Abstract
Purpose
To determine the added value of quality of life (QOL) as a prognostic factor for overall survival (OS) in patients with locally advanced non–small-cell lung cancer (NSCLC) treated on Radiation Therapy Oncology Group RTOG-9801.
Patients and Methods
Two hundred forty-three patients with stage II/IIIAB NSCLC received induction paclitaxel and carboplatin (PC) and then concurrent weekly PC and hyperfractionated radiation (to 69.6 Gy). Patients were randomly assigned to amifostine (AM) or no AM during chemoradiotherapy. The following pretreatment factors were analyzed as prognostic factors for OS: Karnofsky performance status, stage, sex, age, race, marital status, histology, tumor location, hemoglobin, tobacco use, treatment arm (AM v no AM) and QOL scores (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 [QLQ-C30] and Lung Cancer 13 [LC-13]). A multivariate (MVA) Cox proportional hazards model was performed using a backwards selection process.
Results
Of the 239 analyzable patients, 91% had a baseline global QOL score. Median follow-up time was 59 months for patients still alive and 17 months for all patients. Median baseline QLQ-C30 global QOL score was 66.7 on both treatment arms. Whether the global QOL score was treated as a dichotomized variable (based on the median score) or a continuous variable, all other variables fell out of the MVA for OS. Patients with a global QOL score less than 66.7 had an approximately 70% higher rate of death than patients with scores ≥ 66.7 (P = .004). A 10-point higher baseline global QOL score corresponded to a decrease in the hazard of death by approximately 10% (P = .004). The other independent QOL predictors for OS were the QLQ-C30 physical functioning (P = .011) and LC-13 dyspnea scores (P = .012).
Conclusion
In this analysis, baseline global QOL score replaced known prognostic factors as the sole predictor of long-term OS for patients with locally advanced NSCLC.
INTRODUCTION
Although health-related quality of life (QOL) has been studied for many years in oncology trials,1 its “added value” is still debated among clinical oncologists. Performance status, as defined by the physician, rather than QOL, as self-reported by the patient, continues to be used, not only as a key stratification factor in trials, but also as a routine parameter in clinical decision making. Prior analyses have shown that QOL is an important prognostic factor in patients with cancer in general,2–5 and lung cancer specifically.6–13 However, most of these QOL analyses have been conducted in the context of patients with advanced disease with a focus on short-term survival.6,8–13 To our knowledge, this study is the first analysis of QOL as a prognostic factor for long-term survival among patients with locally advanced/inoperable non–small-cell lung cancer (NSCLC) treated with chemoradiotherapy, the current standard-of-care for this group of patients.14,15
Radiation Therapy Oncology Group (RTOG)–9801 is a phase III randomized study of the radioprotector, amifostine (AM), in patients with favorable-prognosis inoperable stage II to IIIA/B NSCLC receiving sequential induction and concurrent hyperfractionated radiotherapy (RT) with paclitaxel and carboplatin.16 QOL was studied using the validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)17 and its lung module (QLQ LC-13).18 RTOG-9801 is the largest randomized study of AM in lung cancer and the only one that included a prospective, valid, and reliable QOL instrument. The results of this randomized trial have previously been published, demonstrating that amifostine (AM) did not significantly reduce severe esophagitis in patients receiving hyperfractionated RT and chemotherapy, nor did it influence survival.16 However, patients' self-assessments, including swallowing diaries and QOL, suggested a possible advantage for AM.16 This disconnect between the objective toxicity end point and the subjective patient-reported outcome (PRO) is the topic of another article.19 The purpose of this analysis is to investigate whether any associations exist between baseline QOL and either overall survival (OS) or disease-free survival (DFS).
PATIENTS AND METHODS
The details regarding patient eligibility and treatment have previously been described.16 Briefly, eligibility stipulated unresectable and/or locoregionally advanced NSCLC (stages II, IIIA, or IIIB), age ≥ 18 years, Karnofsky performance score (KPS) ≥ 70, and weight loss ≤ 5% in the prior 3 months. All patients signed an institutional review board–approved, study-specific consent form. In addition to standard pretreatment evaluations, a QOL instrument was completed before the initiation of treatment. Patients were stratified by stage (II v IIIA v IIIB), KPS (70 to 80 v 90 to 100), and age (≤ 70 v ≥ 70 years).
Treatment began with two cycles of induction chemotherapy. Paclitaxel 225 mg/m2 intravenously (IV) was followed by carboplatin (area under the curve 6) on days 1 and 22. This was followed by concurrent weekly paclitaxel (50 mg/m2 IV) and carboplatin (area under the curve 2) during hyperfractionated RT starting on day 43. RT was administered 1.2 Gy twice daily to 69.6 Gy. At registration, patients were randomly assigned to receive or not receive AM. In the AM arm, AM (500 mg IV) over 5 minutes was generally administered before the afternoon treatment (4 days per week).
Quality of Life
PROs were captured prospectively using the EORTC QLQ-C30 questionnaire version 3.017 and QLQ LC-13,18 a lung cancer–specific instrument. These instruments together take approximately 10 to 15 minutes to complete. The QLQ-C30 is a 30-item self-report which assesses multidimensional QOL, including physical, emotional, role, social and cognitive functioning, and symptom scales. Global QOL was evaluated by two items assessing ratings of overall health and QOL on a 7-point scale (1, “very poor,” to 7, “excellent”). Symptoms were further assessed with the QLQ LC-13 scales (ie, dyspnea, cough, hemoptysis, sore mouth, dysphagia, peripheral neuropathy, alopecia, and pain “in chest, in arm or shoulder, in other parts”). Patients were asked to report severity of symptoms experienced during the previous week on a 4-point scale (1, “not at all,” to 4, “very much”). All scores on the EORTC QLQ-C30 and the QLQ LC-13 were transformed to a 0 to 100 scale according to the guidelines of EORTC.20 Higher scores in the global QOL indicate better functioning; higher scores for symptoms indicate greater symptom severity. These instruments have undergone psychometric testing with acceptable reliability and validity in lung cancer21 and are able to discriminate among patients by performance status and response.17 A 10-point difference in QOL is equivalent to a “moderate” difference, considered to be clinically meaningful.22
Statistical Methods
This was an exploratory analysis, evaluating whether associations exist between baseline QOL and either OS or DFS. Summary statistics (eg, percentages, means, SEs, and odds ratios) were presented for both categoric and continuous data. Tests of association between categoric variables were conducted using the χ2 test. t tests were used to compare the mean QOL and symptom measure scores between treatment arms. The protocol was originally designed to detect a 10-point difference in EORTC QLQ-C30 scores using a two-sided type I error = 0.05% and 80% power, requiring 116 patients per arm. In this analysis, there was a minimum of 99 patients and a maximum of 112 patients per arm, which provides 73% to 78% power to detect a 10-point difference.23
In all analyses involving the aforementioned QOL and symptom measures, all unadjusted P values were reported, but to guard against false-positive significant results in the situation of multiple comparisons, the α level was set at a conservative .01 for determining statistical significance.24 For all other analyses, which do not include the QOL and symptom measures, the α level was set at the standard level of .05.
The Kaplan-Meier method25 was used to estimate OS and DFS rates, and the log-rank test was used to compare these estimates between patient groups. An OS event was death from any cause. A DFS event was progression or death from any cause. OS and DFS were estimated from the date of randomization.
Univariate Cox proportional hazards26 models were used to identify the impact of global QOL and QLQ-C30 and LC-13 scores on OS and DFS. For each end point, two models were built with each measure. The first model analyzed the QOL measure as a continuous variable, and the second model dichotomized the measure by using its median value as the cut point (0, better QOL/less symptom severity v 1, worse QOL/greater symptom severity). For each individual QOL and symptom measure, a multivariate Cox proportional hazards model was built using that measure along with the following pretreatment factors: age (continuous), treatment arm (AM v no AM), KPS (70 to 80 v 90 to 100), histology (squamous v nonsquamous), sex, tumor location (lower/multiple v not), marital status (married/partner v single/divorced/widowed), race (white v nonwhite), American Joint Committee on Cancer stage (II/IIIA v IIIB), hemoglobin (< 12 v ≥ 12 g/dL), and smoking status (current v not). These multivariate models were built using a backwards selection process that eliminates variables with a P value more than .01. Due to missing marital (n = 6) and smoking status (n = 13) data, these multivariate models were built on slightly smaller sample sizes. Separate models not including QOL and symptom measures were built using a backwards selection process that eliminates variables with a P value more than .05. All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC).
RESULTS
This study accrued 243 patients from September 1998 through March 2002: 120 patients to the AM arm and 123 patients to the no AM arm. Two patients were ineligible on each arm. Patient characteristics, shown in Table 1, were not significantly different by treatment arm.
Table 1.
Pretreatment Characteristics by Treatment Arm
| Characteristic | Amifostine (n = 118) | No Amifostine (n = 121) |
|---|---|---|
| Age, years | ||
| ≤ 70 | 84 | 85 |
| > 70 | 16 | 15 |
| Sex | ||
| Male | 59 | 65 |
| Female | 41 | 35 |
| Race | ||
| White | 81 | 88 |
| Nonwhite | 19 | 12 |
| Hemoglobin, g% | ||
| ≥ 12 | 81 | 81 |
| < 12 | 19 | 19 |
| Karnofsky performance status | ||
| 70-80 | 28 | 21 |
| 90-100 | 72 | 79 |
| Histology | ||
| Nonsquamous | 63 | 67 |
| Squamous | 37 | 33 |
| AJCC Stage | ||
| IIA/B | 7 | 6 |
| IIIA | 46 | 45 |
| IIIB | 47 | 50 |
| Tumor location | ||
| Not lower/multiple lobes | 39 | 43 |
| Lower/multiple lobes | 61 | 57 |
| Tobacco use | ||
| Never/former | 70 | 78 |
| Currently smoke | 23 | 17 |
| Unknown | 7 | 6 |
| Marital status | ||
| Married or other live-in relationship | 69 | 74 |
| Single, divorced, separated, or widowed | 29 | 23 |
| Unknown | 3 | 3 |
Abbreviation: AJCC, American Joint Committee on Cancer.
Of the 118 patients on the AM arm, 105 patients (89%) had a baseline global QOL score compared with 112 patients (93%) on the no AM arm (P = .339). Institutional error was the most frequent reason given for these missing QOL scores (54%, AM; 67%, no AM). The baseline scores of the EORTC QLQ-C30 and EORTC QLQ LC-13 tools were equally distributed between the treatment arms (Table 2). The median baseline global QOL score was 66.7 on both arms (range, 16.7 to 100 on the AM arm and 0 to 100 on the no AM arm). There were no significant differences observed in the means of the baseline QOL measures between the treatment arms. Median follow-up for patients still alive was 59 months for patients with a global QOL score ≥ 66.7 and 55.3 months for those with scores less than 66.7. Median time from study registration was 26.8 months for all patients with a global QOL score ≥ 66.7 and 15.4 months for those with scores less than 66.7.
Table 2.
Baseline EORTC Scores
| QOL Scale | Amifostine (n = 105) |
No Amifostine (n = 112) |
P† | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No.* | Median | Mean | SE | Min | Q1 | Q3 | Max | No.* | Median | Mean | SE | Min | Q1 | Q3 | Max | ||
| EORTC QLQ-C30 | |||||||||||||||||
| Global health status/QOL | 105 | 66.7 | 62.6 | 2.29 | 16.7 | 50 | 83.3 | 100 | 112 | 66.7 | 64.7 | 2.16 | 0 | 50 | 83.3 | 100 | .503 |
| Functional scales | |||||||||||||||||
| Physical functioning | 104 | 86.7 | 82.4 | 1.69 | 20 | 73.3 | 93.3 | 100 | 112 | 86.7 | 83.8 | 1.75 | 26.7 | 73.3 | 100 | 100 | .568 |
| Role functioning | 102 | 83.3 | 77.5 | 2.65 | 0 | 66.7 | 100 | 100 | 112 | 100 | 78.7 | 2.77 | 0 | 66.7 | 100 | 100 | .742 |
| Emotional functioning | 104 | 75 | 73.2 | 2.14 | 8.3 | 66.7 | 91.7 | 100 | 112 | 75 | 71.2 | 2.16 | 0 | 58.3 | 91.7 | 100 | .506 |
| Cognitive functioning | 104 | 100 | 85.7 | 1.84 | 16.7 | 83.3 | 100 | 100 | 112 | 100 | 84.5 | 2.26 | 0 | 83.3 | 100 | 100 | .678 |
| Social functioning | 104 | 100 | 81.4 | 2.34 | 0 | 66.7 | 100 | 100 | 112 | 83.3 | 79.2 | 2.17 | 0 | 66.7 | 100 | 100 | .482 |
| Symptom scales | |||||||||||||||||
| Fatigue | 102 | 22.2 | 28.5 | 2.43 | 0 | 11.1 | 33.3 | 100 | 112 | 33.3 | 30.6 | 2.46 | 0 | 11.1 | 33.3 | 100 | .561 |
| Nausea and vomiting | 103 | 0 | 6.5 | 1.31 | 0 | 0 | 0 | 66.7 | 112 | 0 | 4.2 | 1.00 | 0 | 0 | 0 | 66.7 | .163 |
| Pain | 104 | 16.7 | 21.0 | 2.25 | 0 | 0 | 33.3 | 100 | 112 | 16.7 | 21.3 | 2.56 | 0 | 0 | 33.3 | 100 | .934 |
| Dyspnea | 103 | 33.3 | 31.4 | 2.51 | 0 | 0 | 33.3 | 100 | 112 | 33.3 | 31.0 | 2.86 | 0 | 0 | 33.3 | 100 | .909 |
| Insomnia | 102 | 33.3 | 28.1 | 2.85 | 0 | 0 | 33.3 | 100 | 111 | 33.3 | 30.0 | 2.86 | 0 | 0 | 33.3 | 100 | .635 |
| Appetite loss | 102 | 0 | 19.0 | 2.81 | 0 | 0 | 33.3 | 100 | 111 | 0 | 15.9 | 2.41 | 0 | 0 | 33.3 | 100 | .410 |
| Constipation | 102 | 0 | 11.4 | 2.15 | 0 | 0 | 33.3 | 100 | 112 | 0 | 8.3 | 1.77 | 0 | 0 | 0 | 100 | .263 |
| Diarrhea | 103 | 0 | 5.5 | 1.46 | 0 | 0 | 0 | 66.7 | 112 | 0 | 3.0 | 0.90 | 0 | 0 | 0 | 33.3 | .143 |
| Financial difficulties | 104 | 0 | 20.8 | 3.03 | 0 | 0 | 33.3 | 100 | 111 | 0 | 20.7 | 2.70 | 0 | 0 | 33.3 | 100 | .978 |
| EORTC QLQ LC-13 symptom scales | |||||||||||||||||
| Dyspnea | 102 | 22.2 | 24.2 | 2.02 | 0 | 11.1 | 33.3 | 88.9 | 108 | 22.2 | 24.7 | 2.36 | 0 | 11.1 | 33.3 | 100 | .871 |
| Coughing | 104 | 33.3 | 46.8 | 2.71 | 0 | 33.3 | 66.7 | 100 | 112 | 33.3 | 42.9 | 2.59 | 0 | 33.3 | 66.7 | 100 | .295 |
| Hemoptysis | 105 | 0 | 8.3 | 2.01 | 0 | 0 | 0 | 100 | 112 | 0 | 6.0 | 1.35 | 0 | 0 | 0 | 66.7 | .343 |
| Sore mouth | 105 | 0 | 2.9 | 1.02 | 0 | 0 | 0 | 66.7 | 112 | 0 | 6.3 | 1.72 | 0 | 0 | 0 | 100 | .091 |
| Dysphagia | 105 | 0 | 3.5 | 1.10 | 0 | 0 | 0 | 66.7 | 112 | 0 | 6.5 | 1.51 | 0 | 0 | 0 | 66.7 | .104 |
| Peripheral neuropathy | 105 | 0 | 8.6 | 1.97 | 0 | 0 | 0 | 100 | 109 | 0 | 6.4 | 1.71 | 0 | 0 | 0 | 100 | .409 |
| Alopecia | 105 | 0 | 1.3 | 0.63 | 0 | 0 | 0 | 33 | 112 | 0 | 1.8 | 0.83 | 0 | 0 | 0 | 66.7 | .620 |
| Pain in chest | 102 | 33.3 | 19.6 | 2.10 | 0 | 0 | 33.3 | 100 | 112 | 0 | 20.2 | 2.65 | 0 | 0 | 33.3 | 100 | .852 |
| Pain in arm/shoulder | 101 | 0 | 14.5 | 2.54 | 0 | 0 | 33.3 | 100 | 111 | 0 | 15.3 | 2.25 | 0 | 0 | 33.3 | 100 | .815 |
| Pain in other parts | 99 | 0 | 16.5 | 2.68 | 0 | 0 | 33.3 | 100 | 107 | 0 | 14.0 | 2.08 | 0 | 0 | 33.3 | 100 | .466 |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; QOL, quality of life; Min, minimum value; Q1, lower quartile value; Q3, upper quartile value; Max, maximum value; QLQ, Quality of Life Questionnaire; LC-13, Lung Cancer 13.
Some patients skipped questions. The No. reported for each question reflects the number of patients who answered the question.
t test.
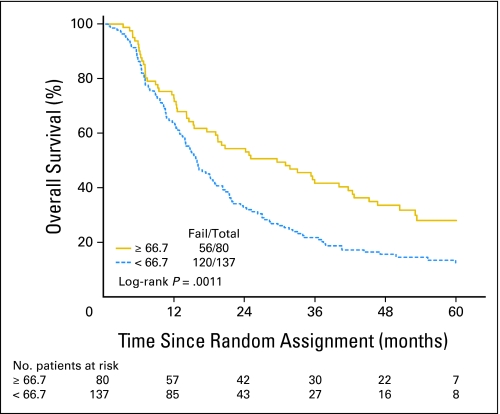
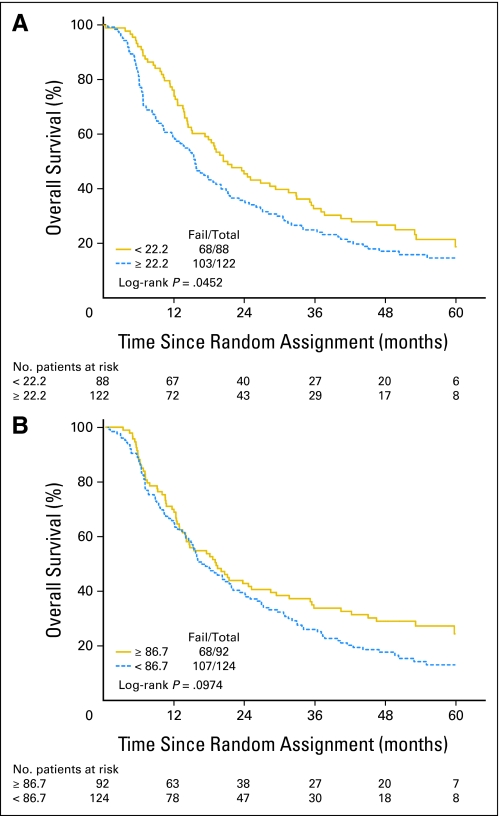
There were statistically significant associations between the baseline global QOL score and KPS, marital status, and sex. A higher global QOL score was associated with a higher KPS, as was being married/partnered, particularly for married females (Table 3). In the univariate Cox proportional hazards models for OS (Table 4), there was a statistically significant difference in OS by global QOL score (log-rank P = .001), with median survival time of 27.1 months and 5-year survival of 27% for patients with global QOL scores ≥ 66.7 compared to a median survival time of 15.4 months and 5-year survival of 11% for patients with global-QOL scores less than 66.7 (Fig 1). Patients with a global QOL score less than 66.7 had a 70% higher risk of death than patients with scores ≥ 66.7 (hazard ratio [HR] = 1.69; 95% CI, 1.23 to 2.33; P = .001). In terms of the baseline QOL subscales, there were associations seen in OS by the dichotomized LC-13 dyspnea score (Table 4, Fig 2A) and by the dichotomized QLQ-C30 physical functioning score (Table 4, Fig 2B). Baseline KPS also significantly predicted OS in univariate analysis (Table 4). Similarly, in the univariate Cox proportional hazards models for DFS, there was a statistically significant difference in DFS between patients with global QOL score ≥ 66.7 versus less than 66.7 (HR = 1.41; 95% CI, 1.04 to 1.89; P = .026). There were no other significant associations between the QOL subscales and DFS. KPS (70 to 80 v 90 to 100) was statistically significant for DFS (HR = 1.47; 95% CI, 1.06 to 2.05; P = .022).
Table 3.
Correlation of Baseline EORTC QLQ-C30 Global QOL Scores With Other Covariates
| Covariate | % Patients With Global QOL ≥ Median Score* | OR | 95% CI | χ2P |
|---|---|---|---|---|
| KPS | 5.84† | 2.36 to 14.44 | < .0001 | |
| 70-80 | 12 | |||
| 90-100 | 44 | |||
| Marital status | 2.76 | 1.35 to 5.63 | .004 | |
| Single, divorced, widowed | 21 | |||
| Married, partnered | 43 | |||
| Marital status and sex | 4.79 | 1.41 to 16.27 | .008 | |
| Single male | 16 | |||
| Married female | 48 |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; QLQ, Quality of Life Questionnaire; QOL, quality of life; OR, odds ratio; KPS, Karnofsky performance score.
The median global QOL score was 66.7.
Interpretation: The odds are 5.8 times higher of having a global QOL ≥ 66.7 in the KPS 90-100 group than in the KPS 70-80 group.
Table 4.
Results of Univariate Analysis for Overall Survival
| Covariate | Median Score | Median Survival Time (months) | 5-Year Overall Survival |
Hazard Ratio | 95% CI | χ2P | |
|---|---|---|---|---|---|---|---|
| % | 95% CI | ||||||
| EORTC QLQ-C30: global QOL score (n = 217) | < 66.7 | 15.4 | 11 | 6 to 17 | 1.69 | 1.23 to 2.33 | .001† |
| ≥ 66.7* | 27.1 | 27 | 17 to 38 | ||||
| EORTC LC-13: dyspnea score (n = 210‡) | < 22.2* | 20.8 | 19 | 10 to 29 | 1.37 | 1.01 to 1.86 | .046 |
| ≥ 22.2 | 15.6 | 15 | 9 to 22 | ||||
| EORTC QLQ-C30: physical functioning (n = 216§) | < 86.7 | 16.2 | 12 | 7 to 19 | 1.29 | 0.95 to 1.75 | .098 |
| ≥ 86.7* | 18.8 | 23 | 14 to 34 | ||||
| KPS (n = 217) | 70-80 | 14.1 | 14 | 6 to 25 | 1.47 | 1.05 to 2.08 | .026‖ |
| 90-100* | 19.8 | 17 | 12 to 24 | ||||
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; QLQ, Quality of Life Questionnaire; QOL, quality of life; LC-13, Lung Cancer 13; KPS, Karnofsky performance score.
Reference level: better functioning/less symptom severity.
Statistically significant using α = .01.
Seven patients did not answer this question.
One patient did not answer this question.
Statistically significant using α = .05.
Fig 1.
Overall survival rates based on the baseline European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 global quality-of-life (QOL) score (5-year overall survival of 27% v 11% for global QOL scores above and below the median level, respectively).
Fig 2.
(A) Overall survival (OS) rates based on the baseline European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Lung Cancer 13 (EORTC QLQ LC-13) dyspnea score (5-year OS of 15% v 19% for EORTC QLQ LC-13 dyspnea scores above and below the median level, respectively). (B) OS rates based on the baseline EORTC QLQ-C30 physical functioning score (5-year OS of 23% v 12% for EORTC QLQ-C30 physical functioning scores above and below the median level, respectively).
The results of the multivariate Cox proportional hazards models showed that when no QOL scores were included in the model, KPS was significantly associated with OS. Patients with KPS 70 to 80 had a 50% higher risk of death than patients with KPS 90 to 100 (HR = 1.47; 95% CI, 1.04 to 2.08; P = .029). When global QOL, QLQ-C30, and LC-13 scores were individually added to the model, the global QOL score, C30 physical functioning score, and LC-13 dyspnea scores were each shown to have significant associations with OS (with P ≤ .01). When the global QOL score was treated as either a dichotomized variable or a continuous variable, all other variables except the global QOL score fell out of the model for OS. Patients with a global QOL score less than 66.7 had an approximately nearly 70% higher risk of death than patients with scores ≥ 66.7 (HR = 1.64; 95% CI, 1.18 to 2.28; P = .004). Likewise, higher baseline global QOL scores of 10 points translated into a decrease in the hazard of death by 9% (HR = 0.990; 95% CI, 0.984 to 0.997; P = .004). When the C30 physical functioning score was treated as a continuous variable, all other variables fell out of the model for OS. Higher baseline physical functioning scores decreased the risk of death (HR = 0.989; 95% CI, 0.981 to 0.998; P = .011). When the LC-13 dyspnea score was treated as a continuous variable, all other variables fell out of the model for OS. Higher baseline dyspnea scores increased the risk of death (for a 10-point increase: HR = 1.088; 95% CI, 1.019 to 1.162; P = .012). Only global QOL score, as a continuous variable, showed a statistical significant association with DFS. Higher baseline global QOL scores decreased the risk of failure (HR = 0.993; 95% CI, 0.986 to 0.999; P = .031). Higher baseline global QOL score of at least 10 points translated into a decrease in the risk of treatment failure by 7%.
DISCUSSION
This analysis demonstrates that for patients with locally advanced NSCLC treated with chemoradiotherapy, the patients' appraisal of their QOL was an independent prognostic factor for survival outcomes. QOL scores replaced the classic prognosticators for long-term survival in this setting, including KPS and stage. Although a clear association between global QOL score and KPS exists (P < .0001), global QOL replaced KPS in the overall model. This does not seem to simply be due to the smaller scope of the KPS scale; the C30 physical functioning scale and the LC-13 dyspnea scores also replaced KPS in the model. Thus PROs seem to be more relevant and powerful as prognostic factors than standard measures assigned by health care providers. These findings may indicate a population in need of more intensive support. Additionally, although corroboratory studies are required, this analysis suggests, as previously pointed out,4,27 that patient-reported QOL may be a better stratification factor in future lung cancer trials than KPS.
Prior studies have similarly shown that overall QOL, or one if its subscales, is an important prognostic factor for survival in lung cancer.6–13 However, the vast majority have been conducted in the context of patients with advanced disease, such that QOL predicted for short-term survival.6,8–13 For example, in patients with advanced lung cancer, Montezari et al6 reported that the initial global QOL, on multivariate analysis, was the most significant predictor of survival at 3 months (P < .02), whereas performance status and weight loss were not. It has been suggested that pretreatment QOL may in fact be more relevant as a short-term prognostic indicator in patients with advanced disease who have a limited prognosis, but not necessarily in those with localized disease.28 There are only a handful of studies of QOL as a prognostic factor in patients with local-only disease (eg, stage III) who have the potential for long-term survival, yet most of these have been done in the palliative setting.29 In one prior study of patients with inoperable NSCLC treated with radiation alone, Langedijk et al7 reported that the pretreatment QOL, as measured by EORTC QLQ-C30, was the strongest prognostic factor for 3-year survival (on multivariate analysis), whereas performance status was not significant. To our knowledge, RTOG-9801 is the first study to confirm this key finding in patients with locally advanced NSCLC treated in the modern era using concomitant chemoradiotherapy.14,15 The 5-year survival was 27% in patients with baseline global QOL scores above the median level versus only 11% for those with scores below the median (log-rank P = .001). Further studies should be done to determine whether patients with lower pretreatment QOL could derive long-term benefit from such intensive treatment regimens. Studies are also needed to address the benefit of more supportive interventions (eg, symptom management and social support) for this high-risk group.
A limitation of this QOL analysis is that it included a fairly limited group of patients who required a good performance status (KPS ≥ 70) and minimal weight loss (≤ 5%) to be eligible. Nevertheless, even in this setting of higher functioning patients, QOL scores had substantial variability and still turned out to be a robust predictor of outcome. As similarly demonstrated by Ganz et al,12 this result suggests that QOL is a more sensitive and powerful predictor of outcome than standard prognosticators. Other studies have shown similar results in more heterogeneous settings among patients with widely varying performance status levels and cancer stages,2,3,6,9 including several recent large analyses.4,30,31 Although all these analyses, including the current one, suggest an association between QOL and survival, they do not demonstrate an underlying causal relationship. Because QOL is a secondary end point of this study, the results of this analysis should be considered exploratory. Furthermore, as recommended for QOL analyses with multiple comparisons, the α level was set at a conservative .01.24 Another limitation is the absence of baseline data on comorbidity in RTOG-9801. Of note, studies including comorbidities within QOL analyses have demonstrated mixed results.9,32 A key strength of this analysis is that the chemoradiotherapy regimen was uniform, thereby eliminating treatment variables as a potential confounding factor. Moreover, unlike other QOL analyses, which were often based on only a subset of the overall treated population, this analysis involved the vast majority (90%) of the patients treated on this protocol.
Other than the global QOL score, the only other QOL measures found to be independently significant were the C30 physical functioning and LC-13 dyspnea scores. As dyspnea can be a key factor affecting physical functioning in patients with lung cancer, this finding underscores the importance of these parameters that may be “drivers” of perceptions of overall QOL. Other studies have similarly shown that physical functioning is often the key QOL domain. For example, RTOG reported that the baseline Functional Assessment of Cancer Therapy–General score independently predicted local control, a key outcome measure in locally advanced head and neck cancer, and that physical functioning was the most important domain.33 These results suggest that QOL should include symptom assessment, such as dyspnea, which may be important in contributing to global QOL appraisals and determining outcomes.
It is often challenging for clinicians to interpret the clinical importance of statistically significant differences based on QOL scores. For this reason, QOL investigators have defined strategies to determine clinically meaningful differences in the QOL scores that are clinically relevant. For the EORTC-QLQ instrument,21 a clinically meaningful difference has been defined as ≥ 10 points (of 100). In this analysis, higher baseline global QOL scores of more than 10 points translated into a commensurate decrease in the hazard of death by approximately 10%. Not only is this an easy guideline to remember, but it also confirms the premise that a clinically meaningful difference in global QOL score (≥ 10 points) translates into different outcomes that are clinically relevant. Critical thresholds for QOL scores need further study.
The influence of sex and marital status on QOL outcomes deserves further study. In this analysis, patients who were married or had a partner had higher global QOL scores than those who did not (P = .004). Moreover, married female patients had significantly higher baseline global QOL scores than single males (P = .008). Siddiqui et al34 recently demonstrated differences in pretreatment QOL between female and male patients with locally advanced NSCLC. In another RTOG analysis, Konski et al35 reported a dramatic disadvantage in survival for men living alone who were treated on RTOG head and neck trials. The QOL findings related to the interaction of sex and marital status in the survival analysis provide further support for the importance of these characteristics.
There are a variety of challenges that must be addressed to successfully implement QOL routinely into clinical practice.36,37 One key issue is the ability to consistently and efficiently collect QOL forms from patients. Newer electronic methods are now becoming available to help reduce missing QOL data. For example, RTOG-0828 is a pilot study to assess the ability of Visiontree, a privacy-secure web-based program, to allow patients to complete QOL forms via the Internet. Second, more abbreviated QOL instruments are currently being developed to facilitate the implementation of QOL into clinical practice.38 For example, the Patient Reported Outcomes Measurement Information System, an initiative of the National Institutes of Health, is focused on developing brief instruments for measurement of PROs.39 Another critical question to address is whether and how QOL scores can be changed. If patient concerns are addressed promptly and symptoms ameliorated, will the prognostic power of baseline QOL remain? RTOG plans to conduct focus groups to further explore this issue and look for potential interventions for patients with cancer who are at high risk for poor outcomes. On a fundamental level, more research needs to be done to better define and ultimately explain the association between QOL and survival. A recent intriguing hypothesis relates to possible links between QOL and underlying biologic/genetic factors.40,41 Studies have shown that both patients and physicians value having this QOL information available as part of routine clinical practice.42,43 QOL will only become part of routine clinical practice when physicians recognize its added value and demand that this critical information be made available to them.
Footnotes
Supported by Grants No. U10 CA21661, U10 CA37422, and U10 CA32115 from the National Cancer Institute and Medimmune Oncology.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Todd Wasserman, Radiation Therapy Oncology Group (U) Consultant or Advisory Role: Corey Langer, Bristol-Myers Squibb (C); Todd Wasserman, Varian Medical Systems (C) Stock Ownership: None Honoraria: Mitchell Machtay, Medimmune, Bristol-Myers Squibb Research Funding: Corey Langer, Bristol-Myers Squibb; Mitchell Machtay, Medimmune, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin Movsas, Linda Sarna, Corey Langer, Maria Werner-Wasik, Ritsuko Komaki, Mitchell Machtay, Todd Wasserman, Deborah Watkins Bruner
Administrative support: Benjamin Movsas, Mitchell Machtay
Provision of study materials or patients: Benjamin Movsas, Corey Langer, Maria Werner-Wasik, Nicos Nicolaou, Ritsuko Komaki, Mitchell Machtay
Collection and assembly of data: Benjamin Movsas, Jennifer Moughan, Corey Langer
Data analysis and interpretation: Benjamin Movsas, Jennifer Moughan, Linda Sarna, Corey Langer, Maria Werner-Wasik, Mitchell Machtay, Todd Wasserman, Deborah Watkins Bruner
Manuscript writing: Benjamin Movsas, Jennifer Moughan, Linda Sarna, Corey Langer, Mitchell Machtay, Todd Wasserman, Deborah Watkins Bruner
Final approval of manuscript: Benjamin Movsas, Jennifer Moughan, Linda Sarna, Corey Langer, Maria Werner-Wasik, Nicos Nicolaou, Ritsuko Komaki, Mitchell Machtay, Todd Wasserman, Deborah Watkins Bruner
REFERENCES
- 1.Siddiqui F, Kachnic LA, Movsas B. Quality-of-life outcomes in oncology. Hematol Oncol Clin North Am. 2006;20:165–185. doi: 10.1016/j.hoc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: Prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33:1025–1030. doi: 10.1016/s0959-8049(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 3.Maisey NR, Norman A, Watson M, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38:1351–1357. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 4.Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 5.Dancey J, Zee B, Osoba D, et al. Quality of life scores: An independent prognostic variable in a general population of cancer patients receiving chemotherapy. Qual Life Res. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 6.Montazeri A, Milroy R, Hole D, et al. Quality of life in lung cancer patients: As an important prognostic factor. Lung Cancer. 2001;31:233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 7.Langendijk H, Aaronson NK, de Jong JM, et al. The prognostic impact of quality of life assessed with the EROTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. 2000;55:19–25. doi: 10.1016/s0167-8140(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 8.Efficace F, Bottomley A, Smit EF, et al. Is a patient's self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann Oncol. 2006;17:1698–1704. doi: 10.1093/annonc/mdl183. [DOI] [PubMed] [Google Scholar]
- 9.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non–small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 10.Herndon JE, Fleishman S, Kornblith AB, et al. Is quality of life predictive of the survival of patients with advanced non-small cell lung carcinoma? Cancer. 1999;85:333–340. doi: 10.1002/(sici)1097-0142(19990115)85:2<333::aid-cncr10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Brown J, Thorpe H, Napp V, et al. Assessment of quality of life in the supportive care setting of the big lung trial in non–small-cell lung cancer. J Clin Oncol. 2005;23:7417–7427. doi: 10.1200/JCO.2005.09.158. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Lee JJ, Siau J. Quality of life assessment. An independent prognostic variable for survival in lung cancer. Cancer. 1991;67:3131–3135. doi: 10.1002/1097-0142(19910615)67:12<3131::aid-cncr2820671232>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Eton DT, Fairclough DL, Cella D, et al. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: Results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–1543. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 14.Furuse K, Fukuoka K, Takada Y, et al. Phase III study of concurrent vs. sequential thoracic radiotherapy (TRT) in combination with mitomycin (M), vindesine (V) and cisplatin (P) in unresectable stage III non-small cell lung cancer (NSCLC) J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 15.Curran WJ, Jr, Scott C, Langer C, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:621. abstr 2499. [Google Scholar]
- 16.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non–small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation Therapy Oncology Group Trial 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Berman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC-13: A modular supplement to the EORTC core quality of life questionnaire (QLC- C30) for use in lung cancer clinical trials-EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 19.Sarna L, Swann S, Langer C, et al. Clinically meaningful differences in patient- reported outcomes with amifostine in combination with chemoradiation for locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. Int J Radiat Oncol Biol Phys. 2008;72:1378–1384. doi: 10.1016/j.ijrobp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Fayers P, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 Scoring Manual. ed 3. Brussels, Belgium: EROTC Publications; 2001. [Google Scholar]
- 21.Osoba D, Zee B, Pater J, et al. Psychometric properties and responsiveness of the EORTC Quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian, and lung cancer. Qual Life Res. 1994;3:353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 22.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 23.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 24.Fayers P. Future directions for health related quality of life. In: Lenderking WR, Revicki DA, editors. Advancing Health Outcomes Research Methods and Clinical Applications. McLean, VA: Degnon Associates; 2005. pp. 369–386. [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 27.Sarna L, Riedinger MS. Assessment of quality of life and symptom improvement in lung cancer clinical trials. Semin Oncol. 2004;31(suppl 9):1–10. doi: 10.1053/j.seminoncol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Osoba D. Lessons learned from measuring health-related quality of life in oncology. J Clin Oncol. 1994;12:608–616. doi: 10.1200/JCO.1994.12.3.608. [DOI] [PubMed] [Google Scholar]
- 29.Movsas B, Scott C. Quality-of-life trials in lung cancer: Past achievements and future challenges. Hematol Oncol Clin North Am. 2004;18:161–186. doi: 10.1016/s0889-8588(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 30.Tan AD, Novotny PJ, Kaur JS, et al. A patient-level meta-analytic investigation of the prognostic significance of baseline quality of life (QOL) for overall survival (OS) among 3,704 patients participating in 24 North Central Cancer Treatment Group (NCCTG) and Mayo Clinic Cancer Center (MC) oncology clinical trials. J Clin Oncol. 2008;26(suppl):505s. abstr 9515. [Google Scholar]
- 31.Quinten C, Coens C, Mauer M, et al. An examination into quality of life as a prognostic survival indicator. Results of meta-analysis of over 10,000 patients covering 30 EORTC clinical trials. J Clin Oncol. 2008;26(suppl):505s. abstr 9516. [Google Scholar]
- 32.Jacot W, Colinet B, Bertrand D, et al. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol. 2008;19:1458–1464. doi: 10.1093/annonc/mdn064. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui F, Pajak RF, Watkins-Bruner D, et al. Pretreatment quality of life predicts for locoregional control in head and neck cancer patients: A radiation therapy oncology group analysis. Int J Radiat Oncol Biol Phys. 2008;70:353–360. doi: 10.1016/j.ijrobp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui F, Kohl R, Swann S, et al. Gender differences in pretreatment quality of life in a prospective lung cancer trial. J Support Oncol. 2008;6:33–39. [PubMed] [Google Scholar]
- 35.Konski AA, Pajak TF, Movsas B, et al. Disadvantage of men living alone participating in Radiation Therapy Oncology Group head and neck trials. J Clin Oncol. 2006;24:4177–4183. doi: 10.1200/JCO.2006.06.2901. [DOI] [PubMed] [Google Scholar]
- 36.Frost MH, Bonomi AE, Cappelleri JC, et al. Applying quality-of-life data formally and systematically into clinical practice. Mayo Clin Proc. 2007;82:1214–1228. doi: 10.4065/82.10.1214. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, Estwing-Ferrans C, Halyard MY, et al. Exploration of the value of health-related quality-of-life information from clinical research and into clinical practice. Mayo Clin Proc. 2007;82:1229–1239. doi: 10.4065/82.10.1229. [DOI] [PubMed] [Google Scholar]
- 38.Hahn EA, Cella D, Chassany O, et al. Precision of health-related quality-of-life data compared with other clinical measures. Mayo Clin Proc. 2007;82:1244–1254. doi: 10.4065/82.10.1244. [DOI] [PubMed] [Google Scholar]
- 39.Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: A patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25:5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 40.Sprangers MA, Sloan JA, Veenhoven R, et al. The establishment of the GENEQOL consortium to investigate the genetic disposition of patient-reported quality-of-life outcomes. Twin Res Hum Genet. 2009;12:301–311. doi: 10.1375/twin.12.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyerhardt JA, Sloan JA, Sargent DJ, et al. Associations between plasma insulin-like growth factor proteins and C-peptide and quality of life in patients with metastatic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1402–1410. doi: 10.1158/1055-9965.EPI-04-0862. [DOI] [PubMed] [Google Scholar]
- 42.Detmar SB, Aaronson NK, Wever LD, et al. How are you feeling? Who wants to know? Patients' and oncologists' preferences for discussing health-related quality-of-life issues. J Clin Oncol. 2000;18:3295–3301. doi: 10.1200/JCO.2000.18.18.3295. [DOI] [PubMed] [Google Scholar]
- 43.Detmar SB, Muller MJ, Schornagel JH, et al. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]