Abstract
Purpose
Increased mammographic density is associated with increased breast cancer risk and reduced sensitivity of screening mammography and is related to hormone exposure. However, the effects of conjugated equine estrogens (CEEs) alone on mammographic density in diverse racial/ethnic populations are not established. We examined the effect of CEE alone on mammographic density in a subsample of the Women's Health Initiative (WHI) clinical trial participants.
Patients and Methods
In the WHI trial, women were randomly assigned to daily CEE 0.625 mg or placebo. The effect of CEE on mammographic percent density was determined over 1 and 2 years in a stratified random sample of 435 racially and ethnically diverse participants from 15 of 40 WHI clinics.
Results
Use of CEE resulted in mean increase in mammographic percent density of 1.6 percentage points (95% CI, 0.8 to 2.4) at year 1 compared with a mean decrease of 1.0 percentage point (95% CI, −1.7 to −0.4) in the placebo group (P < .001). The effect persisted for 2 years, with a mean increase of 1.7 percentage points (95% CI, 0.7 to 2.7) versus a mean decrease of 1.2 percentage points (95% CI, −1.8 to −0.5; P < .001) in the hormone and placebo groups, respectively. These effects were greater in women age 60 to 79 years (P = .03 for interaction across age).
Conclusion
Use of CEE results in a modest but statistically significant increase in mammographic density that is sustained over at least a 2-year period. The clinical significance of the CEE effect on mammographic density remains to be determined.
INTRODUCTION
Mammographic density is positively associated with breast cancer risk.1–5 The decrease in mammographic density after menopause6 suggests a hormonal influence, and observational and short-term clinical trials have shown an association between menopausal hormone therapy use and increased mammographic density.7–11 A relationship between an intervention's influence on breast density and its influence on breast cancer risk has been proposed.12 However, the effects of menopausal hormone therapy on breast density in racial/ethnic populations and duration effects are not established.
The Women's Health Initiative (WHI) reported that combined conjugated equine estrogens (CEEs) plus medroxyprogesterone acetate (MPA) doubled mammographic density9 and significantly increased breast cancer incidence in postmenopausal women with no prior hysterectomy.13 However, CEE alone did not increase breast cancer incidence but suggested a decrease in incidence.14 We evaluated the effect of CEE alone on mammographic density over 2 years in a randomly identified subsample of WHI CEE clinical trial participants, with over-sampling of women from racial/ethnic minorities.
PATIENTS AND METHODS
WHI Study Design
The WHI CEE-alone trial enrolled 10,739 postmenopausal women from 1993 to 1998 at 40 clinical centers.15,16 The study was approved by human subjects committees at each institution. Briefly, women recruited by multiple methods17 were eligible if they were between age 50 and 79 years at entry and were postmenopausal, had prior hysterectomy, and provided written informed consent. Women with prior breast cancer or those with medical conditions likely to result in death within 3 years were excluded. Prior menopausal hormone use required a 3-month washout period before baseline testing (mammographic density has been shown to decrease within a few weeks of stopping menopausal hormone use).18 Before random assignment, 46% had used estrogen alone and 5% had used estrogen plus progesterone. Baseline mammogram and clinical breast examination within 6 months before random assignment were required. Women were randomly assigned to daily CEE 0.625 mg in a single tablet (Premarin; Wyeth Ayerst, Philadelphia, PA), or an identical-appearing placebo. Participants had follow-up clinic visits every 6 months, where pill adherence was obtained from pill counts, potential adverse effects were determined, and exposure covariates were updated.14 During follow-up, women received annual clinical breast examinations and screening mammograms. After a mean of 6.8 years of follow-up, the trial was stopped early on the basis of an increased risk of stroke and no reduction in coronary heart disease risk.15
Mammogram Density Ancillary Study
Fifteen of 40 WHI clinical centers participated in the separately funded Mammogram Density Ancillary Study. The Clinical Coordinating Center identified a stratified random sample of women in the WHI CEE trial to be approached for participation, with the goal of sampling 150 women within each of the following groups: African Americans, Asian/Pacific Islanders, Hispanics, and non-Hispanic whites. A 10% over-sample was selected to allow for those electing not to participate. Inclusion criteria included availability of a prerandomization (baseline) mammogram plus at least one follow-up mammogram after 1 or 2 years. Sample size was based on the primary study aim—an anticipated mammographic density difference of 8% (standard deviation [SD], 10%) between CEE and placebo groups after 1 year of treatment, with the assumption of 33% loss to follow-up/nonadherence. A secondary aim of the study was to determine whether a hormone effect exists within different race/ethnic groups; therefore, the study was powered to assess effects on density within the four race/ethnic groups. Two years of follow-up was the time frame chosen to determine the longer-term effects of CEE on density.
Data Collection
Our mammographic density measurement methods have been previously published9 and have been validated across all mammographic density types (fatty to dense) and all age groups, including older women.19 Briefly, films were digitized on a Lumisys 85 laser digitizer (Eastman Kodak Company, Rochester, NY), and the files were converted to bitmap format suitable for display and density measurement. Mammographic density was assessed with a previously validated20 computer-assisted interactive thresholding technique using Imaging Research Program software (Sunnybrook Health Science Centre, Toronto, Ontario, Canada). After identifying thresholds, the software was used to calculate the total breast area, the total area of density, and percent mammographic density (ratio of dense breast area to total breast area). The right craniocaudal view was measured, unless it was unavailable, in which case the left craniocaudal view was used. Measurements were done in batches of 30 to 40 films selected without regard to whether they were before random assignment or follow-up films, and the films were sorted in random order. Baseline and follow-up films from the same participant were not necessarily included in the same batch, and batches for each observer were generated independently.
Two trained investigators (C.M. and J.D.P.), blind to treatment arm and film time sequence, performed density measurements on all films, with high reliability (intraclass correlation coefficients > 0.92). We calculated percent density for each film as the mean of both investigators' measures for that film.
Statistical Methods
All primary analyses focused on changes in mean percent density at baseline compared with years 1 and 2 by CEE random assignment. Mixed-effects (repeated measures) models were used to formally test whether treatment affected longitudinal density change, whether this relationship depended on race/ethnicity, mammogram sequence (baseline or follow-up), or baseline characteristics. Log-transformed percent density values were used in these models to reduce skewness of the percent density data. We also present differences over time (eg, year 1 – baseline) that are not skewed. Change in weight and physical activity were determined at each follow-up interval chosen because of their potential associations with mammographic density.21 We assessed several baseline characteristics as potential effect modifiers, including age (50 to 59, 60 to 69, and 70 to 79 years), race/ethnicity (non-Hispanic white, African American, white), body mass index (BMI; < 25.0, 25.0 to 29.9, ≥ 30 kg/m2), smoking status (past, never, current), and parity (0, 1 to 2, 3 to 4, ≥ 5), and Gail risk score22 (5-year risk, < 1.7% v ≥ 1.7% [the cutoff used in the breast cancer chemoprevention trials]23,24). Statistical significance of interactions between random assignment and baseline characteristics was judged by a Bonferroni-corrected alpha to account for 19 tests (.05/19 = .003). CIs, based on the t distribution, are presented for changes in mammographic percent density. Comparisons of baseline characteristics by random assignment were made by χ2 tests of association. All primary analyses were based on the intention-to-treat principle, and all statistical tests were two-sided.
RESULTS
Demographics
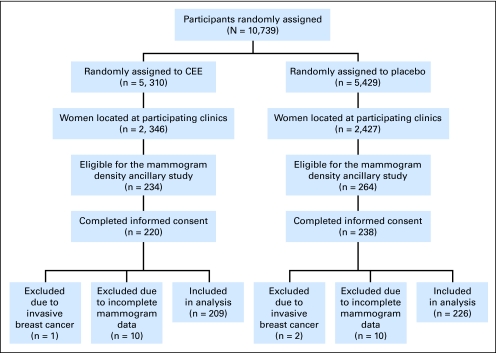
A total of 498 women in the WHI CEE-alone clinical trial were randomly selected for participation in the Mammogram Density Ancillary Study. Forty declined participation, 458 were eligible and consented, and mammograms were received and digitized for 438. Three women developed invasive breast cancer during the study period and were excluded, resulting in 435 (209 CEE, 226 placebo) participants (Fig 1). Baseline mammograms were available within 6 months before random assignment for all except three women whose baseline mammograms occurred more than 6 months before random assignment (mean time from baseline mammogram to random assignment was 367.3 days; SD, 187.1 days). Two of the women reported never using hormone therapy before entering the trial, and one reported prior use. These women were included in the analysis. Including these three women in the analysis, the mean time between baseline mammogram and random assignment was 45.4 days (SD, 35.9 days), and excluding them it was 43.2 days (SD, 20.2 days).
Fig 1.
Flow diagram of the Mammogram Density Ancillary Study of the Conjugated Equine Estrogen (CEE) Component of the Women's Health Initiative Study.
The mean age of participants was 62.2 years (SD, 8.0 years). Ancillary study participants were younger, more likely to be black or Hispanic (by design), fewer years past menopause, less likely to have used hormone therapy, less physically active, reporting less alcohol consumption, and more likely to have never smoked (all P < .01; data not shown), compared with the overall trial participants. Most ancillary study participants were non-Hispanic white (40.2%) or African American (42.8%), 16.1% were Hispanic, and 0.9% were Asian/Pacific Islander (Table 1). There were no statistically significant baseline differences (P < .05) between CEE and placebo participants on demographic and health factors. However, several factors differed between treatment groups by ≥ 5% including age, ethnicity, years since menopause, years since hysterectomy, parity, oral contraception use, BMI, and smoking. Therefore, these factors were evaluated as potential confounders in analyses.
Table 1.
Baseline Characteristics of the 435 Women in the Women's Health Initiative Mammogram Density Ancillary Study, CEE Trial Subsample, by Random Assignment
| Characteristic | CEE(n = 209) |
CEE Placebo(n = 226) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age group at screening, years | .36 | ||||
| 50-59 | 74 | 35.4 | 94 | 41.6 | |
| 60-69 | 87 | 41.6 | 89 | 39.4 | |
| 70-79 | 48 | 23.0 | 43 | 19.0 | |
| Ethnicity | .21 | ||||
| White | 91 | 43.5 | 84 | 37.2 | |
| Black | 80 | 38.3 | 106 | 46.9 | |
| Hispanic | 37 | 17.7 | 33 | 14.6 | |
| Asian/Pacific Islander | 1 | 0.5 | 3 | 1.3 | |
| Education | .69 | ||||
| High school diploma/GED or less | 66 | 32.0 | 79 | 35.4 | |
| School after high school | 93 | 45.1 | 92 | 41.3 | |
| College or higher | 47 | 22.8 | 52 | 23.3 | |
| Income, $ | .73 | ||||
| < 35,000 | 119 | 60.7 | 131 | 62.4 | |
| ≥ 35,000 | 77 | 39.3 | 79 | 37.6 | |
| Years since menopause | .14 | ||||
| < 5 | 24 | 13.7 | 18 | 9.7 | |
| 5-< 10 | 23 | 13.1 | 20 | 10.8 | |
| 10-< 15 | 27 | 15.4 | 19 | 10.2 | |
| ≥ 15 | 101 | 57.7 | 129 | 69.4 | |
| Years since hysterectomy | .06 | ||||
| < 18 | 66 | 31.9 | 61 | 27.2 | |
| 18-< 25 | 60 | 29.0 | 89 | 39.7 | |
| ≥ 25 | 81 | 39.1 | 74 | 33.0 | |
| Age at menarche, years | .97 | ||||
| ≤ 12 | 103 | 49.3 | 110 | 49.1 | |
| > 12 | 106 | 50.7 | 114 | 50.9 | |
| No. of term pregnancies | .22 | ||||
| Never pregnant/never had term pregnancy | 20 | 9.8 | 22 | 9.8 | |
| 1-2 | 53 | 25.9 | 72 | 32.1 | |
| 3-4 | 88 | 42.9 | 75 | 33.5 | |
| 5+ | 44 | 21.5 | 55 | 24.6 | |
| Duration of oral contraceptive use, years | .65 | ||||
| Nonuser | 132 | 63.2 | 132 | 58.4 | |
| < 5 | 48 | 23.0 | 55 | 24.3 | |
| 5-< 10 | 15 | 7.2 | 23 | 10.2 | |
| 10+ | 14 | 6.7 | 16 | 7.1 | |
| Lifetime HRT duration, years | .84 | ||||
| Never used | 128 | 61.2 | 137 | 60.6 | |
| < 5 | 41 | 19.6 | 49 | 21.7 | |
| ≥ 5 | 40 | 19.1 | 40 | 17.7 | |
| Years since last HRT use | .55 | ||||
| Never used HRT | 128 | 61.2 | 137 | 60.6 | |
| Current user/≤ 1 | 30 | 14.4 | 26 | 11.5 | |
| > 1 | 51 | 24.4 | 63 | 27.9 | |
| Family history of breast cancer (female) | 31 | 16.1 | 28 | 13.1 | .39 |
| Prior bilateral oophorectomy | 69 | 35.9 | 76 | 38.0 | .67 |
| BMI, kg/m2 | .59 | ||||
| Normal (< 25.0) | 36 | 17.2 | 36 | 16.1 | |
| Overweight (25.0-29.9) | 74 | 35.4 | 71 | 31.7 | |
| Obese (≥ 30.0) | 99 | 47.4 | 117 | 52.2 | |
| Physical activity (METs/wk) | .83 | ||||
| Lower tertile | 98 | 51.9 | 109 | 52.4 | |
| Middle tertile | 54 | 28.6 | 63 | 30.3 | |
| Upper tertile | 37 | 19.6 | 36 | 17.3 | |
| Smoking | .06 | ||||
| Never smoked | 116 | 55.5 | 128 | 57.4 | |
| Past smoker | 73 | 34.9 | 60 | 26.9 | |
| Current smoker | 20 | 9.6 | 35 | 15.7 | |
| Alcohol intake, g/d | .64 | ||||
| Nondrinker | 125 | 62.8 | 128 | 59.8 | |
| < 2.7 | 38 | 19.1 | 49 | 22.9 | |
| ≥ 2.7 | 36 | 18.1 | 37 | 17.3 | |
NOTE. There were no statistically significant differences between treatment arms. Categories were chosen on the basis of distribution of the variable in the entire Women's Health Initiative data set.
Abbreviations: CEE, conjugated equine estrogen; GED, General Equivalency Diploma; HRT, hormone replacement therapy; BMI, body mass index; MET, metabolic equivalent tasks.
All participants had at least one, and 93% had two, follow-up mammograms. Mean time was 1.2 years (SD, 0.4 years) between the baseline and first follow-up mammogram, and 1.1 years (SD, 0.3 years) between the first and second follow-up mammograms. Eleven women developed breast cancer from the end of the mammogram density measurement period to the end of the trial.
Baseline Mammographic Percent Density
Baseline mammographic percent density ranged between 0% and 69.4% with a mean of 7.3% (95% CI, 6.4% to 8.2%) and similar distributions within each treatment group (mean, 6.8%; 95% CI, 5.6% to 8.0% in CEE; and mean, 7.7%; 95% CI, 6.3% to 9.0% in placebo; P = .80; Table 2). BMI was inversely related to baseline mammographic percent density, with overweight women having lower baseline percent density (P < .001; data not shown). Age had modest inverse relationships with percent density (P = .02; data not shown). Baseline percent density differed slightly, but not statistically significantly, among the four ethnic groups (P = .11). Mean percent density was 7.7% (median, 6.8%) for the four Asian-American women, 9.7% (median, 5.5%) for the 70 Hispanics, 6.2% (median, 2.8%) for the 175 non-Hispanic whites, and 7.3% (median, 3.7%) for the 186 African Americans. After adjusting for age and BMI, these slight differences remained (P = .09). Women who had used menopausal hormone therapy within 6 months before random assignment (eg, just before the required 3-month washout period; n = 38) had a mean percent density of 7.2% (95% CI, 4.4% to 10.0%) compared with 7.3% (95% CI, 6.3% to 8.2%) for nonusers. Adjustment for age and BMI did not change these results.
Table 2.
MPD at Baseline, Year 1, and Year 2, by Treatment Assignment
| Measurement | CEE MPD |
Placebo MPD |
P* | P† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Median | Mean | 95% CI | No. | Median | Mean | 95% CI | |||
| ITT | ||||||||||
| Baseline | 209 | 3.4 | 6.8 | 5.6 to 8.0 | 226 | 3.4 | 7.7 | 6.3 to 9.0 | .80 | |
| Year 1 | 209 | 4.6 | 8.4 | 7.2 to 9.7 | 226 | 3.1 | 6.6 | 5.5 to 7.8 | ||
| Year 2 | 193 | 5.2 | 8.8 | 7.5 to 10.1 | 213 | 2.9 | 6.6 | 5.4 to 7.8 | ||
| Year 1−baseline | 209 | 0.2 | 1.6 | 0.8 to 2.4 | 226 | −0.2 | −1.0 | −1.7 to −0.4 | < .001 | < .001 |
| Year 2−baseline | 193 | 0.3 | 1.7 | 0.7 to 2.7 | 213 | −0.3 | −1.2 | −1.8 to −0.5 | < .001 | |
| Adjusted for adherence | ||||||||||
| Baseline | 209 | 3.4 | 6.8 | 5.6 to 8.0 | 226 | 3.4 | 7.7 | 6.3 to 9.0 | ||
| Year 1 | 149 | 5.3 | 8.8 | 7.3 to 10.4 | 184 | 3.0 | 6.3 | 5.1 to 7.6 | ||
| Year 2 | 113 | 5.9 | 9.1 | 7.3 to 10.8 | 138 | 2.9 | 6.4 | 5.0 to 7.8 | ||
| Year 1−baseline | 149 | 0.2 | 1.6 | 0.6 to 2.5 | 184 | −0.2 | −0.9 | −1.6 to −0.2 | .002 | < .001 |
| Year 2−baseline | 113 | 0.5 | 1.8 | 0.6 to 3.1 | 138 | −0.3 | −0.7 | −1.4 to −0.1 | < .001 | |
Abbreviations: MPD, mammographic percent density; CEE, conjugated equine estrogen; ITT, intention to treat.
P value of main effect of CEE, by visit, is based on a two-sided t test from a repeated measures model with log (percent density + 0.001) as the response. All statistical tests were two-sided.
P value of overall main effect of CEE at follow-up is based on an F test from a repeated measures model with log (percent density + 0.001) as the response.
Effect of CEEs on Mammographic Percent Density
After 1 year, women in the CEE group had a mean percent density increase of 1.6 percentage points (95% CI, 0.8 to 2.4) compared with a mean 1.0 percentage point decrease (95% CI, −1.7 to −0.4) in the placebo group (P < .001; Table 2). The increase in density with CEE use persisted to year 2, with a mean increase of 1.7 percentage points (95% CI, 0.7 to 2.7) from baseline compared with a mean decrease of 1.2 percentage points (95% CI, −1.8 to −0.5) in the placebo group (P < .001). The increase in density from year 1 to year 2 among CEE users was not statistically significant (P = .44). Relative to baseline, 56% and 55% of CEE-treated participants experienced an increase in percent density at year 1 and year 2, respectively (data not shown).
Effect of Adherence to Study Medications
To adjust for study medication adherence, percent density measurements at follow-up were censored when a participant became nonadherent (stopped taking study drugs, used < 80% of study drugs over a 6-month interval or, if in the placebo group, started nonprotocol hormone therapy). The compliance effect of CEE was similar to that shown in the intention-to-treat analyses. Among women who were adherent to treatment, the CEE group had a mean of 1.6 percentage points (95% CI, 0.6 to 2.5) increase at year 1 versus a 0.9 percentage points (95% CI, −1.6 to −0.2) decrease in placebo-treated women (Table 2). While percent density decreased by a mean 0.4 percentage points (95% CI, −1.2 to 2.1) from year 1 to year 2 among women on CEE who became nonadherent within the first year of the trial, it was not statistically significant.
Subgroup Analyses
CEE-related increase in percent density was consistent between non-Hispanic white and African American women, while no increase in percent density with CEE versus placebo was observed in Hispanic women (P = .09 for test of heterogeneity; Table 3). This association of race/ethnicity with change in density by CEE versus placebo remained after censoring for nonadherence (data not shown). The greatest increase in percent density with CEE use was seen in women age 70 to 79 years (P = .03 for interaction). The increase in mammographic density in CEE versus placebo participants was greater in normal-weight women (BMI < 25.0 kg/m2) compared with heavier participants (P = .003 for interaction), and results were similar when weight was used as a measure of adiposity (data not shown). The increase in percent density in CEE versus placebo participants was greater in women who were not currently smokers compared with current or previous smokers (P < .001), and in those with three or more births compared with women of lower parity (P = .04). We assessed the effect of prior hormone use on trial results by stratifying participants into three groups—those who had never used hormone therapy, those who used hormone therapy ≤ 1 year before random assignment, and those with last use more than 1 year before random assignment—and assessed change in percent density in CEE versus placebo participants in these three groups. The test for interaction was not statistically significant (P = .87).
Table 3.
MPD at Baseline, Year 1, and Year 2, by Baseline Characteristics and Treatment Assignment
| Characteristic | CEE MPD |
Placebo MPD |
P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Median | Mean | 95% CI | No. | Median | Mean | 95% CI | ||
| Race | .09 | ||||||||
| Non-Hispanic white | |||||||||
| Baseline | 91 | 2.8 | 5.3 | 4.0 to 6.5 | 84 | 2.6 | 7.2 | 5.1 to 9.2 | |
| Year 1−baseline | 91 | 0.6 | 2.3 | 1.2 to 3.5 | 84 | −0.2 | −1.0 | −1.8 to −0.3 | |
| Year 2−baseline | 88 | 0.6 | 2.7 | 1.3 to 4.1 | 82 | −0.3 | −0.9 | −1.7 to −0.1 | |
| African American | |||||||||
| Baseline | 80 | 3.9 | 7.2 | 5.3 to 9.1 | 106 | 3.4 | 7.4 | 5.6 to 9.3 | |
| Year 1−baseline | 80 | 0.1 | 1.7 | 0.2 to 3.2 | 106 | −0.1 | −0.4 | −1.5 to 0.6 | |
| Year 2−baseline | 75 | 0.2 | 1.3 | −0.3 to 2.9 | 102 | 0.0 | −0.7 | −1.6 to 0.2 | |
| Hispanic | |||||||||
| Baseline | 37 | 4.3 | 9.5 | 5.3 to 13.7 | 33 | 5.6 | 10.0 | 5.0 to 14.9 | |
| Year 1−baseline | 37 | 0.1 | −0.2 | −2.0 to 1.5 | 33 | −1.4 | −3.2 | −5.3 to −1.1 | |
| Year 2−baseline | 29 | −0.2 | −0.5 | −3.4 to 2.3 | 26 | −2.1 | −3.7 | −6.3 to −1.1 | |
| Age, years | .03 | ||||||||
| 50-59 | |||||||||
| Baseline | 74 | 5.0 | 9.0 | 6.6 to 11.5 | 94 | 4.6 | 7.9 | 6.0 to 9.9 | |
| Year 1−baseline | 74 | −0.1 | 0.4 | −1.0 to 1.9 | 94 | −0.6 | −1.4 | −2.3 to −0.5 | |
| Year 2−baseline | 69 | −0.5 | −0.2 | −1.8 to 1.5 | 91 | −0.7 | −2.0 | −3.0 to −0.9 | |
| 60-69 | |||||||||
| Baseline | 87 | 2.8 | 5.9 | 4.3 to 7.5 | 89 | 2.5 | 7.5 | 5.2 to 9.8 | |
| Year 1−baseline | 87 | 0.3 | 1.9 | 0.8 to 3.0 | 89 | −0.1 | −0.6 | −1.7 to 0.6 | |
| Year 2−baseline | 79 | 0.5 | 2.5 | 1.0 to 4.1 | 82 | −0.2 | −0.7 | −1.6 to 0.2 | |
| 70-79 | |||||||||
| Baseline | 48 | 1.9 | 5.0 | 3.2 to 6.8 | 43 | 3.3 | 7.4 | 4.2 to 10.6 | |
| Year 1−baseline | 48 | 0.4 | 2.9 | 1.0 to 4.8 | 43 | −0.1 | −1.2 | −2.4 to −0.1 | |
| Year 2−baseline | 45 | 1.2 | 3.0 | 1.1 to 5.0 | 40 | −0.0 | −0.2 | −1.4 to 1.0 | |
| BMI, kg/m2 | .003 | ||||||||
| Normal (< 25) | |||||||||
| Baseline | 36 | 5.6 | 9.4 | 5.9 to 13.0 | 36 | 7.1 | 11.8 | 6.9 to 16.6 | |
| Year 1−baseline | 36 | 2.2 | 3.2 | 0.5 to 5.8 | 36 | −1.0 | −1.0 | −3.2 to 1.2 | |
| Year 2−baseline | 35 | 1.8 | 3.4 | 0.0 to 6.7 | 35 | −0.6 | −0.3 | −2.3 to 1.7 | |
| Overweight (25-30) | |||||||||
| Baseline | 74 | 3.8 | 7.7 | 5.7 to 9.7 | 71 | 5.2 | 9.7 | 7.0 to 12.4 | |
| Year 1−baseline | 74 | 0.9 | 2.8 | 1.5 to 4.2 | 71 | −0.6 | −2.1 | −3.2 to −0.9 | |
| Year 2−baseline | 67 | 0.8 | 2.7 | 1.0 to 4.3 | 67 | −1.0 | −2.6 | −3.9 to −1.2 | |
| Obese (> 30) | |||||||||
| Baseline | 99 | 2.5 | 5.2 | 3.7 to 6.7 | 117 | 2.4 | 5.3 | 4.0 to 6.5 | |
| Year 1−baseline | 99 | 0.0 | 0.2 | −0.8 to 1.1 | 117 | −0.1 | −0.4 | −1.2 to 0.3 | |
| Year 2−baseline | 91 | 0.2 | 0.3 | −0.9 to 1.5 | 109 | 0.0 | −0.6 | −1.2 to 0.0 | |
| Smoking | < .001 | ||||||||
| Never | |||||||||
| Baseline | 116 | 3.6 | 7.0 | 5.4 to 8.6 | 128 | 3.5 | 7.1 | 5.6 to 8.7 | |
| Year 1−baseline | 116 | 0.1 | 1.4 | 0.3 to 2.4 | 128 | −0.1 | −0.8 | −1.5 to −0.1 | |
| Year 2−baseline | 108 | 0.5 | 2.0 | 0.7 to 3.2 | 123 | −0.2 | −1.0 | −1.8 to −0.2 | |
| Past | |||||||||
| Baseline | 73 | 2.0 | 6.0 | 4.1 to 7.9 | 60 | 5.5 | 8.4 | 5.8 to 11.0 | |
| Year 1−baseline | 73 | 0.4 | 2.4 | 0.8 to 4.0 | 60 | −1.1 | −1.8 | −2.9 to −0.7 | |
| Year 2−baseline | 68 | 0.3 | 2.1 | 0.3 to 3.9 | 56 | −0.7 | −1.2 | −2.4 to −0.0 | |
| Current | |||||||||
| Baseline | 20 | 5.6 | 8.7 | 4.4 to 13.1 | 35 | 1.7 | 8.4 | 3.4 to 13.5 | |
| Year 1−baseline | 20 | −0.0 | 0.5 | −1.1 to 2.0 | 35 | −0.2 | −0.5 | −3.1 to 2.1 | |
| Year 2−baseline | 17 | −1.3 | −1.7 | −4.8 to 1.4 | 31 | 0.1 | −1.5 | −3.5 to 0.5 | |
| Parity | .04 | ||||||||
| Never | |||||||||
| Baseline | 20 | 10.6 | 10.8 | 7.0 to 14.7 | 22 | 8.4 | 12.6 | 7.2 to 18.1 | |
| Year 1−baseline | 20 | −0.3 | −0.2 | −1.9 to 1.6 | 22 | 0.4 | −0.8 | −3.3 to 1.6 | |
| Year 2−baseline | 20 | 0.5 | −0.5 | −3.0 to 2.0 | 22 | −0.4 | −0.9 | −3.0 to 1.1 | |
| 1 to 2 | |||||||||
| Baseline | 53 | 3.8 | 7.6 | 4.8 to 10.4 | 72 | 2.7 | 6.6 | 4.4 to 8.8 | |
| Year 1−baseline | 53 | −0.0 | 0.4 | −1.1 to 1.9 | 72 | −0.3 | −0.7 | −1.9 to 0.5 | |
| Year 2−baseline | 50 | −0.2 | −0.1 | −1.8 to 1.7 | 67 | −0.4 | −1.6 | −2.6 to −0.6 | |
| 3 to 4 | |||||||||
| Baseline | 88 | 3.2 | 6.7 | 4.9 to 8.6 | 75 | 3.7 | 8.8 | 6.0 to 11.6 | |
| Year 1−baseline | 88 | 0.3 | 2.2 | 0.8 to 3.7 | 75 | −0.1 | −1.4 | −2.6 to −0.2 | |
| Year 2−baseline | 78 | 0.6 | 2.4 | 0.6 to 4.2 | 72 | −0.6 | −1.5 | −2.7 to −0.3 | |
| ≥ 5 | |||||||||
| Baseline | 44 | 2.0 | 4.3 | 2.6 to 5.9 | 55 | 3.1 | 5.8 | 4.1 to 7.5 | |
| Year 1−baseline | 44 | 0.3 | 2.2 | 0.9 to 3.6 | 55 | −0.8 | −1.2 | −2.2 to −0.2 | |
| Year 2−baseline | 41 | 0.5 | 2.9 | 1.0 to 4.8 | 50 | −0.2 | −0.4 | −1.6 to 0.9 | |
NOTE. P values for interactions between CEE and subgroups are based on an F test from a repeated measures model with log (percent density + 0.001) as the response.
Abbreviations: MPD, mammographic percent density; BMI, body mass index; CEE, conjugated equine estrogen.
Women with a Gail risk score ≥ 1.7% experienced a greater increase in percent density if they were randomly assigned to CEE versus placebo compared with women with Gail risk score less than 1.7% (P = .02 for interaction; Table 4). When we investigated effects of individual components of the Gail risk score as potential effect modifiers (age, age at menarche, age at first full-term pregnancy, history of breast biopsy, and history of atypical hyperplasia), only age modified the CEE effect on mammographic density (for age, see Table 3; other data not shown). None of the other baseline characteristics, including baseline percent density (Tables 3 and 5), were statistically significant effect modifiers of the CEE effect (at the Bonferroni adjusted or unadjusted 0.05 level).
Table 4.
MPD at Baseline, Year 1, and Year 2, by Treatment Assignment and Gail Risk
| Group | CEE MPD |
Placebo MPD |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | Median | Mean | 95% CI | No. | Median | Mean | 95% CI | |
| Low (< 1.7%) | ||||||||
| Baseline | 166 | 3.2 | 6.9 | 5.5 to 8.3 | 189 | 2.9 | 7.4 | 5.9 to 8.9 |
| Year 1 | 166 | 3.8 | 8.2 | 6.7 to 9.7 | 189 | 2.9 | 6.4 | 5.1 to 7.7 |
| Year 2 | 151 | 4.7 | 8.6 | 7.1 to 10.0 | 177 | 2.7 | 6.5 | 5.2 to 7.9 |
| Year 1−baseline | 166 | 0.1 | 1.3 | 0.4 to 2.1 | 189 | −0.2 | −1.0 | −1.7 to −0.3 |
| Year 2−baseline | 151 | 0.3 | 1.2 | 0.1 to 2.3 | 177 | −0.2 | −1.1 | −1.8 to −0.4 |
| High | ||||||||
| Baseline | 43 | 3.8 | 6.3 | 4.4 to 8.2 | 37 | 5.5 | 9.1 | 5.8 to 12.3 |
| Year 1 | 43 | 7.7 | 9.3 | 6.9 to 11.8 | 37 | 3.9 | 7.9 | 5.2 to 10.5 |
| Year 2 | 42 | 6.7 | 9.6 | 6.7 to 12.4 | 36 | 4.8 | 6.8 | 4.4 to 9.3 |
| Year 1−baseline | 43 | 1.3 | 3.0 | 1.0 to 5.1 | 37 | −0.3 | −1.2 | −2.8 to 0.4 |
| Year 2−baseline | 42 | 0.9 | 3.4 | 1.0 to 5.8 | 36 | −0.5 | −1.5 | −2.7 to −0.3 |
NOTE. P = .02 for interaction between estrogen and menopausal hormone therapy is based on a two-sided F test from a repeated measures model with log (percent density + 0.001) as the response.
Abbreviations: MPD, mammographic percent density; CEE, conjugated equine estrogen.
Table 5.
MPD at Baseline, Year 1, and Year 2, by Treatment Assignment and Percent Density at Baseline
| Group | CEE MPD |
Placebo MPD |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | Median | Mean | 95% CI | No. | Median | Mean | 95% CI | |
| MPD first tertile < 1.5% | ||||||||
| Baseline | 66 | 0.5 | 0.6 | 0.5 to 0.7 | 77 | 0.5 | 0.6 | 0.5 to 0.7 |
| Year 1 | 66 | 0.7 | 2.5 | 1.3 to 3.8 | 77 | 0.7 | 1.0 | 0.8 to 1.3 |
| Year 2 | 60 | 0.9 | 2.5 | 1.4 to 3.5 | 71 | 0.7 | 1.1 | 0.9 to 1.4 |
| Year 1−baseline | 66 | 0.2 | 2.0 | 0.7 to 3.2 | 77 | 0.2 | 0.5 | 0.3 to 0.6 |
| Year 2−baseline | 60 | 0.5 | 1.9 | 0.9 to 2.9 | 71 | 0.3 | 0.6 | 0.3 to 0.9 |
| MPD second tertile 1.5%-7.35% | ||||||||
| Baseline | 80 | 3.5 | 3.8 | 3.4 to 4.2 | 68 | 3.2 | 3.7 | 3.3 to 4.1 |
| Year 1 | 80 | 3.3 | 5.9 | 4.5 to 7.2 | 68 | 3.0 | 3.8 | 2.7 to 4.8 |
| Year 2 | 72 | 4.1 | 6.8 | 5.1 to 8.4 | 64 | 2.1 | 3.3 | 2.5 to 4.0 |
| Year 1−baseline | 80 | −0.0 | 2.0 | 0.8 to 3.3 | 68 | −0.8 | 0.1 | −1.0 to 1.1 |
| Year 2−baseline | 72 | 0.5 | 2.9 | 1.4 to 4.5 | 64 | −1.0 | −0.5 | −1.1 to 0.2 |
| MPD third tertile ≥ 7.35% | ||||||||
| Baseline | 63 | 14.5 | 17.1 | 14.9 to 19.4 | 81 | 13.9 | 17.7 | 15.2 to 20.2 |
| Year 1 | 63 | 16.8 | 17.9 | 15.7 to 20.1 | 81 | 11.2 | 14.4 | 12.1 to 16.6 |
| Year 2 | 61 | 16.7 | 17.3 | 15.0 to 19.7 | 78 | 11.5 | 14.2 | 11.8 to 16.6 |
| Year 1−baseline | 63 | 0.2 | 0.8 | −1.0 to 2.6 | 81 | −3.4 | −3.4 | −4.8 to −2.0 |
| Year 2−baseline | 61 | −1.0 | 0.0 | −2.3 to 2.4 | 78 | −3.6 | −3.3 | −4.8 to −1.9 |
NOTE. P = .38 for the interactiovisuln between CEE and baseline percent density is based on an F test from a repeated measures model with log (percent density + 0.001) as the response.
Abbreviations: MPD, mammographic percent density; CEE, conjugated equine estrogen.
DISCUSSION
In an ancillary study of the WHI randomized clinical trial, CEE resulted in a higher mammographic percent density compared with placebo after use for 1 year (2.6 percentage point difference; 95% CI, 1.0 to 2.7) and 2 years (2.9 percentage point difference; 95% CI, 0.6 to 3.1). Similar effects were observed in African American and non-Hispanic white women.
These results, suggesting that the effect of CEE on percent density is maintained over a 2-year period, extend the findings from the Postmenopausal Estrogen and Progesterone Intervention (PEPI) trial in which using CEE 0.625 mg/d for 1 year increased mammographic density by an absolute mean of 1.2% versus a decrease of 0.1% in placebo-treated women.8 In a small trial, postmenopausal women randomly assigned to CEE 0.625 mg/d (n = 36) had an absolute mean increase in mammographic density over 2 years of 1.2% versus a 1.3% decrease in women randomly assigned to placebo (n = 45; P < .01).7 A report that included women from two trials found that postmenopausal women randomly assigned to 1 mg/d of micronized 17β-estradiol (n = 104) had an adjusted absolute mean increase in mammographic density of 4.6% after 12 months versus a 0.02% increase in women assigned to placebo (n = 93).25
Our finding of a 2.6 percentage point difference in percent density in women randomly assigned to CEE versus placebo is lower than the 6.9 percentage point difference observed for CEE plus MPA 2.5 mg/d in WHI, where CEE plus MPA increased percent density by a mean of 6.0 percentage points (95% CI, 4.6 to 7.5) at year 1 compared with a mean decrease of 0.9 percentage points (95% CI, −0.2 to −1.5) in the placebo group (P < .001), with differences largely persisting after 2 years. These results suggest a sizeable role for progestin on mammographic density, but our results suggest that CEE alone also can influence mammographic density.
Unlike a prior report by Ursin et al4 of substantially lower density in African American women, mammographic breast density was not substantially or significantly lower in African Americans relative to Hispanic and non-Hispanic whites in our study population. This inconsistency could be attributed to racial/ethnic variations by age or menopausal status, given that the Ursin et al study was in younger women (ages 35 to 64 years) and included 44% premenopausal women. The effect of CEE in our study on increasing percent density was consistent between non-Hispanic whites and African American women but was not observed in Hispanic women.
Breast cancer risk increases by ≈15% with each 10% increase in percent density,3 and a meta-analysis including more than 14,000 patients confirmed a strong relationship between percent density and breast cancer risk.26 Too few breast cancer cases (n = 11) occurred in this study to assess the relationship between the small hormone-associated increase in mammographic density and individual breast cancer risk.
One hypothesis is that mammographic density change may predict an intervention's effect on breast cancer risk.9,12 Combined hormone therapy increases mammographic density9 and breast cancer risk,13 while tamoxifen reduces mammographic density27,28 and breast cancer risk.24,29 However, raloxifene, which reduces invasive breast cancer risk23 has not consistently been reported to decrease breast density.7,30 Similarly, data currently do not support an effect of aromatase inhibitors on breast density,31 although these medications reduce new contralateral breast cancers.24 In the WHI hormone therapy trials, use of CEE plus MPA increased breast density9 and breast cancer risk,12 while CEE alone, which increased breast density, did not increase breast cancer incidence but rather showed a trend toward decreased risk.14 It remains to be determined whether an individual's change in mammographic density represents a breast cancer risk factor for that woman.
Strengths of this report include the double-blind randomized design; the quantitative, quality-controlled mammographic density assessment; the 2-year follow-up period, which provides new information on duration and the cumulative nature of CEE effects; and the over-representation of women from several race and ethnic minority groups. Study limitations include the evaluation of a single formulation, dose and schedule of hormone therapy, and a population that is older than women typically beginning hormone therapy.
In conclusion, use of CEE alone results in a modest but statistically significant increase in mammographic percent density which is sustained over at least a 2-year period. The clinical significance of the CEE effect on mammographic density remains to be determined.
Appendix
Women's Health Initiative Investigators
Program Office. National Heart, Lung, and Blood Institute, Bethesda, MD: Barbara Alving, Jacques Rossouw, Linda Pottern.
Clinical Coordinating Centers. Fred Hutchinson Cancer Research Center, Seattle, WA: Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth E. Patterson, Anne McTiernan; Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker, Pentti Rautaharju; Medical Research Laboratories, Highland Heights, KY: Evan Stein; University of California at San Francisco, San Francisco, CA: Steven Cummings; University of Minnesota, Minneapolis, MN: John Himes; University of Washington, Seattle, WA: Bruce Psaty.
Clinical Centers. Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, TX: Jennifer Hays; Brigham and Women's Hospital, Harvard Medical School, Boston, MA: JoAnn Manson; Brown University, Providence, RI: Annlouise R. Assaf; Emory University, Atlanta, GA: Lawrence Phillips; Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley Beresford; George Washington University Medical Center, Washington, DC: Judith Hsia; Harbor-University of California, Los Angeles Research and Education Institute, Torrance, CA: Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, OR: Cheryl Ritenbaugh; Kaiser Permanente Division of Research, Oakland, CA: Bette Caan; Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen; MedStar Research Institute/Howard University, Washington, DC: Barbara V. Howard; Northwestern University, Chicago/Evanston, IL: Linda Van Horn; Rush-Presbyterian St. Luke's Medical Center, Chicago, IL: Henry Black; Stanford Center for Research in Disease Prevention, Stanford University, Stanford, CA: Marcia L. Stefanick; State University of New York at Stony Brook, Stony Brook, NY: Dorothy Lane; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Alabama at Birmingham, Birmingham, AL: Cora Beth Lewis; University of Arizona, Tucson/Phoenix, AZ: Tamsen Bassford; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of California at Davis, Sacramento, CA: John Robbins; University of California at Irvine, Orange, CA: Allan Hubbell; University of California at Los Angeles, Los Angeles, CA: Howard Judd; University of California at San Diego, La Jolla/Chula Vista, CA: Robert D. Langer; University of Cincinnati, Cincinnati, OH: Margery Gass; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Hawaii, Honolulu, HI: David Curb; University of Iowa, Iowa City/Davenport, IA: Robert Wallace; University of Massachusetts/Fallon Clinic, Worcester, MA: Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, NJ: Norman Lasser; University of Miami, Miami, FL: Mary Jo O'Sullivan; University of Minnesota, Minneapolis, MN: Karen Margolis; University of Nevada, Reno, NV: Robert Brunner; University of North Carolina, Chapel Hill, NC: Gerardo Heiss; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; University of Tennessee, Memphis, TN: Karen C. Johnson; University of Texas Health Science Center, San Antonio, TX: Robert Brzyski; University of Wisconsin, Madison, WI: Gloria Sarto; Wake Forest University School of Medicine, Winston-Salem, NC: Denise Bonds; Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI: Susan Hendrix.
Mammogram Density Ancillary Study Investigators
Coordinating Center: (University of North Carolina) Gerardo Heiss, Barbara Hulka, Christopher Martin, Jennifer Peck, Etta Pisano.
WHI Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Anne McTiernan, C.Y. Wang.
Clinical Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora Beth Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University of California at Davis, Sacramento, CA) John Robbins; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds.
Footnotes
Written on behalf of the Women's Health Initiative Mammographic Density Study Investigators.
Supported by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services (Women's Health Initiative study), by the National Cancer Institute Grant No. R01 CA76017-04 (Mammogram Density Ancillary Study), and by Wyeth-Ayerst Research Laboratories (active study drug and placebo).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Etta D. Pisano, GE, Siemens, Sectra, VuComp, R2 (Hologic), ICAD, Konica-Minolta and Naviscan (U) Stock Ownership: None Honoraria: None Research Funding: Etta D. Pisano, GE, Siemens, Sectra, VuComp, R2 (Hologic), Konica-Minolta and Naciscan Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Anne McTiernan, Etta D. Pisano, JoAnn E. Manson
Administrative support: Anne McTiernan, Etta D. Pisano, Gerardo Heiss
Provision of study materials or patients: Etta D. Pisano, Karen C. Johnson, JoAnn E. Manson, Mara Z. Vitolins, Gerardo Heiss
Collection and assembly of data: Jennifer David Peck, Aaron Aragaki, Etta D. Pisano, Karen C. Johnson, JoAnn E. Manson, Gerardo Heiss
Data analysis and interpretation: Anne McTiernan, Rowan T. Chlebowski, Christopher Martin, Aaron Aragaki, Etta D. Pisano, C.Y. Wang, Karen C. Johnson, JoAnn E. Manson, Robert B. Wallace
Manuscript writing: Anne McTiernan, Jennifer David Peck, Aaron Aragaki, Etta D. Pisano, Robert B. Wallace, Mara Z. Vitolins
Final approval of manuscript: Anne McTiernan, Rowan T. Chlebowski, Christopher Martin, Jennifer David Peck, Aaron Aragaki, Etta D. Pisano, Karen C. Johnson, JoAnn E. Manson, Robert B. Wallace, Mara Z. Vitolins, Gerardo Heiss
REFERENCES
- 1.Boyd NF, Lockwood GA, Byng JW, et al. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- 2.Byrne C, Schairer C, Brinton LA, et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States) Cancer Causes Control. 2001;12:103–110. doi: 10.1023/a:1008935821885. [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Schairer C, Wolfe J, et al. Mammographic features and breast cancer risk: Effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 4.Ursin G, Ma H, Wu AH, et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12:332–338. [PubMed] [Google Scholar]
- 5.Ziv E, Tice J, Smith-Bindman R, et al. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2090–2095. [PubMed] [Google Scholar]
- 6.Boyd N, Martin L, Stone J, et al. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048–1053. [PubMed] [Google Scholar]
- 7.Freedman M, San Martin J, O'Gorman J, et al. Digitized mammography: A clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst. 2001;93:51–56. doi: 10.1093/jnci/93.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Greendale GA, Reboussin BA, Slone S, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A, Martin CF, Peck JD, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women's Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–1376. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 10.Persson I, Thurfjell E, Holmberg L. Effect of estrogen and estrogen-progestin replacement regimens on mammographic breast parenchymal density. J Clin Oncol. 1997;15:3201–3207. doi: 10.1200/JCO.1997.15.10.3201. [DOI] [PubMed] [Google Scholar]
- 11.Rutter CM, Mandelson MT, Laya MB, et al. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 12.Chlebowski RT, McTiernan A. Biological significance of interventions that change breast density. J Natl Cancer Inst. 2003;95:4–5. doi: 10.1093/jnci/95.1.4. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 14.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 16.Design of the Women's Health Initiative clinical trial and observational study: The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Hays T, Hunt JR, Hubbel A, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Colacurci N, Fornaro F, De Franciscis P, et al. Effects of a short-term suspension of hormone replacement therapy on mammographic density. Fertil Steril. 2001;76:451–455. doi: 10.1016/s0015-0282(01)01967-7. [DOI] [PubMed] [Google Scholar]
- 19.Stone J, Gunasekara A, Martin LJ, et al. The detection of change in mammographic density. CEBP. 2003;12:625–630. [PubMed] [Google Scholar]
- 20.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 21.Titus-Ernstoff L, Tosteson AN, Kasales C, et al. Breast cancer risk factors in relation to breast density (United States) Cancer Causes Control. 2006;17:1281–1290. doi: 10.1007/s10552-006-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 23.Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Constantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 25.Lord SJ, Mack WJ, Van Den Berg D, et al. Polymorphisms in genes involved in estrogen and progesterone metabolism and mammographic density changes in women randomized to postmenopausal hormone therapy: Results from a pilot study. Breast Cancer Res. 2005;7:R336–R344. doi: 10.1186/bcr999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 27.Brisson J, Brisson B, Cote G, et al. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–915. [PubMed] [Google Scholar]
- 28.Chow CK, Venzon D, Jones EC, et al. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9:917–921. [PubMed] [Google Scholar]
- 29.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 30.Christodoulakos GE, Lambrinoudaki IV, Vourtsi AD, et al. Mammographic changes associated with raloxifene and tibolone therapy in postmenopausal women: A prospective study. Menopause. 2002;9:110–116. doi: 10.1097/00042192-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Becker S, Kaaks R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: Can mammographic density be considered an intermediate marker of risk?—Recent results. Cancer Res. 2009;181:135–157. doi: 10.1007/978-3-540-69297-3_14. [DOI] [PubMed] [Google Scholar]