Abstract
Understanding how immaturities in the reward system affect decision-making can inform us on adolescent vulnerabilities to risk-taking, which is a primary contributor to mortality and substance abuse in this age group. In this paper, we review the literature characterizing the neurodevelopment of reward and cognitive control and propose a model for adolescent reward processing. While the functional neuroanatomy of the mature reward system has been well-delineated, adolescent reward processing is just beginning to be understood. Results indicate that adolescents relative to adults demonstrate decreased anticipatory processing and assessment of risk, but an increased consummatory response. Such differences could result in suboptimal representations of reward valence and value and bias adolescent decision-making. These functional differences in reward processing occur in parallel with on-going structural and pharmacological maturation in the adolescent brain. In addition to limitations in incentive processing, basic cognitive control abilities, including working memory and inhibitory control, continue to mature during adolescence. Consequently, adolescents may be limited, relative to adults, in their abilities to inhibit impulsive behaviors and reliably hold ‘on-line’ comparisons of potential rewards/punishments during decision-making.
Keywords: Rewards, Adolescence, Cognitive control, fMRI, Dopamine
1. Introduction
Adolescence refers to the developmental time period between childhood and adulthood, generally considered to encompass ages 12–17 in humans, taking into account variability in factors such as puberty and gender (Spear, 2000; Dahl, 2004). In parallel with obvious pubertal changes (e.g., increases in height, weight, and secondary sex characteristics), a number of characteristic behaviors emerge during adolescence, including heightened sensation- and novelty-seeking and increased behavioral impulsivity (Arnett, 1992; Spear, 2000). These changes appear to be highly conserved behavioral traits, as they have been observed across cultures and even species (Spear, 2000; Laviola et al., 2003). On one hand, normative increases in these behaviors have been proposed to serve an adaptive function in that they promote exploration of the environment and the development of skills necessary for independence in adulthood (Kelley et al., 2004). On the other hand, such behaviors, particularly when coupled with immature cognitive control abilities, may increase the likelihood of engaging in risky and reckless behaviors, which can undermine survival (Zuckerman, 1979; Arnett, 1992; Spear, 2000; Zuckerman, 1994). Risk-taking is broadly defined here as engaging in behaviors that may be high in subjective desirability (i.e., associated with high sensation, novelty, or perceived reward) but exposes the individual to potential injury or loss. Examples of risk-taking include initiating use of addictive drugs, driving at excessive speeds, and engaging in unprotected sex (Arnett, 1992; Silveri et al., 2004; Dahl, 2004). Negative outcomes associated with adolescent risk taking are a major health concern for this age group (Spear, 2000; Dahl, 2004), resulting in dramatic increases in mortality rates despite peaks in other measurable aspects of physical health (Resnick et al., 1997; Call et al., 2002; Dahl, 2004).
A primary component of heightened sensation/novelty seeking and risk-taking in adolescence is immature brain circuitry mediating incentive (i.e., reward and punishment) processing (Arnett, 1992; Spear, 2000; Chambers et al., 2003; Ernst et al., 2006). Immaturities in incentive-related circuitry could, for example, lead to misevaluation of the value or predicted consequences associated with a given stimulus or action thereby biasing decision making. As an example, an adolescent with a still-maturing incentive processing system might decide that jumping his/her skateboard down a steep flight of stairs is highly rewarding, particularly if friends are watching, while not giving equal weight to the associated risk (e.g., the severe pain associated with a broken ankle) as might most adults. Characterization of the neurodevelopment of the reward system would promote understanding of adolescent risky behaviors and advance educational and intervention strategies for this age group (Dahl, 2004).
In addition to insight on risk-taking, our understanding of the etiology of mood and substance abuse disorders would be informed by the characterization of incentive processing during adolescence. Schizophrenia and depression, for instance, often emerge during the adolescent years (Sweeney et al., 2004; Everling and Fischer, 1998; Chau et al., 2004) and exhibit co-morbid abnormalities in incentive processing (Chau et al., 2004).
The normative maturation of incentive-related brain circuitry through adolescence is just beginning to be investigated in humans. Current data indicate that adolescents process incentives differently than adults, yet the nature, and more specifically the directionality of such differences remains uncertain (Chambers et al., 2003; Ernst et al., 2006; Spear, 2000) (see below). Furthermore, a mechanistic understanding of the interaction of adolescent incentive processing and other functional networks contributing to risk-taking is currently under-specified. That is, while immature incentive processing expectedly plays a primary role in these behaviors, additional functional brain systems including those mediating core aspects of cognitive control are critically intertwined and need to be jointly considered.
In this paper, we review the literature on the maturation of incentive processing and basic components of cognitive control as an initial step towards generating a clearer picture of adolescent behavior and vulnerabilities to risk-taking. We begin by highlighting two broad theoretical models that posit how adolescent incentive processing differs from adults. We then provide well-characterized evidence describing primary elements of the adult reward system followed by a review on what is currently known regarding the adolescent system. A description of brain maturation and cognitive development follows in order to provide an overall picture of the collective limitations that affect the motivation and decision-making systems during adolescence.
2. Models of adolescent incentive processing
Two models emerge from the adolescent reward literature which characterize how incentive processing is different in adolescents compared to adults, and how such processing may contribute to risk-taking (Spear, 2000; Chambers et al., 2003; Ernst et al., 2006). Both models agree that adolescents recruit a similar underlying brain circuitry and that there is a fundamental difference in the way that the adolescent brain processes incentives relative to adults. The models diverge, however, in terms of the directionality of this difference.
One model suggests that the adolescent incentive processing system is hypo-active relative to adults and results in reduced motivation (Spear, 2000). In other words, those brain areas that process incentives are not recruited as strongly or to the same degree as they are in adults given equivalent reward contingency. In this model, risk-taking is explained as adolescents seeking out experiences with high reward values because those with more modest value are not sufficiently appetitive or enticing enough to drive a normatively under-active reward system, specifically the ventral striatum (Spear, 2000). As a consequence, adolescents may be more vulnerable to drug addiction, for example, because they require quantitatively more drug per use to drive a hypo-responsive reward system. This model shares general similarity to accounts of adult dopamine (DA) hypo-function (Spear, 2000) and a model of ADHD (Castellanos and Tannock, 2002) (see below).
In contrast, a second model suggests that adolescents are hyper-responsive to incentives. That is, adolescents demonstrate a heightened sensitivity to rewards and over-activate incentive-related brain circuitry compared to adults given the same reward contingency (Chambers et al., 2003; Ernst et al., 2006). Chambers et al. (2003), for example, point out that normative maturational increases in mono-aminergic (dopamine) neurotransmitter activity in the fronto-striatal ‘motivational’ system compared to relatively lower levels of inhibitory (e.g., serotoninergic) mechanisms contribute to increased reward sensitivity in adolescents (Chambers et al., 2003). In typical development, increased activity in motivational circuitry serves an important adaptive function in that it leads to adolescents engaging in novelty and sensation-seeking behaviors which may promote independent skills necessary for survival in adulthood (Kelley et al., 2004). However, this increased activity could also confer vulnerability in adolescents in the form of a heightened sensitivity to the dependency producing effects of addictive drugs.
Hyper-active incentive processing is also central to a recently proposed triadic model (Ernst et al., 2006). This model suggests that during adolescence a normative imbalance exists between a hyperactive reward-driven system (e.g., ventral striatum-mediated) and limited harm-avoidant (e.g., amygdala-mediated) and regulatory/executive control (e.g., prefrontal cortex-mediated) circuitries. Behaviorally, adolescents are more ‘reward-driven’ (i.e., respond more strongly to rewards than adults) due to the interactions between these systems. The triadic model shares similarities with the model suggested by Chambers et al. (2003) in that there is an imbalance in reward and inhibitory circuitries during adolescence and that increased sensitivity to rewarding stimuli is hypothesized, particularly in the ventral striatum. The triadic model is novel in terms of emphasizing the notion of functional interconnectivity among multiple related circuitries including executive control to explain risk-taking.
The hypo- and hyper-active reward system models lead to contrasting predictions of neural activation and behavior in adolescents. In the following sections, we examine how the adolescent reward system may demonstrate both over- and under-active responses to a reward. We begin with a brief overview of the mature system as this establishes a useful framework for studying adolescents.
3. Adult incentive processing
Incentive processing in the mature brain is supported by a relatively well-delineated circuitry. Single-cell studies in non-human primates have demonstrated that incentives modulate neuronal activity in several regions, including (but not limited to) the dorsal and ventral striatum VS; including (nucleus accumbens, NAcc), midbrain (ventral tegmental area, substantia nigra pars compacta), amygdala, orbitofrontal cortex (OFC), medial and lateral prefrontal cortex, and posterior parietal cortex (Apicella et al., 1991; Hikosaka et al., 2006; Schultz, 2000; Roesch and Olson, 2003; Wise, 2002; Roesch and Olson, 2004). Neuroimaging studies in humans have identified similar regions in adults (Thut et al., 1997; O’Doherty, 2004; McClure et al., 2004; Delgado et al., 2000; Knutson et al., 2000; Breiter et al., 2001; Delgado et al., 2003; Elliott et al., 2003).
Importantly, the temporal resolution afforded by single-cell and event-related functional magnetic resonance imaging (fMRI) studies have lead to the observation that specific brain regions carry temporally distinct information or ‘signals’ related to rewards (Schultz et al., 2000; O’Doherty, 2004). Fig. 1 schematically represents a sample of these reward-related signals, brain regions identified as subserving them, and their temporal relation with respect to incentive delivery. In this model, incentive signals are broadly categorized as those occurring prior to or after incentive delivery. Distinguishable signals occurring prior to incentive delivery include reward detection, as well as estimation of the valence and anticipated value of a future incentive (O’Doherty et al., 2002; Knutson and Cooper, 2005). The term ‘value’ is inconsistently defined in the literature and often used interchangeably with ‘expected value’, the magnitude of a reward X probability of its attainment (Schultz, 2004). Here, value is conceptualized as a complex interaction between an incentive’s magnitude (i.e., amount of reward available) (Leon and Shadlen, 1999; Roesch and Olson, 2004; Wallis and Miller, 2003; Delgado et al., 2003), probability of attainment (O’Doherty, 2004), the time between action and incentive delivery (Tsujimoto and Sawaguchi, 2005), an animal’s state of satiety (Critchley and Rolls, 1996), and subjective preference (Tremblay and Schultz, 1999; Hassani et al., 2001). Signals occurring after incentive delivery include, for example, those related to the magnitude and valence of the received incentive (Delgado et al., 2003; Delgado et al., 2000; Rolls, 2000; O’Doherty et al., 2001), as well as those corresponding to whether or not the outcome matched up with predictions (‘prediction error’ signals) (Schultz, 2000; Schultz and Dickenson, 2000; Hare et al., 2008). Importantly, several brain regions including the OFC, VS, and medial prefrontal cortex are consistently engaged and support computations that underlie these multiple incentive signals. For example, the OFC has been implicated in executive assessment of rewards including representations of subjective preference (Hare et al., 2008; Kringelbach, 2005), while the ventral striatum (VS) contributes to anticipatory processing, including initial reward detection and prediction (Knutson and Cooper, 2005). Thus, characterizing how these regions develop, in particular, is central to understanding limitations in specific aspects of reward system function during adolescence.
Fig. 1.
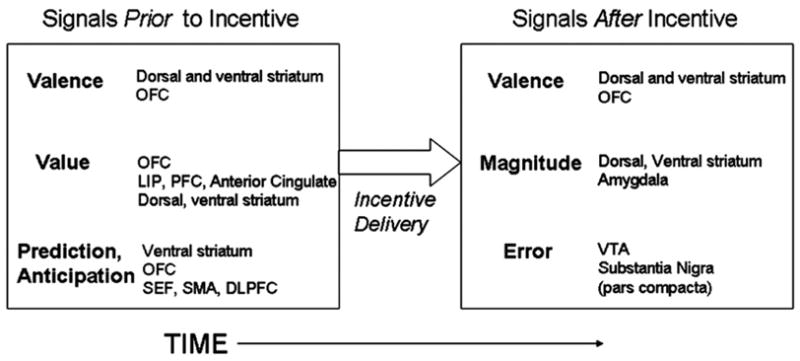
Examples of dissociable incentive-related ‘signals’ and contributing brain regions. Incentive signals can be broadly categorized as those occurring prior to (e.g., reward detection, value; ‘anticipatory processing’) and after (e.g., prediction error signals; ‘consummatory processing’) incentive delivery (see text for references).
The discernable signals and the temporal nature of reward processing observed in the mature system form a useful framework in which to consider data generated on the adolescent reward system, which is discussed next.
4. Adolescent incentive processing
In contrast to the extensive literatures exploring the neural basis of mature incentive processing in non-human primates and human adults, fewer studies have specifically focused on the development of this system through adolescence in humans (May et al., 2004; van Leijenhorst et al., 2006; Bjork et al., 2007; Bjork et al., 2004; Ernst et al., 2005; Eshel et al., 2007; Galvan et al., 2006). Collectively, studies indicate that adolescent incentive processing is supported by a similar neural circuitry as adults, including orbitofrontal cortex, basal ganglia (dorsal and ventral striatum, including nucleus accumbens), amygdala, and medial prefrontal cortex. However, as will be illustrated below, the manner in which these regions are recruited by adolescents differs during the course of incentive processing.
May et al. (2004) found that children and adolescents recruit ventral striatum and orbital frontal cortex (similar to non-human primate reports) during the anticipation of reward or loss in a gambling task. This study was the first to apply event-related functional neuroimaging methods to child and adolescent incentive processing, but did not have an adult comparison group allowing for developmental comparisons to be made in terms of the recruitment of these primary regions. Studies which have investigated developmental differences between adolescents and adults in incentive processing have focused on different temporal aspects of incentive processing, leading to disparate conclusions. For example, Bjork et al. (2004) compared blood oxygenation level dependent (BOLD) changes during an anticipatory period (i.e., before responding to receive incentive) in adolescents and adults using the monetary incentive delay (MID) task (Knutson et al., 2000), a rewarded reaction time task. Briefly, in this task subjects first saw one of several geometric shapes, each of which was uniquely associated with a different magnitude of reward (money) available at trial end. Subjects then fixated a white crosshair for a variable delay period (i.e., the ‘anticipation’ period) after which they had to quickly respond via button press when a white square was flashed on the screen. If subjects responded while the square was still visible, they earned the promised reward. While adolescents performed similarly to adults on this task (by design), adolescents exhibited significantly less activation in the right ventral striatum (nucleus accumbens, NAcc) and extended-amygdala while anticipating responding for a reward (versus a condition where no reward was available). Ernst et al. (2005) using fMRI examined changes in the BOLD response as subjects performed a rewarded decision-making task—the ‘wheel of fortune’ task. In this task, subjects had to choose via button press which half of a colored wheel they thought would be randomly picked by the computer (referred to as the ‘choice’ epoch). Each colored side was associated with a different magnitude of reward (win money) or punishment (lose money). Following a brief anticipation phase, subjects were presented with feedback about what color the computer selected (unbeknownst to the subjects, the color choice was selected at random but at a predetermined probability) and what incentive they received. During this feedback epoch (i.e., consummatory processing), adolescents demonstrated enhanced activity in the left nucleus accumbens, whereas adults exhibited more activity in the left amygdala, suggesting that adolescents are more sensitive to rewards (associated with NAcc) and adults are more sensitive to punishments (associated with amygdala) (Ernst et al., 2006). Subsequent work manipulated the probability of receiving a reward by changing the relative size of the colored wheel slices in the Wheel of Fortune task (Eshel et al., 2007). In this study, BOLD activity unique to the ‘choice’ epoch was investigated. Although behavioral performance did not differ across ages, adults activated OFC/VLPFC (BA 47, 10) and dorsal ACC (BA 32) significantly more than adolescents when making risky selections. These regions are known to contribute to aspects of cognitive control (Casey et al., 2001) as well as the monitoring and resolution of conflicting decisions (Carter et al., 1998). Results thus indicate that adolescents do not engage prefrontal regulatory mechanisms as much as adults when making risky choices. In a recent study, Bjork et al. (2007) investigated the circuitry supporting rewarded decision-making using a novel monetary game of ‘chicken’ in which subjects had to choose when to bank accumulating rewards before the trial unpredictably terminated. Trials varied in terms of the penalty associated with losing (failing to bank winnings before trial stopped). Adolescents activated posterior mesofrontal cortex, a region reported to be recruited during pre-response conflict and during the monitoring and avoidance of errors (Ridderinkhof et al., 2004), in a similar manner compared to adults in cases when a severe threat of loss was clear. However, under milder and more ambiguous conditions of risk, adolescents under-activated this region. Similarly, children (9 to 12 year-olds) compared to adults (18–26 year-olds) were found to recruit the anterior cingulate cortex more during high risk decision-making and engaged lateral orbitofrontal cortex more in response to negative compared to positive feedback (van Leijenhorst et al., 2006). These results suggest that younger subjects have limitations in reward assessment that may underlie their apparent under-activity of rewards when valence is harder to assess.
Galvan et al. (2006) using fMRI investigated BOLD differences in subjects performing a rewarded match-to-sample paradigm. Briefly, subjects saw one of three different visual cues (pictures of cartoon pirates) presented to the left or right of fixation, each of which was associated with a distinct reward value (different amounts of money). Following a brief delay, subjects saw two images of treasure chests to the left of right of fixation and were instructed to select (via button press and within 2 s) which chest appeared on the same side as the previous pirate picture. Subjects were then given feedback indicating if and how much they had won. Adolescents demonstrated an exaggerated response (higher magnitude of BOLD response) in NAcc relative to children or adults during the reward receipt epoch for large rewards. Furthermore, the extent (number of significantly active voxels) of NAcc activity in adolescents looked more like adults than children, overall. In OFC, adolescents looked more like children in terms of both extent and magnitude of activation. Results from this study were interpreted as reflecting a protracted development of OFC relative to NAcc and suggest that adolescents have limitations in the executive assessment of rewards and an overactive reward system.
Collectively, the studies suggest that the predictions of the hypo-and hyper-active models may not be mutually exclusive. For instance, Bjork et al. (2004) found under-activity in ventral striatum during a period when adolescents anticipated responding for rewards. This is a temporally distinct phase of incentive processing than that explored by Ernst et al. (2005) and Galvan et al. (2006), studies which report adolescents had increased activity when receiving reward. Thus, an important factor contributing to the hypo- versus hyper-active distinction may be the temporal stage of incentive processing under scrutiny—that is, distinct phases of incentive processing result in different patterns of activations.
Interestingly, Bjork et al. (2004) did not observe significant differences in the ventral striatum between adolescents and adults performing the MID task during reward receipt, an epoch more directly comparable with Ernst et al. (2005). One factor that may underlie these contradictory results is a difference in the levels of cognitive load demanded by the different tasks. Bjork et al. (2004) used a simple reaction time task where subjects simply responded to the appearance of a target, while the paradigms used by Galvan et al. (2006) and Ernst et al. (2006) required that subjects assess different responses and invoke working memory for instructions and past performance. More cognitively demanding tasks have been shown to recruit additional brain areas and/or increased activity within a single area (Rubia et al., 2000) and may increase the likelihood of recruiting reward-related brain areas.
Finally, we note that conclusions based on comparison of BOLD responses across different age groups are a common concern. The challenge put forth by neuroscientists investigating the adult system is that it is not straightforward if BOLD activity changes in fMRI studies are due to actual differences in neuronal computations or an isolated artifact due to immaturities in the vasculature or gross head size differences. Counter to these arguments, however, we note that brain size is adult-like early childhood (see Brain Maturation during Adolescence, below) and that the feasibility of comparing BOLD responses across developmental age groups transformed into a common stereotaxic space has been well established (Brown et al., 2005; Kang et al., 2003; Wenger et el., 2004). An additional concern is that performance differences in the scanner may lead to different levels or patterns of BOLD activity. We agree that this may be an effect in some studies. However, pediatric imaging studies frequently employ simple tasks easily performed by children (Luna et al., 2004a; Galvan et al., 2006) minimizing performance differences. Furthermore, when performance is equated across age groups (Bjork et al., 2004; Schlaggar et al., 2002), age-related functional differences are still observed.
Below, we next address why adolescents may demonstrate these particular patterns of functional brain activity—that is, what underlying brain mechanisms support these types of responses? From adolescence to adulthood, important brain structural and physiological changes occur with significant effects on brain function. Differences in brain maturational state, including thinning gray matter (e.g., synaptic pruning), increases in white matter (e.g., myelination), and neurotransmitter system differences, likely contribute to the particular functional patterns observed in adolescents and adults and are examined below.
5. Brain maturation during adolescence
Overall size, weight, cortical folding, and regional functional specialization of the human brain is adult-like by early childhood (Armstrong et al.,1995; Caviness et al.,1996; Giedd et al.,1996a; Giedd et al., 1996b; Reiss et al., 1996). While basic aspects of brain development are in place early, key processes continue to refine the basic structure to fit the biological and external environments. Two such processes include synaptic pruning and increased myelination (Huttenlocher, 1990; Jernigan et al., 1991; Pfefferbaum et al., 1994; Giedd et al., 1999b), which are critical to the developmental progression of the functional integration of frontal regions with the rest of the brain (Thatcher et al., 1987; Luna and Sweeney, 2004b; Chugani, 1998). These processes enhance neuronal processing and support mature cognitive control of behavior (Luna et al., 2004a).
5.1. Age-related gray matter reductions
Recent structural imaging studies with large subject pools indicate continued, non-linear reductions in gray matter through adolescence in cortical areas (Gogtay et al., 2004; Toga et al., 2006; Paus et al.,1999; Sowell et al., 1999a; Giedd et al., 1999a), as well as the basal ganglia (Sowell et al., 1999b). Such reduction in gray matter is largely due to the loss of weak or unused synapses via synaptic pruning (though other maturational processes such as glial cell changes, dendritic arborization, and vascular changes also contribute to this decline) (Gogtay et al., 2004). Synaptic pruning promotes enhanced information processing capacity, speed, and overall efficiency and supports complex computations within regional circuitry.
Gogtay et al. (2004) demonstrated a progressive decline of gray matter density throughout neocortex with increasing age. Notably, higher-order ‘association’ cortical areas including orbitofrontal cortex, dorsolateral prefrontal cortex, and the lateral temporal lobes, show persistent decreases in gray matter volume through adolescence (Gogtay et al., 2004). Evidence from post-mortem histological studies confirms a protracted rate of regional gray matter reduction with age that differs by region (Huttenlocher, 1990). For example, the middle frontal gyrus in prefrontal cortex continues to mature into adolescence, as opposed to visual cortex, which stabilizes near adult levels during childhood (Huttenlocher, 1990).
The basal ganglia (including dorsal and ventral striatum) and prefrontal areas, notably the orbitofrontal and dorsolateral prefrontal cortex, demonstrate comparably late maturation (Sowell et al., 1999b; Gogtay et al., 2004; Giedd, 2004). This observation has important ramifications for incentive processing during adolescence. As mentioned above, these regions underlie multiple incentive-related signals in adults. Immaturities in these areas would thus be expected to result in a limited ability to efficiently and accurately form representations of key signals like incentive valence and value. Furthermore, immaturities in the OFC and dorsal and ventral striatum would be expected to affect an adolescent’s ability to generate reliable predictions of incentive outcome and perhaps feedback-based learning computations.
5.2. Age-related white matter increases
Myelination enhances the efficiency of information processing by increasing the speed and fidelity of distal neuronal transmission, aiding the functional integration of widely distributed circuitry, critical for the emergence of complex cognitive behavior (Goldman-Rakic et al., 1992; Luna and Sweeney, 2004b). Myelination increases in a linear fashion throughout development and occurs in parallel to the non-linear gray matter reductions described above (Yakovlev and Lecours, 1967). Similar to findings regarding gray matter, myelination does not occur last in frontal regions but throughout the brain. Frontal, temporal and parietal association areas continue to myelinate through adolescence compared to earlier maturation in occipital regions. Recent studies using diffusion tensor imaging (DTI), which measures the integrity of white matter presumed to mostly reflect myelination, substantiate previous histological work and, collectively, indicate a continued increase in measures of frontal white matter anisotropy throughout childhood and into adulthood, evidence for continued white matter integrity (myelination) with age (Klingberg et al., 1999; Barnea-Goraly et al., 2005; Mukherjee and McKinstry, 2006; Huppi and Dubois, 2006).
As noted above, a distributed yet limited number of brain areas are consistently activated during incentive processing, including spatially distant regions like the orbitofrontal cortex, basal ganglia (dorsal and ventral striatum/nucleus accumbens), amygdala, and lateral prefrontal cortex. The inter-connectivity of these brain regions has been well characterized (Alexander et al., 1986; Middleton and Strick, 1994; Middleton and Strick, 2000; Middleton and Strick, 2002; Carmichael and Price, 1995; Haber et al., 1995; Haber et al., 2000; Groenewegen et al., 1997). Importantly, accumulating evidence in human and animal studies suggests that pathways within and between these regions are not yet fully myelinated during adolescence. For example, Klingberg et al. (1999) demonstrated with DTI that fiber tracts throughout frontal cortex continue to myelinate well into the second decade of life. In another study, Olesen et al. (2003) combined DTI (structural) and fMRI (functional) analyses in 8–18 year olds and demonstrated that enhanced integrity of connections between superior frontal sulcus, inferior parietal lobe, and caudate were found to correlate with BOLD response and visual-spatial working memory performance. The Olesen et al. study importantly links brain structure with function, supporting the notion that increased myelination of pathways contributes to improved working memory abilities (Luna et al., 2004a; Demetriou et al., 2002). Similarly, Liston et al. (2006) demonstrated that enhanced integrity of fronto-striatal tracts correlated with improved performance on a go/no go task and with age. The fronto-striatal tract is a crucial communication route for top-down cognitive control mechanisms like response inhibition as well as incentive processing. Converging evidence of continued myelination in the developing brain also comes from the animal literature. For example, amygdalo-cortical pathways in rat continue to myelinate through adolescence (Benes et al., 1994). The progressive maturation of amygdalo-cortical pathways could provide one plausible mechanism for increasingly more inhibitory control affecting reward processing with age.
A normatively under-myelinated brain would be expected to undermine adolescents’ ability to have efficient and rapid access to incentive signals as well as limit how rapidly these signals may be integrated and used to inform decision-making and guide behavior. Further, given that the overall value of an incentive is complex and may emerge from different processes (e.g., magnitude, delay to receipt, etc.), and that evidence suggests that these components are coded by distributed brain areas, accurate value representations, in particular, may rest on efficient functional connectivity between regions aided by myelination. Importantly, under-myelination would also make top-down, prefrontal cortex mediated cognitive control mechanisms like response inhibition (Liston et al., 2006) inefficient (see below) and may confer vulnerability to impulsive behaviors.
In addition to brain structural changes, important changes occur in key neurotransmitter systems during adolescence. Evidence for ongoing changes in dopamine signaling during adolescence will be briefly considered next.
5.3. Maturation of dopamine signaling
Dopamine (DA), a key monoamine neurotransmitter modulating reward circuitry (Kirsch et al., 2006), has been associated with multiple aspects of reward processing, including the hedonic value associated with rewards, motivation, and the reinforcement of rewarded behavior (Wise, 2004). Dopamine cells primarily originate from the zona compacta of the substantia nigra and the ventral tegmental area (VTA) and are known to project to components of the basal ganglia (nigrostriatal system), the limbic system, including hippocampus, amygdala, and nucleus accumbens (mesolimbic system), as well as to widespread areas of the frontal lobe (mesocortical system). Converging evidence from human and animal models indicates that the mechanisms underlying dopamine neurotransmission in striatal and cortical systems continue to mature during adolescence in a number of ways (Spear, 2000; Andersen, 2003; Crews et al., 2007). For example, human nigrostriatal DA neurons show the highest tyrosine hydroxylase (the rate limiting enzyme in dopamine synthesis) activity in childhood, followed by an exponential decrease during the next first three decades of life (Segawa, 2000). In rat striatum, D1 and D2 receptors levels are greater during adolescence compared to adulthood (Seeman et al., 1987). In addition to changing receptor levels, activity levels appear to change as well, with D1 and D2 receptor binding in the rat striatum peaking during adolescence (post-natal day 40) at levels that are 30–40% greater than in adults (Seeman et al., 1987; Spear, 2000). The density of dopamine transporters, which function to remove DA from the synapse, has also been shown to peak during adolescence in the striatum (Meng et al., 1999). Furthermore, evidence indicates that during adolescence, there is relatively greater activity in dopamine systems than in inhibitory serotonin (5-HT) systems, potentially resulting in an imbalance in reward (DA-mediated) and suppression (5-HT-mediated) mechanisms (Takeuchi et al., 2000; Lambe et al., 2000; Ernst et al., 2006; Spear, 2000). In mesocortical pathways, non-human primate work has shown that DA inputs to prefrontal cortex (PFC) peak in adolescence (Rosenberg and Lewis, 1994; Rosenberg and Lewis, 1995; Spear, 2000). In rats, DA fiber density to PFC also increases in adolescents relative to adults (Kalsbeek et al., 1988).
Developmental changes in dopamine signaling may provide insight on the functional differences observed between adolescent and adult incentive processing. First, as noted above there is a peak in the number of dopamine transporters in adolescence, which function to remove DA from the synapse. An increase in the number of transporters could lead to limitations in the ability to maintain motivation over a delay or anticipation period compared to adults. Indeed, a recent model of attention deficit hyperactivity disorder (ADHD) suggests that the premature removal of synaptic DA may lead to impairment in the ability to sustain motivation for a delayed reward (Castellanos and Tannock, 2002). As a behavioral consequence, short-term rewards may be favored over long-term rewards in individuals with ADHD (Krain and Castellanos, 2006). A peak in DAT resulting in normative limitations sustaining motivation across an anticipatory delay may explain adolescents’ decreased activity in the nucleus accumbens as indicated in Bjork et al. (2004). Second, as demonstrated by Segawa (2000), nigrostriatal DA neurons and components of the basal ganglia show higher activity during adolescence than adulthood. Increased dopaminergic activity, coupled with thicker gray matter (and perhaps more synapses) in adolescents than in adults (Sowell et al., 1999b), may partially explain adolescents’ enhanced response in the nucleus accumbens to the receipt of a reward—particularly when there is no delay before receiving it (and thus the increased transporters are not a factor).
6. Maturation of cognitive control
In parallel with functional changes in reward processing and ongoing structural and neurotransmitter differences, aspects of cognitive control also show protracted development through adolescence. The maturation of these cognitive control processes, including working memory and voluntary response suppression, may play significant roles in how incentives guide behavior by regulating what incentive-related information is accessible during decision-making. The maturation of voluntary response suppression and working memory, and their proposed relations to incentive-related processing and behavior, are discussed below.
6.1. Maturation of voluntary response suppression
Voluntary response suppression (also referred to as response inhibition) refers to the ability to inhibit task irrelevant responses to prepotent or salient stimuli in favor of goal-appropriate action. Inhibitory control is engaged when deciding among competing alternatives during decision making (Hooper et al., 2004; Pierrot-Deseilligny et al., 2003). As such, this system expectedly serves an important regulatory role in incentive-based decision-making. An immature voluntary response suppression system may bias an adolescent to respond to an immediate reward, even if that means neglecting a larger reward that is delivered later (i.e., delay discounting) (Yarkoni et al., 2005; Hariri et al., 2006).
A distributed neural circuitry underlies voluntary response suppression in adults, including dorsolateral prefrontal cortex (DLPFC), the cortical eye fields, anterior cingulate cortex, basal ganglia, superior colliculus, and thalamus, among others, as indicated by non-human primate electrophysiology (Munoz and Everling, 2004; Funahashi et al., 1993) and functional imaging work in human (Brown et al., 2006; Luna et al., 2001; Connolly et al., 2002; Ford et al., 2005).
Converging evidence from several studies demonstrates that inhibitory control of behavior continues to improve throughout childhood and well into adolescence. Compared to children, adolescents exhibit improved inhibitory performance during the Go-No-Go, Stroop, Flanker, and Stop signal tasks, and are able to more reliably hold fixation in the presence of visual distractors (Levin et al., 1991; Williams et al., 1999; Liston et al., 2006; Ridderinkhof et al., 1999; Paus et al.,1990; Luciana and Nelson, 1998; Tipper et al.,1989; Ridderinkhof et al., 1997). Work from our laboratory and others using the antisaccade task (Hallett, 1978), which measures the ability to halt an impending saccade to a suddenly appearing stimulus, indicates continued improvements in response suppression during adolescence, with adult-like levels of control stabilizing by mid-adolescence (Fischer et al., 1997; Fukushima et al., 2000; Klein and Foerster, 2001; Luna et al., 2004a; Munoz et al., 1998).
Although adolescents may appear to behave like adults on this task, they engage a different neural circuitry to do so. Our previous developmental antisaccade fMRI study indicated that performance on the antisaccade task is supported by the establishment of a widely distributed neural circuitry that shows continued refinement through adolescence (Luna et al., 2001; Luna et al., 2004a). Adolescents rely more heavily on less mature regions like the dorsolateral prefrontal cortex (DLPFC) while showing reduced involvement in inhibitory control areas like the cortical eye fields (FEF, SEF) (Luna et al., 2001) These data support other studies consistently indicating protracted development of inhibitory control circuitry (Rubia et al., 2000; Durston et al., 2006; Casey et al., 1997; Rubia et al., 2006; Rubia et al., 2007; Bunge et al., 2002; Adleman et al., 2002; Tamm et al., 2002; Marsh et al., 2006; Luna et al., 2001).
6.2. Maturation of working memory
Working memory refers to the ability to maintain and, when necessary, manipulate information on-line (Baddeley, 1983; Baddeley, 1992; Fuster, 1997). Working memory improvement throughout adolescence is important for the emergence of adult-level higher-order cognition (Nelson et al., 2000; Bjorklund and Harnishfeger, 1990); (Dempster, 1981; Dempster, 1981; Case, 1992). Immaturities in working memory would be predicted to limit adolescents’ ability to maintain critical incentive related information (i.e., estimated reward value, probability of reward receipt, previous reward history, etc.), particularly when there are multiple and/or competing incentive stimuli, during decision-making.
Widely distributed brain areas are known to underlie working memory. In non-human primates, such areas include prefrontal cortex (Funahashi et al., 1997; Funahashi et al., 1993), frontal eye field (FEF) (Funahashi et al., 1989), supplementary eye field (SEF) (Hanes et al., 1995), inferior parietal lobule (Colby et al., 1996; Gnadt and Andersen, 1988), caudate nucleus (Hikosaka et al., 1989), and substantia nigra pars reticulata (SNpr) (Hikosaka and Wurtz, 1983). Functional imaging studies with humans implicate the dorsolateral prefrontal cortex (DLPFC), FEF, SEF, inferior parietal sulcus (IPS), cingulate cortex, basal ganglia, and lateral cerebellum (Brown et al., 2004; Cabeza and Nyberg, 2000; Petit et al., 1998; Curtis et al., 2004; LaBar et al., 1999; Passingham and Sakai, 2004; Postle et al., 2000; Geier et al., 2007; Postle et al., 2000; Sweeney et al., 1996; Wager and Smith, 2003).
Similar to voluntary response suppression, evidence suggests a prolonged development of working memory into adolescence (Swanson, 1999; Olesen et al., 2003; Luna et al., 2004a; Luciana and Nelson, 1998; Demetriou et al., 2002). Performance on spatial working memory tasks, for example, where subjects must remember the location of a briefly appearing target in space, continues to improve from childhood through adolescence (Zald and Iacono, 1998; Geier et al., 2009; Luna et al., 2004a; Scherf et al., 2006). Improvements in controlling interference may also contribute to increased efficiency of working memory in development (Bjorklund and Harnishfeger, 1990; Sakai et al., 2002). Although adolescents recruit a more specialized network of brain regions than children during spatial working memory tasks, they are not yet at adult levels of specificity (Scherf et al., 2006; Geier et al., 2009). Further, adolescents appear to necessitate more prefrontal activity (specifically right DLPFC) to achieve similar levels of behavioral performance (Scherf et al., 2006; Luna et al., 2008).
7. Incentive processing and cognitive control
Immature incentive processing is likely not the exclusive determinant of adolescent decision making leading to risk-taking. Rather, other functional circuitries including those mediating cognitive control are critically involved (Steinberg, 2004; Ernst et al., 2006). We propose a framework for advancing current understanding of adolescent incentive processing and risk-taking which emphasizes that incentive-related signals and core aspects of cognitive control, specifically response inhibition/inhibitory control and working memory, function together during decision-making. In this model, risk-taking behavior reflects the outcome of one or more suboptimal decisions (Ernst et al., 2006; Eshel et al., 2007). Contributing to suboptimal decision-making is the interaction of immature reward processing and inconsistencies/limitations in the cognitive control of behavior. Returning to a previous example, consider again the adolescent deciding whether or not to jump his skateboard down the stairs. Immature processing in regions like the orbitofrontal cortex, for example, may lead to an enhanced value estimation of landing the jump relative to sustaining an injury, and thus bias the adolescent to engage in the behavior. Fig. 2 schematically depicts a proposed relationship between incentive processing, cognitive control abilities, and behavioral outcome.
Fig. 2.
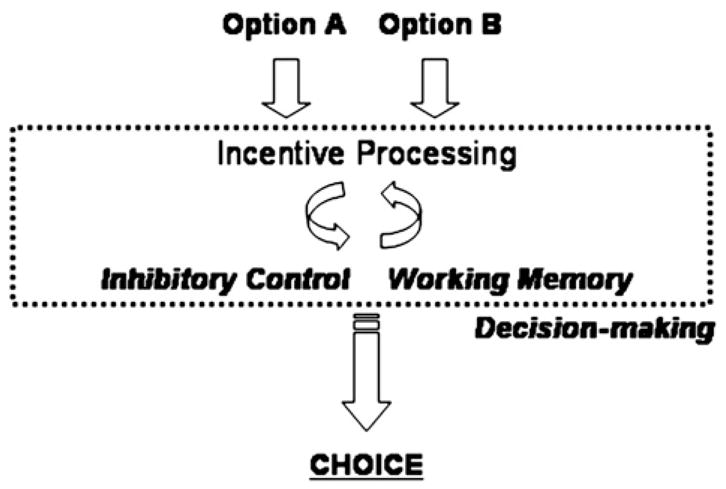
A simple model emphasizing the interaction between incentive processing and basic cognitive control abilities in decision-making. Suboptimal decision-making has been suggested to contribute to risk-taking behavior. Immaturities in brain systems supporting how incentives are represented in the brain as well as in specific cognitive control systems like working memory and inhibitory control are proposed to underlie poor decision-making.
Numerous factors including cognitive, emotional, and social processes influence decision making and risk taking behavior (e.g., computational capacity, abstract thinking abilities, social context, time estimation, etc.). Our model focuses specifically on the influence of limited incentive processing in adolescence in the context of a still developing cognitive control system. While these elements are not the only ones at play in adolescent risk taking, delineating their limitations can help us begin to understand the platform where risk taking can emerge and where other factors can then also play a role. We propose that the increased yet short lived DA processing as well as immaturities in the local circuitries and connectivity of reward related regions result in an overactive system that is biased towards short term goals. These factors can then undermine a still immature cognitive control system that can either be enhanced by the added activation of the incentive processing or distracted from considering alternatives which could result in risk taking behavior. An increased incentive system can enhance areas that support the behavior that is related to receiving the reward which can result in adaptive behavior if the decision at hand is appropriate (performing an innocuous choice in a scientific experiment) or maladaptive behavior if the reward contingent behavior has immediate rewards (social approval from doing a risky skateboarding trick).
One assumption of the proposed model is that incentives should affect performance on tasks designed to probe working memory and inhibitory control. Indeed, recent work has shown incentive-related modulation of performance in working memory (Krawczyk et al., 2007) and response suppression (Duka and Lupp, 1997; Jazbec et al., 2006; Blaukopf and DiGirolamo, 2006) in adults, and, importantly, that there are developmental differences in how rewards affect basic aspects of cognitive control (Jazbec et al., 2006). Using a rewarded antisaccade task, Jazbec and colleagues have shown that adolescents demonstrate shorter antisaccade latencies and higher peak velocities on rewarded trials compared adults, who did not modulate saccadic parameters in this task based on reward contingency. These results suggest a fundamental difference in sensitivity to the effects of incentives on inhibitory behavior in adolescents compared to adults. Importantly, this work also highlights the notion that the relationship between incentives and cognitive control processes like response suppression may be bidirectional and complex. That is, on one hand, what incentive information decision-making brain regions use may be regulated by cognitive control mechanisms. On the other hand, incentives may also enhance aspects of cognitive control (e.g., inhibitory control). One possible explanation for this enhancement may be the dopamine system biasing collicular activity (Hikosaka et al., 2000).
8. Summary and conclusions
Adolescence is a transitional developmental period marked by normative increases in risk taking, which can oftentimes lead to maladaptive outcomes. In this paper, we reviewed the literature on brain systems supporting incentive processing and basic aspects of cognitive control including working memory and response inhibition as an initial step towards gaining insight on the neurobiological mechanisms underlying risk taking behavior. Current evidence indicates that adolescents relative to adults demonstrate under- or over-activity at different stages of reward processing such as early hypo-responsiveness in the executive assessment of rewards and later hyper-activity in consummatory responses. In parallel with these functional differences are on-going brain maturational processes like synaptic pruning and myelination, as well as regional changes in dopamine neurotransmission. A simple model of adolescent risk taking was presented which emphasized the need to consider the role of immature working memory and inhibitory control systems jointly with incentive processing during decision making. In sum, risk-taking behavior in adolescence may best be understood as an emergent property of a still-maturing brain still learning to integrate external and internal drives.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop Color-Word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–73. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Philosophical Transactions of the Royal Society of London. Series B Biol Sci. 1983;B302:311–24. [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–49. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The resources construct in cognitive development: diverse sources of evidence and a theory of inefficient inhibition. Dev Rev. 1990;10:48–71. [Google Scholar]
- Blaukopf CL, DiGirolamo GJ. Differential effects of reward and punishment on conscious and unconscious eye movements. Exp Brain Res. 2006;174:786–92. doi: 10.1007/s00221-006-0685-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brown JW, Bullock D, Grossberg S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Networks: the official journal of the International Neural Network Society. 2004;17:471–510. doi: 10.1016/j.neunet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–59. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Call KT, Reidel AA, Hein K, McLoyd V, Petersen A, Kipke M. Adolescent health and well-being in the twenty-first century: a global perspective. J Res Adolesc. 2002;12:69–98. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Case R. The role of the frontal lobes in the regulation of cognitive development. Brain Cogn. 1992;20:51–73. doi: 10.1016/0278-2626(92)90061-p. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, et al. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, ystrom LE, iedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9:835–47. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–28. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Bates JF, Makris N. The developing human brain: a morphometric profile. In: Thatcher RW, Reid Lyon G, Rumsey J, Krasnegor NA, editors. Developmental Neuroimaging: Mapping The Development of Brain and Behavior. New York: Academic Press; 1996. pp. 3–14. [Google Scholar]
- Chambers RA, Taylor JR, Petenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatr. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6:391–9. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–52. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–52. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–86. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–52. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: efficiency, working memory, and thinking. Monogr Soc Res Child Dev. 2002;67:1–155. [discussion 156] [PubMed] [Google Scholar]
- Dempster FN. Memory span: sources of individual and developmental differences. Psychol Bull. 1981;89:63–100. [Google Scholar]
- Duka T, Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved. Behav Pharmacol. 1997;8:373–82. [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–7. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–99. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Res. 1997;754:285–97. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–40. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain Develop. 2000;22:173–80. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–6. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-related activity in the primate prefrontal cortex during sequential reaching tasks with delay. Neurosci Res. 1993;18:171–5. doi: 10.1016/0168-0102(93)90019-m. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behav Brain Res. 1997;84:203–23. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 3. New York: Raven Press; 1997. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–2692. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuoimage. 2007;35:904–15. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. The Development of Working Memory Maintenance. Journal of Neurophysiology. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999a;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999b;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996a;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996b;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–20. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S Am. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Bates JF, Chafee MV. The prefrontal cortex and internally generated motor acts. Curr Opin Neurobiol. 1992;2:830–5. doi: 10.1016/0959-4388(92)90141-7. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HBM. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–67. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Thompson KG, Schall JD. Relationship of presaccadic activity in frontal eye field and supplementary eye field to saccade initiation in macaque: Poisson spike train analysis. Exp Brain Res. 1995;103:85–96. doi: 10.1007/BF00241967. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–7. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol. 2001;85:2477–89. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–84. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakumura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–84. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movement. J Neurophysiol. 1989;61:780–98. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–78. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Dev Psychol. 2004;40:1148–58. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–27. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Exp Brain Res. 2006;174:754–62. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–49. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlagger BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuro-Image. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, et al. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–89. [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–21. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–44. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–77. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–7. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–25. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–95. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson C. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–93. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004a;75:1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Cognitive development: fMRI studies. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. London: Cambridge University Press; 2004b. pp. 45–68. [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–93. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier CF. Development of eye-movement control. Brain Cogn. 2008;68:293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–63. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–66. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res. 1999;843:136–44. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–61. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘Projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–35. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwitt CL. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36:109–16. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward andpunishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Diechmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol. 2004;14:163–8. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Paus T, Babenko V, Radil T. Development of an ability to maintain verbally instructed central gaze fixation studied in 8- to 10-year-old children. Int J Psychophysiol. 1990;10:53–61. doi: 10.1016/0167-8760(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby J. Sustained activity in the medial wall during working memory delays. J Neurosci. 1998;18:9429–37. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–73. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D’Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci. 2000;12:2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D’Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Protoc. 2000;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, et al. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA. 1997;278:823–32. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychol. 1999;101:315–37. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GP, Bashore TR. Sources of interference from irrelevant information: a developmental study. J Exp Child Psychol. 1997;65:315–41. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–89. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–10. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–7. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–9. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–77. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–584. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci. 2006;18:1045–58. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–9. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–47. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickenson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowj NH, Fuan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Segawa M. Development of the nigrostriatal dopamine neuron and the pathways in the basal ganglia. Brain Develop. 2000;22:S1–4. doi: 10.1016/s0387-7604(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci. 2004;1021:363–70. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999a;9:597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999b;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–8. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Swanson HL. What develops in working memory? A life span perspective. Dev Psychol. 1999;35:986–1000. doi: 10.1037//0012-1649.35.4.986. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75:454–68. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, Minshew NJ. Eye movements in neurodevelopmental disorders. Curr Opin Neurol. 2004;17:37–42. doi: 10.1097/00019052-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Matsushita H, Sakai H, Kawano H, Yoshimoto K, Sawada T. Developmental changes in cerebrospinal fluid concentrations of monoamine-related substances revealed with a Coulochem electrode array system. J Child Neurol. 2000;15:267–70. doi: 10.1177/088307380001500415. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psych. 2002;41:1231–8. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–3. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. J Exp Child Psychol. 1989;48:353–78. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Neuronal activity representing temporal precition of reward in the primate prefrontal cortex. J Neurophysiol. 2005;93:3687–92. doi: 10.1152/jn.01149.2004. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–70. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Visscher KM, Miezin FM, Petersen SE, Schlaggar BL. Comparison of sustained and transient activity in children and adults using a mixed blocked/event-related fMRI design. NeuroImage. 2004;22:975–85. doi: 10.1016/j.neuroimage.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–13. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Yarkoni T, Braver TS, Gray JR, Green L. Prefrontal Brain Activity Predicts Temporally Extended Decision-Making Behavior. J Int Neuropsychol Soc. 2005;84:537–54. doi: 10.1901/jeab.2005.121-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Iacono WG. The development of spatial working memory abilities. Develop Neuropsychol. 1998;14:563–78. [Google Scholar]
- Zuckerman M. Sensation seeking: beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum; 1979. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]


