to vary according to factors such as ethnicity,5 the presence of adenotonsillar hypertrophy,6 and socioeconomic status.7
In adults, there appears to be a much stronger association between body mass and OSAS, as compared with in children.8,9 In addition, there is an apparent discrepancy in the strength of the association between studies of older versus younger children,5 and the impact of developmental changes in the association among children has been recently considered.10–12 The limited data to date suggest little association between body mass and upper airway obstruction among Caucasian children aged 2 to 12 years; however, differences between children and adolescents have not been investigated, and the age at which increased body mass predisposes children to upper airway obstruction during sleep currently remains unknown. Although the prevalence of upper airway obstruction among adolescents may be similar to that of younger children, the risk due to obesity may be much greater.13,14 Reductions in upper airway tone and changes to anatomic structures may play a significant mediating role in any change in association between body mass and upper airway obstruction with age.15–17
We have previously reported on the association between obesity and upper airway obstruction among a group of Australian Caucasian children reported to snore and aged 4 to 12 years.11 The aim of this study was to expand on the previous findings and examine changes in the association of body mass and the severity of sleep-related upper airway obstruction between children and adolescents.
METHODS
Participants
This was an extension of our previous study examining the association between body mass and OSAS among 4- to 12-year-old children.11 All children aged 2 to 18 years undergoing their first overnight polysomnogram for evaluation of snoring and possible OSAS at the Sleep Disorders Unit, Women's and Children Hospital, Adelaide, South Australia, between October 1999 and May 2007 were initially identified. A total of 631 cases were identified. Non-Caucasian children; children with previous craniofacial surgery, adenoidectomy or tonsillectomy; and those with specific syndromes (such as Prader-Willi syndrome and muscular dystrophy) or taking medications known to potentially disrupt sleep and impact on respiratory dynamics were further excluded from the initial sample. A total of 220 cases were therefore retained for statistical analyses. The children were classified by age into 6 groups: 2 to 3.9 years, 4 to 5.9 years, 6 to 7.9 years, 8 to 9.9 years, 10 to 11.9 years, and 12+ years (adolescents). Due to the relatively low number of eligible adolescents, children aged 12 to 18 years who had received their first polysomnogram between June 2007 and October 2008 were also identified. Of 33 potential extra adolescents, 14 were included. All children were reported to snore frequently by a parent ( ≥ 1 night/week). Established growth charts corrected for age and sex were used to determine body mass index (BMI) z-scores and classify overweight and obesity (BMI ≥ 85th and 95th percentile, respectively).18,19 A measure of socioeconomic status (SES) was derived from the Australian Bureau of Statistics' Index of Relative Socio-economic Advantage/Disadvantage national census data. A higher score on this index indicates increased income and occupational skills and/or training within the geographic area of residence, with a national mean of 1000 and SD of 100. This study was approved by the Human Ethics Committee, Child Youth and Women's Health Service, South Australia.
Polysomnography
Overnight polysomnography was conducted without sedation or sleep deprivation and began at each child's usual bedtime. A parent accompanied each child throughout the procedure. Polysomnography was performed using a computerized sleep data-acquisition system (Compumedics S-Series and E-Series Sleepwatch System, Melbourne, Australia). The following standard parameters were measured and recorded continuously utilizing the appropriate signal sampling and filtering protocols: electroencephalogram (C3-A2 or C4-A1), left and right electrooculogram, submental and intercostal electromyogram with skin-surface electrodes, leg movements by piezoelectric motion detection, heart rate by electrocardiogram, oronasal airflow by thermistor and nasal pressure, respiratory movements of the chest and abdominal wall using uncalibrated respiratory inductive plethysmography, arterial oxygen saturation by pulse oximetry (Nellcor N2000 and 595, Boulder, USA, using 2- to 3-second averaging time) and transcutaneous CO2 using a heated (41oC) transcutaneous electrode (TINA, Radiometer Pacific, Mt Waverley, Australia). All data were digitized and stored on computer disk for subsequent analysis. Each child was continuously monitored and observed via infrared camera by a pediatric sleep technician who also documented observations of sleep behavior, including the presence or absence of snoring.
Data Analysis
All polysomnograms were analyzed and scored manually by a sleep technician experienced and trained in analyzing pediatric sleep studies. Sleep stages were scored in 30-second epochs according to Rechtschaffen and Kales criteria.20
Respiratory variables were scored according to standard guidelines recommended for pediatric sleep studies.21 Obstructive apneas were defined as the absence of airflow associated with continued chest and abdominal wall movement for duration of 2 or more respiratory cycles. Obstructive hypopneas were defined as a 50% to 80% reduction in the amplitude of the respiratory inductance plethysmography and/or airflow signal associated with paradoxical chest/abdominal wall movement for duration of 2 or more respiratory cycles and associated with a 3% or greater oxygen desaturation. The presence of any other supportive data such as increased intercostal or submental electromyographic activity was also used to distinguish between obstructive and central hypopneas. Central apneas were scored if there was an absence of respiratory effort as determined by respiratory inductance plethysmography and/or intercostal electromyogram in association with an absence of airflow for duration of 2 or more respiratory cycles and associated with at least a 3% oxygen desaturation. Central apneas were also scored if the event lasted at least 20 seconds. Central hypopneas were defined as a 50% to 80% reduction in airflow from baseline in association with a 50% to 80% reduction in respiratory effort from baseline. Apnea events that included both central and obstructive components were scored as a mixed apnea.
The obstructive apnea and hypopnea index (OAHI) was calculated as the total number of obstructive apneas and obstructive hypopneas, divided by the total sleep time and expressed as the number of events per hour of sleep. An OAHI of 1 or greater was considered indicative of OSAS. A central apnea and hypopnea index was calculated as the total number of central apneas and central hypopneas divided by the total sleep time and expressed as the number of events per hour of sleep (CAHI). The total apnea and hypopnea index (AHI) was calculated as OAHI plus CAHI.
Spontaneous and respiratory arousals were scored according to the criteria of the American Sleep Disorders Task Force and are expressed as the total number of arousals per hour of sleep (spontaneous arousal index, SAI, and respiratory arousal index, RAI).22
Statistical Analysis
Statistical analyses were conducted using SPSS version 15.0 for Windows (SPSS, Inc., Chicago, IL). All p values reported are 2 tailed, with significance determined at α = 0.05. Data are presented as mean ± SD unless otherwise stated.
Group differences in demographics, body mass measures, and polysomnography results were determined using 1-way analysis of variance for continuous data, and Games-Howell tests were used for posthoc analyses. χ2 analyses were used to determine group differences for categorical data. All continuous variables were normally distributed with the exception of AHI, OAHI, CAHI, and RAI. Inverse transformation was required to reduce positive skew before entering variables into analyses [i.e., 1/(x+c), where x = data value and c = 1].
Risk of OSAS due to age and BMI z-score was determined using logistic regression. OSAS status was entered as the dependent variable, and age group (adolescents vs younger children), BMI z-score, and age group × BMI z-score were entered as predictors.
RESULTS
A total of 234 Caucasian patients between 2 and 18 years of age with full polysomnographic data were included in analyses. Patient characteristics and body mass measures are presented by age group in Table 1. Despite a trend toward greater BMI z-score and obesity among older children and adolescents, compared with younger children, groups did not differ statistically for sex, SES, body mass, and percentage of overweight and obese individuals.
Table 1.
Patient Characteristics and Body Measurements by Age Group
| Age group, y |
p value | ||||||
|---|---|---|---|---|---|---|---|
| 2-3.9 | 4-5.9 | 6-7.9 | 8-9.9 | 10-11.9 | 12-18 | ||
| No. | 34 | 44 | 50 | 38 | 30 | 38 | 0.259 |
| Age, y | 3.0 ± 0.6 | 5.0 ± 0.5 | 7.1 ± 0.6 | 9.0 ± 0.6 | 11.0 ± 0.7 | 15.4 ± 1.5 | |
| Males | 25 (73.5) | 23 (52.3) | 34 (68.0) | 20 (52.6) | 15 (50.0) | 27 (71.1) | 0.111 |
| SES | 973.5 ± 73.0 | 979.5 ± 87.5 | 973.2 ± 75.8 | 967.4 ± 88.4 | 940.3 ± 87.5 | 946.0 ± 67.0 | 0.198 |
| BMI | |||||||
| Percentile | 62.6 ± 27.1 | 63.0 ± 28.0 | 72.4 ± 26.7 | 73.8 ± 24.7 | 75.9 ± 24.5 | 75.8 ± 33.1 | 0.099 |
| z-score | 0.58 ± 1.1 | 0.56 ± 1.1 | 0.92 ± 1.2 | 0.99 ± 1.1 | 1.05 ± 1.0 | 1.28 ± 1.4 | 0.055 |
| Weight category | |||||||
| Normal weight | 24 (70.6) | 32 (72.7) | 30 (60.0) | 21 (55.3) | 17 (56.7) | 13 (34.2) | 0.099 |
| Overweight | 3 (8.8) | 3 (6.8) | 4 (8.0) | 3 (7.9) | 2 (6.7) | 5 (13.2) | |
| Obese | 7 (20.6) | 9 (20.5) | 16 (32.0) | 14 (36.8) | 11 (36.7) | 20 (52.6) | |
Data are presented as mean ± SD or number (%). Significant p values are in bold. SES refers to socioeconomic status; BMI, body mass index.
Results from overnight polysomnography are presented in Table 2. Differences between age groups were found for total sleep time; percentage of stage 2 sleep, slow wave sleep (SWS), and REM sleep; REM latency; and frequency of spontaneous arousals. Specifically, adolescents (age ≥ 12 years) demonstrated reduced sleep time and a lower percentage of SWS compared with all other age groups. Adolescents also demonstrated a greater percentage of stage 2 sleep compared with all other age groups, a reduced percentage of rapid eye movement (REM) sleep compared with all age groups except 10- to 11.9-year-olds, an increased REM latency compared with 2- to 3.9-year-olds, and an increased frequency of spontaneous arousals (SAI) compared with 4- to 5.6-year-olds and 8- to 9.9-year-olds. Despite the apparent greater range in OAHI and RAI among adolescents, groups were not statistically different for measures of OSAS severity (OAHI, RAI, and arterial oxygen saturation nadir) or for percentage of individuals classified as having OSAS.
Table 2.
Polysomnography Results by Age Group
| Age group, y |
p value | Posthoc | ||||||
|---|---|---|---|---|---|---|---|---|
| 2-3.9 | 4-5.9 | 6-7.9 | 8-9.9 | 10-11.9 | 12-18 | |||
| TST, min | 430.4 ± 53.6 | 427.0 ± 44.6 | 424.5 ± 54.0 | 428.5 ± 44.5 | 429.4 ± 54.4 | 332.3 ± 66.8 | < 0.001 | 1–5 > 6 |
| Sleep stage, % | ||||||||
| 1 | 4.6 ± 2.5 | 3.6 ± 2.3 | 4.0 ± 3.2 | 4.0 ± 3.3 | 3.9 ± 2.8 | 5.5 ± 4.4 | 0.128 | |
| 2 | 43.7 ± 5.6 | 44.1 ± 5.9 | 43.6 ± 7.2 | 43.6 ± 7.3 | 46.2 ± 6.7 | 53.8 ± 7.1 | < 0.001 | 1–5 < 6 |
| SWS | 29.9 ± 6.2 | 30.4 ± 5.9 | 31.4 ± 7.8 | 30.9 ± 4.1 | 30.2 ± 6.7 | 24.1 ± 7.7 | < 0.001 | 1–5 > 6 |
| REM | 22.0 ± 4.9 | 22.1 ± 4.2 | 20.5 ± 5.3 | 21.1 ± 5.0 | 19.7 ± 4.7 | 16.5 ± 4.3 | < 0.001 | 1–4 > 6 |
| REM latency, min | 97.6 ± 48.5 | 110.2 ± 58.5 | 109.7 ± 54.3 | 112.8 ± 52.6 | 113.2 (57.4) | 156.4 ± 94.5 | 0.002 | 1 < 6 |
| AHIa | 4.8 ± 9.7 | 3.4 ± 8.7 | 5.4 ± 9.4 | 3.1 ± 4.7 | 4.2 ± 6.6 | 14.3 ± 34.3 | 0.890 | |
| OAHIb | 1.5 ± 2.4 | 1.9 ± 7.4 | 2.3 ± 4.3 | 1.0 ± 2.2 | 2.1 ± 5.3 | 11.9 ± 32.6 | 0.311 | |
| CAHIa | 3.3 ± 8.4 | 1.4 ± 2.0 | 3.1 ± 5.8 | 2.1 ± 3.9 | 2.0 ± 3.7 | 2.4 ± 3.4 | 0.657 | |
| OSAS | 13 (38.2) | 11 (25.0) | 19 (38.0) | 6 (15.8) | 6 (20.0) | 13 (34.2) | 0.126 | |
| RAIa | 2.7 ± 2.5 | 1.9 ± 2.9 | 3.7 ± 6.8 | 2.5 ± 3.5 | 2.5 ± 4.3 | 10.2 ± 27.3 | 0.394 | |
| SAI | 9.4 ± 5.0 | 8.4 ± 3.7 | 9.0 ± 5.8 | 8.8 ± 4.9 | 8.9 ± 5.5 | 13.2 ± 7.6 | 0.002 | 2,4 < 6 |
| Sao2nadir | 90.8 ± 6.0 | 91.5 ± 4.3 | 90.9 ± 4.3 | 92.0 ± 3.0 | 90.5 ± 6.5 | 90.5 ± 6.6 | 0.781 | |
Data are presented as mean ± SD or number (%). For posthoc results 1 = 2- to 3.9-y age group, 2 = 4- to 5.6-y age group, 3 = 6- to 7.9-y age group, 4 = 8- to 9.9-y age group, 5 = 10- to 11.9-y age group, 6 = 12- to 18-y age group (adolescents). TST refers to total sleep time; SWS, slow wave sleep; OAHI, obstructive apnea and hypopnea index; CAHI, central apnea and hypopnea index; OSAS, obstructive sleep apnea syndrome; RAI, respiratory arousal index; SAI, spontaneous arousal index.
Analysis conducted using transformed values; untransformed mean ± SD values are presented in the table.
Analysis conducted using median test due to extreme skew; mean ± SD values are presented in the table.
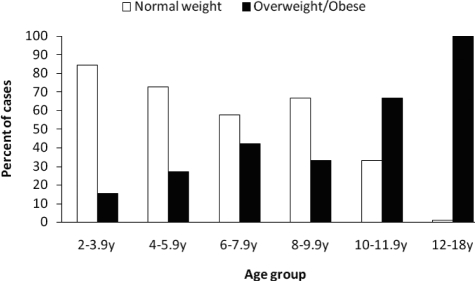
When considering only children demonstrating OSAS, there was a clear increase in the proportion of individuals who were overweight and obese with increasing age (Figure 1). Across the total group, stepwise regression assessing impact of BMI z-score and age on each of the respiratory indexes was performed. Age and BMI z-score were entered at the first step, followed by an age-by-BMI z-score factor. BMI z-score but not age was a mild yet significant contributor to the variance in AHI at step 1 (β = 0.16, t = 2.4, p < 0.05). Addition of the age-by-BMI z-score factor at step 2 resulted in a significant increment in R2 (R2 = 0.09, F1, 232 = 17.0, p < 0.001), indicating it is the interaction between age and body mass that is the important contributor to respiratory-disturbance severity. A similar pattern was observed when using the OAHI or CAHI as dependent variables. When considering age groups separately, the BMI-z-score but not age or the interaction of age and BMI z-score was a significant predictor of respiratory-disturbance severity among adolescents; however, no effect of any factor was found among children younger than 12 years.
Figure 1.
The percentage of normal-weight vs overweight/obese children classified as having obstructive sleep apnea-hypopnea syndrome (obstructive apnea-hypopnea index ≥ 1) within each age group. There was a significant increase in prevalence of overweight/obese among older children and adolescents, χ2= 79.7, p < 0.001.
To provide some clinically applicable data, logistic regression was also performed. Risk of OSAS alone was not found to be greater among adolescents (age ≥ 12 years) compared with younger children. Similarly, risk of OSAS was not significantly elevated with increasing BMI z-score across the total group. However, the risk of OSAS among adolescents was significantly increased (3.5 times) with each SD increase in BMI z-score (Table 3). These results were unchanged after addition of sleep-architecture differences found between adolescents and younger children (i.e., SAI, SWS percentage, Stage 2 percentage, and REM sleep percentage. See Table 2)
Table 3.
Risk of OSAS by Increasing BMI Z-Score and Adolescence
| Risk factors | ORa | (95% CI)a | p Value |
|---|---|---|---|
| Age ≥ 12 y | 0.16 | (0.02–1.35) | 0.92 |
| BMI z-scoreb | 0.99 | (0.75–1.30) | 0.09 |
| Age ≥ 12 y + BMI z-scoreb | 3.55 | (1.30–9.71) | 0.01 |
OSAS refers to obstructive sleep apnea syndrome.
Data are provided as odds ratio (OR) and 95% confidence intervals (CI).
OR are for each increase in body mass index (BM)I z-score of 1 SD.
DISCUSSION
This study has demonstrated a clear change in the association between body mass increase and risk of OSAS with age. Specifically, the previously reported increased risk of developing OSAS due to being overweight and obese predominates among adolescents, with little increase in risk noted among children younger than 12 years. These results suggest that the increase in risk among overweight and obese adolescents results from developmental changes, with adolescents possibly demonstrating an “adult etiology” irrespective of the initial underlying cause.
Reductions in upper airway tone with age have been reported among children15–17 and suggest a mass effect on the upper airway during sleep may be a greater risk for OSAS among older children and adolescents. In addition, a recent study has shown that although upper airway response to subatmospheric pressure loading during sleep decreases with age, it is not necessarily related to stage of pubertal development.17 We have previously reported that a greater proportion of studies in children of mean age greater than 10 years report obesity as a significant risk for OSAS, compared with similar studies of children with a younger age.5 Three previous studies have directly investigated the impact of age on the association between obesity and OSAS among children. Stepanski et al.,12 in a predominantly African American sample of children aged 0 to 12 years, found OSAS was not more prevalent among those with greater body mass referred for overnight assessment of upper airway obstruction; however, children with OSAS older than 8 years of age were found to be more obese than age-matched normal sleepers. We have previously described a weak association between body mass and upper airway obstruction among Caucasian children 4 to 12 years of age; however, no effect of age was found among this group.11 Finally, risk of OSAS was not related to body mass among Greek children younger than 6 years of age, yet older children (7-15 years) who were obese were twice as likely to have OSAS, as compared with nonobese children of the same age.10 It is difficult to generalize results across ethnic groups5; however, when combined with the results from this study, a change in the risk for OSAS with age due to increased body mass seems apparent. The precise age at which this change occurs is unknown and may vary by ethnicity as well as other risk factors.
Tonsil size is not routinely graded in the respective clinics of the host institution but may also interact with obesity to increase the risk for developing OSAS.24 Furthermore, reductions in upper airway tone with age may further predispose older children and adolescents who are obese and have large tonsils to developing OSAS. Recently, Dayyat et al.23 retrospectively investigated 412 snoring children who had undergone overnight polysomnography. Despite similar degrees of OSAS severity, nonobese children displayed larger adenotonsillar size, compared with obese children. This suggests a smaller change in magnitude of adenotonsillar size is required among obese children for an equivalent change in severity of upper airway obstruction. Whether the discrepancy reported by Dayyat and colleagues between obese and nonobese children changes with age is not known. It will be informative for future studies to assess tonsil size; however, the present results would suggest obesity among snoring adolescents should still be considered grounds for referral independent of tonsil size.
In addition, obesity is thought to increase the risk for residual obstruction following surgical removal of adenoids and tonsils in children24–30; however, results to the contrary have also been reported.31–32 Results from studies reporting increased risk indicate persistent obstruction in 35% to 79% of obese children after treatment. However, Tauman et al.30 found that older children with a greater body mass were most likely to demonstrate persistent obstruction. Recently, Amin et al.28 showed a change in BMI per year, rather than a single BMI measurement, to be a better predictor of OSAS recurrence following treatment. Age was not a significant contributing factor for OSAS recurrence in their study; however, the age range was restricted to 7 to 13 years. Combined with results demonstrating reductions in OSAS severity following surgical weight loss among adolescents,14,33 obesity appears to be of primary importance for initial patient prioritization and follow-up, especially among older children and adolescents.
An important consideration of this study is that all participants were Caucasian, and, as such, interpretation and application of results is potentially relevant to Caucasian children only. Independent of obesity, African American children appear to also be at higher risk of having OSAS.24,27,34,35 In addition, pubertal development may occur earlier among African American compared with Caucasian children.36 Therefore, developmental changes in the association between obesity and OSAS may follow a different age trajectory among African American children and, indeed, among other ethnicities also. In addition, all participants in our study were children referred for overnight evaluation of breathing due to suspected upper airway obstruction, limiting our ability to generalize results across the wider population. Despite this, the results indicate that, when OSAS is suspected, obesity will increase the likelihood of its presence among adolescent children. In regard to sex, the incidence of OSAS among adult men is reported at 3 times that for women9 and is attributed to differences in fat deposition,37,38 upper airway structure, and sex-hormone concentrations (see39,40 for a review). The ratio of males to females was similar across age groups in our study, and an interaction between sex, body mass, and OSAS was not found (results not reported). However, if developmental changes such as sex-hormone concentrations and fat deposition are indeed critical determinants of age-related changes in association between body mass and OSAS, larger studies are needed that also assess these factors. Finally, a selection bias may occur in referral of younger children versus adolescents. In Adelaide, both younger children and adolescents are followed by the same physician under the same referral protocols. However, discrepancies in initial referral indications from a general practitioner cannot be completely ruled out.
Clearly the etiology of OSAS is multifactorial, comprising an interaction of physiologic and anatomic factors. The identification of these factors will continue to be an active and important field of research given the striking morbidity associated with OSAS. However, researchers and clinicians alike should realize that such factors appear to change with age during childhood. In this study, overweight and obese adolescent children ( ≥ 12 years of age) show an increased risk of OSAS, a pattern not found among younger children. Among Caucasian children, being overweight is a significant risk for developing OSAS after age 12 years, especially in combination with other established risk factors, including snoring and adenotonsillar hypertrophy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Ros Lontis, Research Nurse, for assistance in data collection and Dr. Nancy Briggs, statistician, for assistance with data analysis. Many thanks to the staff at the Sleep Disorders Unit, Women's and Children's Hospital. Mark Kohler was supported by NH&MRC project grant 453669.
REFERENCES
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwok KL, Ng DK, Chan CH. Cardiovascular changes in children with snoring and obstructive sleep apnoea. Ann Acad Med Singapore. 2008:715–21. [PubMed] [Google Scholar]
- 4.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29:1115–34. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 5.Kohler MJ, van den Heuvel CJ. Is there a clear link between overweight/obesity and sleep disordered breathing in children? Sleep Med Rev. 2008;12:347–61. doi: 10.1016/j.smrv.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med Rev. 2008;12:339–46. doi: 10.1016/j.smrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Spilsbury JC, Storfer-Isser A, Kirchner L, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 10.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12:25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 11.Kohler M, Lushington K, Couper R, et al. Obesity and risk of sleep related upper airway obstruction in Caucasian children. J Clin Sleep Med. 2008;4:129–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanski E, Zayyad A, Nigro C, Lopata M, Basner R. Sleep-disordered breathing in a predominantly African-American pediatric population. J Sleep Res. 1999;8:65–70. doi: 10.1046/j.1365-2869.1999.00136.x. [DOI] [PubMed] [Google Scholar]
- 13.Bidad K, Anari S, Aghamohamadi A, et al. Prevalence and correlates of snoring in adolescents. Iranian J Allergy Asthma Immunol. 2006;5:127–32. [PubMed] [Google Scholar]
- 14.Kalra M, Mannaa M, Fitz K, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obes Surg. 2008;18:675–9. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Fernandes Do Prado LB, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 16.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 17.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellizzi MC, Dietz WH. Workshop on childhood obesity: summary of the discussion. Am J Clin Nutr. 1999;70:173S–5S. doi: 10.1093/ajcn/70.1.173s. [DOI] [PubMed] [Google Scholar]
- 19.McLennan J. Obesity in children: tackling a growing problem. Aust Fam Physician. 2004;33:33–6. [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: 1968. BIS/BRI. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet M. D., Carley M., Carskadon P, et al. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 23.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–44. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton S, Rosen C, Larkin E, Tishler P, Aylor J, Redline S. Predictors of sleep-disordered breathing in children with a history of tonsillectomy and/or adenoidectomy. Sleep. 2001;24:823–9. doi: 10.1093/sleep/24.7.823. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien LM, Sitha S, Baur LA, Waters KA. Obesity increases the risk for persisting obstructive sleep apnea after treatment in children. Int J Pediatr Otorhinolaryngol. 2006;70:1555–60. doi: 10.1016/j.ijporl.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Shine NP, Lannigan FJ, Coates HL, Wilson A. Adenotonsillectomy for obstructive sleep apnea in obese children: Effects on respiratory parameters and clinical outcome. Arch Otolaryngol Head Neck Surg. 2006;132:1123–7. doi: 10.1001/archotol.132.10.1123. [DOI] [PubMed] [Google Scholar]
- 27.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 28.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177:654–9. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137:43–8. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Tauman R, Gulliver T, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–8. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 31.Suen J, Arnold J, Brooks L. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995;121:525–30. doi: 10.1001/archotol.1995.01890050023005. [DOI] [PubMed] [Google Scholar]
- 32.Nafiu O, Reynolds P, Bamgbade O, Tremper K, Welch K, Kasa-Vubu J. Childhood body mass index and perioperative complications. Paediatr Anaesth. 2007;17:426–30. doi: 10.1111/j.1460-9592.2006.02140.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalra M, Inge T, Garcia V, et al. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–9. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 34.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 35.Rosen CL. Clinical features of obstructive sleep apnea hypoventilation syndrome in otherwise healthy children. Pediatr Pulmonol. 1999;27:403–9. doi: 10.1002/(sici)1099-0496(199906)27:6<403::aid-ppul7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Kaplowitz P. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol. 2006;18:487–91. doi: 10.1097/01.gco.0000242949.02373.09. [DOI] [PubMed] [Google Scholar]
- 37.Dancey DR, Hanly PJ, Soong C, et al. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123:1544–50. doi: 10.1378/chest.123.5.1544. [DOI] [PubMed] [Google Scholar]
- 38.Legato MJ. Gender-specific aspects of obesity. Int J Fertility Women's Med. 1997;42:184–97. [PubMed] [Google Scholar]
- 39.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome. Part 1: clinical features. Sleep. 2002;25:412–9. [PubMed] [Google Scholar]
- 40.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome. Part 2: mechanisms. Sleep. 2002;25:499–506. [PubMed] [Google Scholar]