Abstract
Background
With the availability of new generation sequencing technologies, bacterial genome projects have undergone a major boost. Still, chromosome completion needs a costly and time-consuming gap closure, especially when containing highly repetitive elements. However, incomplete genome data may be sufficiently informative to derive the pursued information. For emerging pathogens, i.e. newly identified pathogens, lack of release of genome data during gap closure stage is clearly medically counterproductive.
Methods/Principal Findings
We thus investigated the feasibility of a dirty genome approach, i.e. the release of unfinished genome sequences to develop serological diagnostic tools. We showed that almost the whole genome sequence of the emerging pathogen Parachlamydia acanthamoebae was retrieved even with relatively short reads from Genome Sequencer 20 and Solexa. The bacterial proteome was analyzed to select immunogenic proteins, which were then expressed and used to elaborate the first steps of an ELISA.
Conclusions/Significance
This work constitutes the proof of principle for a dirty genome approach, i.e. the use of unfinished genome sequences of pathogenic bacteria, coupled with proteomics to rapidly identify new immunogenic proteins useful to develop in the future specific diagnostic tests such as ELISA, immunohistochemistry and direct antigen detection. Although applied here to an emerging pathogen, this combined dirty genome sequencing/proteomic approach may be used for any pathogen for which better diagnostics are needed. These genome sequences may also be very useful to develop DNA based diagnostic tests. All these diagnostic tools will allow further evaluations of the pathogenic potential of this obligate intracellular bacterium.
Introduction
The recent availability of new generation sequencing technologies [1], [2] has provided unprecedented sequencing capacity, enabling the acquisition of genome-scale sequences at an extraordinary fast rate. These innovative techniques provide amazing opportunities for high-throughput structural and functional genomic researches and have been applied to date to a variety of contexts such as whole-genome sequencing [3] and resequencing [4], targeted resequencing [5], non coding RNA [6] or DNA-binding of modified histones [7], [8]. These high-throughput sequencing methods avoid the need for in vivo cloning and achieve a high accuracy. Even homopolymer problems, i.e. the major drawback of 454 pyrosequencing, may be overcome by reaching high sequence coverage [1]. These new technologies greatly reduce the work, time and expenses of such projects.
However, the relative short read length makes genome assembly problematic and their use in bacterial genomics has been fairly restricted to new strains closely related to already sequenced organisms to identify for example virulence factors [9], antibiotic resistance genes [10], or epidemiological markers [11]. Although improved techniques can now achieve paired-read information and longer reads [12], genomes still need a costly and time-consuming gap closure step, especially when containing highly repetitive elements such as transposases and recombination hot spots.
Still, complete genomic information is not necessarily needed and incomplete genome data obtained using high-throughput sequencing methods may potentially be informative enough to derive the pursued information. Moreover, the low time to results of such approaches (about 15 weeks [9]) is especially useful when genomic information are readily needed for instance in case of outbreaks (i) to search for the presence of specific pathogenicity island or virulence genes, (ii) to identify specific or multicopy gene targets in order to rapidly develop a reliable molecular diagnosis test, and (iii) to identify immunogenic proteins to set up a diagnostic tool for sero-epidemiological investigations or to develop a vaccine.
This strategy is particularly interesting for obligate intracellular bacteria such as members of the Chlamydiales order that lack a genetic manipulation system and only replicate within eukaryotic cells of different origins including humans, animals and amoebae [13]. One of them, Parachlamydia acanthamoebae strain Hall's coccus, was initially isolated from the water of an humidifier at the origin of a fever outbreak [14], and since then some evidences have accumulated suggesting the role of this species as an emerging human respiratory pathogen [15]. An emerging pathogen refers here to an agent that has been recently identified as pathogenic. Indeed, several serological and molecular studies supported a role of P. acanthamoebae in patients with community-acquired and aspiration pneumonia [16], [17], [18]. P. acanthamoebae also appeared to possibly cause bronchiolitis in children [19]. Moreover, pneumonia has been reproduced in a murine model following intranasal and intratracheal inoculation with P. acanthamoebae [20], [21]. Finally, the ability of Parachlamydia to resist to human macrophages [22], [23] further supported its human pathogenicity. Besides, the role of P. acanthamoebae in bovine abortion has been clearly demonstrated since the bacteria was detected by PCR, immunohistochemistry and electron microscopy in the placenta of aborted bovines [24]. The pathogenic potential of Parachlamydia towards humans and animals still remains largely unexplored since this strict intracellular bacterium does not grow on media routinely used for the detection of pathogens. To date, there are only few strains of Parachlamydia acanthamoebae available worldwide. Moreover, little information is available about strains from cattle and other animals, since no Parachlamydia strains have been isolated from animal samples by cell culture. It is thus important to develop new diagnostic approaches for P. acanthamoebae to better understand its epidemiological and pathogenic potentials in various human and animal diseases.
In this work, we undertook a proof of principle project that investigated the feasibility of combining genomic and proteomic approaches to rapidly identify immunogenic proteins. We showed that, even with relatively short reads from Genome Sequencer 20 (GS20) and after homopolymer correction through Solexa, we can gather almost the whole genome sequence of an emerging pathogen, allowing to analyze the proteome and to elaborate the first steps of an ELISA test, thus enabling to further evaluate its pathogenic role.
Results
Genome Sequencing
The pyrosequencing of P. acanthamoebae genomic DNA by two runs of GS20 yielded 566'453 reads of an average length of 111 nucleotides. In order to correct eventual frameshifts due to homopolymer errors, genomic DNA was also sequenced with Solexa technology, which produced 1'655'941 short reads of 36 bp that could be assembled in 8616 contigs. The latters were assembled with GS20 reads in 95 contigs larger than 500 bp, with a N50 size of 101'998 bp. The coverage obtained with 454 reads was of 17x whereas that obtained with Solexa reads was of 12x. The 95 contigs represents approximately 97% of the total genome and as many as 99.99% of all the non-repeated regions, i.e. when excluding contigs exhibiting a sequence depth higher than 30x with 454. As indicated by the total length of the contigs, the complete genome of P. acanthamoebae Hall's coccus stands around 3 Mb and was predicted to contain 4798 open reading frames larger than 90 nucleotides. More than 91% of the large contigs were covered with Solexa. The 1037 differences between Solexa and 454 were manually inspected. As many as 405 differences could be attributed to the presence of homopolymers and were corrected according to Solexa whereas the remaining 632 differences were mainly due to inaccurate Solexa contig ends and were not corrected.
Identification of Immunoreactive Proteins
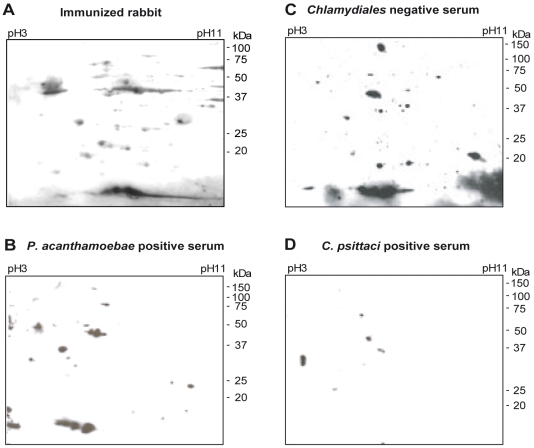
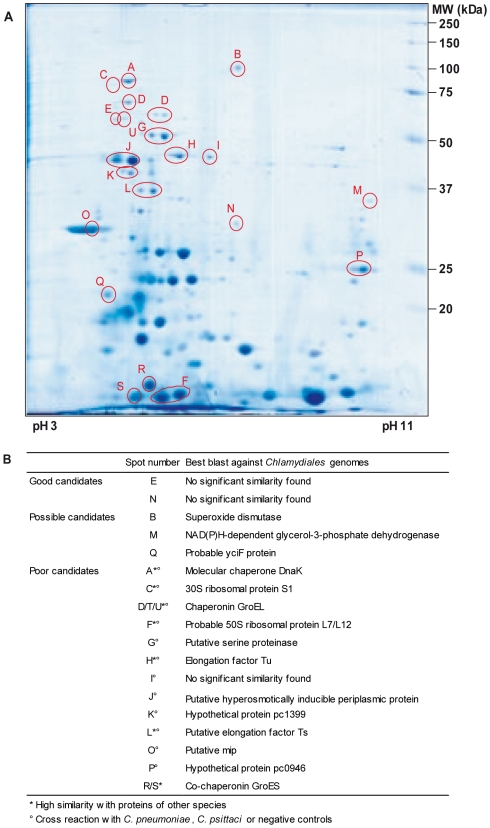
To identify immunoreactive proteins that could be used in a diagnostic test, total proteins of P. acanthamoebae elementary bodies were separated by 2D gel electrophoresis and either Coomassie blue-stained or transferred onto nitrocellulose membranes. Immunoblots were performed with sera of rabbits immunized with P. acanthamoebae and with human P. acanthamoebae positive sera ( Fig. 1A,C ). Spots corresponding to immunogenic proteins reacting with at least one rabbit anti-Parachlamydia serum were selected by computer-assisted matching of the Coomassie blue-stained gel and immunoblots, and further analyzed by mass spectrometry. Eighteen different proteins were identified ( Fig. 2A ), out of which 5 reacted only against sera from immunized rabbits and 13 reacted with both rabbit and human Parachlamydia positive sera. Some of these proteins, such as chaperonin GroEL (Hsp60), DnaK (Hsp70), elongation factor Tu and the ribosomal proteins S1 and L7/L12, were already known to be antigenic [25] ( Fig. 2B and Table S1). Some classical Chlamydiales immunogenic proteins, such as 60 kDa cysteine-rich OMP, LcrE or CPAF protease [25], [26], [27] were not detected. Since the corresponding genes were found in our contig assembly, these proteins are likely poorly expressed in elementary bodies or when the bacteria are co-cultivated in amoebae. Membranes were also probed with control human sera, i.e. either completely negative for any member of the Chlamydiales order ( Fig. 1B ), or positive only for C. psittaci ( Fig. 1D ) or C. pneumoniae (see Table S2). Based on 2D immunoblots with control sera and blast analysis of the MS identified proteins, the best candidates for a diagnostic assay of P. acanthamoebae infection were determined (see Table S2). Antigens displaying a high sequence homology with similar proteins in other species as well as proteins cross-reacting with non specific or negative sera were discarded. The two best candidate proteins were selected for evaluation in an ELISA test ( Fig. 2B ).
Figure 1. 2D patterns of the immunoreactive proteins of P. acanthamoebae.
Proteins of P. acanthamoebae separated by 2D gel electrophoresis were probed with (A) serum from immunized rabbit #1, (B) a Chlamydiales negative human serum, (C) a P. acanthamoebae positive human serum, and, (D) a C. psittaci positive human serum. Five immunogenic proteins are numbered in reference to the following figures.
Figure 2. 2D map and identification of P. acanthamoebae immunogenic proteins.
A. Proteins reacting with at least one rabbit anti-Parachlamydia serum were excised from gel and analysed by MALDI TOF mass spectrometry. Spots successfully identified are numbered. Molecular mass standards are indicated on the right side of the gel. B. The potential of 18 immunogenic proteins for use in a serological diagnostic test was evaluated based on their reactivity with control sera and on their sequence similarity in BLASTP results (see Table S2 for detailed analysis).
Western-Blot and ELISA of Proteins E and N
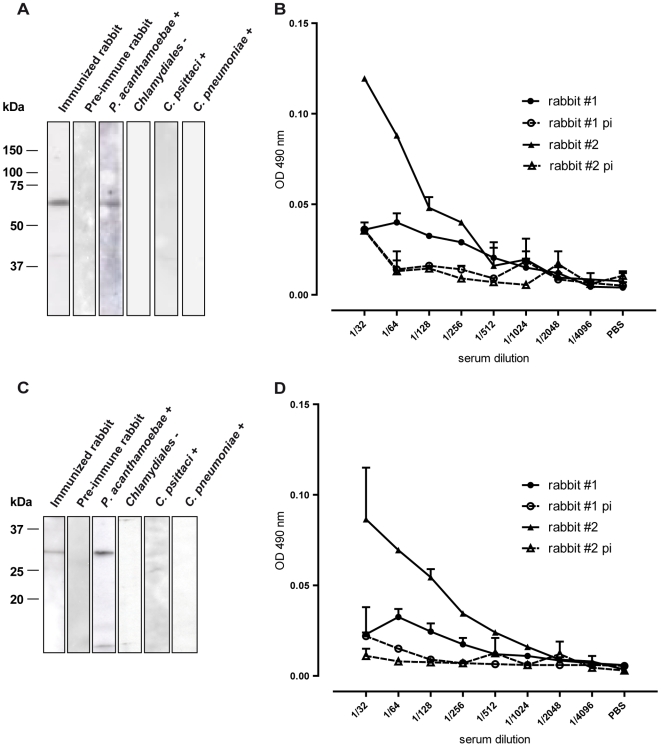
The parachlamydial protein E and N, which have no sequence homology with any known protein, were chosen to develop serological diagnostic tools. Recombinant proteins E (MW ∼58 kDA) and N (MW ∼30 kDA) were expressed in E. coli and purified thanks to a 6His tail fused to their N-terminal end. The purified recombinant proteins were detected by western blot with a rabbit anti-Parachlamydia serum or with a human P. acanthamoebae positive serum ( Fig. 3A,C ). Lower molecular weight bands also visible on these blots probably correspond to degradation products. Moreover, faint bands were detectable when protein E was probed with a C. psittaci positive serum indicating a low level of cross reaction with this organism. For both proteins, no signal was obtained when Chlamydiales negative or C. pneumoniae positive sera were tested.
Figure 3. Western blot and ELISA with recombinant proteins E and N.
Purified recombinant protein E (A) and N (C) were blotted on a nitrocellulose membrane and probed with a rabbit anti-Parachlamydia serum (rabbit #1), a rabbit pre-immune serum (rabbit #1), a P. acanthamoebae positive human serum, a Chlamydiales negative human serum, a C. psittaci positive human serum and a C. pneumoniae positive human serum. Proteins E (B) and N (D) were used as antigen in a direct ELISA. Sera from 2 rabbits immunized with P. acanthamoebae and pre-immune (pi) sera were tested in duplicates.
When used as antigen in a direct ELISA assay, purified proteins E and N were detected by the sera of two rabbits immunized with P. acanthamoebae until a dilution value of 1/256 while only background reaction is observed with pre-immune sera at this and lower dilutions ( Fig. 3B,D ). For both proteins, a significant difference was observed in the level of reactivity of the two sera. However, both rabbit sera exhibited good antigen reactivity when tested by western-blot. Overall, these data demonstrate the potential of immunogenic proteins E and N for serological diagnostic tests that could be developed in the future.
Comparison between Combined or Separated 454 and Solexa Approaches to Identify Proteins
In addition to identifying immunogenic proteins, the most abundantly expressed proteins of P. acanthamoebae elementary bodies were also analyzed. A total of 95 Coomassie blue-stained spots were analyzed by mass spectrometry and a reliable protein identification was obtained for 85 of them using the combined GS20 and Solexa sequences. Identification failed for 2 proteins due to the absence of signal by mass spectrometry and for 8 proteins due to the absence of hits in the genome-derived protein database. In many cases multiple spots on the gel corresponded to a single protein so that 61 different proteins were identified, including the immunoreactive proteins described above (see Fig. S1).
All 61 proteins identified using combined GS20 and Solexa sequences would also have been identified when using only the GS20 sequences-derived protein database. However, with uncorrected GS20 sequences, 4 ORFs presented a frameshift leading twice to a longer protein and twice to a premature end of the protein, i.e. splitting the ORF in two parts. Only 5 of the 61 proteins identified using combined GS20 and Solexa sequences were identical to the predicted ORFs using only Solexa sequences. The remaining 56 proteins were split between two to seven different small contigs preventing their accurate identification by Mascot. The limited performance of Solexa technology as compared to 454 is likely due to the short Solexa reads and the relative low sequence depth obtained.
Most Abundantly Expressed Proteins and Virulence Genes
By BLAST against nr database, a function could be derived for half of the 61 proteins identified by mass spectrometry (for details, see Table S1 and Table S3), whereas one fourth have homologs of unknown function in Protochlamydia amoebophila genome [33] or in other organisms. Finally, the remaining proteins exhibit no significant homology with any known amino acid sequence.
Our assembly also enabled the identification of several virulence genes present in P. acanthamoebae genome. In addition to the previously mentioned LcrE and CPAF protease, P. acanthamoebae encodes a complete type three secretion system (T3SS), including components of the secretory apparatus, translocators, T3SS specific chaperones and effectors [28]. Like in other Chlamydiales species, T3SS genes are spread in distinct conserved genomic clusters (see Fig. S2). Interestingly, sctJ and sctC, two genes encoding components of the secretory apparatus, are duplicated in the genome. Moreover, four clear homologs to Protochlamydia amoebophila nucleotide transporters (ntt_1, ntt_2, ntt_3 and ntt_4) which play a key feature in energy parasitism [29], [30] have been identified in P. acanthamoebae. A fifth putative ADP/ATP translocase candidate was also detected but homologies were not sufficient to establish the substrate transport specificity. Finally, four genes belonging to the F-like conjugative DNA transfer operon located on the genomic island of Protochlamydia amoebophila [31] were also detected in P. acanthamoebae (traU, traN, traF and pc1435).
Discussion
In case of outbreak due to a new pathogen, diagnostic tests must be developed rapidly. Genome sequence is an important resource to develop various tools for molecular and serological diagnostic, specific monoclonal antibodies production or vaccine design. With the availability of high throughput sequencing strategies such as the widely used GS20/GSFLX [1], large sequence datasets are now obtained within very short time. However, the costly and time-consuming follow up necessary to close the gaps delays the release and accessibility of most genome sequences. As shown for Francisella tularensis, a rapid comparative genome analysis can be successfully applied on unfinished contigs enabling to uncover genomic rearrangements or gene mutations that could be involved in an increased strain virulence and resistance [9]. A similar approach was also proposed to study the role of Helicobacter pylori in chronic gastric infection by analyzing genetic changes in this species over time or between infected humans [32].
A rapid and public availability of raw genome data from an emerging pathogen at the origin of an outbreak is critical to permit the development of various diagnostic tools by medical microbiologists. The delay before genome release is especially crucial in case of new pathogenic agents with the absence of available closely related genomes, i.e. absence of scaffold that may be used to facilitate assembly and gap closure steps. This problem was faced here for P. acanthamoebae with the availability of a single published genome within the Parachlamydiacaeae family, that of Protochlamydia amoebophila [33]. The presence of repeated elements in the genome significantly increases the number of contigs obtained, thus prolonging the gap closure. Although Chlamydiaceae genomes do not contain many transposases, the genome sequence of Protochlamydia amoebophila was much more invaded by such repeated components [33]. This suggests that sequence repetitions probably account for a large number of gaps in our own Parachlamydia genome project. Nevertheless, if these repeated elements can prevent an assembly in one unique contig, they do not hinder the availability of most coding sequences. Indeed, 90% of analyzed proteins could be identified, the remaining 10% being uncharacterized due either to the lack of mass spectrometry signal or to the lack of hits in the ORF database. Thus, although we could not determine the exact number of immunogenic proteins that have been missed due to the presence of the remaining gaps, we may estimate that only few (<10%) additional immunogenic proteins would have been identified if a complete genome sequence was available.
Our proteomic approach allowed us to detect 18 immunogenic proteins among which are several antigens already described as highly immunoreactive such as GroEL, DnaK or elongation factor Tu [25]. Five proteins represented good/possible candidates to develop a diagnostic test since exhibiting significant reactivity to sera taken from humans infected by Parachlamydia as well as from immunized rabbits and no cross-reactivity to sera from humans infected with C. pneumoniae, C. psittaci and negative controls. Then, we focused on only two of these five proteins, E and N, displaying no significant homology with any known amino acid sequence. Their potential to develop vaccine or diagnostic tools was suggested by western-blot and by preliminary ELISA tests despite the absence, in the heterologous protein expression system used, of post-translational modifications such as glycosylation or phosphorylation that might have resulted in poor serum recognition. Given its 96-well format, the ELISA test, once developed, would be very useful in large epidemiological studies to assess the precise seroprevalence of Parachlamydia antibodies in human population and to confirm the pathogenic role of this intracellular bacterium in human lower respiratory tract infections and in bovine abortion. Moreover, an ELISA based on a given immunogenic protein will be more specific than diagnostic microimmunofluorescence and western-blot assays based on whole bacterial proteins.
Interestingly, among the 85 ORFs identified using our dirty genome sequences, only 9 proteins could have been identified by Mascot versus protein sequences of the closest related bacteria available to date, Protochlamydia amoebophila, all of which are very conserved and, if immunogenic, would likely produce strong cross reactions when used in serological tests. In addition, among the five immunogenic proteins selected as good and possible candidates for the development of a diagnostic ELISA, none have been identified by Mascot versus SPTrembl database because of the differences between the peptides identified and the protein database. Moreover, the two proteins we considered as the best candidates have no homologs in other genomes and their sequences could not be derived from those of any related or unrelated bacteria.
This further emphasizes the need for a protein database directly derived from genome sequences of the studied emerging pathogen. Besides, rapid genome sequencing provides information useful not only for proteomics but also for comparative genomics, transcriptomics, cell biology and molecular biology. The availability of most genome sequences of a new emerging pathogen isolated during an outbreak may also be important to design molecular diagnostic tools, to define epidemiological marker as well as to identify virulence genetic traits and antibiotic resistance determinants.
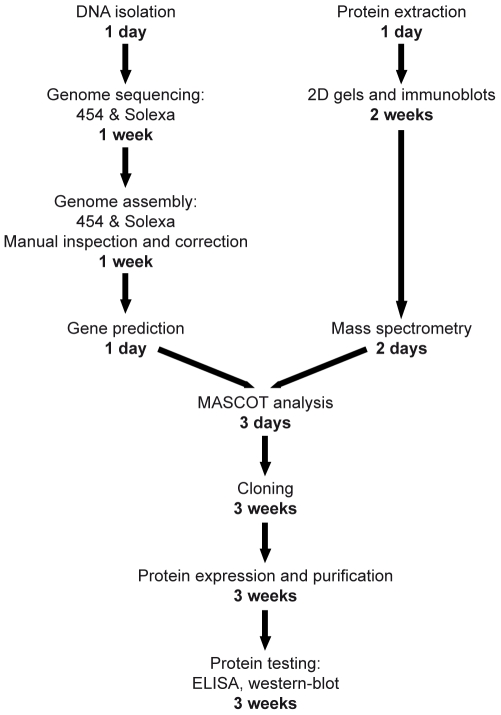
The advantages of using mass spectrometry associated with an unfinished genome to identify immunogenic proteins, compared to other approaches such as phage display library [34], [35], comparative genomic [36] or systematic expression of all ORFs [37], resides mainly in the minimal necessary workload and in the rapidity of the method. Indeed, the whole process can take place in less than 4 months, with only 2 weeks necessary to obtain the contigs ( Fig. 4 ). In addition, with the lowering of sequencing costs, the price of such an approach is highly competitive. Furthermore, constructing random expression libraries by fractionation of whole bacterial DNA would likely identify less immunogenic proteins since any plasmid carrying ORFs whose product is toxic will not be successfully expressed.
Figure 4. Time scale of a dirty genome approach combined with proteomics to develop serological diagnostic tools.
Schematic representation of the main steps of genome sequencing, immunogenic proteins identification and testing of candidate proteins in an ELISA. In bold, approximate time necessary to complete each step.
The 4 months that it takes to develop an ELISA may seem long as compared to the few weeks needed to develop a DNA-based test. However, detecting proteins represents a distinct advantage over detecting unique DNA sequences, and the availability of a serological test may especially prove very useful (i) to confirm positive PCR results (that may be false positive due to PCR contamination) and to better document a given case, (ii) to perform large seroepidemiological studies in order to precise the mode of transmission of a new pathogen and (iii) to investigate the possible role of a new bacterial pathogen in different clinical settings, such as pericarditis and endocarditis, for which valvular/pericardial fluid samples are not easily available. Similarly, when investigating patients with atypical pneumonia, serum samples are easier to obtain than lower respiratory tract specimen, especially when patients present a non-productive cough. Moreover, for several fastidious intracellular bacteria, even highly sensitive PCR tests may fail in detecting the agent at the infection site (i) due to relatively low bacterial load, e.g. sensitivity of only 50% to detect Borrelia in cerebrospinal fluid taken from patient with neurological Lyme disease [38], (ii) due to the presence of inhibiting molecules present in clinical samples, or (iii) due to “sampling bias” of PCRs tests performed on tissue samples, e.g. sensitivity of 60% of PCR on valve samples taken from patients with definite endocarditis [39].
Furthermore, the identification of immunogenic proteins also allows the development of species specific immunohistochemistry, that is useful (i) to confirm the presence of the pathogen in the tissue lesions, (ii) to analyze retrospectively various biopsy samples taken from patients with infection of unknown etiology and (iii) to shed some light on the underlying pathogenesis in vivo, by precising which cells are infected using double staining.
In summary, this work constitutes the proof of principle for a dirty genome approach, i.e. the use of unfinished genome sequences of pathogenic bacteria, coupled with proteomics to rapidly identify immunogenic proteins useful to develop a specific diagnostic test. Indeed, genomic information concerning new emerging pathogens must be placed at the scientific community's disposal as soon as possible, since their retention in order to close all gaps before genome publication is clearly medically counterproductive. This work demonstrated that 454/Solexa combined dirty genomes are sufficient and useful for medical downstream applications.
Methods
Ethics Statement
Human sera used in this work (see below) have been obtained from patients and control subjects, as part of prospective studies. These clinical studies have been accepted by the local ethical committee of the University of Lausanne. Both patients and controls gave their informed consent for various serological investigations including study of their serum reactivity against Parachlamydia acanthamoebae.
Cultivation and Purification of P. acanthamoebae
Parachlamydia acanthamoebae strain Hall's coccus was grown in Acanthamoeba castellanii strain ATCC 30010 in peptone yeast-extract glucose broth (PYG) and purified using a sucrose barrier and a gastrographin gradient, as previously described [22].
Chromosome Sequencing and Assembly
Purified P. acanthamoebae elementary bodies resuspended in PBS were lysed for DNA extraction with QIAamp DNA mini kit (Qiagen) according to the manufacturer protocol. Genomic DNA was pyrosequenced by two runs of Genome Sequencer 20 [1]. Genomic DNA was also sequenced using Solexa technology in Illumina Genome Analyzer [2]. Solexa sequences were assembled using Edena software [40] with parameter m = 16. Both GS20 runs only or GS20 runs and Solexa contigs were compiled in one assembly using Newbler software V1.1.02 with default parameters except for overlap size (45 nt) and identity score (95%). Differences between 454 and solexa contigs were manually inspected and corrected when necessary. In case of homopolymer discrepancy preference was generally given to Solexa when correcting frameshifts in protein coding region and in potentially non coding regions.
Gene Prediction and Annotation
To improve the prediction of incomplete genes at contig ends, a stop encoding tag “CTAGCTAGCTAG” was added at both extremities of each contig. A reference proteome was created with all open reading frames (stop to stop ORF) for peptide identification. Besides, locally installed Glimmer v3.02 [41] trained on long ORFs of the concatenated contigs was then applied to predict gene position on contigs longer than 500 bp. All reported ORFs larger than 100 nt were submitted to BLASTP versus nr database and local InterProScan search. Finally, tRNAscan-SE [42] and RNAmmer [43] were used to find structural RNAs. Genes of special importance for this study were manually annotated. This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession ACZE00000000. The version described in this paper is the first version, ACZE01000000.
Crude Extract Sample Preparation and 2D Gel Electrophoresis
Bacterial cells resuspended in PBS were washed in 10 mM Tris, 5 mM MgAc, pH 8.0 and then lysed by 5 cycles of short-pulse sonication in lysis buffer (30 mM Tris, 7 M urea, 2 M Thiourea, 4% CHAPS, pH 8.5). Proteins were recovered by centrifugation at 6'000 g and their concentration determined using a Bradford assay (Quick Start™ Bradford Protein Assay, Biorad, Hercules, USA).
Two dimensional gel electrophoresis was performed as described by Centeno et al. [44] using approximately 150 µg (mini gels) or 600 µg (midi-gels) of total elementary bodies proteins. Proteins were visualized by Coomassie Blue staining or transferred to nitrocellulose for subsequent immunoblot analysis (see Methods S1).
Human Sera
P. acanthamoebae positive human sera were described in previous studies where their positivity was assessed by immunofluorescence and western-blot [17], [45]. Control sera were taken from women with at term uneventful pregnancy [45]. Chlamydiales negative sera were tested negative by immunofluorescence for reactivity against various members of the Chlamydiales order (P. acanthamoebae, W. chondrophila, S. negevensis, N. hartmannellae, C. trachomatis, C. pneumoniae and C. psittaci), C. pneumoniae positive sera were positive for C. pneumoniae but negative for all other Chlamydiales tested. C. psittaci positive human serum was taken from a patient who suffered from well-documented psittacosis [46].
ELISA
Proteins E and N cloned and expressed in E. coli were purified thanks to a 6His tail (see Methods S1 ). Then, 96-well ELISA microplates were coated with 100 ng of purified E or N proteins in carbonate buffer pH 9.6 and incubated overnight at 4°C. After blocking with 3% non-fat dry-milk in PBST (PBS + 0.1% Tween 20) during 1 hour at 37°C, plates were washed with PBST and incubated 2 hours at 37°C with serial two-fold dilutions, in PBST+1% non-fat dry-milk, of sera from 2 rabbits immunized with P. acanthamoebae and of corresponding pre-immune sera. After 3 subsequent washes with PBST, plates were incubated 1 hour at 37°C with horseradish peroxidase-conjugated anti-rabbit IgG (Cell Signaling, Allschwill, Switzerland) diluted 1∶1000 in PBS + 1% non-fat dry-milk. Plates were washed 5 more times with PBST. O-phenylenediamine dihydrochloride (OPD) in citrate buffer was used as substrate for the peroxydase. After 15 minutes incubation, the optical density was read at 492 nm using an ELISA reader (Multiskan Ascent, Thermo Scientific, Waltham, USA).
Additional Methods
Descriptions of sera from immunized rabbits, immunoblot analysis, mass spectrometry and cloning, expression and purification of immunogenic proteins E and N are available in Methods S1.
Supporting Information
Supplementary Methods
(0.03 MB DOC)
Identification of P. acanthamoebae immunogenic proteins. Putative protein description of each immunogenic protein identified by mass spectrometry, according to BLASTP results against nr database or Chlamydiales genomes, respectively. Note that one protein spot, as for exemple F or P, can match with similar MS scores with two different ORFs, resulting in two different protein descriptions. Moreover, different spots such as D, T and U, can match with different ORFs which have the same protein description and thus correspond to orthologous or paralogous proteins.
(0.03 MB XLS)
Western blot recognition of identified immunogenic proteins with human sera. Immunogenic proteins identified by mass spectrometry (see Table S1) were tested by western blotting of 2D gels with two sera positive for P. acanthamoebae, two sera positive for C. pneumoniae, one serum positive for C. psittaci and two sera negative for most Chlamydiales. Results are evaluated in the perspective of a Parachlamydia specific serological diagnostic test.
(0.03 MB XLS)
Identification of P. acanthamoebae most abundantly expressed proteins. Putative protein description of each open reading frame identified by mass spectrometry (see Figure S1), according to BLASTP results against nr database or Chlamydiales genomes, respectively. Note that one protein spot, as for exemple 20 and 29, can match with similar MS scores with two different ORFs, resulting in two different protein descriptions. Moreover, different spots such as 35 and 36, can match with different ORFs which have the same protein description and thus correspond to orthologous or paralogous proteins.
(0.05 MB XLS)
2D map of most abundantly expressed P. acanthamoebae proteins. Proteins of P. acanthamoebae elementary bodies were separated by 2D gel electrophoresis and stained with Coomassie blue. Spots successfully identified by mass spectrometry are numbered A–S for immunogenic proteins and 1–29 for non immunogenic proteins (See Table S1 and Table S3).
(5.01 MB TIF)
Genetic organization of identified T3SS genes. The conserved genes are represented by different colors according to their respective functions. Hypothetical proteins are represented in light gray and genes encoding for proteins with identified functions likely not involved in T3SS are represented in dark gray. Capital letters refer to sct gene names according to the unified nomenclature suggested by Hueck in 1998 (Microbiol Mol Biol Rev 62: 379–433). sycE and sycD: genes encoding for SycE-like and SycD/LcrH-like T3SS chaperones. All SycD/LcrH predicted T3SS chaperones contain conserved tetratricopeptide repeats domains (TPRs).
(6.98 MB TIF)
Acknowledgments
We thank C. Robert (Unité des Rickettsies, Marseille) for the technical realization of the GS20 sequences as well as M. Quadroni and the proteomic facility of the University of Lausanne for assisting with mass spectrometry analyses. We also thank I. Riederer (Proteomics Unit, Prilly-Lausanne) and F. Auderset (Institute of Microbiology, Lausanne) for technical help, as well as L. Farinelli (Fasteris, Plan-les-Ouates), J. Schrenzel and D. Hernandez (University of Geneva) for helpful discussions. Gilbert Greub is supported by the Leenards Foundation through a career award entitled “Bourse Leenards pour la relève académique en médecine clinique à Lausanne”.
Footnotes
Competing Interests: The antigenic proteins and corresponding polypeptides identified as immunogenic thanks to this work have been patented for the detection of antibodies directed against Parachlamydia acanthamoebae as well as for their use in related diagnostic tests (i.e. immunohistochemistry) and vaccines (European patent n° 08172133.4-1223, 12th December 2008). This patent does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials, and the authors of the present work will make freely available any materials and information associated with their publication that are reasonably requested by others for the purpose of academic, non-commercial research.
Funding: This project was mainly funded by a grant from the Swiss National Science Foundation no. 3200BO-116445. This project was also partially supported by a grant from the Infectigen Foundation (In010) and by the CNRS (UPRESA 6020). Gilbert Greub is supported by the Leenards Foundation through a career award entitled “Bourse Leenards pour la relève académique en médecine clinique à Lausanne”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett S. Solexa Ltd. Pharmacogenomics. 2004;5:433–438. doi: 10.1517/14622416.5.4.433. [DOI] [PubMed] [Google Scholar]
- 3.Pol A, Heijmans K, Harhangi HR, Tedesco D, Jetten MS, et al. Methanotrophy below pH 1 by a new Verrucomicrobia species. Nature. 2007;450:874–878. doi: 10.1038/nature06222. [DOI] [PubMed] [Google Scholar]
- 4.Korbel JO, Urban AE, Affourtit JP, Godwin B, Grubert F, et al. Paired-end mapping reveals extensive structural variation in the human genome. Science. 2007;318:420–426. doi: 10.1126/science.1149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert TJ, Molla MN, Muzny DM, Nazareth L, Wheeler D, et al. Direct selection of human genomic loci by microarray hybridization. Nat Methods. 2007;4:903–905. doi: 10.1038/nmeth1111. [DOI] [PubMed] [Google Scholar]
- 6.Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38(Suppl):S2–7. doi: 10.1038/ng1794. [DOI] [PubMed] [Google Scholar]
- 7.Taylor KH, Kramer RS, Davis JW, Guo J, Duff DJ, et al. Ultradeep bisulfite sequencing analysis of DNA methylation patterns in multiple gene promoters by 454 sequencing. Cancer Res. 2007;67:8511–8518. doi: 10.1158/0008-5472.CAN-07-1016. [DOI] [PubMed] [Google Scholar]
- 8.Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Scola B, Elkarkouri K, Li W, Wahab T, Fournous G, et al. Rapid comparative genomic analysis for clinical microbiology: the Francisella tularensis paradigm. Genome Res. 2008;18:742–750. doi: 10.1101/gr.071266.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolain JM, Francois P, Hernandez D, Bittar F, Richet H, et al. Genomic analysis of an emerging multiresistant Staphylococcus aureus strain rapidly spreading in cystic fibrosis patients revealed the presence of an antibiotic inducible bacteriophage. Biol Direct. 2009;4:1. doi: 10.1186/1745-6150-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francois P, Hochmann A, Huyghe A, Bonetti EJ, Renzi G, et al. Rapid and high-throughput genotyping of Staphylococcus epidermidis isolates by automated multilocus variable-number of tandem repeats: a tool for real-time epidemiology. J Microbiol Methods. 2008;72:296–305. doi: 10.1016/j.mimet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Jarvie T, Harkins T. De novo assembly and genomic structural variation analysis with genome sequencer FLX 3K long-tag paired end reads. Biotechniques. 2008;44:829–831. doi: 10.2144/000112894. [DOI] [PubMed] [Google Scholar]
- 13.Corsaro D, Greub G. Pathogenic potential of novel Chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clin Microbiol Rev. 2006;19:283–297. doi: 10.1128/CMR.19.2.283-297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birtles RJ, Rowbotham TJ, Storey C, Marrie TJ, Raoult D. Chlamydia-like obligate parasite of free-living amoebae. Lancet. 1997;349:925–926. doi: 10.1016/s0140-6736(05)62701-8. [DOI] [PubMed] [Google Scholar]
- 15.Greub G. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin Microbiol Infect. 2009;15:18–28. doi: 10.1111/j.1469-0691.2008.02633.x. [DOI] [PubMed] [Google Scholar]
- 16.Greub G, Berger P, Papazian L, Raoult D. Parachlamydiaceae as rare agents of pneumonia. Emerg Infect Dis. 2003;9:755–756. doi: 10.3201/eid0906.020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greub G, Boyadjiev I, La Scola B, Raoult D, Martin C. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann N Y Acad Sci. 2003;990:311–319. doi: 10.1111/j.1749-6632.2003.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 18.Marrie TJ, Raoult D, La Scola B, Birtles RJ, de Carolis E. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg Infect Dis. 2001;7:1026–1029. doi: 10.3201/eid0706.010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casson N, Posfay-Barbe KM, Gervaix A, Greub G. New diagnostic real-time PCR for specific detection of Parachlamydia acanthamoebae DNA in clinical samples. J Clin Microbiol. 2008;46:1491–1493. doi: 10.1128/JCM.02302-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casson NE, JM, Borel N, Pospischil A, Greub G. A mice model of lung infection by Parachlamydia acanthamoebae. Microbial pathogenesis 2008. 2008;45:92–97. doi: 10.1016/j.micpath.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Casson NS, K, Klos A, Stehle J-C, Pusztaszeri M, Greub G. Intranasal murine model of infections by Parachlamydia acanthamoebae. Proceedings of the Annual Meeting of the Swiss Society for Infectious Diseases. 2008:17. [Google Scholar]
- 22.Greub G, Mege JL, Raoult D. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis. Infect Immun. 2003;71:5979–5985. doi: 10.1128/IAI.71.10.5979-5985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greub G, Mege JL, Gorvel JP, Raoult D, Meresse S. Intracellular trafficking of Parachlamydia acanthamoebae. Cell Microbiol. 2005;7:581–589. doi: 10.1111/j.1462-5822.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 24.Borel N, Ruhl S, Casson N, Kaiser C, Pospischil A, et al. Parachlamydia spp. and related Chlamydia-like organisms and bovine abortion. Emerg Infect Dis. 2007;13:1904–1907. doi: 10.3201/eid1312.070655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Campillo M, Bini L, Comanducci M, Raggiaschi R, Marzocchi B, et al. Identification of immunoreactive proteins of Chlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis. 1999;20:2269–2279. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2269::AID-ELPS2269>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun. 2004;72:7164–7171. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma J, Zhong Y, Dong F, Piper JM, Wang G, et al. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun. 2006;74:1490–1499. doi: 10.1128/IAI.74.3.1490-1499.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Haferkamp I, Schmitz-Esser S, Wagner M, Neigel N, Horn M, et al. Tapping the nucleotide pool of the host: novel nucleotide carrier proteins of Protochlamydia amoebophila. Mol Microbiol. 2006;60:1534–1545. doi: 10.1111/j.1365-2958.2006.05193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haferkamp I, Schmitz-Esser S, Linka N, Urbany C, Collingro A, et al. A candidate NAD+ transporter in an intracellular bacterial symbiont related to Chlamydiae. Nature. 2004;432:622–625. doi: 10.1038/nature03131. [DOI] [PubMed] [Google Scholar]
- 31.Greub G, Collyn F, Guy L, Roten CA. A genomic island present along the bacterial chromosome of the Parachlamydiaceae UWE25, an obligate amoebal endosymbiont, encodes a potentially functional F-like conjugative DNA transfer system. BMC Microbiol. 2004;4:48. doi: 10.1186/1471-2180-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh JD, Kling-Backhed H, Giannakis M, Xu J, Fulton RS, et al. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci U S A. 2006;103:9999–10004. doi: 10.1073/pnas.0603784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn M, Collingro A, Schmitz-Esser S, Beier CL, Purkhold U, et al. Illuminating the evolutionary history of chlamydiae. Science. 2004;304:728–730. doi: 10.1126/science.1096330. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava N, Zeiler JL, Smithson SL, Carlone GM, Ades EW, et al. Selection of an immunogenic and protective epitope of the PsaA protein of Streptococcus pneumoniae using a phage display library. Hybridoma. 2000;19:23–31. doi: 10.1089/027245700315761. [DOI] [PubMed] [Google Scholar]
- 35.Naidu BR, Ngeow YF, Wang LF, Chan L, Yao ZJ, et al. An immunogenic epitope of Chlamydia pneumoniae from a random phage display peptide library is reactive with both monoclonal antibody and patients sera. Immunol Lett. 1998;62:111–115. doi: 10.1016/s0165-2478(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 36.Araoz R, Honore N, Cho S, Kim JP, Cho SN, et al. Antigen discovery: a postgenomic approach to leprosy diagnosis. Infect Immun. 2006;74:175–182. doi: 10.1128/IAI.74.1.175-182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKevitt M, Patel K, Smajs D, Marsh M, McLoughlin M, et al. Systematic cloning of Treponema pallidum open reading frames for protein expression and antigen discovery. Genome Res. 2003;13:1665–1674. doi: 10.1101/gr.288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooskens J, Templeton KE, Claas EC, van Dam AP. Evaluation of an internally controlled real-time PCR targeting the ospA gene for detection of Borrelia burgdorferi sensu lato DNA in cerebrospinal fluid. Clin Microbiol Infect. 2006;12:894–900. doi: 10.1111/j.1469-0691.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 39.Greub G, Lepidi H, Rovery C, Casalta JP, Habib G, et al. Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am J Med. 2005;118:230–238. doi: 10.1016/j.amjmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez D, Francois P, Farinelli L, Osteras M, Schrenzel J. De novo bacterial genome sequencing: Millions of very short reads assembled on a desktop computer. Genome Res. 2008;18:802–809. doi: 10.1101/gr.072033.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centeno C, Repici M, Chatton JY, Riederer BM, Bonny C, et al. Role of the JNK pathway in NMDA-mediated excitotoxicity of cortical neurons. Cell Death Differ. 2007;14:240–253. doi: 10.1038/sj.cdd.4401988. [DOI] [PubMed] [Google Scholar]
- 45.Baud D, Thomas V, Arafa A, Regan L, Greub G. Waddlia chondrophila, a potential agent of human fetal death. Emerg Infect Dis. 2007;13:1239–1243. doi: 10.3201/eid1308.070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senn L, Greub G. Local newspaper as a diagnostic aid for psittacosis: a case report. Clin Infect Dis. 2008;46:1931–1932. doi: 10.1086/588562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
(0.03 MB DOC)
Identification of P. acanthamoebae immunogenic proteins. Putative protein description of each immunogenic protein identified by mass spectrometry, according to BLASTP results against nr database or Chlamydiales genomes, respectively. Note that one protein spot, as for exemple F or P, can match with similar MS scores with two different ORFs, resulting in two different protein descriptions. Moreover, different spots such as D, T and U, can match with different ORFs which have the same protein description and thus correspond to orthologous or paralogous proteins.
(0.03 MB XLS)
Western blot recognition of identified immunogenic proteins with human sera. Immunogenic proteins identified by mass spectrometry (see Table S1) were tested by western blotting of 2D gels with two sera positive for P. acanthamoebae, two sera positive for C. pneumoniae, one serum positive for C. psittaci and two sera negative for most Chlamydiales. Results are evaluated in the perspective of a Parachlamydia specific serological diagnostic test.
(0.03 MB XLS)
Identification of P. acanthamoebae most abundantly expressed proteins. Putative protein description of each open reading frame identified by mass spectrometry (see Figure S1), according to BLASTP results against nr database or Chlamydiales genomes, respectively. Note that one protein spot, as for exemple 20 and 29, can match with similar MS scores with two different ORFs, resulting in two different protein descriptions. Moreover, different spots such as 35 and 36, can match with different ORFs which have the same protein description and thus correspond to orthologous or paralogous proteins.
(0.05 MB XLS)
2D map of most abundantly expressed P. acanthamoebae proteins. Proteins of P. acanthamoebae elementary bodies were separated by 2D gel electrophoresis and stained with Coomassie blue. Spots successfully identified by mass spectrometry are numbered A–S for immunogenic proteins and 1–29 for non immunogenic proteins (See Table S1 and Table S3).
(5.01 MB TIF)
Genetic organization of identified T3SS genes. The conserved genes are represented by different colors according to their respective functions. Hypothetical proteins are represented in light gray and genes encoding for proteins with identified functions likely not involved in T3SS are represented in dark gray. Capital letters refer to sct gene names according to the unified nomenclature suggested by Hueck in 1998 (Microbiol Mol Biol Rev 62: 379–433). sycE and sycD: genes encoding for SycE-like and SycD/LcrH-like T3SS chaperones. All SycD/LcrH predicted T3SS chaperones contain conserved tetratricopeptide repeats domains (TPRs).
(6.98 MB TIF)