Abstract
Purpose
To evaluate the risk of recurrence in women diagnosed with T1a and T1b, node-negative, human epidermal growth factor receptor 2 (HER2) –positive breast cancer.
Methods
We reviewed 965 T1a,bN0M0 breast cancers diagnosed at our institution between 1990 and 2002. Dedicated breast pathologists confirmed HER2 positivity if 3+ by immunohistochemistry or if it had a ratio of 2.0 or greater by fluorescence in situ hybridization (FISH). Patients who received adjuvant chemotherapy or trastuzumab were excluded. Kaplan-Meier product was used to calculate recurrence-free survival (RFS) and distant recurrence–free survival (DRFS). Cox proportional hazard models were fit to determine associations between HER2 status and survival after adjustment for patient and disease characteristics. Additionally, 350 breast cancers from two other institutions were used for validation.
Results
Ten percent of patients had HER2-positive tumors. At a median follow-up of 74 months, there were 72 recurrences. The 5-year RFS rates were 77.1% and 93.7% in patients with HER2-positive and HER2-negative tumors, respectively (P < .001). The 5-year DRFS rates were 86.4% and 97.2% in patients with HER2-positive and HER2-negative tumors, respectively (P < .001). In multivariate analysis, patients with HER2-positive tumors had higher risks of recurrence (hazard ratio [HR], 2.68; 95% CI, 1.44 to 5.0; P = .002) and distant recurrence (HR, 5.3; 95% CI, 2.23 to 12.62; P < .001) than those with HER2-negative tumors. Patients with HER2-positive tumors had 5.09 times (95% CI, 2.56 to 10.14; P < .0001) the rate of recurrences and 7.81 times (95% CI, 3.17 to 19.22; P < .0001) the rate of distant recurrences at 5 years compared with patients who had hormone receptor–positive tumors.
Conclusion
Patients with HER2-positive T1abN0M0 tumors have a significant risk of relapse and should be considered for systemic, anti-HER2, adjuvant therapy.
INTRODUCTION
Approximately 25% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2), and overexpression has been associated with worse disease-free and overall survivals.1–4 The HER2-positive phenotype correlates with poorly differentiated tumors, markers of high proliferative rate, and lack of expression of estrogen and progesterone receptors. In addition, many studies suggest that HER2 positivity is an independent predictor of disease recurrence and breast cancer–related mortality.1–3
Five randomized, phase III, clinical trials reported significant improvement in disease-free and overall survivals with trastuzumab administered in conjunction with adjuvant chemotherapy for early-stage, HER2-positive breast cancer.5–8 However, these studies included principally node-positive occurrences and, with the exception of BCIRG-006, excluded patients with tumors 1 cm or smaller that were node negative.5–8
In the setting of node-negative small tumors (ie, 1 cm or less), available data regarding HER2-positive disease recurrence at 5 and 10 years is limited.9 Consensus guidelines, such as those of the National Comprehensive Cancer Network, do not recommend systemic anti-HER2 therapy for tumors less than 1 cm because of the lack of supportive information.10 Mammography has facilitated the detection of smaller tumors; therefore, our experience in managing such tumors is limited.11,12 The purpose of this study was to evaluate the risk of recurrence in women diagnosed with T1a and T1b, node-negative, HER2-positive breast cancer.
METHODS
The Breast Cancer Management System database of The University of Texas M. D. Anderson Cancer Center (MDACC) identified women who were diagnosed before May 2002 with newly diagnosed, node-negative, invasive breast cancers that were 1 cm or smaller. Patients with ductal carcinoma in situ and microinvasion, and patients with recurrent breast cancer at presentation, were excluded. Of the 1,390 patients identified, 425 were excluded from the analysis because of male sex (n = 1), lack of receptor information (n = 237), treatment with adjuvant chemotherapy (n = 138), non–T1ab stage (n = 2), or diagnosis before 1990 (n = 47). A total of 965 patients were eligible: 77% had hormone receptor (HR) –positive tumors, 13% had triple-receptor–negative tumors (TNs), and 10% had HER2-positive tumors. Five hundred twenty-six patients (55%) received adjuvant hormonal therapy. None received adjuvant trastuzumab therapy.
A second set of patients with the same inclusion criteria and similar follow-up time was obtained from collaborators at the General Hospital Leoben, Austria, and at the Institute Jules Bordet, Brussels, Belgium. These 350 tumors were used to validate the findings of the MDACC population. The institutional review boards at the three different institutions reviewed and approved this research.
Pathology Methods
Dedicated breast pathologists at the three institutions reviewed all pathologic specimens. Immunohistochemical (IHC) analysis to determine HR status was performed by using standard procedures on 4-μm sections of paraffin-embedded tissues stained with monoclonal antibodies for estrogen and progesterone receptors. Nuclear staining ≥ 10% of either estrogen receptor or progesterone receptor was considered a positive result. HER2 status was evaluated by IHC or by fluorescence in situ hybridization (FISH). HER2 positivity was defined as 3+ receptor overexpression on IHC staining (ie, strong membranous staining in at least 10% of cells) and/or as gene amplification found on FISH. A gene copy-to–CEP-17 ratio greater than 2.0 was considered amplified.
Statistical Methods
Patients were categorized according to the HER2 status. Patient characteristics were tabulated by median and range and were compared between groups with the χ2 test or Wilcoxon rank sum test, as appropriate. Time to recurrence was measured from the date of diagnosis to the date of first local or distant disease recurrence or to last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their dates of death. Time to distant recurrence was measured from the date of diagnosis to the date of first distant recurrence or to last follow-up. Patients who died before experiencing a distant recurrence were considered censored at their dates of death, and patients who experienced local recurrence as the first recurrence were considered censored at their dates of local recurrence. Because the at-risk proportion of patients became too small at 5 years, and because most of the recurrences occurred by then, follow-up time was truncated at 5 years. Time to recurrence and time to distant recurrence were estimated according to the Kaplan-Meier method and were compared between groups with the log-rank statistic. Cox proportional hazards models were fit to determine the association of HER2 status with the risk of recurrence after adjustment for other patient and disease characteristics. Each model contained terms for HER2 status, HR status, age at diagnosis, nuclear grade, and T stage. Analyses were also performed that considered HR and HER2 status combined into one of three groups: HER2-positive status (regardless of HR status), HR-positive status (and HER2-negative status), and TN (ie, HR- and HER2-negative status). P values less than .05 were considered statistically significant. SAS 9.1 (SAS Institute, Cary, NC) was used.
The primary analyses considered patients diagnosed and treated at MDACC. Patients diagnosed and treated at other institutions were used to confirm the MDACC results. Because there were few recurrence events in the confirmatory cohort, multivariate models were not fit.
We also considered the cumulative incidence of any recurrence given death as a competing risk, we considered the cumulative incidence of distant recurrence given both death and local recurrence as competing risks. However, both the estimates and the inference tended to be similar to those obtained by the Kaplan-Meier method; therefore, only the survival analyses are presented.13,14
RESULTS
MDACC
Patient characteristics are listed in Table 1. Median age was 57 years (range, 26 to 87 years). Ten percent of patients had HER2-positive tumors, 68% had HR-positive disease, and 23% had TN breast cancer. Compared with patients who had HER2-negative tumors, those who had HER2-positive tumors were younger (P = .001), presented more frequently with T1a tumors (P = .001), had HR-positive disease less frequently (P < .001), and had tumors with higher nuclear grade (P < .001). Sixty patients were both HER2 positive and HR positive, and 31 patients received either adjuvant tamoxifen or an aromatase inhibitor.
Table 1.
MDACC Patient Demographic and Clinical Characteristics by HER2 Status
| Characteristic | HER2 Negative (n = 867) |
HER2 Positive (n = 98) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Minimal | 26 | 28 | |||
| Median | 57 | 51.5 | |||
| Maximal | 87 | 78 | < .0001 | ||
| Ethnicity | |||||
| Black | 61 | 7.0 | 9 | 9.2 | |
| Hispanic | 78 | 9.0 | 10 | 10.2 | |
| Other | 35 | 4.0 | 4 | 4.1 | |
| White | 693 | 79.9 | 75 | 76.5 | .843 |
| Menopausal status | |||||
| Premenopausal | 201 | 23.2 | 43 | 43.9 | |
| Postmenopausal | 665 | 76.8 | 55 | 56.1 | < .0001 |
| Histology | |||||
| Other | 206 | 23.8 | 8 | 8.2 | |
| Ductal | 661 | 76.2 | 90 | 91.8 | .0004 |
| T stage | |||||
| Ia | 280 | 32.3 | 43 | 43.9 | |
| Ib | 587 | 67.7 | 55 | 56.1 | .021 |
| Hormone receptor status | |||||
| Negative | 125 | 14.4 | 38 | 38.8 | |
| Positive | 742 | 85.6 | 60 | 61.2 | < .0001 |
| Nuclear grade | |||||
| 1 | 116 | 17.3 | 1 | 1.5 | |
| 2 | 386 | 57.6 | 17 | 25.4 | |
| 3 | 168 | 25.1 | 49 | 73.1 | < .0001 |
Abbreviations: MDACC, M. D. Anderson Cancer Center; HER2, human epidermal growth factor receptor 2.
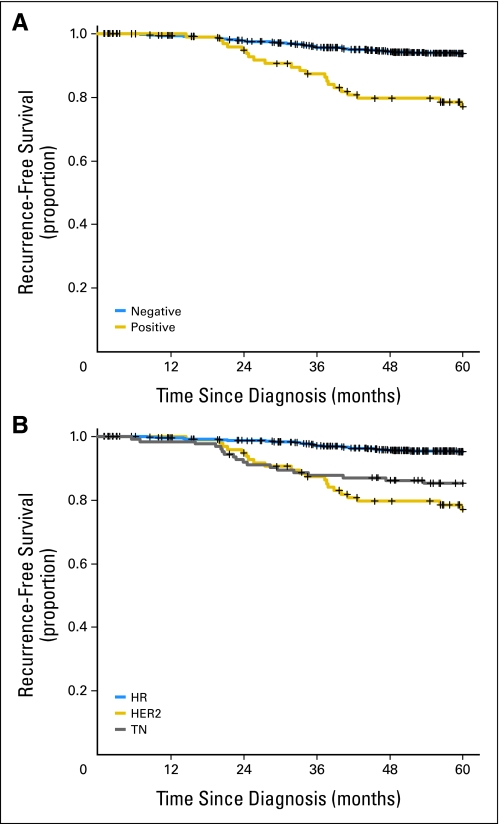
Within maximum follow-up time of 5 years, there were 72 recurrences, including 34 distant recurrences. The 5-year recurrence-free survival (RFS) estimates are listed in Table 2. Among all patients, 5-year RFS was 92.0% (95% CI, 90.1%, 93.6%). Patients who had HER2-positive breast cancer had worse RFS than patients who had HER2-negative breast cancer (77.1% v 93.7% at 5 years; P < .0001; Fig 1A). HR status, age, menopausal status, and nuclear grade also were significantly associated with RFS among these patients (Table 2). When patients were considered in groups according to HER2 and HR status, patients who had HER2-positive breast cancer had worse RFS than patients who had either HR-positive breast cancer or TN breast cancer (P < .0001; Fig 1B). There were no differences in RFS estimates in patients who had HER2-positive and HR-negative tumors compared with patients who had HER2-positive and HR-positive tumors. There were 21 recurrences in the patients who had HER2-positive disease; 13 (21%) of 60 patients who had HER2-positive and HR-positive tumors experienced recurrences; and eight (21%) of 38 patients who had HER2-positive and HR-negative tumors experienced recurrences. In addition, in the group of 60 patients who had both HER2- and HR-positive tumors, there were 13 recurrences; seven of the recurrences were in the no–hormonal therapy group, and six were in the tamoxifen or aromatase inhibitor group.
Table 2.
MDACC Survival Estimates
| Variable by Type of Survival | Survival Estimates and Analyses |
||||
|---|---|---|---|---|---|
| No. at Risk | No. of Events | 5-Year Estimate (%) | 95% CI (%) | P | |
| Recurrence-free survival | |||||
| All patients | 965 | 72 | 92.0 | 90.1 to 93.6 | |
| HER2 status | |||||
| Negative | 867 | 51 | 93.7 | 91.8 to 95.2 | |
| Positive | 98 | 21 | 77.1 | 67 to 84.5 | < .0001 |
| HR status | |||||
| Negative | 163 | 26 | 83.3 | 76.3 to 88.3 | |
| Positive | 802 | 46 | 93.9 | 91.9 to 95.4 | < .0001 |
| Breast cancer subgroup | |||||
| HER2 positive | 98 | 21 | 77.1 | 67 to 84.5 | |
| HR positive | 742 | 33 | 95.2 | 93.3 to 96.6 | |
| Triple-receptor negative | 125 | 18 | 85.2 | 77.6 to 90.4 | < .0001 |
| Age, years | |||||
| ≤ 50 | 437 | 42 | 85.0 | 80.3 to 88.7 | |
| > 50 | 528 | 30 | 95.2 | 93.2 to 96.6 | < .0001 |
| Menopausal status | |||||
| Premenopausal | 244 | 33 | 85.8 | 80.6 to 89.7 | |
| Postmenopausal | 720 | 39 | 94.2 | 92.1 to 95.7 | < .0001 |
| Histology | |||||
| Other | 214 | 14 | 93.0 | 88.4 to 95.8 | |
| Ductal | 751 | 58 | 91.8 | 89.5 to 93.6 | .547 |
| T stage | |||||
| Ia | 323 | 23 | 92.5 | 88.9 to 94.9 | |
| Ib | 642 | 49 | 91.8 | 89.3 to 93.8 | .723 |
| Grade | |||||
| 1-2 | 520 | 29 | 93.8 | 91.1 to 95.6 | |
| 3 | 217 | 30 | 85.4 | 79.8 to 89.6 | < .0001 |
| Distant recurrence–free survival | |||||
| All patients | 965 | 34 | 96.2 | 94.7 to 97.2 | |
| HER2 status | |||||
| Negative | 867 | 22 | 97.2 | 95.8 to 98.2 | |
| Positive | 98 | 12 | 86.4 | 77.3 to 92.1 | < .0001 |
| HR status | |||||
| Negative | 163 | 9 | 93.9 | 88.5 to 96.8 | |
| Positive | 802 | 25 | 96.6 | 95 to 97.7 | .111 |
| Breast cancer subgroup | |||||
| HER2 positive | 98 | 12 | 86.4 | 77.3 to 92.1 | |
| HR positive | 742 | 17 | 97.5 | 96 to 98.4 | |
| Triple-receptor negative | 125 | 5 | 95.6 | 89.8 to 98.2 | < .0001 |
| Age, years | |||||
| ≤ 50 | 437 | 19 | 92.9 | 89.1 to 95.4 | |
| > 50 | 528 | 15 | 97.6 | 96 to 98.5 | .001 |
| Menopausal status | |||||
| Premenopausal | 244 | 13 | 94.2 | 90.2 to 96.6 | |
| Postmenopausal | 720 | 21 | 96.8 | 95.1 to 97.9 | .069 |
| Histology | |||||
| Other | 214 | 8 | 95.9 | 92 to 97.9 | |
| Ductal | 751 | 26 | 96.2 | 94.5 to 97.4 | .882 |
| T stage | |||||
| Ia | 323 | 10 | 96.6 | 93.8 to 98.2 | |
| Ib | 642 | 24 | 95.9 | 94 to 97.3 | .576 |
| Grade | 94.7 to 97.2 | ||||
| 1-2 | 520 | 19 | 96.0 | 93.7 to 97.4 | |
| 3 | 217 | 12 | 94.0 | 89.6 to 96.5 | .188 |
Abbreviations: MDACC, M. D. Anderson Cancer Center; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Fig 1.
Recurrence-free survival by (A) human epidermal growth factor receptor 2 status and (B) breast cancer subtype.
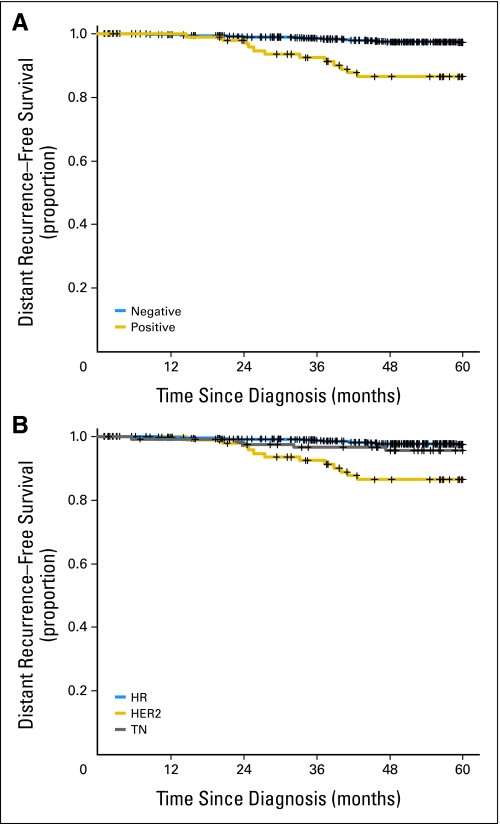
Distant recurrence–free survival (DRFS) estimates are listed in Table 2. Among all patients, DRFS at 5 years was 96.2% (95% CI, 94.7% to 97.2%). Patients who had HER2-positive breast cancer had worse DRFS than patients who had HER2-negative breast cancer (86.4% v 97.2% at 5 years; P < .0001; Fig 2A). In addition to HER2, only age was significantly associated with DRFS (P = .001). There were trends towards a significant association with HR, grade, and menopausal status. When patients were considered in groups according to both HER2 and HR status, patients who had HER2-positive breast cancer had worse DRFS than patients who had either HR-positive disease or TN disease (P < .0001; Fig 2B).
Fig 2.
Distant recurrence–free survival by (A) human epidermal growth factor receptor 2 (HER2) status and (B) breast cancer subtype. HR, hormone receptor–positive status; HER2, HER2-positive status; TN, triple-receptor–negative status.
Table 3 lists the results of the multivariable models for RFS and DRFS. After adjustment for HR status, age at diagnosis, T stage, and nuclear grade, patients who had HER2-positive breast cancer had a significantly increased risk of both recurrence (hazard ratio [HR], 2.68; 95% CI, 1.44 to 5; P = .002) and distant recurrence (HR, 5.3; 95% CI, 2.23 to 12.62; P = .0002) compared with patients who had HER2-negative breast cancer. Similarly, when patients were grouped according to both HER2 and HR status, patients with HER2-positive breast cancer had 5.09 times (95% CI, 2.56 to 10.14; P < .0001) the risk of disease recurrence and 7.81 times (95% CI, 3.17 to 19.22; P < .0001) the risk of distant recurrence compared with patients who had HR-positive disease. Patients who had TN breast cancer had 3.89 times (95% CI, 2.56 to 10.14; P < .0001) the risk of recurrence and 2.84 times (95% CI, 0.99 to 8.14; P = .053) the risk of distant recurrence compared with patients who had HR-positive breast cancer.
Table 3.
MDACC Multivariable Models
| Comparative Variable | Multivariable Analyses by Survival Status |
|||||
|---|---|---|---|---|---|---|
| Recurrence Free |
Distant Recurrence–Free |
|||||
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| HER2 status | ||||||
| Positive v negative | 2.68 | 1.44 to 5 | .002 | 5.30 | 2.23 to 12.62 | .0002 |
| Hormone receptor status | ||||||
| Positive v negative | 0.41 | 0.23 to 0.72 | .002 | 0.59 | 0.25 to 1.37 | .219 |
| Age at diagnosis, years* | 0.96 | 0.94 to 0.98 | .001 | 0.73 | 0.32 to 1.7 | .467 |
| Tumor grade | ||||||
| 3 v 1-2 | 1.34 | 0.75 to 2.41 | .320 | 0.97 | 0.94 to 1 | .080 |
| Stage | ||||||
| Ib v Ia | 1.59 | 0.91 to 2.78 | .103 | 1.47 | 0.68 to 3.18 | .329 |
Abbreviations: MDACC, M. D. Anderson Cancer Center; HER2, human epidermal growth factor receptor 2.
Continuous variable.
Other Institutions
Data on 350 additional patients treated at two other institutions were obtained and used to show reproducibility. In this cohort of patients, 21 (6%) were HER2 positive. The median age was 60 years (range, 29 to 88 years); 79% were postmenopausal; 86% had T1b primaries; 48% had HR-positive tumors; and 86% had nuclear grade 1 or 2 disease. At 5 years, only 10 patients had experienced a recurrence, and RFS was 96.4% (95% CI, 93.4% to 98.1%). The 5-year RFS rates were 87.4% (95% CI, 57.7% to 96.8%) and 97.0% (95% CI, 93.9% to 98.5%) for patients who had HER2-positive and HER2-negative tumors, respectively (P = .043). In addition, 5-year RFS were 99.3% (95% CI, 95.1% to 99.9%) and 94.7% (95% CI, 88.8% to 97.5%) for patients who had HR-positive and TN breast cancer, respectively. Patients who had HER2-positive disease had significantly worse RFS than patients with HR-positive or TN disease (P = .002). Nine of the 10 recurrence events in this group of patients were distant recurrences. Although the trend of the results remained consistent with the MDACC analyses, statistical significance was not attained. The 5-year DRFS rates were 92.3% (95% CI, 56.6% to 98.9%) and 97.0% (95% CI, 93.9% to 98.5%) for patients who had HER2-positive and HER2-negative tumors, respectively (P = .449). Among patients who had HR-positive breast cancer, the 5-year DRFS rate was 99.3% (95% CI, 95.1% to 99.9%), and the 5-year DRFS rate among patients who had TN tumors was 94.7% (95% CI, 89.0% to 97.5%).
DISCUSSION
Increased expression of HER2 or amplification of the HER2/neu gene has been associated with a more aggressive phenotype of early-stage breast cancer.11 Several large trials that incorporated the humanized monoclonal antibody against HER2, trastuzumab, into adjuvant chemotherapy regimens have demonstrated marked improvements in both disease-free survival and overall survival in patients who had HER2-positive disease. However, most of these trials consistently excluded patients with node-negative tumors that were 1 cm or smaller.5–8 Data from these studies were summarized in a meta-analysis, which demonstrated that patients who had HER2-positive breast cancer had approximately a 50% reduction in the risk of early recurrence and mortality, irrespective of nodal status. Therefore, it is likely that patients who have T1a,bN0 tumors would benefit similarly from treatment with trastuzumab.9 Guidelines generally have not recommended treatment of such patients, in part because unselected patients with T1a,bN0 tumors have an excellent prognosis and because the uncertainty if HER2 is a powerful independent unfavorable prognostic factor for patients with these otherwise low-risk cancers.10 Our analysis suggests that HER2 is a powerful negative prognostic factor for patients with T1a,bN0 tumors. These results are consistent with evolving literature that addresses this question. Joensuu et al11 described a large cohort of 852 unilateral pathologic T1N0 tumors, 313 of which were 1 cm or smaller. The rate of HER2 amplification or overexpression was 12%, similar to the 10% that we report. When this group evaluated the effect of HER2 positivity on the entire 852 patients, there was an increased risk of recurrence (HR, 2.56; 95% CI, 1.05 to 6.23; P = .04). Interestingly, in these and other small-tumor cohorts, HER2 positivity appears to be less frequent than in cohorts of larger, node-positive tumors.11,12
We have collaborated with two additional institutions to additionally examine the generalizability of these observations. Although the CIs for RFS in the confirmatory cohort are wider than the MDACC group—because there are both fewer patients and fewer recurrence events—the results of the analysis of this additional cohort appear consistent with our primary results.
Our findings show that patients who have HER2-positive tumors 1 cm or smaller have a high risk of relapse and likely should be considered for future clinical trials that include adjuvant anti-HER2 therapy, given that it will be difficult to do a clinical trial exclusively including these patients. Two trastuzumab-based adjuvant trials already have shown significant improvement in DFS when compared with chemotherapy alone in stages I to II, node-negative, HER2-positive breast cancer.6,8 In the absence of direct evidence to support such intervention, and in the absence of randomized, clinical trials to test the hypothesis, patients who have HER2-positive, T1a,bN0 tumors should be informed about the risk of recurrence and the availability of HER2-directed therapy, and there should be a clear discussion of potential risks, adverse effects, and benefits.15
Limitations of this study include its retrospective nature. However, HER2 status, in many cases, was not known and was tested only later, after the cohort was identified. For patients whose HER2 status was known, those given systemic chemotherapy or anti-HER2 therapy were excluded. Another important bias is that patients may have presented to our center with recurrence as their initial presentation, thereby artificially raising the recurrence rate. We accounted for this bias by excluding patients that initially presented to our institution with recurrent disease. It is also important to comment on the issue that patients with HER2-positive disease were more likely to have smaller tumors (ie, T1aN0); this may be explain by the selection bias, in which patients with T1b disease were treated with adjuvant chemotherapy and were excluded from the analysis.
Given the known, aggressive nature of HER2-positive tumors, clinicians struggle with therapeutic decisions because of a scarcity of clinical data in patients with tumors 1 cm or smaller. This large analysis was conducted in an effort to address such uncertainty in this population of patients with breast cancer. With the advent of digital mammography and MRI as screening modalities for breast cancer, the T1ab population could additionally increase, which would make this data even more powerful in the future as early detection improves.16 With the incidence of T1ab tumors increasing, our results strongly suggest that a large number of women may benefit from adjuvant anti-HER2 therapy. This ultimately may translate into more cures of breast cancer, if the curative efficacy of adjuvant trastuzumab therapy in this setting truly is validated.
In summary, we report that HER2 is a powerful independent prognostic factor in T1a,bN0 breast cancer. These findings, together with the evolving literature, suggest that a change in current guidelines would be appropriate and that systemic treatment with anti-HER2 therapies are worthy of consideration in the T1a,bN0 population. In addition, clinical trials to determine the benefit-to-risk ratio of adjuvant anti-HER2 therapy in this group of patients are needed.
Acknowledgment
We thank Antonio Wolff, MD, for helpful discussions, and Shu-Wan Kau, Informatics Manager for the Breast Cancer Management System database, for assistance with our data.
Footnotes
Supported in part by an American Society of Clinical Oncology Career Development Award (A.M.G.); by National Cancer Institute Grant No. 1K23CA121994-01 (A.M.G.); and by the Nellie B. Connally Breast Cancer Research Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Martine Piccart-Gebhart, AstraZeneca (C), Bristol-Myers Squibb (C), GlaxoSmithKline (C), Novartis (C), Pfizer (C), Roche (C), sanofi-aventis (C), Schering-Plough (C); Gabriel N. Hortobagyi, Bristol-Myers Squibb (C), Novartis (U) Stock Ownership: None Honoraria: Martine Piccart-Gebhart, Amgen, AstraZeneca, Eli Lilly, Novartis, Pfizer, Roche, sanofi-aventis, Schering-Plough; Gabriel N. Hortobagyi, Bristol-Myers Squibb Research Funding: Martine Piccart-Gebhart, Bristol-Myers Squibb, Pfizer, Roche; Gabriel N. Hortobagyi, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ana M. Gonzalez-Angulo, Jennifer K. Litton, Kristine R. Broglio, Funda Meric-Bernstam, Fatima Cardoso, Peter M. Ravdin, Gabriel N. Hortobagyi
Financial support: Ana M. Gonzalez-Angulo, Gabriel N. Hortobagyi
Provision of study materials or patients: Ana M. Gonzalez-Angulo, Funda Meric-Bernstam, Fatima Cardoso, Florentia Peintinger, Aysegul Sahin, Merih Guray, Denis Larsimont, Francesco Feoli
Collection and assembly of data: Ana M. Gonzalez-Angulo, Jennifer K. Litton, Ronjay Rakkhit, Fatima Cardoso, Florentia Peintinger, Emer O. Hanrahan, Heidi Stranzl
Data analysis and interpretation: Ana M. Gonzalez-Angulo, Jennifer K. Litton, Kristine R. Broglio, Funda Meric-Bernstam, Fatima Cardoso, Thomas A. Buchholz, Vicente Valero, Richard Theriault, Martine Piccart-Gebhart, Peter M. Ravdin, Donald A. Berry, Gabriel N. Hortobagyi
Manuscript writing: Ana M. Gonzalez-Angulo, Jennifer K. Litton, Kristine R. Broglio, Funda Meric-Bernstam, Ronjay Rakkhit, Fatima Cardoso, Thomas A. Buchholz, Martine Piccart-Gebhart, Donald A. Berry, Gabriel N. Hortobagyi
Final approval of manuscript: Ana M. Gonzalez-Angulo, Jennifer K. Litton, Kristine R. Broglio, Funda Meric-Bernstam, Ronjay Rakkhit, Fatima Cardoso, Florentia Peintinger, Emer O. Hanrahan, Aysegul Sahin, Merih Guray, Denis Larsimont, Francesco Feoli, Heidi Stranzl, Thomas A. Buchholz, Vicente Valero, Richard Theriault, Martine Piccart-Gebhart, Peter M. Ravdin, Donald A. Berry, Gabriel N. Hortobagyi
REFERENCES
- 1.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Ravdin PM, Chamness GC. The c-erbB-2 proto-oncogene as a prognostic and predictive marker in breast cancer: A paradigm for the development of other macromolecular markers—A review. Gene. 1995;159:19–27. doi: 10.1016/0378-1119(94)00866-q. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Slamon D, Eiermann W, Robert N, et al. BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2/neu positive early breast cancer patients. Presented at the 29th Annual San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. [Google Scholar]
- 9.Viani G, Afonso S, Stefano E, et al. Adjuvant trastuzumab in the treatment of HER-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Guidelines for Treatment of Cancer by Site. Jenkintown, PA: National Comprehensive Cancer Network; 2008. [Google Scholar]
- 11.Joensuu H, Isola J, Lundin M, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: A nationwide population-based study. Clin Cancer Res. 2003;9:923–930. [PubMed] [Google Scholar]
- 12.Norris B, Chia S, Cheang M, et al. Poor 10-year breast cancer–specific survival and relapse-free survival for HER2-positive T1N0 tumors. Presented at the 29th Annual San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. [Google Scholar]
- 13.Fine J, RJ G. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 14.Gray R. A Class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 15.Hudis CA. Trastuzumab: Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 16.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]