Abstract
Objective:
The aim of this study was to investigate the effects of obstructive sleep apnea (OSA) on procedural and declarative memory encoding in the evening prior to sleep, on memory consolidation during subsequent sleep, and on retrieval in the morning after sleep.
Methods:
Memory performance (procedural mirror-tracing task, declarative visual and verbal memory task) and general neuropsychological performance were assessed before and after one night of polysomnographic monitoring in 15 patients with moderate OSA and 20 age-, sex-, and IQ-matched healthy subjects.
Results:
Encoding levels prior to sleep were similar across groups for all tasks. Conventional analyses of averaged mirror tracing performance suggested a significantly reduced overnight improvement in OSA patients. Single trial analyses, however, revealed that this effect was due to significantly flattened learning curves in the evening and morning session in OSA patients. OSA patients showed a significantly lower verbal retention rate and a non-significantly reduced visual retention rate after sleep compared to healthy subjects. Polysomnography revealed a significantly reduced REM density, increased frequency of micro-arousals, elevated apnea-hypopnea index, and subjectively disturbed sleep quality in OSA patients compared to healthy subjects.
Conclusions:
The results suggest that moderate OSA is associated with a significant impairment of procedural and verbal declarative memory. Future work is needed to further determine the contribution of structural or functional alterations in brain circuits relevant for memory, and to test whether OSA treatment improves or normalizes the observed deficits in learning.
Citation:
Kloepfer C; Riemann D; Nofzinger EA; Feige B; Unterrainer J; O'Hara R; Sorichter S; Nissen C. Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med 2009;5(6):540-548.
Keywords: Obstructive sleep apnea, memory, plasticity, procedural, declarative
Converging evidence from the molecular to the behavioral level indicates that sleep is critically implicated in the activity-dependent reorganization of neural networks and related memory consolidation.1,2 Sleep has been demonstrated to enhance procedural memories for skills3,4 as well as declarative memories for hippocampus-dependent fact-based information.5 Despite compelling evidence indicating that healthy sleep facilitates memory consolidation, insights into the effects of clinically relevant sleep disorders on memory functioning are just beginning to evolve. Preliminary findings suggest that sleep-related consolidation of procedural6 and declarative memories7 is impaired in patients with primary insomnia compared to healthy subjects. To our knowledge, the current study is the first to assess memory functioning before and after night time sleep in patients with obstructive sleep apnea (OSA) in comparison to healthy subjects.
OSA is a prevalent health problem affecting around 2% of women and 4% of men aged 25 years and over.8 The disorder is caused by a complete or partial collapse of the upper airways during sleep resulting in apnea/hypopnea-induced oxygen desaturation, repetitive micro-arousals, and non-restorative sleep.9 In addition to its detrimental effects on physical health, such as increased risk for cardiovascular morbidity and mortality,10 and a high socioeconomic burden,11 patients with severe OSA exhibit prominent cognitive alterations including deficits in memory functioning—the ability to register, store, and retrieve information.
Deficits in learning during daytime waking periods have been demonstrated for both major systems in OSA, declarative memory (explicit, hippocampus-dependent memory12) and procedural memory (implicit, hippocampus-independent memory13). These deficits might be due to functional or structural alterations in brain circuits critical for memory14 or to increased sleepiness and fatigue related to chronic OSA.15 In addition to daytime dysfunction, nightly sleep disruptions or hypoxemia in OSA might interfere with uniquely sleep-related brain activity implicated in the consolidation of labile memory traces acquired during preceding wakefulness, such as neuronal reactivation16 or synaptic downscaling during sleep.17
In this study, we investigated whether initial memory performance in the evening, consolidation during subsequent sleep, or retrieval in the morning were impaired in patients with OSA. Since patients with severe OSA frequently suffer from concomitant medical disorders including cerebrovascular disease and excessive daytime sleepiness that are known to interfere with our primary measures, only strictly selected patients with moderate OSA were included to reduce the impact of these confounding factors.
METHODS
Subjects
Fifteen patients with moderate OSA and 20 healthy subjects were studied. Demographical and clinical characteristics of OSA patients and healthy subjects are listed in Table 1. All participants were informed in detail and provided written consent prior to the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the IRB of the University of Freiburg Medical Center.
Table 1.
Demographic and Clinical Parameters for patients with Moderate OSA and Healthy Comparison Subjects
| OSA patients (N = 15) | Comparison subjects (N = 20) | t | p | |
|---|---|---|---|---|
| Men/women | 10/5 | 12/8 | – | – |
| Age in years | 46.4 ± 5.9 | 47.4 ± 5.6 | 0.5 | 0.631 |
| Years in school | 10.6 ± 1.6 | 11.6 ± 1.6 | 1.7 | 0.096 |
| IQ | 99.0 ± 12.6 | 103.7 ± 13.3 | 1.1 | 0.303 |
| SMQ | 50.9 ± 23.3 | 53.4 ± 21.8 | 0.3 | 0.754 |
| PSQI | 6.0 ± 2.5 | 3.3 ± 1.8 | −3.7 | 0.001 |
| ESS | 11.1 ± 6.1 | 5.8 ± 3.9 | −3.0 | 0.007 |
| SFA, SQ | 2.6 ± 0.9 | 3.4 ± 0.8 | 3.0 | 0.005 |
Data are presented as mean ± SD. SMQ, subjective memory questionnaire refers to the subjective memory of the last 4 weeks, measured with a visual analogue scale from 0 (poor) to 100 (excellent). PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; SF-A, SQ, sleep questionnaire by Görtelmeyer (sleep quality). T-Test for independent samples.
Patients referred for evaluation of OSA were invited to participate in the study. Patients who met International Classification of Sleep Disorders criteria18 for obstructive sleep apnea syndrome (ICSD: 780.53-0), as assessed by sleep questionnaires and polysomnography (apnea-hypopnea index [AHI] > 5 per hour) were included in the study. The diagnosis of sleep apnea was made by a sleep specialist (MD, respiratory physician) in the sleep laboratory at the University of Freiburg Medical Center not involved in the analysis of the data. Mean estimated time from onset of illness (based on patients subjective symptom reports) to study entry was 5.1 ± 4.4 years, the mean nadir of oxygen saturation (SpO2) during all-night polysomnography was 79.6%. All OSA patients were untreated prior to and during the experimental night in the sleep laboratory.
Healthy comparison subjects matched for age, sex and IQ were recruited from the community. Their good-sleeper status was ensured by sleep questionnaires and sleep diaries for 2 weeks. All participants underwent an extensive examination to rule out any comorbid medical or psychiatric disorder that might affect sleep or cognitive functioning. This examination included a clinical medical investigation, routine blood tests, and the Composite International Diagnostic Interview.19 All subjects were free of any medication that might affect sleep or cognition for at least 2 weeks prior to the study and did not consume alcohol or caffeine ≥ 24 h prior to or during the study. All subjects were right handed and nonsmokers. A urine drug screening after the sleep laboratory night demonstrated that all participants were free of benzodiazepines, barbiturates, amphetamines, and opiates. OSA patients and healthy controls kept a regular sleep/ wake rhythm (±1 h 22:30, ±1 h 06:30) within 2 weeks prior to the study as monitored by sleep diaries.
Subjective memory estimation within 4 weeks prior to the study (standardized visual analogue scale, from 0 (poor) to 100 (excellent); unpublished Kloepfer 2004) did not differ between groups. As expected, healthy subjects reported better subjective sleep quality within 2 weeks prior to the study (Pittsburgh Sleep Quality Index, PSQI20), reduced daytime sleepiness (Epworth Sleepiness Scale, ESS21), and better sleep quality in the sleep laboratory night (German Standardized Sleep Questionnaire, SF-A22).
Experimental Design
All subjects spent one night in the sleep laboratory at the University of Freiburg Medical Center with polysomnography recording from 22:30 to 06:30. In addition to general neuropsychological performance, declarative and procedural memory performance was assessed at 19:00 to 20:30 prior to sleep (learning session) and at 07:00 to 08:30 after sleep (recall session). In order to control for sequence effects, the memory tasks were administered in a randomized order.
Procedural Mirror Tracing Task
To assess procedural memory, a mirror tracing (MT) task was used.3,6 In this task, subjects were required to trace different line drawn stimuli with an electronic stylus as quickly and accurately as possible. Visual access to the stimuli was provided only indirectly via a mirror. The light sensor measured draw time, error count (number of deviations from the line), and error time (time out of the stimulus line). In the evening learning session, subjects traced a star until a criterion of < 15 errors was reached. After these training trials, subjects traced 6 different line drawn figures, one after the other (evening test trials). In the morning recall session, subjects traced the star one single time to keep conditions comparable. Then, the 6 figures of the preceding evening were traced in a randomized order (morning test trials). In the evening, learning was assessed by registering the number of trials to criterion for mirror tracing the star, and measuring mean draw time, error count, and error time for tracing the 6 figures. In addition, based on theoretical considerations (trade-off between time and number of errors) and empirical findings (significant correlation between time and error count, r = −0.432, p = 0.01, slope 1.9), MT capacity was calculated as MT time plus a penalty of 1.9 s per error. In the following morning, the same parameters were assessed for tracing the 6 figures. Overnight memory formation was expressed as the percentage of improvement in draw time, error count, error time, and capacity.
As shown by Manoach,23 repetitions of a procedural task are characterized by a continuous improvement, while the amplitude of this improvement decreases with each repetition. In addition to the analysis of means across all evening and morning trials patterned after Plihal and Born,3 we also analyzed the enhancement across all 2 × 6 trials, allowing for a separate effect of the intervening night. To do this, we focused on the single parameter of capacity change in % relative to the evening mean. This normalization to the individual evening mean ensures that the values are compatible with the procedure used for the morning means; furthermore, it leverages interindividual performance differences.
The model formula, obtained by integrating a 1/x decrease of enhancement across trials, contains 4 parameters: An offset y0 (value at first trial), a slope parameter dy0, a scale parameter and an additional night effect: c(t) = y0+dy0*ln(scale*t+1)/ scale+(night effect if t > = 6) with t ranging from 0 to 11. The parameters were determined by nonlinear optimization using the method of Byrd et al.24 The optimization was performed using the statistical software “R”.25 In a first step, the scale parameter was fitted globally; individual fits for the remaining parameters were then obtained for every subject. Due to the normalization to baseline mean, a fit can be described by 2 parameters: the slope in the middle of the evening session and the night effect.
Declarative Visual and Verbal Learning Task
To measure declarative memory, the visual and verbal memory task (VVM) has been chosen as a standardized task for verbal and visual memory.26 In the visual task, subjects were required to memorize a line-drawn path on a map. In the verbal task, subjects learned information about the construction of a building provided in a text, such as names, numbers, and propositional content. Visual and verbal recall was assessed immediately after the initial learning session in the evening and, without further presentation of the material, in the following morning (correctly retrieved sections of the map, correct answers to text questions). Overnight memory formation was expressed as the retention rate in percent in the morning in reference to the evening session.
Neuropsychological Test Battery
The Standard Progressive Matrices test (SPM27; adopted short-version containing 32 homogeneous items) was used in the screening session to estimate general intelligence (factor “g” and “fluid” intelligence) and logic thinking that might confound memory performance.28
A standardized test battery was used in the encoding and retrieval session to assess the effects of alertness, divided attention and psychomotor speed on declarative and procedural memory. The alertness task29 measured simple reaction time (in milliseconds) to visual stimuli that were presented with a preceding auditory signal. To assess divided attention,29 visual stimuli (squares) and auditory stimuli (sounds) were presented simultaneously. Participants were asked to respond as fast as possible to relevant stimuli with reaction being registered. Psychomotor speed was quantified by asking the participants to connect digits from 1 to 25 with a continuous line in the Trail Making Test.30 Outcome measure was the time (in seconds) needed to complete the trail.
Sleep Questionnaires
The German version of the Pittsburgh Sleep Quality Index20 was used to assess subjective sleep quality and disturbances over a 2-week time interval prior to the experimental night. Values ≥ 5 indicated disturbed sleep. The “Schlaffragebogen-A”22 is a self-rating questionnaire that assesses sleep quality of the preceding night and was administered in the morning after the experimental night. The Epworth Sleepiness Scale21 was used to measuring the general level of daytime sleepiness. Values > 10 indicated elevated daytime sleepiness.
Sleep Recordings
Sleep recordings were performed from 22:30 to 06:30 and scored according to Rechtschaffen and Kales standard criteria31 by 3 experienced and blinded raters. All raters participated in weekly meetings discussing and solving scoring problems. Interrater reliability was checked every second month. Coefficients of agreement between 2 raters were required to be > 85%.
A digital video-polysomnography system was used for recording (Leonardo-Polygraph by Sagura Medical Equipment). Electroencephalogram (EEG) derivations C3-A2 and C4-A1 as defined by the international 10-20 system were used, sampled at 250 Hz. Electromyographic (EMG) activity was recorded by submental electrodes. The vertical and horizontal electrooculogram (EOG) was recorded by 2 horizontal electrodes and one vertical electrode. Continuous electrocardiogram (ECG), plethysmography, EMG recordings of the legs, and 3 recordings of breathing (chest and abdominal excursions, nose-mouth airflow), together with saturation of oxygen in arterial blood flow (SpO2, via finger pulse oximeter), and body position were registered. Video from an infrared camera and sound recorded in synchrony with the polysomnography were available in the scoring process for better judgment of body movements and sleep disordered breathing.
Respiratory events were classified as obstructive or central based on thoracic/ abdominal effort, airflow, and pulse transit time. OSA was defined as > 5 pharyngeal obstructions with hypopnea, subsequent arousals, or apneas per hour. According to the guidelines of the German Society of Sleep Research and Sleep Medicine, hypopnea was defined as a reduction of respiratory amplitude or frequency with a subsequent decline in oxygen saturation ≥ 3% or a subsequent arousal. Apnea was defined as a cessation of breathing ≥ 10 s.32 The number of inspiratory flow-limitation episodes per hour of sleep (apneas and hypopneas) was calculated to obtain the apnea-hypopnea index (AHI). In addition, the total counts of apneas without and with arousal during sleep period time were measured. Mild OSA was defined as AHI 5–15, moderate OSA as AHI 15–25, and severe OSA as AHI > 25; only moderate OSA patients were included in the study. The following variables of sleep continuity and architecture were assessed: sleep onset latency (SOL), defined as the period between when the lights were turned off and the first 30-s epoch of sleep stage 2, 3, 4, or REM sleep; sleep period time (SPT), defined as the period between sleep onset and the final awakening; total sleep time (TST), defined as SPT minus waking epochs, sleep efficiency (SE), defined as the ratio of total sleep time to time in bed × 100%; time spent in waking referred to SPT in percent (waking%); time spent in sleep stages 1, 2, slow wave sleep (SWS, combined stages 3 and 4), and REM sleep referred to SPT in percent (stage 1%, stage 2%, SWS%, REM%). REM sleep latency was defined as the period between sleep onset and the occurrence of the first 30-s epoch of REM sleep (REM latency). REM density was defined as the percentage (%) of 3-s mini-epochs containing rapid eye movements referred to the total number of 3-s mini-epochs of REM sleep. Arousals were defined according to American Sleep Disorders Association criteria.33 In addition to the arousal index pertaining to the whole sleep period, the arousal indices specific to sleep stages 2, SWS, and REM were calculated as number of arousals per hour of the respective stages. REM sleep cycles were defined as the total number of NREM-REM sleep cycles per night. Intervening periods of other sleep stages or wake periods had to persist ≥ 15 min.34
Statistical Analysis
Descriptive presentation of the data includes mean values and standard deviations. Levene's test indicated homogeneity of variance between the groups. T-tests for independent samples were used to evaluate demographic and clinical differences between patients and comparison subjects, and to analyze the number of trials to criterion (mirror tracing). A multivariate analysis of variances (MANCOVA) with factor group (OSA patients and healthy subjects) was used to test for differences in polysomnographic parameters and overnight memory formation (%). Repeated measurement MANCOVAs with the repeated measurement factor test session (learning and recall), between-subject factor group (OSA patients and healthy subjects) and covariate age were used to test for differences in memory performance and general neuropsychological performance. The covariate age was included because of its known effect of age on sleep and cognitive functioning. Univariate tests were Greenhouse-Geisser corrected. MANCOVA effects were quantified with partial ETA squared (pETAsq) effect sizes (small, 0.01; medium, 0.06; large, 0.12). Exploratory Pearson r correlations were used to examine associations between sleep and memory parameters. The level of significance was set at p < 0.05 (2-tailed). The analyses were performed using SPSS for Windows, Version 15.0.
RESULTS
Sleep
Results from the sleep recordings are summarized in Table 2. Compared to healthy subjects, OSA patients showed a significantly lower REM density and an elevated arousal index within the sleep period time and in stage 2 sleep, reflecting fragmented sleep. Furthermore, OSA patients displayed a significantly higher AHI, a higher number of apneas with and without arousals, and lower mean oxygen saturation compared to healthy subjects. Consistent with a first night sleep laboratory effect,35 healthy subjects showed relatively poor sleep in the experimental night. No significant differences in other standard sleep parameters were observed in the current sample of healthy subjects and patients with moderate OSA.
Table 2.
Polysomnographic Parameters for Moderate OSA Patients and Healthy Comparison Subjects
| OSA patients (N = 15) | Comparison subjects (N = 20) | F | p | pETAsq | ||
|---|---|---|---|---|---|---|
| Sleep latency, min | 29.5 ± 18.3 | 26.3 ± 20.4 | 0.2 | 0.650 | 0.01 | |
| Sleep period time, min | 416.6 ± 28.8 | 428.6 ± 28.1 | 1.7 | 0.206 | 0.05 | |
| Total sleep time | 358.9 ± 56.2 | 355.2 ± 28.9 | < 0.1 | 0.848 | < 0.01 | |
| Sleep efficiency, % | 78.4 ± 11.1 | 77.2 ± 8.9 | 0.1 | 0.762 | < 0.01 | |
| Arousal Index/h SPT | 22.1 ± 7.6 | 16.3 ± 7.5 | 4.9 | 0.035 | 0.14 | |
| Arousal Index/h stage 2 | 19.1 ± 8.8 | 13.1 ± 6.9 | 5.3 | 0.029 | 0.14 | |
| REM density, % | 20.6 ± 10.9 | 27.5 ± 7.7 | 4.9 | 0.035 | 0.13 | |
| REM latency, min | 91.3 ± 61.4 | 110.7 ± 49.6 | 0.9 | 0.363 | 0.03 | |
| No REM cycles | 3.7 ± 1.3 | 3.8 ± 1.0 | 0.2 | 0.678 | 0.01 | |
| Sleep stage, as percentage of sleep period time | ||||||
| Waking% | 14.2 ± 10.1 | 16.8 ± 8.2 | 0.7 | 0.411 | 0.02 | |
| Stage 1% | 12.9 ± 5.7 | 11.6 ± 5.4 | 0.6 | 0.428 | 0.02 | |
| Stage 2% | 47.2 ± 11.4 | 53.2 ± 8.2 | 3.3 | 0.081 | 0.09 | |
| SWS% | 5.0 ± 4.6 | 2.7 ± 4.2 | 2.2 | 0.151 | 0.06 | |
| REM% | 14.9 ± 6.7 | 15.2 ± 4.3 | < 0.1 | 0.877 | < 0.01 | |
| Respiration parameters | ||||||
| AHI /h | 19.7 ± 13.7 | 2.0 ± 2.5 | 31.0 | < 0.001 | 0.49 | |
| No apneas | 73.4 ± 88.4 | 5.0 ± 8.3 | 11.8 | 0.002 | 0.27 | |
| No apneas with arousal | 59.2 ± 81.3 | 3.7 ± 7.5 | 9.2 | 0.005 | 0.22 | |
| Mean SpO2 (SPT),% | 93.0 ± 2.6 | 95.1 ± 1.2 | 10.4 | 0.003 | 0.25 | |
Data are presented as mean ± SD. SWS, slow wave sleep; AHI, apnea/ hypopnea index; No Apneas, total number of apneas during sleep period time; Mean SpO2 (SPT)%, mean oxygen saturation (SPT)%; pETAsq, partial ETA squared. MANCOVA with factor group and age as covariate.
Procedural and Declarative Memory
The repeated measures MANCOVA (Table 3) showed significant main effects for the factor test session in the variables MT capacity and MT draw time. In addition, significant test session × group interactions in MT capacity and MT draw time were observed, but no significant main effects for the factor group.
Table 3.
Memory Performance for Patients with Moderate OSA and Healthy Comparison Subjects
| OSA patients (N=15) |
Comparison subjects (N=20) |
Test session |
Group |
Session * Group |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Evening | Morning | Evening | Morning | F | p | F | p | F | p | |
| Procedural memory | ||||||||||
| MT trials star | 6.1 ± 4.4 | 1.0 | 4.9 ± 4.7 | 1.0 | – | 1.4 | 0.254 | – | ||
| MT capacity | 131.5 ± 41.3 | 90.9 ± 28.1 | 151.9 ± 44.9 | 89.8 ± 25.2 | 6.9 | 0.013 | 0.6 | 0.432 | 6.0 | 0.020 |
| MT draw time | 88.3 ± 38.6 | 67.0 ± 28.0 | 113.2 ± 50.9 | 73.9 ± 29.0 | 6.2 | 0.018 | 1.8 | 0.194 | 5.6 | 0.024 |
| MT error count | 22.8 ± 10.9 | 12.7 ± 7.3 | 20.4 ± 10.7 | 8.4 ± 7.7 | 0.8 | 0.382 | 2.2 | 0.147 | 0.5 | 0.474 |
| MT error time | 16.5 ± 8.4 | 11.2 ± 9.1 | 11.6 ± 9.3 | 7.4 ± 8.3 | 0.4 | 0.529 | 3.2 | 0.084 | 0.4 | 0.515 |
| Declarative memory | ||||||||||
| Verbal memory | 12.6 ± 3.8 | 10.5 ± 4.4 | 14.3 ± 4.5 | 12.7 ± 4.5 | 0.3 | 0.619 | 1.9 | 0.175 | 1.8 | 0.195 |
| Visual memory | 21.8 ± 3.8 | 18.1 ± 4.9 | 23.1 ± 4.3 | 20.6 ± 4.9 | 4.2 | 0.050 | 1.8 | 0.186 | 0.7 | 0.419 |
Data are presented as mean ± SD. Times are given in seconds. MT, mirror tracing. The values for verbal and visual memory represent the number of correctly retrieved items. Rm-MANCOVA with repeated measures factor test session, factor group and age as covariate.
In the encoding session prior to sleep, no significant differences in declarative or procedural memory performance (averaged across the 6 tests trials) were observed between patients and healthy subjects (MT capacity: F4,30 = 1.9, p = 0.178, MT draw time: F4,30 = 2.5, p = 0.123; MT error count: F4,30 = 0.4, p = 0.522; MT error time: F4,30 = 2.6, p = 0.117; verbal memory: F4,30 = 1.2, p = 0.285; visual memory: F4,30 = 0.8, p = 0.373). The number of MT training trials to criterion did not differ between OSA patients (6.1±4.4) and healthy subjects (4.9±4.7, t-test t = −0.8, p = 0.419).
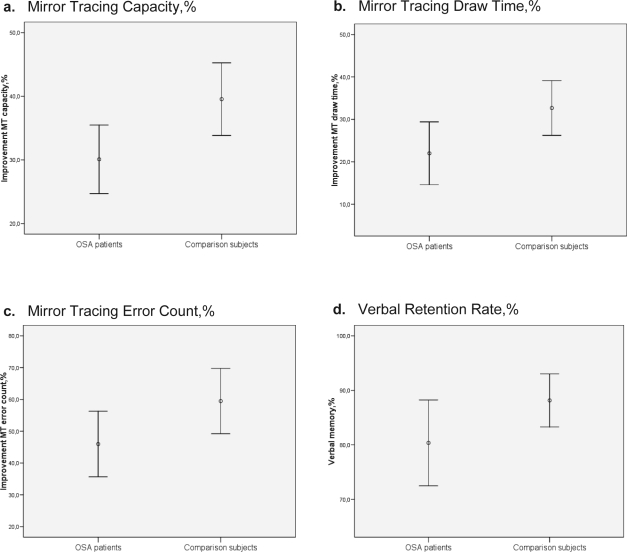
Overnight consolidation rates for procedural and declarative memories are shown in Table 4 and Figures. 1 a to d. Regarding procedural memory, the analysis comparing averaged evening and morning performances demonstrated a significantly lower overnight improvement of MT capacity in OSA patients (30.1%±9.7%) than healthy subjects (39.5%±12.2%, Figure 1a, large effect size). This effect was mainly driven by a significantly reduced improvement in MT draw time in OSA patients (22.0%±13.4%) compared to healthy subjects (32.7%±13.8%, Figure 1b, large effect size), and a significantly reduced improvement in MT error count in OSA patients (46%±18.6%) compared to healthy subjects (59.5%±22.0%, Figure 1c, large effect size). No significant group differences were observed for the improvement in MT error time. Regarding declarative memory (Table 4), OSA patients showed a significantly reduced verbal retention rate after sleep (80.4%±13.6%) compared to healthy subjects (88.2%±10.4%, Figure 1d, large effect size). Without reaching significance, patients with OSA also tended to show a lower retention rate of declarative visual memory (medium effect size).
Table 4.
Overnight Memory Formation for Patients with Moderate OSA and Healthy Comparison Subjects
| OSA patients (N=15) | Comparison subjects (N=20) | F | p | pETAsq | |
|---|---|---|---|---|---|
| Procedural memory, improvement % | |||||
| MT capacity | 30.1 ± 9.7 | 39.5 ± 12.2 | 8.5 | 0.007 | 0.21 |
| MT draw time | 22.0 ± 13.4 | 32.7 ± 13.8 | 6.6 | 0.015 | 0.17 |
| MT error count | 46.0 ± 18.6 | 59.5 ± 22.0 | 4.7 | 0.037 | 0.13 |
| MT error time | 38.4 ± 24.8 | 40.0 ± 45.6 | 0.1 | 0.765 | <0.01 |
| Declarative memory, retention rate % | |||||
| Verbal memory | 80.4 ± 13.6 | 88.2 ± 10.4 | 4.3 | 0.046 | 0.12 |
| Visual memory | 82.0 ± 16.3 | 90.1 ± 20.3 | 1.3 | 0.255 | 0.04 |
Data are presented as mean ± SD. Times are given in seconds. MT, mirror tracing. The values for verbal and visual memory represent the number of correctly retrieved items. MANCOVA with factor group and age as covariate. pETAsq, partial ETA squared.
Figures 1a-d.
Overnight memory formation for patients with moderate OSA and healthy comparison subjects, MANCOVA with factor group (OSA patients and healthy subjects). Error bars represent mean with 95% confidence intervals. a. Mirror Tracing Capacity, %. b. Mirror Tracing Draw Time, %. c. Mirror Tracing Error Count, %. d. Verbal Retention Rate %.
Mirror Tracing Capacity Single Trial Analysis
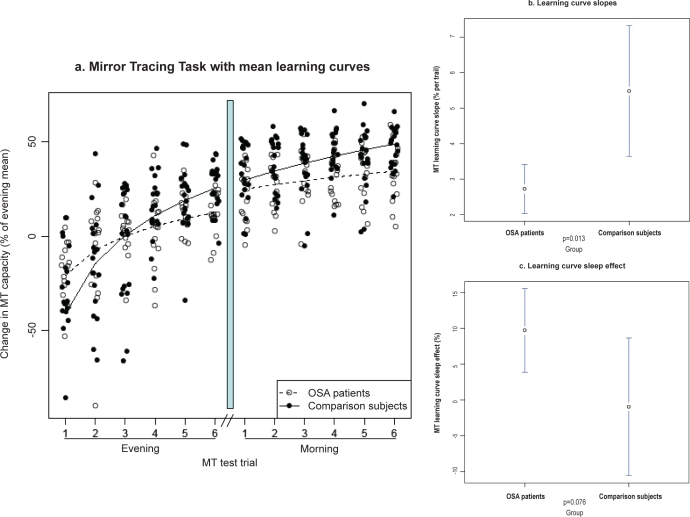
To further assess whether the observed overnight group differences in MT (based on averaged evening and morning trial values) were a result of overnight consolidation or, alternatively, different learning curves during the evening and morning test sessions, an additional single MT trial analysis was performed (Figure 2a). The value of the global scale parameter of the learning curve model fit (obtained across all subjects) was 1.15. This value was not treated separately for the 2 groups. It defined how strongly the trial-by-trial enhancement declined across trials.
Figure 2a-c.
Mirror Tracing Task performance for patients with moderate OSA and healthy comparison subjects across single trails for the morning and evening session. a. Mirror Tracing Task with mean learning curves. b. Learning curve slopes. c. Learning curve sleep effect.
The individual slope parameter was 2.7±1.2 for OSA patients vs. 5.5±3.9 for healthy subjects (group difference test t = 2.6. p = 0.013, Figure 2b). The sleep effect was 9.7±10.6 for OSA patients (test against zero: t = 3.6, p = 0.003) vs. −1.0±20.5 for healthy subjects (test against zero: t = −0.2, p = 0.8; group difference test t = 1.8, p = 0.076, Figure 2c). This implies that the trial-by-trial enhancement was significantly stronger in healthy subjects than in OSA patients, while the additional enhancement across sleep tended to be larger in OSA patients. The absolute enhancement across sleep was significant in OSA patients, but not in healthy controls. Since different slopes could be caused by different absolute performance at the start of baseline measurement, we explicitly tested the group difference in the first MT trial. The difference was not significant (p > 0.1).
Relationships between Sleep and Memory Parameters
Exploratory correlation analyses between sleep variables and parameters for overnight memory consolidation (sleep effect parameter derived from single trial analyses for MT, retention rates for VVM) were conducted separately for OSA patients and healthy comparison subjects. In OSA patients, a positive correlation between the verbal retention rate (%) and the number of REM cycles was observed (r = 0.52, p = 0.046). No other significant correlation was observed, including no significant correlation with respiratory parameters (p > 0.1, results not listed).
General Neuropsychological Performance
Table 5 summarizes the results of general neurocognitive performance. In the current sample, patients with moderate OSA and healthy subjects did not differ significantly in their performance on any task (alertness, divided attention, psychomotor speed).
Table 5.
General Neuropsychological Performance for Patients with Moderate OSA and Healthy Comparison Subjects
| OSA patients (N = 15) |
Comparison subjects (N = 20) |
Test Session |
Group |
Session * Group |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Evening | Morning | Evening | Morning | F | p | F | p | F | p | |
| Alertness [ms] | 235.4 ± 39.2 | 234.5 ± 40.0 | 242.3 ± 39.6 | 237.9 ± 39.3 | <0.1 | 0.838 | 0.1 | 0.742 | 0.1 | 0.743 |
| Divided attention [ms] | 715.4 ± 72.5 | 713.4 ± 77.1 | 719.2 ± 91.2 | 706.2 ± 73.2 | 1.4 | 0.245 | <0.1 | 0.952 | 0.9 | 0.357 |
| Psychomotor speed [s] | 34.7 ± 6.0 | 30.5 ± 5.5 | 30.1 ± 9.5 | 29.1 ± 8.3 | <0.1 | 0.860 | 2.3 | 0.143 | 1.7 | 0.197 |
Data are presented as mean ± SD. Rm-MANCOVA with repeated-measures factor test session, factor group and age as covariate.
DISCUSSION
The results of the present study provide additional evidence for the initial hypothesis that declarative and procedural memory processes are impaired in patients with OSA compared to healthy subjects. In extension to previous work focussing on memory consolidation across periods of daytime wakefulness, the current study assessed memory encoding in the evening, consolidation during subsequent sleep, and retrieval in the morning. Thus, the study was designed to further distinguish between memory deficits evident already under baseline conditions prior to sleep and potential deficits in memory consolidation during sleep in OSA.
Regarding procedural memory, conventional analyses of averaged mirror tracing suggested a significantly reduced overnight improvement in OSA patients. However, subsequent single trial analyses based on recent studies of motor learning36 revealed that this effect was due to significantly flattened learning curves in the evening and morning session in OSA patients—an observation that is in line with previous daytime findings of significant deficits in motor learning in OSA.13 In contrast, the lack of difference in overnight improvement does not support the initial hypothesis of disrupted procedural memory consolidation during sleep in OSA and highlights the importance of single trial analyses across test sessions when assessing motor learning. The overnight improvement itself was significant in OSA patients but not in healthy controls. This unexpected result would suggest that sleep in our OSA patients could actually compensate for parts of the daytime learning deficits.
In line with previous studies reporting beneficial effects of sleep on declarative memory consolidation,36 we observed following similar encoding, a significant decrease in overnight verbal memory consolidation in patients with OSA compared to healthy subjects. This finding would be consistent with the concept of deficits in sleep related memory consolidation in OSA. Yet importantly, an additional daytime control condition would be needed to demonstrate that these deficits are specific to nighttime sleep and not only time related. The verbal retention rates observed in the current study are similar to those Ellenbogen et al.37 found for word pair retention after 12 h of nighttime sleep compared to 12 h of daytime wakefulness. No significant differences were observed for visual declarative memory.
Whereas most previous studies on the interplay between sleep and declarative memory used associated word pairs,3 in the current study, the Visual and Verbal Memory Task (VVM) has been chosen as a standardized task for the assessment of declarative verbal and visual memory.26 The VVM comprises practice and every day relevant material that has to be stored within 2 minutes. The concept of the VVM comes close to that of the subtests for logical memory as provided by the Wechsler Memory Scale.38 Future research might include different modes of encoding and different levels of association of declarative material to further elucidate the relationship between sleep and declarative memory in OSA.
The reported correlation between overnight declarative memory consolidation and the number of completed sleep cycles suggests that declarative memories might specifically benefit from cyclic alterations between NREM and REM sleep, allowing a transition from labile memory traces initially stored in the hippocampus to the neocortex for long-term consolidation.39
Although we carefully restricted our sample of patients to a moderate degree of OSA, long-term sleep disruptions or hypoxia might contribute to neural dysfunction or structural alterations in OSA patients. Preclinical studies show that hypoxic episodes lead to an elevation of neurotoxic substances, e.g., reactive oxygen species, resulting in neural dysfunction and potentially brain lesion.40 Sensitive brain structures comprise those important for declarative and procedural memory consolidation, such as the mesial temporal lobe, basal ganglia, and the neocortex.41
Pilot findings in patients with primary insomnia suggest that, in addition to neurotoxic effects of hypoxia, chronically disturbed sleep itself might lead to altered brain plasticity ultimately resulting in reduced brain volumes, particularly in highly plastic brain areas such as the hippocampus.42 Recently, a number of brain imaging studies in OSA patients have provided initial evidence for alterations in brain structures that relate to memory, planning and affect.14 Interestingly, greater numbers of respiratory events negatively impacted memory in carriers of the apolipoprotein (APOE) ε4 allele only, suggesting an interaction of genetic susceptibility and OSA on memory.43
A number of additional issues need to be considered. Engleman and Joffe44 reported attenuated attention, psychomotor performance, and executive functioning in patients with severe OSA. In the current sample of patients with moderate OSA, we did not observe significant differences in alertness, divided attention, and psychomotor speed. The absence of significantly impaired general cognitive functioning in the current sample may result from the strict selection criteria for moderate OSA, in particular from the exclusion of significant comorbidities as secondary causes of cognitive deterioration. Future studies should incorporate objective measures of sleepiness, such as the multiple sleep latency test (MSLT).
In addition, it is noteworthy that in the current study, healthy subjects showed a clear “first-night effect,”35 characterized by disrupted NREM sleep, a sleep stage that might be critical for effective memory consolidation.3 In patients with OSA as assessed in the current study, continuous sleep disturbances may cause an increase in sleep pressure,45 shifting NREM sleep related indicators of sleep quality (excluding the arousal index) towards those of healthy controls as also reported in other studies.46 Thus, larger deficits in memory consolidation in OSA might emerge after adaptation in a second or subsequent sleep laboratory night. Finally, since encoding and retrieval were at different circadian phases it is possible that differences in circadian factors may have contributed to the group differences. Yet at this time, there is, to our knowledge, no evidence for altered circadian processes in OSA. Since testing started half an hour after wake-up, sleep inertia might also have influenced the results.
In sum, we observed flattened MT learning curves in the evening and morning and a significantly lower verbal retention rate from the evening to the morning in OSA patients compared to healthy subjects. Remarkably, impaired memory was observed in a sample of patients with only moderate OSA. An additional daytime control condition is needed to investigate the extent to which the observed deficits are specific to sleep and not only time related. Future research is also needed to determine whether short- or long-term treatment, including CPAP therapy, alleviates or even normalizes memory deficits in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was funded by Freiburg University Medical Center intramural funds. The authors have indicated no financial conflicts of interest. Christoph Nissen has been supported by research fellowships provided by the German Research Foundation (Ni 924/1-1) and the University of Freiburg Research Committee.
ACKNOWLEDGMENTS
The authors thank Mark H. Sanders, M.D., at the University of Pittsburgh Medical Center for helpful comments on the manuscript and the sleep technicians and doctoral students at the University Medical Center Freiburg for their help in conducting the study.
REFERENCES
- 1.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 3.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–48. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 2004;11:679–85. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nissen C, Kloepfer C, Nofzinger EA, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia - a pilot study. Sleep. 2006;29:1068–73. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- 7.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine. ICSD-2 - International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 10.Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res. 2007;6:128–34. doi: 10.1111/j.1365-2869.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 11.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 12.Décary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23:369–81. [PubMed] [Google Scholar]
- 13.Rouleau I, Décary A, Chicoine AJ, Montplaisir J. Procedural skill learning in obstructive sleep apnea syndrome. Sleep. 2002;25:401–11. [PubMed] [Google Scholar]
- 14.Macey PM, Kumar R, Woo MA, Valladares BS, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Mills PJ, Kim JH, Bardwell W, Hong S, Dimsdale JE. Predictors of fatigue in obstructive sleep apnea. Sleep Breath. 2008;12:397–9. doi: 10.1007/s11325-008-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–85. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 17.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.American Sleep Disorders Association. The International Classification of Sleep Disorders, revised. Diagnostic and Coding Manual. Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- 19.Wittchen HU, Pfister H. Instruktionsmanual zur Durchführung von DIA-X-Interviews. Frankfurt am Main: Swets & Zeitlinger; 1997. DIA-X- Interview. [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgher Schlafqualitätsindex (PSQI) Weinheim: Psychologie Verlags Union; 1989. [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale (ESS) Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Görtelmeyer R. Collegium Internationale Psychiatriae Scalarum (CIPS). Internationale Skalen fur Psychiatrie. Weinheim: Beltz; 1981. Schlaffragebogen SF-A und SF-B. [Google Scholar]
- 23.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–6. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Byrd RH, Lu P, Nocedal J, Zhu C. A limited memory algorithm for bound constrained optimization. SIAM J Scientific Computing. 1995;16:1190–208. [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 26.Schellig D, Schächtele B. Visueller und Verbaler Merkfähigkeitstest (VVM) Frankfurt am Main: Swets Test Services; 2001. [Google Scholar]
- 27.Raven JC, Court JH. Raven’s Progressive Matrices (SPM) Frankfurt am Main: Swets Test Services; 1999. [Google Scholar]
- 28.Warner MH, Ernst J, Townes BD, Peel J, Preston M. Relationships between IQ and neuropsychological measures in neuropsychiatric populations: within-laboratory and cross-cultural replications using WAIS and WAIS-R. J Clin Exp Neuropsychol. 1987;9:545–62. doi: 10.1080/01688638708410768. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprufung (TAP) Herzogenrath: Fimm, V./ Psytest; 2000. [Google Scholar]
- 30.Reitan RM. Validity of the trail making test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 31.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, California: Brain Research Institute, UCLA; 1973. [Google Scholar]
- 32.Hein H, Raschke F, Kohler D, Mayer G, Peter JH, Ruhle KH. Guideline on diagnostics and treatment of sleep-related respiratory disorders in adults. Pneumologie. 2001;55:339–42. doi: 10.1055/s-2001-15613. [DOI] [PubMed] [Google Scholar]
- 33.American Sleep Disorders Association and Sleep Research Society. ASDA Report. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 34.Le Bon O, Staner L, Rivelli SK, Hoffmann G, Pelc I, Linkowski P. Correlations using the NREM-REM sleep cycle frequency support distinct regulation mechanisms for REM and NREM sleep. J Appl Physiol. 2002;93:141–6. doi: 10.1152/japplphysiol.00917.2001. [DOI] [PubMed] [Google Scholar]
- 35.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 36.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 37.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104:7723–8. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler D, Härting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K. Deutsche Adapta-tion der Wechsler-Memory-Scale (WMS-R) Bern: Verlag Hans Huber; 2000. Wechsler-Gedächtnistest - Revidierte Fassung. dt.Version. [Google Scholar]
- 39.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–9. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 41.Cervos-Navarro J, Diemer NH. Selective vulnerability in brain hypoxia. Crit Rev Neurobiol. 1991;6:149–82. [PubMed] [Google Scholar]
- 42.Riemann D, Voderholzer U, Spiegelhalder K, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65:642–4. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 44.Engleman H, Joffe D. Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev. 1999;3:59–78. doi: 10.1016/s1087-0792(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 45.Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, Kushida C. Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome. Eur Respir J. 2001;17:838–47. doi: 10.1183/09031936.01.17508380. [DOI] [PubMed] [Google Scholar]
- 46.Tauman R, O’Brien LM, Barbé F, Iyer VG, Gozal D. Reciprocal interactions between spontaneous and respiratory arousals in adults with suspected sleep-disordered breathing. Sleep Med. 2006;7:229–34. doi: 10.1016/j.sleep.2005.09.009. [DOI] [PubMed] [Google Scholar]