Abstract
Aims
Long-term endurance sport practice has been increasingly recognized as a risk factor for lone atrial fibrillation (AF). However, data on the outcome of circumferential pulmonary vein ablation (CPVA) in endurance athletes are scarce. The aim of the study was to evaluate the efficacy of CPVA in AF secondary to endurance sport practice.
Methods and results
Patients submitted to CPVA answered a questionnaire about lifetime history of endurance sport practice. Endurance athletes were defined as those who engaged in >3 h per week of high-intensity exercise for at least the 10 years immediately preceding their AF diagnosis. A series of 182 consecutive patients was included (51 ± 11 years, 65% with paroxysmal AF, 81% men, 42 ± 6 mm mean left atrial diameter); 107 (59%) patients had lone AF, and 42 of them (23% of the study population) were classified as endurance athletes (lone AF sport group). Freedom from arrhythmia after a single CPVA was similar in the lone AF sport group compared with the remaining patients (P = 0.446). Left atrial size and long-standing AF were the only independent predictors for arrhythmia recurrence after ablation.
Conclusion
Circumferential pulmonary vein ablation was as effective in AF secondary to endurance sport practice as in other aetiologies of AF.
Keywords: Atrial fibrillation, Catheter ablation, Endurance sport, Recurrence, Athletes
Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm disorder in clinical practice. The estimated prevalence of AF is 0.4–1% in the general population,1 increasing with age to 8% in those older than 80 years.2 The recognized risk factors for developing AF include age, hypertension, structural heart disease, diabetes mellitus, and hyperthyroidism.3 However, in a significant number of patients younger than age 60, no cardiovascular disease or any other known causal factor is present and the aetiology remains unclear. This condition is termed lone AF,4 and may be responsible for as many as 30% of patients with paroxysmal AF seeking medical attention.5,6 In recent years, long-term endurance sport practice has been recognized as a risk factor for AF.7–14 On the other hand, AF ablation has emerged as an effective and safe invasive treatment of drug-refractory AF.15–21 However, little is known about the outcome of this therapy in AF secondary to endurance sport practice.22 The aim of the present study was to analyse the efficacy of circumferential pulmonary vein ablation (CPVA) in endurance athletes with lone AF referred to AF ablation.
Methods
Study population
A series of 182 consecutive patients submitted to a first CPVA procedure because of symptomatic and drug-refractory AF between February 2003 and October 2006 was included in the study. Patients were asked about their lifetime physical activity and about the occurrence of AF episodes.
Prior to the procedure, all patients underwent transthoracic echocardiography to rule out structural heart disease and to measure the cardiac chambers and left ventricular ejection fraction. Transoesophageal echocardiography was performed 1–5 days before ablation to exclude the presence of intracavitary thrombus, as was magnetic resonance angiography of the left atrium (LA) and pulmonary veins (PVs) which, when possible, merged into the navigation system to achieve better spatial resolution and anatomical definition.
Anti-arrhythmic drug therapy was stopped at least five half-lives before the ablation, except in patients receiving amiodarone, to try to unmask the potential triggers of AF, such as supraventricular tachycardias (AV nodal re-entry or accessory pathway-mediated atrioventricular reciprocating tachycardia). Patients on oral anticoagulation stopped medication 3 days prior to the procedure, and low-molecular-weight heparin was administered until the day before the ablation. All ablation procedures were performed by experienced operators. Written informed consent was obtained from all the participants. The protocol was approved by the Institutional Ethics Committee.
Definitions
Atrial fibrillation was classified as paroxysmal, persistent, or long-standing, following the definition established by AF ablation consensus.23
Lone AF was defined as AF presenting in individuals younger than 60 years without clinical or echocardiographic evidence of cardiopulmonary disease, including hypertension, or other identifiable cause for the arrhythmia such as hyperthyroidism or alcohol abuse.4
Athletes were defined as those who performed regular endurance sport activity (cycling, jogging, swimming, soccer, rowing, etc.) for at least 3 h per week for at least the 10 years immediately preceding the arrhythmia diagnosis. This cut-off point was chosen because it represents a lifetime sport practice of at least 1500 h, which, as previously described,8 is associated with a higher risk of lone AF.
Vagally induced AF was defined as AF occurring at least 80% of the time during sleep, after heavy meals or in the hours immediately following exercise. Adrenergically induced AF was defined as AF episodes that started during waking hours at least 80% of the time and during strenuous exercise or other states of adrenergic activation.10,24 The remaining patients were classified as having AF of unknown origin.
Circumferential pulmonary vein ablation
Catheters were introduced percutaneously through the femoral vein; a transseptal puncture was performed to access the LA. After transseptal access, a bolus of heparin was administered (5000–6000 IU, according to patient weight), followed by additional boluses to maintain an activated clotting time of 200–250 s. Ablation was performed under intravenous sedation with midazolam and analgesia with meperidin and phentanyl. Oxygen saturation and invasive arterial blood pressure were monitored throughout the procedure. A three-dimensional map was constructed using an electro-anatomical mapping system (CARTO, Biosense-Webster Waterloo, Belgium or NAVX, St Jude Corporation, St Paul, MN, USA) to support the creation and validation of radiofrequency (RF) lesions. Continuous RF lesions surrounding each ipsilateral PV were delivered as described previously.25 Ablation lines were also deployed along the LA roof and LA posterior wall joining contralateral encircling lesions, and along the mitral isthmus. Mitral isthmus ablation was performed in all patients by creating an RF line from the inferior-lateral aspect of the left PV lesions to the mitral annulus. This line was anatomically performed, and no electrical block was assessed. Then, an RF line was created connecting contralateral PV-encircling lesions through the LA roof, and the LA posterior wall was electrically excluded by adding another ablation line, on top of the roof line, connecting the inferior aspect of the contralateral PV. The isolation of the posterior wall was confirmed by the absence of electrical activity and the inability to capture with pacing inside the whole encircled LA posterior region. All patients got ablation lines, regardless of AF type.
Radiofrequency was delivered through an 8 mm or irrigated tip thermocouple-equipped catheter, using a target temperature of 55°C at a maximum output of 60 W for the 8 mm tip catheter and 48°C at 40 W for the irrigated tip catheter.
The endpoint was a reduction of local electrogram to <0.15 mV.19
Follow-up
Patients were followed up at the outpatient clinic at 1, 4, and 7 months after the ablation procedure and every 6 months thereafter. Routine 24 or 48 h Holter monitoring was performed before each appointment, and a 12-lead electrocardiogram was obtained at each visit. Patients were asked to report to the emergency room or our arrhythmia unit for an ECG if any symptom suggestive of recurrence occurred between scheduled visits.
After the ablation procedure, all patients received anti-arrhythmic treatment for at least 1 month to protect against early recurrences and continued oral anticoagulation for a minimum of 2 months to maintain an international normalized ratio between 2.0 and 3.0. Additionally, magnetic resonance angiography was repeated at 3–6 months after the procedure to evaluate the presence of PV stenosis.
Arrhythmia recurrence was defined as a documented AF or atrial flutter episode of >30 s. Arrhythmic episodes within the first 3 months after the CPVA (healing period) were not considered in the evaluation of final success rates because they are often described as transient recurrences related to atrial inflammatory processes following RF lesions.26
The endpoint of the study was freedom from arrhythmia recurrence after a single CPVA procedure, without anti-arrhythmic medication.
A minimum follow-up of 3 months was required.
Statistical analysis
Continuous variables were expressed as mean ± SD. Comparisons were made using Student's t-test or χ2 analysis as appropriate. The relationship between baseline variables and time to recurrence during follow-up was evaluated using survival Cox analysis methodology; relationships with P < 0.10 were included as covariates in the multivariable analysis. Predictors of arrhythmia recurrence were assessed with a Cox regression model using a backward stepwise procedure with criteria of P < 0.05 for inclusion and P > 0.10 for removal from the model. A two-sided P-value ≤0.05 was considered statistically significant. Analyses were performed using the SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A series of 182 consecutive patients with symptomatic and drug refractory AF was included in the study. Their baseline characteristics are shown in Table 1. The majority (81%) of patients were male, younger than 75 years with a mean age of 51 ± 11 years, and without a markedly dilated LA. A total of 120 (66%) patients had paroxysmal AF; AF was persistent in 39 patients (21%), and the remaining 23 patients (13%) had long-standing AF.
Table 1.
Clinical and echocardiographic parameters at baseline
| Baseline characteristics of patients | n = 182 |
|---|---|
| Age (years) | 51 ± 11 |
| Male gender | 148 (81%) |
| Hypertension | 59 (32%) |
| Type of AF | |
| Paroxysmal | 120 (66%) |
| Persistent | 39 (21%) |
| Long-standing | 23 (13%) |
| Structural heart disease | 32 (18%) |
| Lone AF | 107 (59%) |
| Endurance athletesa | 64 (35%) |
| Lone AF sport groupb | 42 (23%) |
| AF origin | |
| Vagal | 64 (35%) |
| Adrenergic | 5 (3%) |
| Unknown | 113 (62%) |
| AF duration (years) | 6.1 ± 5.0 |
| Echocardiography | |
| LAD (mm) | 41.0 ± 5.9 |
| LVEDD (mm) | 51.4 ± 5.2 |
| LVESD (mm) | 32.9± 5.8 |
| LVEF (%) | 60.7 ± 10.3 |
Data are expressed as mean ± SD or number (%) of patients. AF, atrial fibrillation; LAD, anteroposterior left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a>3 h/week >10 years when arrhythmia was diagnosed (with or without any other risk factors for AF).
b>3 h/week >10 years when arrhythmia was diagnosed and without any other risk factors for AF (i.e. athletes with lone AF).
Lone AF was diagnosed in 107 (58%) patients; 42 of these patients (23% of the total study population) were identified as endurance athletes and included in the lone AF sport group.
The clinical and echocardiographic characteristics of the lone sport AF group vs. patients with lone AF and no history of endurance sport practice and the remaining patients (the control group) are given in Table 2. In the lone AF sport group, there was a higher proportion of male patients, and the left ventricular end-systolic diameter was smaller than in the control group.
Table 2.
Clinical and echocardiographic characteristics of control group, lone AF sport group, and patients with lone AF and no history of endurance sport practice
| Control group | Athletes (lone AF sport group) | Lone AF | P-valuea | P-valueb | |
|---|---|---|---|---|---|
| LAD (mm) | 41.0 ± 6.2 | 41.1 ± 4.4 | 39 ± 6 | 0.883 | 0.174 |
| LVEDD (mm) | 51.7 ± 5.3 | 50.0 ± 4.3 | 51 ± 4.46 | 0.233 | 0.288 |
| LVESD (mm) | 33.5 ± 6.0 | 30.4 ± 4.7 | 32.5 ± 4.37 | 0.027 | 0.060 |
| LVEF (%) | 60.1 ± 10.6 | 62.9 ± 8.9 | 61.7 ± 8.5 | 0.152 | 0.610 |
| Age (years) | 52.1 ± 10.8 | 48.5 ± 11.0 | 47.3 ± 10.5 | 0.057 | 0.585 |
| Paroxysmal AF | 90 (64%) | 31 (74%) | 48 (74%) | 0.251 | 0.603 |
| Vagal AF | 48 (34%) | 16 (38%) | 19 (36%) | 0.650 | 0.545 |
| Hypertension | 60 (43%) | 0 (0%) | 0 (0%) | <0.001 | |
| Male gender | 111 (79%) | 39 (93%) | 50 (77%) | 0.0427 | 0.031 |
| Structural heart disease | 32 (23%) | 0 (0%) | 0 (0%) | <0.001 |
AF, atrial fibrillation; LAD, left atrial anteroposterior diameter; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; LVEF, left ventricle ejection fraction.
aP-value: control group vs. lone AF sport group.
bP-value: lone AF sport group vs. lone AF (without history of sport practice) group.
When we analyse baseline characteristics of the lone AF and lone AF sport groups, there are no statistically significant differences, except for a higher proportion of male patients in the lone AF sport group (P = 0.031).
Outcomes after circumferential pulmonary vein ablation
The mean follow-up was 18.69 ± 11.7 months.
Ablation was performed using 35.7 ± 14.8 min of RF with total fluoroscopic and procedural durations of 26.0 ± 9.3 and 122.3 ± 33.1 min, respectively. The procedural duration of ablation comprised the time between the vascular puncture and the withdrawal of the catheters (skin-to-skin time). The complications rate in the lone AF sport group was comparable with the control group (7.1 vs. 4.3%; P = 0.45). Overall, four patients (2.2%) suffered a transient cerebrovascular ischaemia, which was resolved under heparin with normal computed tomography scanning; three patients (1.6%) showed transient inferior myocardial ischaemia during the procedure, secondary to air embolism, resolved with sublingual NTG within a few minutes and without consequences; and two patients (1.1%) had cardiac tamponade during transseptal puncture, resolved by pericardiocentesis.
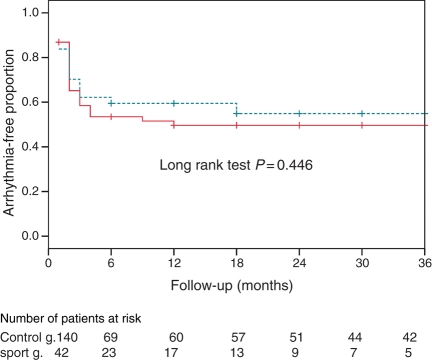
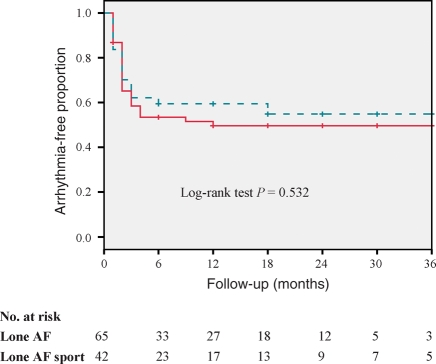
The probability of remaining free from arrhythmia at 1-year follow-up after a single CPVA procedure, based on Kaplan–Meier estimates, was similar in the lone AF sport group and controls: 59 and 48%, respectively, log-rank test P = 0.44 (Figure 1). On the other hand, the probability of remaining free from arrhythmia at 1-year follow-up after a single CPVA procedure was similar in the lone AF sport group compared with other patients with lone AF and no history of endurance sport activity (59 vs. 47%, log-rank test P = 0.532) (Figure 2).
Figure 1.
Kaplan–Meier curves for long-term freedom from recurrent arrhythmias after a single ablation procedure in the lone AF sport group (dashed line) and the control group (solid line).
Figure 2.
Kaplan–Meier curves for long-term freedom from recurrent arrhythmias after a single ablation procedure in the lone AF sport group (dashed line) and patients with lone AF and no history of exercise activity (solid line).
Table 3 shows the relationship between each baseline variable and arrhythmia recurrence. In the Cox regression multivariable analysis, after adjusting for the type of AF (paroxysmal, persistent, or long-standing), age, HTA, LA dimension, EF, LV dimensions, presence of structural cardiac disease, and regular sport practice, only LA anteroposterior diameter and long-standing AF were independent predictors of arrhythmia recurrence (Table 4). The ejection fraction was a predictor of AF recurrence in the univariable analysis. However, it had no independent predictive value for recurrence in the multivariable analysis.
Table 3.
Relationship between each baseline variable and arrhythmia recurrence after a single ablation procedure
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Age (years) | 1.004 (0.983–1.025) | 0.742 |
| Male gender | 1.048 (0.598–1.838) | 0.869 |
| Hypertension | 1.181 (0.743–1.877) | 0.482 |
| Paroxysmal AF | 0.535 (0.344–0.831) | 0.005 |
| Structural heart disease | 0.931 (0.501–1.729) | 0.821 |
| AF duration (months) | 1.00 (0.997–1.003) | 0.940 |
| LAD (mm) | 1.057 (1.013–1.104) | 0.011 |
| LVEDD (mm) | 1.014 (0.962–1.069) | 0.609 |
| LVESD (mm) | 1.036 (0.997–1.077) | 0.070 |
| LVEF (%) | 0.974 (0.953–0.996) | 0.020 |
| Sport practice | 0.821 (0.475–1.419) | 0.479 |
AF, atrial fibrillation; LAD, left atrial anteroposterior diameter; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; LVEF, left ventricle ejection fraction.
Table 4.
Final model of the Cox regression for arrhythmia recurrence after a single ablation procedure
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| AF | ||
| Paroxysmal | 1 (—) | — |
| Persistent | 1.819 (0.990–3.340) | 0.054 |
| Long-standing | 2.297 (1.090–4.839) | 0.029 |
| LAD (mm) | 1.069 (1.018–1.122) | 0.007 |
Ablation was repeated in 67 patients of this series (37%), achieving a 72% overall probability of freedom from arrhythmia recurrence at 1 year without anti-arrhythmic drugs. There were no statistically significant differences in the proportion of redo procedures between the lone AF sport group (40.5%) and the control group (37.3%) (P = 0.587) and between the lone AF (36.4%) and the lone AF sport group (P = 0.540). The majority of these patients had AF recurrences (62%), atrial flutter was present in 12%, and the remaining patients had AF and atrial flutter recurrences (26%). All patients showed reconduction into the prior PV-encircling areas.
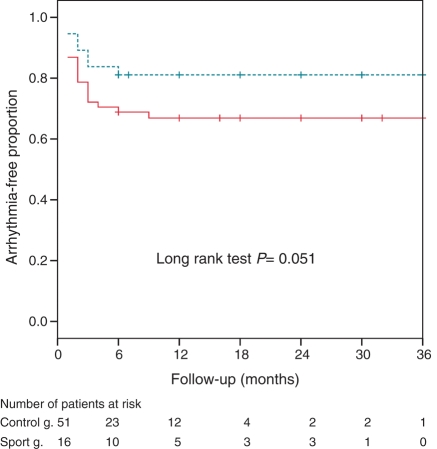
The Kaplan–Meier probability of remaining arrhythmia-free after repeated ablations was higher in the lone AF sport group (81%) at 1 year compared with the control group (63%, P = 0.051) (Figure 3).
Figure 3.
Kaplan–Meier curves for long-term freedom from recurrent arrhythmias after repeated ablation procedures in the lone AF sport group (dashed line) and the control group (solid line).
Discussion
The main finding of the present study is that the probability of remaining free of arrhythmia recurrences after a single CPVA was similar in athletes compared with the control group. Furthermore, LA diameter and long-standing AF were the only independent predictors of recurrence.
Recent studies have shown that the prevalence of AF is higher in individuals who regularly engage in endurance sports than in control populations.7–13 Additionally, Heidbüchel et al.27 reported that a history of endurance sport activity is associated with the development of AF after ablation of atrial flutter. In contrast with these previous studies, Pelliccia et al.28 reported that the incidence of lone AF among competitive athletes was uncommon and similar to that observed in the general population. However, the study was performed in young athletes at the moment of highest activity. Studies supporting the association have been performed in middle-aged individuals, after many years of sport practice. The most recent and largest epidemiological study14 supports that this association is not a matter of selection because despite adjustment for multiple potentially confounding lifestyle factors and health conditions, vigorous exercise activity was associated with an increased risk of developing AF.
The pathophysiological mechanisms responsible for increased AF risk in individuals who practice an endurance sport remain unclear. However, it is well known that the athlete's heart is characterized by increased LA size and left ventricular mass, adaptations that may create the substrate for developing AF. Another proposed mechanism is increased vagal tone, resulting in a short refractory atrial period.29 Experimental animal models have demonstrated that AF can be induced by acetylcholine,30 and that increasing vagal tone shortens the atrial refractory period, which, combined with atrial stimulation, induces AF.31
In the present study, we did not find differences in LA or LV size between the lone AF sport group and the control group. We think the explanation could be that exercise training leads to LA and LV enlargement similar to that seen in patients with other causes of AF, such as increased afterload in hypertension.
It is worth mentioning that our data showed that a high proportion of men with AF submitted to the ablation procedure are engaged in regular endurance sport practice (35%). This proportion is significantly higher than that of males of the same age in the general population of Catalonia (15.4%), according to the data from the REGICOR study.32 This finding is in agreement with previous studies7–14 and further supports the association between long-term endurance sport practice and AF.
We included highly symptomatic patients younger than 75 years and without a markedly dilated LA. Therefore, this selection procedure may explain the high proportion of lone AF among our patients. Nevertheless, we think that it represents the clinical practice, as our findings are consistent with the previous ablation studies which show that 50–60% of patients do not have structural heart disease.33–35
Circumferential pulmonary vein ablation is established as an effective and safe treatment of AF, with success rates ranging from 30 to 85%.20 To date, one small study by Furlanello et al.22 has demonstrated high efficacy of repeated ablation procedures in a population of elite athletes; however, no data comparing endurance athletes and a control population are available. Theoretically, the effectiveness of this procedure in athletes, who are usually a younger population, could be associated with higher success rates than in the general population with AF on the basis of age. On the other hand, the pathophysiological mechanisms of AF in endurance athletes could be responsible for reducing the success of CPVA.
In the present study, we observed no difference in the effectiveness of CPVA between a population of endurance athletes with lone AF and other patients with AF after a first CPVA procedure, and found that endurance sport practice is not a predictor of the results of the ablation procedure.
Data obtained after repeated ablation procedures probably reflect a selection bias and should be interpreted with caution, since repeated ablation procedures were not performed in patients with a low probability of success.
Earlier studies reported LA anteroposterior diameter prior to the ablation procedure, paroxysmal vs. persistent AF, early recurrence of AF, age, and hypertension as independent predictors of AF recurrences.25,35,36 Our study confirmed LA diameter and long-standing AF as the most powerful predictors of AF recurrence after CPVA; in addition, persistent AF showed a trend towards a higher probability of recurrence (P = 0.054).
Study limitations
One limitation of the study is the follow-up method of scheduled 24 or 48 h Holter recordings. If asymptomatic arrhythmias or non-documented symptomatic episodes occurred between routine follow-up visits, recurrence after ablation might have been missed in these patients, as previous studies have demonstrated.37 This could have affected athletes differently than the control group, since athletes are typically well trained in monitoring heart rate and are more used to detecting pulse abnormalities. Theoretically, asymptomatic arrhythmia episodes could have been more frequently detected among athletes. However, this potential bias is unlikely to be significant, since our data showed a trend towards fewer recurrence episodes among athletes. Another limitation is that both study groups included endurance athletes because athletes with any other identified risk factor, such as hypertension, were included in the control group. However, the main purpose of the study was to analyse the efficacy of the CPVA in ‘purely’ endurance sport related arrhythmia. Finally, the number of athletes with lone AF is relatively small. Therefore, further studies including a larger number of patients are required to confirm our results.
Conclusions
Circumferential pulmonary vein ablation seems to be as effective in endurance athletes as in other aetiologies of AF.
Conflict of interest: none declared.
Funding
This work was supported by a grant awarded by a Thematic Networks in Health Cooperative Research grant (REDSINCOR RD 06/0003/008) and by the FIS grant (05/0881) from the Spanish Health Ministry, Madrid, Spain. D.T. was supported by a grant from the Institut d'Investigació Biomèdica August Pi i Sunyer (IDIBAPS) and from the Agencia de Gestió d'Ajuts Universitaris i de Recerca—AGAUR (2005SGR00497), Spain. E.G. was supported by a grant from Hospital Clínic, Barcelona. Funding to pay the Open Access publication charges for this article was provided by Fundació Privada Clinic per a la Recerca Biomèdica.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation—Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Planas F, Romero-Menor C, Gabriel Vázquez-Oliva JS, Poblet T, Navarro-López F on behalf of the FAP Study researchers. Perfil clínico de la fibrilación auricular paroxística primaria (registro FAP) Rev Esp Cardiol. 2001;54:838–44. doi: 10.1016/s0300-8932(01)76409-3. [DOI] [PubMed] [Google Scholar]
- 6.Lévy S, Maarek M, Coumel P, Guize L, Lekieffre J, Medvedowsky JL, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99:3028–35. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 7.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case–control study. BMJ. 1998;316:1784–5. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. Long lasting sport practice and atrial fibrillation. Eur Heart J. 2002;23:477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 9.Hoogsteen J, Schepb G, van Hemelc NM, van der Walld EE. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace. 2004;6:222e–8e. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Elosua R, Arquer A, Mont L, Sambola A, Molina L, Garcia-Moran E, et al. Sport practice and the risk of lone atrial fibrillation: a case–control study. Int J Cardiol. 2006;108:332–7. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, et al. Sinus node disease and arrhythmias in the long term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–8. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 12.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, et al. on behalf of the GIRAFA (Grup Integrat de Recerca en Fibril-lacio Auricular) Investigators. Physical activity, height and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 13.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, et al. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–23. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 14.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–7. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haïssaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 17.Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–81. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 18.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled non-randomized long-term study. J Am Coll Cardiol. 2003;42:185–97. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 19.Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 20.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 21.Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 22.Furlanello F, Lupo P, Pittalis M, Foresti S, Vitali-Serdoz L, Francia P, et al. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J Cardiovasc Electrophysiol. 2008;19:457–62. doi: 10.1111/j.1540-8167.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 23.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-Up: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation Developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS) Europace. 2007;9:335–79. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 24.Oral H, Chugh A, Scharf C, Hall B, Cheung P, Veerareddy S, et al. Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:402–6. doi: 10.1046/j.1540-8167.2004.03432.x. [DOI] [PubMed] [Google Scholar]
- 25.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–41. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 26.Natale A, Raviele A, Arentz T, Calkins H, Chen SA, Haïssaguerre M, et al. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18:560–80. doi: 10.1111/j.1540-8167.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 27.Heidbüchel H, Anne W, Willems R, Adriaenssens B, Van de Werf F, Ector H. Endurance sport is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–6. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 30.Hoff HE, Geddes LA. Cholinergic factor in atrial fibrillation. J Appl Physiol. 1955;8:177–92. doi: 10.1152/jappl.1955.8.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Alessie R, Nusynowitz M, Abildskow J, Moe GK. Non-uniform distribution of vagal effects on the atrial refractory period. Am J Physiol. 1958;194:406–10. doi: 10.1152/ajplegacy.1958.194.2.406. [DOI] [PubMed] [Google Scholar]
- 32.Masiá R, Pena A, Marrugat J, Sala J, Vila J, Pavesi M, et al. High prevalence of cardiovascular risk factors in Gerona, Spain, a province with low myocardial infarction incidence. REGICOR Investigators. J Epidemiol Community Health. 1998;52:707–15. doi: 10.1136/jech.52.11.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chugh A, Oral H, Lemola K, Hall B, Cheung P, Good E, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2:464–71. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Wazni O, Marrouche NF, Martin DO, Gillinov AM, Saliba W, Saad E, et al. Randomized study comparing combined pulmonary vein–left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein–left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108:2479–83. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 35.Vassamreddy CR, Lickfett L, Jayam VK, Nasir K, Bradley D, Eldadah Z, et al. Predictors of recurrence following catheter ablation of atrial fibrillation using an irrigated-tip ablation catheter. J Cardiovasc Electrophysiol. 2004;15:692–7. doi: 10.1046/j.1540-8167.2004.03538.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee S-H, Tai C-T, Hsieh M-H, Tsai C-F, Lin Y-K, Tsao H-M, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2004;10:221–6. doi: 10.1023/B:JICE.0000026915.02503.92. [DOI] [PubMed] [Google Scholar]
- 37.Dagres N, Kottkamp H, Piorkowski C, Weis S, Arya A, Sommer P, et al. Influence of the duration of Holter monitoring on the detection of arrhythmia recurrences after catheter ablation of atrial fibrillation: implications for patient follow-up. Int J Cardiol. doi: 10.1016/j.ijcard.2008.10.004. Advance access publication 4 November 2008, doi: 10.1016/j.ijcard.2008.10.004. [DOI] [PubMed] [Google Scholar]