Abstract
Purpose
Mantle-cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin's lymphoma with a poor prognosis. We explored the feasibility, safety, and effectiveness of an aggressive immunochemotherapy treatment program that included autologous stem-cell transplantation (ASCT) for patients up to age 69 years with newly diagnosed MCL.
Patients and Methods
The primary end point was 2-year progression-free survival (PFS). A successful trial would yield a 2-year PFS of at least 50% and an event rate (early progression plus nonrelapse mortality) less than 20% at day +100 following ASCT. Seventy-eight patients were treated with two or three cycles of rituximab combined with methotrexate and augmented CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). This treatment was followed by intensification with high doses of cytarabine and etoposide combined with rituximab and filgrastim to mobilize autologous peripheral-blood stem cells. Patients then received high doses of carmustine, etoposide, and cyclophosphamide followed by ASCT and two doses of rituximab.
Results
There were two nonrelapse mortalities, neither during ASCT. With a median follow-up of 4.7 years, the 2-year PFS was 76% (95% CI, 64% to 85%), and the 5-year PFS was 56% (95% CI, 43% to 68%). The 5-year overall survival was 64% (95% CI, 50% to 75%). The event rate by day +100 of ASCT was 5.1%.
Conclusion
The Cancer and Leukemia Group B 59909 regimen is feasible, safe, and effective in patients with newly diagnosed MCL. The incorporation of rituximab with aggressive chemotherapy and ASCT may be responsible for the encouraging outcomes demonstrated in this study, which produced results comparable to similar treatment regimens.
INTRODUCTION
Mantle-cell lymphoma (MCL) usually exhibits an aggressive clinical course and is characterized by a predominance of males, a tendency to afflict older people, and a propensity for extranodal involvement.1–3 With anthracycline-based chemotherapy regimens, the response rate in MCL is high but the progression-free survival (PFS) and overall survival (OS) are poor (medians of 1.5 and 3 years, respectively).1–8 Treating MCL has become a formidable challenge, especially with regard to the affected age group, and because it currently remains incurable.
MCL cells express CD20 on their surface, providing a target for immunotherapy with rituximab.1–2,7,9 Rituximab produces responses in 22% to 38% of patients with relapsed MCL.10–12 The addition of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy increases the complete remission (CR) rate and time-to-treatment failure but has no impact on either PFS or OS in untreated patients with MCL.7 The addition of rituximab to the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) regimen in MCL patients appeared to improve outcomes compared with Hyper-CVAD followed by autologous stem-cell transplantation (ASCT), but the comparison is compromised by comparing untreated patients with untreated and relapsed patients.13,14 The full impact of adding rituximab to the treatment of MCL remains unclear but may be important.
The role of ASCT in MCL remains controversial.14–20 Most published trials include untreated and relapsed patients with MCL, rendering conclusions of the effectiveness of ASCT in these studies uncertain.14,15 Other trials of ASCT in first-remission MCL patients suggest improved outcomes compared with historical non-ASCT outcomes.16–19 A prospective randomized trial of ASCT versus alpha-interferon in first-remission patients found that ASCT improved remission duration and, with long follow-up, OS as well.20,21 The role of ASCT in untreated MCL may be substantial, but its full contribution is not yet defined.
The Cancer and Leukemia Group B (CALGB) developed a new treatment approach for patients with MCL. CALGB 59909 incorporates high-dose chemotherapy (HDCT) and ASCT with rituximab for MCL, while acknowledging the older age of afflicted patients. Thus, the design of CALGB 59909 is intense, but brief. It incorporates features of traditional chemotherapy for aggressive non-Hodgkin's lymphoma (NHL)22: intense immunochemotherapy mobilization and in vivo purging of autologous peripheral-blood stem cells (PBSCs)16,17,23–25 and post-ASCT rituximab to eliminate remaining lymphoma cells.16,26 CALGB 59909 success was dependent on survival benefits in conjunction with acceptable feasibility and toxicity.
PATIENTS AND METHODS
Eligibility
Patients 18 to 69 years old were eligible provided they had histologic documentation of MCL with at least one of the following confirmatory findings: coexpression of CD20 (or CD19) and CD5 with a lack of CD23 expression by immunophenotyping; immunostaining for cyclin D1; t(11;14)(q13;q32) by standard banding cytogenetic or fluorescent in situ hybridization analysis; or molecular evidence of the bcl-1/IgH rearrangement. The pathology of registered patients underwent central review. Patients with mantle zone histology and those with Ann Arbor stage I or II nodular histology were ineligible because of the relatively good prognosis of these MCL subgroups.27 Other eligibility criteria included measurable disease, no known hypersensitivity to murine products, negative HIV serology, not pregnant or nursing, left ventricular ejection fraction ≥ 45%, and serum creatinine (Cr) ≤ 2 mg/dL. Patients could be enrolled on study if they received a single cycle of chemotherapy and/or a single dose of rituximab. Each participant signed an institutional review board–approved informed consent document in accordance with federal and institutional guidelines. Patients were excluded for symptomatic meningeal or parenchymal brain lymphoma and medical conditions requiring the chronic use of corticosteroids.
On-Study Procedures
At the time of study enrollment, patients underwent history and physical examination; laboratory studies including a complete blood count, differential, and platelet count; serum electrolytes and chemistries; lactate dehydrogenase (LDH); 24-hour urine collection for Cr clearance; ECG; chest radiograph; lumbar puncture: WBC (differential, glucose, protein, and cytology); computed tomography scan or magnetic resonance imaging of chest/abdomen/pelvis; and a unilateral bone marrow aspirate and biopsy (BM-Bx) with cytogenetics. Endoscopy of the GI tract was not routinely performed.
Protocol Treatment
CALGB 59909 has five treatment modules (Table 1). Treatments 1 and 2 are identical, containing rituximab, methotrexate, cyclophosphamide (augmented dose), doxorubicin, vincristine, and prednisone (R-M-CHOP). The initial methotrexate dose was 3 g/m2 to be given on day 1 after rituximab. Because of eight unexpected episodes of nonoliguric acute renal failure in the first 20 patients, the protocol was amended to lower methotrexate to 300 mg/m2 to be given on day 2.
Table 1.
Treatment Details
| Treatment No. | Drugs/Procedures | Doses/Schedules |
|---|---|---|
| 1, 2, 2.5* (R-M-CHOP) | Rituximab† | 375 mg/m2 IV on day 1 |
| Methotrexate | 300 mg/m2 IV over 4 hours on day 2 | |
| Leucovorin | 50 mg/m2 IV every 6 hours for three doses starting 24 hours after completing methotrexate, then 10 mg/m2 IV or PO every 6 hours until serum methotrexate level is < 0.05 μM | |
| Cyclophosphamide | 2,000 mg/m2 IV over 2 hours on day 3 | |
| Doxorubicin | 50 mg/m2 IV on day 3 | |
| Vincristine | 1.4 mg/m2 IV on day 3 (cap the dose at 2 mg if patient is > 40 years old) | |
| Prednisone | 100 mg/m2 PO daily on days 3-7 | |
| G-CSF | 5 μg/kg SQ daily starting on day 4 until neutrophils > 10,000/μL once or > 5,000/μL twice | |
| Levofloxacin | 500 mg PO daily starting on day 6 until neutrophils ≥ 1,500/μL | |
| Fluconazole | 200 mg PO daily starting on day 6 until neutrophils ≥ 1,500/μL | |
| 3 (EAR) | Etoposide | 40 mg/kg IV over 96 hours on days 1-4 |
| Cytarabine | 2,000 mg/m2 IV over 2 hours twice daily on days 1-4 | |
| Rituximab | 375 mg/m2 IV on day 6 and day13 (two total doses) | |
| G-CSF | 10 μg/kg SQ daily starting on day 14 until completion of peripheral blood stem-cell collection | |
| Leukapheresis | Begin daily when WBC is ≥ 5,000/μL | |
| Levofloxacin | 500 mg PO daily starting on day 7 until neutrophils ≥ 500/μL | |
| Fluconazole | 200 mg PO daily starting on day 6 until neutrophils ≥ 500/μL | |
| Acyclovir | 200 mg PO three times daily starting on day 6 to continue until 1 year post-ASCT | |
| 4 (CBV) | Carmustine | 15 mg/kg (maximum, 550 mg/m2) IV over 1 hour on day –6 |
| Etoposide | 60 mg/kg IV over 4 hours on day –4 | |
| Cyclophosphamide | 100 mg/kg IV over 2 hours on day −2 | |
| Infusion of peripheral-blood stem cells | Day 0 | |
| G-CSF | 5 μg/kg SQ daily starting on day +4 until neutrophils > 5,000/μL once or > 1,500/μL twice | |
| Levofloxacin | 500 mg PO daily starting on day +2 until neutrophils ≥ 500/μL | |
| Fluconazole | 200 mg PO daily starting on day +1 until neutrophils ≥ 500/μL | |
| Acyclovir | 200 mg PO three times daily starting on day −2 to continue until 1 year post-ASCT | |
| Trimethoprim/sulfamethoxazole | One double-strength tablet PO twice every Saturday and Sunday to continue until 3 months post-ASCT | |
| 5 (post-ASCT immunotherapy) | Rituximab | 375 mg/m2 IV weekly for two doses in weeks 6 and 7 after ASCT |
NOTE. All chemotherapy doses were based on a corrected body weight (in kilograms) defined as ideal weight +0.25 (actual weight − ideal weight).23 When the actual weight was less than the ideal weight, the corrected weight was the actual weight. For patients of more than 150% of ideal weight, the corrected weight was capped at 112.5% of ideal weight. The median days between start of Treatments were as follows: between Treatments 1 and 2, median = 23 days (range, 16-41 days); between Treatments 2/2.5 and 3, median = 30 days (range, 14-52 days); between Treatments 3 and 4, median = 54 days (range, 38-92 days); and between Treatments 4 and 5, median = 42 days (range, 13-327 days).
Abbreviations: R-M-CHOP, rituximab, methotrexate, cyclophosphamide (augmented dose), doxorubicin, vincristine, and prednisone; IV, intravenous; PO, per os (by mouth); G-CSF, granulocyte-colony stimulating factor (filgrastim); SQ, subcutaneously; EAR, high-dose etoposide and cytarabine with rituximab; ASCT, autologous stem-cell transplantation; CBV, high-dose carmustine, etoposide, and cyclophosphamide.
Treatment 2.5 is given if the pre-Treatment 3 bone marrow biopsy contains > 15% MCL. Ten patients needed Treatment 2.5.
Rituximab is withheld if circulating mantle cells are > 10,000/μL.
Treatment 3, high-dose etoposide and cytarabine with rituximab (EAR),23 began 4 weeks after Treatment 2, provided that a BM-Bx showed ≤ 15% involvement with MCL (otherwise Treatment 2.5 was given, identical to Treatment 2). Patients receiving Treatment 2.5 had a BM-Bx repeated and if it still showed more than 15% involvement, they were removed from the protocol. Treatment 3 functioned as both intense cytoreductive therapy and as a means to mobilize PBSC.23,28 Treatment 3 could start when neutrophils were ≥ 1,000/μL, platelets were ≥ 100,000/μL, Cr was less than 2 mg/dL, total bilirubin was less than two times the upper limit of normal, and AST was less than three times the upper limit of normal. Cytarabine doses were reduced for a rising Cr to minimize the risk of CNS toxicity as previously described.29 Leukapheresis began when the WBC rose to 5,000/μL following the chemotherapy nadir. The target CD34+ cell dose was 5 million/kg (minimal dose for transplantation, 2 million/kg). The collection and cryopreservation of PBSC was per institutional standards.
Treatment 4 was high-dose carmustine, etoposide, and cyclophosphamide30 followed by ASCT and was intended to begin 4 weeks after the collection of PBSC. Treatment 5 included two weekly doses of rituximab to begin 6 weeks after ASCT.16,26
Treatment of involved CSF.
If the cerebrospinal fluid (CSF) showed MCL with a CSF WBC ≤ 5 cells/μL, then the patient received intrathecal methotrexate (12 mg) for 10 instillations spread out over Treatments 1 to 3. Intrathecal methotrexate was not given concurrently with intravenous methotrexate or cytarabine. For a CSF WBC of more than 5 cells/μL, the patient also received 24 Gy cranial radiation in 12 fractions.
Supportive care.
Filgrastim (granulocyte colony-stimulating factor) and bacterial (fluoroquinolone) and fungal (azole) prophylaxis were given during the neutrophil nadirs of Treatments 1 to 4. Pneumocystis carinii prophylaxis with trimethoprim/sulfamethoxazole was started in Treatment 3 and continued until 3 months post-ASCT. Herpes/Varicella zoster prophylaxis with acyclovir started with Treatment 3 and continued for 12 months post-ASCT. Febrile neutropenia and transfusion support were managed according to institutional guidelines. High-dose prednisone (0.5 mg/kg twice daily for 2 weeks, then tapering doses over 4 weeks) was recommended for any patient felt to be experiencing carmustine-induced pneumonitis.31
Documentation of Response
Patients were restaged at 3 months post-ASCT with history and physical examination, LDH, computed tomography scan or magnetic resonance imaging of the chest/abdomen/pelvis, BM-Bx, and lumbar puncture (if previously positive). The International Lymphoma Workshop response criteria were used as previously published.32 Staging procedures were repeated 1 month after demonstration of a CR as confirmation of CR. Following ASCT, patients were seen every 3 months for 2 years, biannually for 3 years, then annually. Imaging studies were repeated only for a clinical suspicion of progression/relapse of MCL. Toxicities throughout the protocol were scored using the National Cancer Institute Common Toxicity Criteria.33
Statistical Considerations
The primary objective of this phase II study was 2-year PFS. Secondary objectives included determination of response rates, event-free survival (EFS), OS, and nonrelapse mortality (NRM). Survivals were measured from study entry and included PFS until documented progression/relapse, EFS until documented progression/relapse, death from any cause or off-protocol treatment for any reason, and OS until death from any cause. The goal was to prolong the PFS of historical controls by one third. Correlations were explored between the International Prognostic Index (IPI) and mantle-cell IPI (MIPI)34 scores and survival. A successful trial was prospectively defined as a 2-year PFS statistically of at least 50% and an event rate (relapse/progression, or NRM) statistically less than 20% at 100 days post-ASCT. Intent-to-treat analysis was performed. A stopping rule was applied to close the protocol early if there was evidence of an event rate greater than 20% at 100 days post-ASCT. With a significance level of 0.17 and a power of 0.86, the protocol would be stopped if an event occurred at 100 days post-ASCT in 5 of 15 patients, 11 of 30 patients, or 18 of 45 patients. The original accrual goal was 45 eligible patients. Because of a protocol amendment involving methotrexate dose (August 15, 2002), the accrual goal was increased to 65 to address protocol end points in at least 45 patients receiving the final treatment design. The Kaplan-Meier method was used to estimate survivals as previously defined.35
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by review of data by CALGB Statistical Center staff and the study chairperson. Statistical analyses were performed by CALGB statisticians.
RESULTS
Patient and Disease Characteristics
Seventy-nine patients were enrolled between June 2001 and October 2004 (Table 2). One patient was removed from analysis because of lack of any protocol treatment. MCL was confirmed in 77 (99%) of 78 of patients: in 60 (77%) by cyclin D1 expression, in seven (9%) by demonstration of t(11;14), and in 10 (13%) by both assays. The median age was 57 years with 82% of the patients being male and 49% having more than one extranodal disease site. One-sixth of patients had a blastic histology. Ninety-four percent of patients were Ann Arbor stage III/IV, and 32% of patients had an elevated LDH.
Table 2.
Patient Demographics and Disease Characteristics
| Characteristic | No. of Patients(n = 78)* | % |
|---|---|---|
| Prior chemotherapy and/or rituximab | 6 | 8 |
| Sex | ||
| Male | 64 | 82 |
| Female | 14 | 18 |
| Age, years | ||
| Median | 57 | |
| Range | 37-69 | |
| Histology | ||
| Blastic | 12 | 15 |
| Diffuse | 37 | 47 |
| Nodular | 21 | 27 |
| Unknown | 8 | 11 |
| B symptoms | ||
| Yes | 25 | 32 |
| No | 53 | 68 |
| LDH, U/L | ||
| Median | 206 | |
| Range | 117-1493 | |
| Elevated LDH | ||
| Yes | 25 | 32 |
| No | 51 | 65 |
| Unknown | 2 | 3 |
| Bone marrow involvement | ||
| Yes | 56 | 72 |
| No | 21 | 27 |
| Unknown | 1 | 1 |
| CSF involvement | ||
| Yes | 4 | 5 |
| No | 72 | 92 |
| Unknown | 2 | 3 |
| IPI score | ||
| Low | 15 | 19 |
| Low-intermediate | 26 | 33 |
| Intermediate-high | 17 | 22 |
| High | 17 | 22 |
| Unknown | 3 | 4 |
| MIPI score34 | ||
| Low | 41 | 53 |
| Intermediate | 24 | 31 |
| High | 12 | 15 |
| Unknown | 1 | 1 |
Abbreviations: LDH, lactate dehydrogenase; IU/L, international units/liter; CSF, cerebrospinal fluid; IPI, International Prognostic Index; MIPI, mantle-cell lymphoma IPI.
Intent-to-treat.
Study Throughput
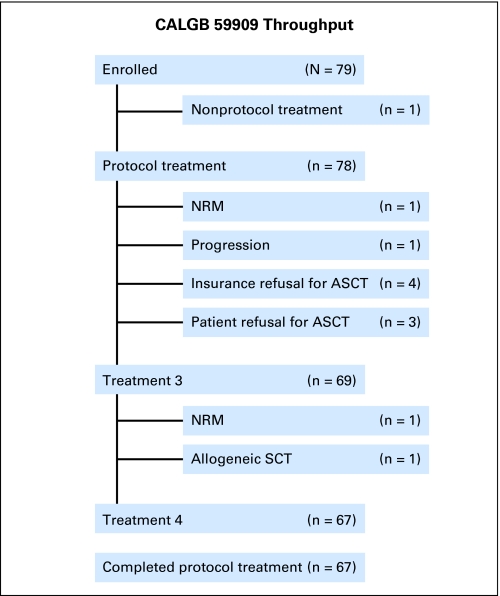
Seventy-seven patients (98%) completed Treatments 1 and 2/2.5, 69 (88%) completed Treatment 3, and 67 (86%) completed Treatment 4 (Fig 1). One study patient was taken off protocol treatment for allogeneic stem-cell transplantation. Four study patients eligible for ASCT did not receive it because of insurance denial of ASCT as a policy benefit (one received an ASCT at a non-CALGB institution). Three otherwise eligible patients refused ASCT. Of the remaining 70 patients, two experienced NRM before ASCT, and one progressed before ASCT.
Fig 1.
The throughput of patients enrolled on Cancer and Leukemia Group B (CALGB) 59909. NRM, nonrelapse mortality; ASCT, autologous stem- cell transplantation.
Autologous PBSC Collection
In Treatment 3, the median day to begin collection of PBSC was day 21 (range, 17 to 57). A median of one stem-cell collection (range, 1 to 6) was needed to acquire the protocol CD34+ cell target. The median number of CD34+ cells collected was 15.9 million/kg (range, 1.3 to 289 million/kg).
Response
The best response was ascertained at 3 months post-ASCT (n = 67). For the 11 patients who did not undergo ASCT, best response was determined at the time of removal from protocol therapy. Response among these 11 patients was CR, two; partial response, three; stable disease, four; and not evaluable for response, two. The overall CR rate was 54 (69%) of 78, the partial response rate was 15 (19%) of 78, the stable disease rate was 7 (9%) of 78, and response was not evaluable in 2 (3%) of 78. For patients receiving the higher methotrexate dose, the CR rate was 66.7%; for those receiving the lower methotrexate dose, the CR rate was 77.8% (P = .56).
Survival
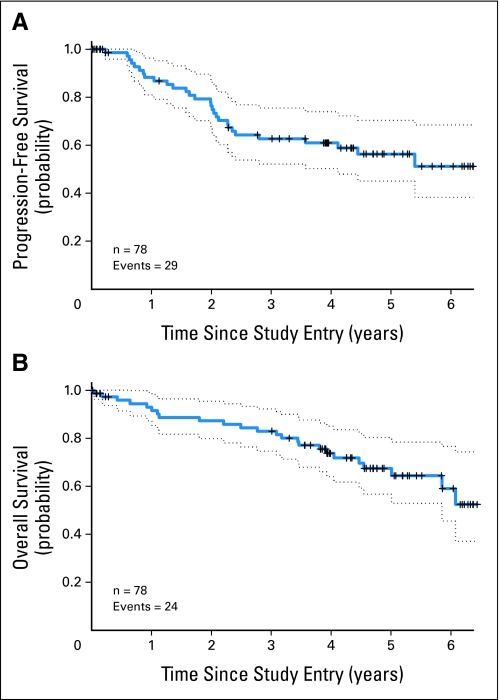
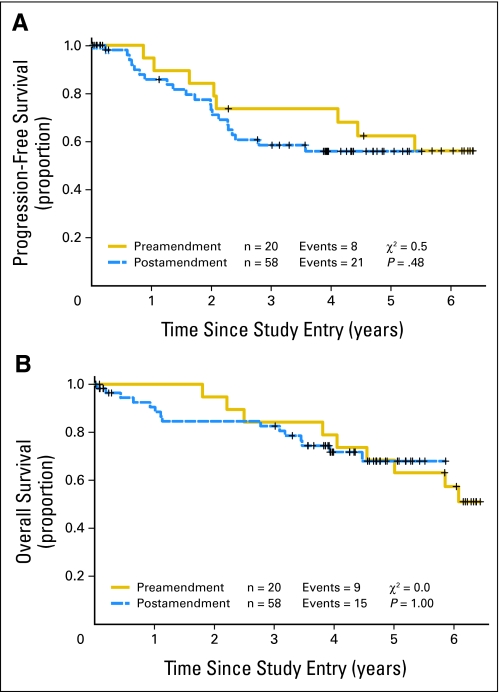
The median follow-up of survivors was 4.7 years (range, 3 to 6.4 years). The 2-year PFS was 76% (95% CI, 64% to 85%). The median PFS was not yet reached (Fig 2A and Table 3). The 5-year PFS was 56% (95% CI, 43% to 68%). The 100-day post-ASCT event rate was 5.1%. The median EFS was 4.4 years (95% CI, 2.4 to 5.4 years). The OS at 2 years was 87% (95% CI, 77% to 93%), with the median not yet reached (Fig 2B). The 5-year OS was 64% (95% CI, 50% to 75%). There was no difference in PFS or OS in patients receiving low-dose methotrexate (n = 58) versus those receiving high-dose methotrexate (n = 20; Appendix Figure A1, online only). The IPI score did not correlate with PFS. The MIPI score did correlate with PFS: 66.7% of high-risk patients progressed compared with 32.3% of low- plus intermediate-risk patients (P < .02). The MIPI score also correlated with OS: 67% of high-risk patients died compared with 25% of low- plus intermediate-risk patients (P < .03).
Fig 2.
(A) Progression-free survival and (B) overall survival by intent-to-treat analysis. Patients were censored at the time of last follow-up without an event or at the time of coming off protocol treatment because of denial of insurance for autologous stem-cell transplantation (ASCT), patient refusal of ASCT, or allogeneic stem-cell transplantation. Dotted lines indicate 95% CIs.
Table 3.
Survival Probabilities
| Survival (year) | Probability | 95% CI |
|---|---|---|
| PFS | ||
| 2 | 0.76 | 0.64 to 0.85 |
| 3 | 0.63 | 0.50 to 0.73 |
| 4 | 0.61 | 0.48 to 0.71 |
| 5 | 0.56 | 0.43 to 0.68 |
| EFS | ||
| 2 | 0.73 | 0.61 to 0.82 |
| 3 | 0.59 | 0.46 to 0.69 |
| 4 | 0.54 | 0.41 to 0.65 |
| 5 | 0.46 | 0.33 to 0.58 |
| OS | ||
| 2 | 0.87 | 0.77 to 0.93 |
| 3 | 0.83 | 0.72 to 0.90 |
| 4 | 0.74 | 0.62 to 0.83 |
| 5 | 0.64 | 0.50 to 0.75 |
Abbreviations: PFS, progression-free survival; EFS, event-free survival; OS, overall survival.
Toxicity
Every patient experienced at least one grade 4 hematologic toxicity during the study (Table 4). Ninety percent of patients experienced a grade 3 or greater nonhematologic adverse event during the study. Unexpectedly, eight of the first 20 patients experienced acute renal failure (Cr ≥ 2 mg/dL) during Treatments 1 and 2 (range, 2.1 to 4.6 mg/dL), prompting a change in methotrexate dose and schedule. Renal failure did not recur after this treatment modification. All cases of renal failure resolved within 4 weeks without the need for hemodialysis. In three patients, the next dose of methotrexate was halved, and in one patient, no further methotrexate was given. Treatment 3 yielded no surprises in terms of toxicity, and it proved to be an excellent mobilizer of autologous PBSC.23,28 Treatment 4 had no NRMs and only one instance of carmustine pneumonitis that was successfully managed with corticosteroids. There were two NRMs in this study (cardiovascular collapse from severe anemia during Treatment 1 and sepsis during Treatment 3).
Table 4.
Toxicity
| Parameter | Treatments 1, 2, and 2.5(n = 78) |
Treatment 3(n = 69) |
Treatment 4(n = 67) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of days for ANC < 500/μL | ||||||
| Median | 5 | 10 | 9 | |||
| Range | 0-16 | 0-16 | 0-39 | |||
| No. of days for platelets < 20,000/μL | ||||||
| Median | 0 | 7 | 6 | |||
| Range | 0-52 | 0-20 | 0-170 | |||
| No. of platelet transfusions | ||||||
| Median | 0 | 7 | 3 | |||
| Range | 0-21 | 0-37 | 0-36 | |||
| No. of RBC units transfused | ||||||
| Median | 0 | 4 | 2 | |||
| Range | 0-23 | 0-11 | 0-22 | |||
| Days of hospitalization | ||||||
| Median | 9 | 13 | 20 | |||
| Range | 0-39 | 3-31 | 0-67 | |||
| No. of days of TPN | ||||||
| Median | 0 | 0 | 0 | |||
| Range | 0-0 | 0-6 | 0-15 | |||
| No. of days of IV narcotics | ||||||
| Median | 0 | 0 | 0 | |||
| Range | 0-6 | 0-11 | 0-61 | |||
| Maximum total bilirubin, mg/dL | ||||||
| Median | 0.8 | 0.9 | 0.9 | |||
| Range | 0.2-2.8 | 0.2-4.7 | 0.3-5.8 | |||
| Maximum alkaline phosphatase, IU/L | ||||||
| Median | 113 | 106 | 109 | |||
| Range | 40-371 | 10-552 | 50-351 | |||
| Maximum creatinine, mg/dL | ||||||
| Median | 1.1 | 1.1 | 1.1 | |||
| Range | 0.7-5.5 | 0.7-2.9 | 0.7-3.2 | |||
| Needed platelet transfusion | 13 | 17 | 67 | 97 | 61 | 91 |
| Needed RBC transfusion | 25 | 32 | 69 | 93 | 54 | 82 |
| Needed hospitalization | 76 | 99 | 69 | 100 | 65 | 97 |
| Needed TPN | 0 | 0 | 3 | 4 | 9 | 13 |
| Needed IV narcotics | 14 | 18 | 24 | 35 | 30 | 45 |
| ≥ Grade 3 (NCI common toxicity criteria) | ||||||
| GI | 10 | 13 | 15 | 22 | 17 | 25 |
| Hepatic | 0 | 0 | 5 | 7 | 3 | 4 |
| Pulmonary | 2 | 3 | 2 | 3 | 1 | 1 |
| Cardiac | 4 | 5 | 3 | 4 | 4 | 6 |
| Cutaneous | 1 | 1 | 6 | 9 | 0 | 0 |
| Infection | 9 | 12 | 21 | 30 | 19 | 28 |
| Febrile neutropenia | 12 | 15 | 28 | 41 | 24 | 36 |
| CNS | 0 | 0 | 3 | 4 | 0 | 0 |
| Peripheral nervous system | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic (Cr) | 2 | 2.5 | 0 | 0 | 0 | 0 |
Abbreviations: ANC, absolute neutrophil count; RBC, red blood cells; TPN, total parenteral nutrition; IV, intravenous; IU/L, international units per liter; NCI, National Cancer Institute; Cr, serum creatinine.
DISCUSSION
MCL represents 6% of all adult NHL, has an aggressive clinical course, and is incurable with standard treatment regimens. CALGB 59909 was designed to be feasible, intense, and brief for untreated patients with MCL, incorporating HDCT and ASCT with rituximab immunotherapy. CALGB 59909 was successful because the 2-year PFS was greater than 50% (observed, 76%), and the probability of an event by day +100 of ASCT was under 20% (observed, 5.1%). The final 58 patients receiving lower-dose methotrexate had a 2-year PFS of 73%, reconfirming the success of this treatment regimen. There is no clear plateau in the PFS curve, so it remains to be seen if any patients are cured with this treatment. The only proven potential cure for MCL at this time remains allogeneic hematopoietic stem-cell transplantation.36
CALGB 59909 produced a high PFS rate. Although relapses continued to occur between 2 and 5 years following treatment, late relapses appeared to be less frequent than those seen with most other treatment approaches, but further follow-up will be necessary to determine the long-term impact of this treatment. The reason for a high PFS is likely a combination of intensified induction chemotherapy, in vivo purging of the autologous PBSC grafts, the use of HDCT and ASCT, and the incorporation of rituximab. Our outcomes are similar to those produced by the M. D. Anderson Cancer Center for MCL with rituximab added to the Hyper-CVAD regimen (R-Hyper-CVAD), which did not incorporate ASCT.13,37 Of note, a Southwest Oncology Group study of R-Hyper-CVAD in MCL patients showed a CR rate of 58%, a 2-year PFS of 64%, and a continuous pattern of relapse, results inferior to those of the M. D. Anderson Cancer Center study.38 Another trial that used R-Hyper-CVAD (without methotrexate/cytarabine) followed by rituximab maintenance produced a 2-year PFS of 60%.39 Thus, the optimal overall treatment strategy for MCL remains undefined.
HDCT and ASCT are important components of curing patients with aggressive NHL after relapse.40 ASCT may be important in the management of patients with MCL as well. Phase II trials involving ASCT for newly diagnosed MCL patients have shown 3-year PFS or EFS rates of 54% or greater, which appear better than most MCL programs not using ASCT.16–20,22,41,42 These data are biased by selecting patients who are candidates for HDCT and ASCT. The Nordic MCL protocol had a treatment design similar to ours.43 With the Nordic MCL protocol, the 4-year PFS and OS were 73% and 81%, respectively, with an apparent plateau to the survival curve beyond 5 years. This study, short of allogeneic stem-cell transplantation, is the only one to demonstrate a plateau in the survival curve, and it led the authors to speculate about cure. However, preemptive rituximab was given for molecular evidence of MCL relapse, not scored as a progression, thus dampening conclusions of curability from the study. Another study prospectively randomly assigned first-remission MCL patients to ASCT or to alpha-interferon.20,21 ASCT resulted in a better median PFS (39 v 17 months), and after a median 6 years of follow-up, improved median OS (7.5 v 5.3 years), but there was no plateau to the OS curve, suggesting a limited benefit from ASCT.21 Our regimen demonstrates results consistent with these other phase II-III trials involving ASCT and supports the use of ASCT in the initial treatment plan of patients under age 70 years with MCL.
The contribution of purging MCL cells from the PBSC graft to improving outcomes in MCL is not certain. At the time of CALGB 59909 design, there were data showing success in purging contaminating NHL cells from autologous PBSC grafts by the in vivo administration of rituximab with chemotherapy in individuals with informative reverse transcriptase polymerase chain reaction for the rearranged bcl-1/IgH or bcl-2/IgH transcripts.24,25 We therefore adopted the emerging concept of in vivo purging and using EAR for MCL.23 We are analyzing the effectiveness of in vivo purging with EAR, and preliminary information suggests that in vivo purging is effective and that the degree of in vivo purging is predictive of relapse.44
Rituximab has shown substantial benefit in patients with low-grade and aggressive NHL.9,10 Rituximab has a modest response rate in patients with relapsed MCL as a single agent.11,12 The contribution of rituximab to our favorable outcomes in untreated MCL cannot be dissected. The German Lymphoma Study Group found that the addition of rituximab to CHOP in untreated MCL improved the CR rate but did not improve either the PFS or the OS.7 Perhaps adding rituximab to HDCT/ASCT is the key to improving outcomes in MCL. It can be argued that more rituximab is needed in treatment regimens like ours, not less, as the Nordic trial suggests. The magnitude of the contribution of rituximab to survival outcomes in CALGB 59909 remains unknown, and the optimal number of rituximab doses is open for debate.
CALGB 59909 is currently one of several effective treatment strategies for MCL. Despite its intensity, it was associated with acceptable morbidity and low NRM. But how do we make further advancement? Bortezomib has activity as a single agent in MCL.45 CALGB 50403 is designed to add maintenance bortezomib for patients with MCL otherwise receiving the backbone treatment of CALGB 59909. The addition of post-ASCT bortezomib might improve survival outcomes compared with those in CALGB 59909, with or without the expectation of cure. With new approaches and novel agents, progress in the management of MCL is being made.
Appendix
The following institutions and members participated in the study: Dana-Farber Cancer Institute, Boston, MA: Eric P. Winer, MD (CA32291); Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH: Marc S. Ernstoff, MD (CA04326); Georgetown University Medical Center, Washington, DC: Minetta C. Liu, MD (CA77597); Illinois Oncology Research Association, Peoria, IL: John W. Kugler, MD (CA35113); Mount Sinai School of Medicine, New York, NY: Lewis R. Silverman, MD (CA04457); North Shore-Long Island Jewish Medical Center, Manhasset, NY: Daniel R. Budman, MD (CA35279); State University of New York Upstate Medical University, Syracuse, NY: Stephen L. Graziano, MD (CA21060); The Ohio State University Medical Center, Columbus, OH: Clara D. Bloomfield, MD (CA77658); University of California at San Diego, San Diego, CA: Barbara A. Parker, MD (CA11789); University of California at San Francisco, San Francisco, CA: Alan P. Venook, MD (CA60138); University of Chicago, Chicago, IL: Gini Fleming, MD (CA41287); University of Vermont, Burlington, VT: Hyman B. Muss, MD (CA77406); Wake Forest University School of Medicine, Winston-Salem, NC: David D. Hurd, MD (CA03927); Washington University School of Medicine, St. Louis, MO: Nancy Bartlett, MD (CA77440); Weill Medical College of Cornell University, New York, NY: John Leonard, MD (CA07968); Western Pennsylvania Cancer Institute, Pittsburgh, PA: Richard K. Shadduck, MD.
Fig A1.
(A) Progression-free survival and (B) overall survival by intent-to-treat analysis, according to patients treated before or after the major protocol amendment involving methotrexate dose and schedule. Patients were censored at the time of last follow-up without an event or at the time of coming off protocol treatment because of denial of insurance for autologous stem-cell transplantation (ASCT), patient refusal of ASCT, or allogeneic stem-cell transplantation. Preamendment is high-dose methotrexate and postamendment is low-dose methotrexate.
Footnotes
Written on behalf of Cancer and Leukemia Group B.
Supported, in part, by Grants No. CA31946 from the National Cancer Institute to the Cancer and Leukemia Group B (CALGB; R.L.S.), CA33601 to the CALGB Statistical Center (S.G. and J.L.J.), and by Amgen. Also supported by Grants No, CA11789 (L.E.D.), CA77597 (D.N.), CA03927 (D.D.H.), CA77440 (N.L.B.), CA77658 (K.A.B.), CA41287 (M.K.), and CA32291 (G.P.C.).
Presented at the 46th Annual Meeting of the American Society of Hematology, December 3-7, 2004, San Diego, CA, and the 48th Annual Meeting of the American Society of Hematology, December 9-12, 2006, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00020943.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: George P. Canellos, Celgene (C) Stock Ownership: Lloyd E. Damon, Genentech Honoraria: None Research Funding: Wendy Stock, Amgen via CALGB Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lloyd E. Damon, Charles A. Linker
Administrative support: Michael Kelly
Provision of study materials or patients: Lloyd E. Damon, Bruce D. Cheson, David D. Hurd, Nancy L. Bartlett, Ann S. LaCasce, Kristie A. Blum, John C. Byrd, Wendy Stock, Charles A. Linker, George P. Canellos
Collection and assembly of data: Jeffrey L. Johnson, Donna Niedzwiecki
Data analysis and interpretation: Lloyd E. Damon, Jeffrey L. Johnson, Donna Niedzwiecki
Manuscript writing: Lloyd E. Damon
Final approval of manuscript: Lloyd E. Damon, Jeffrey L. Johnson, Donna Niedzwiecki, Bruce D. Cheson, David D. Hurd, Nancy L. Bartlett, Ann S. LaCasce, Kristie A. Blum, John C. Byrd, Wendy Stock, Charles A. Linker, George P. Canellos
REFERENCES
- 1.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23:6409–6414. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 2.Brody J, Advani R. Treatment of mantle cell lymphoma: Current approach and future directions. Crit Rev Oncol Hematol. 2006;58:257–265. doi: 10.1016/j.critrevonc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Andersen NS, Jensen MK, de Nully Brown P, et al. A Danish population-based analysis of 105 mantle cell lymphoma patients: Incidences, clinical features, response, and prognostic factors. Eur J Cancer. 2002;38:401–408. doi: 10.1016/s0959-8049(01)00366-5. [DOI] [PubMed] [Google Scholar]
- 4.Fernàndez V, Hartmann E, Ott G, et al. Pathogenesis of mantle-cell lymphoma: All oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Bertoni F, Rinaldi A, Zucca E, et al. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22–27. doi: 10.1002/hon.767. [DOI] [PubMed] [Google Scholar]
- 6.Benn HAN. Mantle cell lymphoma and other t(11;14)-related disorders: From biology to designing therapy. Mol Oncol Rep. 2006;1:42–48. [Google Scholar]
- 7.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 8.Zelenetz AD. Mantle cell lymphoma: An update on management. Ann Oncol. 2006;17:iv12–iv14. doi: 10.1093/annonc/mdj992. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 11.Foran JM, Rohatiner AZ, Cunnigham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–324. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Ghielmini M, Schmitz SF, Bürki K, et al. The effect of Rituximab on patients with follicular and mantle-cell lymphoma. Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2000;11:123–126. [PubMed] [Google Scholar]
- 13.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cyatarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: An active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–3809. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 15.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: A study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 16.Hicks L, Connors JM, Mangel J, et al. Autologous stem-cell transplant with a rituximab purge and maintenance vs. standard chemotherapy for mantle cell lymphoma: Extended follow-up of a matched pair analysis. Blood. 2006;108:868a. abstr 3051. [Google Scholar]
- 17.Cortelazzo S, Magni M, Pintimalli M, et al. Frontline high dose sequential chemotherapy with rituximab (R-HDS) and autologous stem cell transplantation induces high rates of complete response and prolongs survival in mantle cell lymphoma (MCL) Blood. 2006;108:866a. abstr 3045. [Google Scholar]
- 18.Vose J, Loberiza F, Bierman P, et al. Mantle cell lymphoma (MCL): Induction therapy with HyperCVAD/High-dose methotrexate and cytarabine (M-C) (±rituximab) improves results of autologous stem cell transplant in first remission. J Clin Oncol. 2006;24:424s. abstr 7511. [Google Scholar]
- 19.Van't Veer MB, Notenboom A, McKenzie M, et al. First report of NOVON 45: A phase II study with rituximab, high dose Ara-C and autologous stem cell transplantation in the primary treatment of mantle cell lymphoma. Blood. 2006;108:773a. abstr 2734. [Google Scholar]
- 20.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 21.Dreyling MH, Hoster E, Van Hoof A, et al. Early consolidation with myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission in mantle cell lymphoma: Long term follow up of a randomized trial. Blood. 2008;112:285a. doi: 10.1182/blood-2004-10-3883. abstr 769. [DOI] [PubMed] [Google Scholar]
- 22.Soussain C, Patte C, Ostronoff M, et al. Small noncleaved cell lymphoma and leukemia in adults. A retrospective study of 65 adults treated with LMB pediatric protocols. Blood. 1995;85:664–674. [PubMed] [Google Scholar]
- 23.Damon L, Damon LE, Gaensler K, et al. Impact of intensive PBSC mobilization therapy on outcomes following auto-SCT for non-Hodgkin's lymphoma. Bone Marrow Transplant. 2008;42:649–657. doi: 10.1038/bmt.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flinn IW, O'Donnell PV, Goodrich A, et al. Immunotherapy with rituximab during peripheral blood stem cell transplantation for non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2000;6:628–632. doi: 10.1016/s1083-8791(00)70028-0. [DOI] [PubMed] [Google Scholar]
- 25.Arcaini L, Orlandi E, Alessandrino EP, et al. A model of in vivo purging with Rituximab and high-dose Ara-C in follicular and mantle cell lymphoma. Bone Marrow Transplant. 2004;34:175–179. doi: 10.1038/sj.bmt.1704551. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz SM, Negrin RS, Blume KG, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–783. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 27.Majlis A, Pugh W, Rodriguez M, et al. Mantle cell lymphoma: Correlation of clinical outcome and biologic features with three different histologic variants. J Clin Oncol. 1997;15:1664–1671. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- 28.Linker CA, Ries CA, Damon LE, et al. Autologous stem cell transplantation for acute myeloid leukemia in first remission. Biol Blood Marrow Transplant. 2000;6:50–57. doi: 10.1016/s1083-8791(00)70052-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith GA, Damon LE, Rugo HS, et al. High-dose cytarabine dose modification reduces the incidence of neurotoxicity in patients with renal insufficiency. J Clin Oncol. 1997;15:833–839. doi: 10.1200/JCO.1997.15.2.833. [DOI] [PubMed] [Google Scholar]
- 30.Stockerl-Goldstein KE, Horning SJ, Negrin RS, et al. Influence of preparatory regimen and source of hematopoietic cells on outcome of autotransplantation for non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 1996;2:76–85. [PubMed] [Google Scholar]
- 31.Cao TM, Negrin RS, Stockerl-Goldstein KE, et al. Pulmonary toxicity syndrome in breast cancer patients undergoing BCNU-containing high-dose chemotherapy and autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2000;6:387–394. doi: 10.1016/s1083-8791(00)70015-2. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Therapy Evaluation Program. Common Terminology for Adverse Events, version 3.0. 2003. Mar 31, http://ctep.cancer.gov.
- 34.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 35.Altman DG, Gore SM, Gardner MJ, et al. Statistical guidelines for contributors to medical journals. BMJ. 1983;286:1489–1493. doi: 10.1136/bmj.286.6376.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khouri IF. Reduced-intensity regimens in allogeneic stem-cell transplantation for non-hodgkin lymphoma and chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2006:390–397. doi: 10.1182/asheducation-2006.1.390. [DOI] [PubMed] [Google Scholar]
- 37.Romaguera J, Fayad L, Rodriguez A, et al. Rituximab + hyperCVAD alternating with R-methotrexate/cytarabine after 9 years: Continued high rate of failure-free survival in untreated mantle cell lymphoma. Blood. 2008;112:309a. abstr 833. [Google Scholar]
- 38.Epner EM, Unger J, Miller T, et al. A multi center trial of hyperCVAD+Rituxan in patients with newly diagnosed mantle cell lymphoma. Blood. 2007;110:121a. abstr 387. [Google Scholar]
- 39.Kahl BS, Longo WL, Eickhoff JC, et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: A pilot study from the Wisconsin Oncology Network. Ann Oncol. 2006;17:1418–1423. doi: 10.1093/annonc/mdl127. [DOI] [PubMed] [Google Scholar]
- 40.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 41.Delarue R, Haioun C, Ribrag V, et al. RCHOP and RDHAP followed by autologous stem cell transplantation (ASCT) in mantle cell lymphoma (MCL): Final results of a phase II study from the GELA. Blood. 2008;112:218a. doi: 10.1182/blood-2011-09-370320. abstr 581. [DOI] [PubMed] [Google Scholar]
- 42.Cortelazzo S, Magni M, Tarella C, et al. Update of a GITIL cohort study: Frontline high dose sequential chemotherapy with rituximab and autologous stem cell transplantation induces a high rate of long-term remissions in patients with mantle cell lymphoma. Blood. 2007;110:386a. abstr 1282. [Google Scholar]
- 43.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sher D, Johnson J, Siddiqui M, et al. Eradication of minimal residual disease during treatment of mantle cell lymphoma: CALGB 59909. Blood. 2004;104:459a. abstr 1652. [Google Scholar]
- 45.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]