Abstract
Purpose
Granulocyte-macrophage colony-stimulating factor (GM-CSF) –secreting tumor vaccines have demonstrated bioactivity but may be limited by disease burdens and immune tolerance. We tested the hypothesis that cyclophosphamide (CY) and doxorubicin (DOX) can enhance vaccine-induced immunity in patients with breast cancer.
Patients and Methods
We conducted a 3 × 3 factorial (response surface) dose-ranging study of CY, DOX, and an HER2-positive, allogeneic, GM-CSF–secreting tumor vaccine in 28 patients with metastatic breast cancer. Patients received three monthly immunizations, with a boost 6 to 8 months from study entry. Primary objectives included safety and determination of the chemotherapy doses that maximize HER2-specific immunity.
Results
Twenty-eight patients received at least one immunization, and 16 patients received four immunizations. No dose-limiting toxicities were observed. HER2-specific delayed-type hypersensitivity developed in most patients who received vaccine alone or with 200 mg/m2 CY. HER2-specific antibody responses were enhanced by 200 mg/m2 CY and 35 mg/m2 DOX, but higher CY doses suppressed immunity. Analyses revealed that CY at 200 mg/m2 and DOX at 35 mg/m2 is the combination that produced the highest antibody responses.
Conclusion
First, immunotherapy with an allogeneic, HER2-positive, GM-CSF–secreting breast tumor vaccine alone or with CY and DOX is safe and induces HER2-specific immunity in patients with metastatic breast cancer. Second, the immunomodulatory activity of low-dose CY has a narrow therapeutic window, with an optimal dose not exceeding 200 mg/m2. Third, factorial designs provide an opportunity to identify the most active combination of interacting drugs in patients. Further investigation of the impact of chemotherapy on vaccine-induced immunity is warranted.
INTRODUCTION
More effective treatments have led to a clear decrease in breast cancer mortality, but up to 40% of diagnosed patients ultimately relapse.1 The best drugs available have limited impact on the survival of patients with disseminated breast cancer. Innovative treatments that complement existing therapies are urgently needed to improve disease outcomes in advanced, treatment-resistant patients.
Active immune-based therapies, such as vaccines, have several advantages that could complement standard breast cancer treatments. First, they can engage the host antitumor response rather than targeting the tumor directly. Second, the immune system can specifically recognize an unlimited number of target antigens preferentially expressed by diseased cells relative to normal tissue. Third, immunotherapy could yield a durable treatment response due to immunologic memory. Several vaccines for metastatic breast cancer have been tested with modest success.2 These studies demonstrated vaccine safety, but immune responses were frequently inconsistent, observed in small numbers of patients, or not clearly associated with clinical benefit. The lack of clinical success is most likely due to suboptimal immunization strategies that fail to consider immune tolerance and disease burdens, inadequate targets, or both.3
Tumor cells genetically modified to express granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce potent T-cell–dependent immunity capable of curing tumor-bearing mice.4 Early clinical trials of GM-CSF–secreting tumor vaccines in diverse solid tumors demonstrated their safety and bioactivity, with some suggestive evidence of clinical benefit.5–13 However, vaccination alone is unlikely to induce an immune response of sufficient magnitude and potency to cause tumor regression when immune tolerance and measurable tumor burdens are present.
Some chemotherapy drugs can augment immunotherapy when given in proper dose and sequence.14 In the immune tolerant HER2/neu (neu-N) transgenic mouse model of mammary cancer, an HER2-targeted, GM-CSF–secreting vaccine alone is ineffective against established HER2-positive tumors.15 In contrast, sequencing the vaccine with low doses of cyclophosphamide (CY) and doxorubicin (DOX) induces curative HER2-specific immune responses in up to 30% of tumor-bearing neu-N mice.16 CY can abrogate the suppressive influence of CD4+CD25+ regulatory T cells (Tregs), allowing the activation of potent, tumor-specific CD8+ T cells.17 Accumulating data implicate Tregs as a major barrier to effective T-cell immunity in advanced cancer patients.18–23
On the basis of these data, we conducted a clinical evaluation of an allogeneic, HER2-positive GM-CSF–secreting breast tumor vaccine alone or in sequence with low doses of CY and DOX. This phase I study was designed to assess the safety and immunologic activity of chemotherapy-modulated vaccination in patients with stable metastatic breast cancer. Modeling responses to HER2 as a sentinel measure of immunologic activity, the study used a factorial design24 to identify the CY and DOX dose combination that maximizes the vaccine-induced immune response.
PATIENTS AND METHODS
Study Design
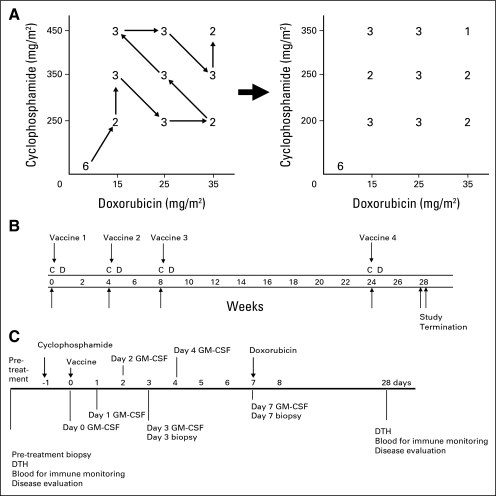
The study protocol has been published.25 This was a dose-ranging study of CY and DOX in a 3 × 3 factorial design to determine the dose combination that maximizes vaccine-induced immunity (Fig 1A). Vaccine alone was first given to six patients; the remaining 22 patients were enrolled in the dosing schema sequencing chemotherapy with vaccination. CY and DOX were each tested at three doses encompassing those efficacious in the preclinical neu-N model, yielding a total of nine design points. Enrollment initially followed a predefined path through the nine design points for safety.
Fig 1.
(A) Three patients each received 5 × 107 or 5 × 108 vaccine cells alone. Chemotherapy with 5 × 108 vaccine cells was given to the remaining patients. (B) Up to four vaccination cycles were given. (C) Cyclophosphamide was given on day −1, vaccine on day 0, and doxorubicin on day +7. Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels and immunity were measured as indicated. C, cyclophosphamide; D, doxorubicin; DTH, delayed-type hypersensitivity.
The study was conducted in accordance with the principles of good clinical practice and the ethical principles stated in the Declaration of Helsinki. It was approved by The Johns Hopkins University School of Medicine Institutional Review Board, the National Institutes of Health Recombinant DNA Advisory Committee, and the US Food and Drug Administration Center for Biologics, Evaluation and Research. A modification to the original design was approved by the Institutional Review Board and the US Food and Drug Administration and was implemented July 6, 2006. This modification, based on early safety and immune data, altered the range of CY doses from 250, 350, and 450 mg/m2 to 200, 250, and 350 mg/m2, allowed flexibility to enter eligible patients onto the design points compatible with their prior cumulative DOX dose, and revised the sample size from 30 to a range of 22 to 30.
Patient Selection
Twenty-eight patients with metastatic breast cancer stable for ≥ 28 days were enrolled at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center between January 15, 2004, and January 9, 2008. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 and a histologic diagnosis of breast cancer; HER2 overexpression was allowed but not required. Prior chemotherapy was allowed but must have been completed ≥ 28 days before vaccination; concurrent endocrine and/or bisphosphonate therapy was allowed. Other requirements included cardiac ejection fraction ≥ 45%, adequate end-organ function, and negative testing for HIV and pregnancy. Stable treated CNS disease was allowed. Key exclusion criteria included a projected lifetime cumulative DOX dose ≥ 450 mg/m2, past/current autoimmune disease, nonprotocol-specific treatment or parenteral steroids within 28 days of vaccination, and past/current second malignancy (except superficial melanoma, bladder cancer, or cervical carcinoma in situ).
Study Plan and Intervention
Eligibility determination.
Written informed consent was obtained from each research participant. Baseline studies included computed tomography, bone scans, complete blood count with differential (CBC), chemistry profile, absolute eosinophil count, and echocardiogram or multiple gated acquisition scan.
Treatment plan.
The intervention and data collection schedule is shown in Figures 1B and 1C. Six patients received vaccine alone, with three each receiving 5 × 107 or 5 × 108 cells. The remaining 22 patients received 5 × 108 cells and chemotherapy, with CY given on day −1, vaccine on day 0, and DOX on day 7. This sequence was repeated every 4 to 6 weeks for three cycles, with a fourth cycle 6 to 8 months after cycle 1. Patients with evidence of disease progression were taken off study.
Vaccinations.
Vaccine development and manufacturing has been published.26 Briefly, the parent cell lines T47D (HER2low) and SKBR3 (HER2high) were genetically modified by plasmid DNA transfection to secrete GM-CSF. Clinical lots were prepared from two subcloned cell lines secreting bioactive levels of GM-CSF, 2T47D-V, and 3SKBR3-7. On day 0, serum-free, cryopreserved, irradiated vaccine cells were thawed and mixed to create an HER2-positive vaccine that secreted GM-CSF levels of 305 ng/106 cells/24 hours.26 Vaccine cells were injected intradermally, evenly distributed over three lymph node areas. Anesthetic lidocaine cream was applied to the injection sites before vaccination.
End Points
Toxicity assessment.
Toxicities were graded using the National Cancer Institute's Clinical Trials Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE v3.0). Toxicity monitoring included clinical assessment and complete blood counts weekly and on day 3 of each cycle; chemistry profiles were measured before and after each cycle and on day 7.
Pharmacokinetic analysis of serum GM-CSF levels.
Serum was collected to measure GM-CSF levels on days 0, 1, 2, 3, 4, and 7 of each cycle, separated from whole blood by centrifugation, and frozen in 1-mL aliquots at −80°C. Serum GM-CSF levels were determined by enzyme-linked immunosorbent assay (Quantikine ELISA, R&D Systems, Minneapolis, MN). Serum GM-CSF levels were determined by using a recombinant GM-CSF standard calibrated against the WHO GM-CSF control standard.
Assessment of delayed-type hypersensitivity (DTH) using HER2 peptides.
One hundred μg each of two major histocompatibility complex class II HER2 epitopes (p369 and p776),27 with mutated k-ras and tetanus toxoid as negative and positive controls, were injected intradermally on the back. Erythema and induration were assessed 2 to 3 days after injection.
Measurement of HER2-specific serum antibody.
The enzyme-linked immunosorbent assay for HER2-specific humoral immunity has been published.28 Briefly, 96-well plates were used for a sandwich assay incorporating the HER2-specific monoclonal antibody 520C9, HER2 antigen derived from SKBR3 cells, and patient serum samples in quadruplicate; serially diluted, purified human immunoglobulin G (IgG) was used as a standard. Plates were developed with a goat antihuman IgG-horseradish-peroxidase conjugate/substrate system.
Statistical Considerations and Data Analysis
The trial database was closed on January 12, 2009. Data from 28 patients across all treatment cycles were used in the analyses. The differences in peak serum GM-CSF levels were analyzed using linear mixed models with CY and DOX doses as predictors. HER2-specific DTH was defined as positive if there was one positive response among the four cycles; HER2-specific humoral immunity was categorized as positive at ≥ 1.13 μg/mL. Statistical significance was assessed by Fisher's exact test. HER2-specific antibody responses were also measured as a quantitative continuous variable. The relationship between quantitative antibody response and CY and DOX drug doses was assessed using a response surface model—an ordinary regression model with antibody response as the dependent variable and CY and DOX doses as independent variables. The model included quadratic (second order) terms for the doses of CY and DOX to permit curvature in the response surface so a maximum antibody response would be clearly evident. A lack of fit test was used to exclude unnecessary terms from the regression model. This response surface analysis provides an established method to select the CY and DOX dose combination that maximizes the immune response (the absolute difference of the median antibody level pre- and postvaccine).24,29–32 All statistical analyses were performed using SAS v. 9.1 (SAS Institute, Cary, NC). Two-sided P values are reported.
RESULTS
Patient Characteristics
Twenty-eight eligible patients were enrolled, with an age range of 36 to 74 years (Table 1). All had estrogen receptor–positive and/or progesterone-positive disease; one patient also had HER2-positive breast cancer. The mean disease-free interval to relapse from first diagnosis was 29 months (range, 0 to 132 months); nine (32%) patients presented initially with metastatic disease. Eight patients (29%) received prior chemotherapy for metastatic disease. All (100%) were on concurrent endocrine therapy, and the majority (71%) received concurrent bisphosphonate therapy for skeletal metastasis.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Total patients | 28 enrolled | |
| 28 evaluable | ||
| Age, years | ||
| Median | 50 | |
| Range | 36-74 | |
| ER-positive or PR-positive tumor | 28 | 100 |
| HER2-positive tumor | 1 | 4 |
| Metastatic disease at diagnosis | 9 | 32 |
| Disease-free interval to relapse, months | ||
| Median | 11 | |
| Range | 0-132 | |
| Mean | 28.8 | |
| Prior chemotherapy for metastatic disease* | 8 | 29 |
| Concurrent endocrine therapy | 28 | 100 |
| Concurrent bisphosphonate therapy† | 20 | 71 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Two patients received consolidation chemotherapy after surgical resection to no evidence of disease, followed by endocrine therapy; three patients received first-line chemotherapy for metastatic disease followed by endocrine therapy; two patients received one or more regimens of salvage chemotherapy after initial endocrine therapy.
Bisphosphonate therapy for skeletal metastasis; one additional patient received oral bisphosphonate for bone health in the absence of skeletal disease.
Almost all dose combinations were evaluated in two or three patients (Fig 1A). All eligible patients (100%) received at least one vaccination, 25 (89%) received at least three vaccinations, and 16 (57%) received all four vaccinations. All off-study events before cycle 4 were due to progressive breast cancer except one; that patient was taken off-study to receive treatment for a preexisting, subclinical thyroid goiter.
Toxicity
No dose-limiting toxicities were observed. The most common adverse events were local vaccine site reactions, including erythema, induration, pruritus, and/or discomfort (Table 2). These self-limited local reactions occurred in all individuals, lasted up to 2 weeks, and typically increased in intensity but not duration with subsequent vaccinations. The most common vaccine-related systemic adverse events were fatigue and flu-like symptoms. Small numbers of patients (7%) developed urticaria or eczema distant from the vaccine site. Cardiac function was followed over time because of the theoretical risk that HER2-specific humoral responses could potentiate DOX-related cardiac dysfunction. There was no statistically significant change in ejection fraction (data not shown). No vaccine-related serious adverse events occurred, and no evidence of autoimmunity was detected.
Table 2.
Summary of Treatment-Related Adverse Events
| Adverse Event | All Grades |
Grade 3 or 4 |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Local vaccine site reactions | ||||
| Erythema/induration | 28 | 100 | 0 | 0 |
| Pruritus | 27 | 96 | 0 | 0 |
| Pain/soreness/tenderness | 27 | 96 | 0 | 0 |
| Blister formation | 7 | 25 | 0 | 0 |
| Hyperpigmentation | 3 | 11 | 0 | 0 |
| Ecchymosis | 2 | 7 | 0 | 0 |
| Local desquamation | 1 | 4 | 0 | 0 |
| Systemic toxicities | ||||
| Fatigue/malaise | 11 | 39 | 0 | 0 |
| Flu-like symptoms/myalgia | 10 | 32 | 0 | 0 |
| Fever | 7 | 25 | 0 | 0 |
| Chills | 6 | 21 | 0 | 0 |
| Headache | 5 | 18 | 0 | 0 |
| Urticaria | 2 | 7 | 0 | 0 |
| Eczema | 2 | 7 | 0 | 0 |
| Lymphadenopathy | 2 | 7 | 0 | 0 |
| Lymph node pain | 2 | 7 | 0 | 0 |
| Pruritus (distant from vaccine site) | 1 | 4 | 0 | 0 |
| Rash (distant from vaccine site) | 1 | 4 | 0 | 0 |
| Anorexia | 1 | 4 | 0 | 0 |
| Nausea | 1 | 4 | 0 | 0 |
| Anxiety | 1 | 4 | 0 | 0 |
NOTE. Data are given as any incident per patient, for a maximum of 28 counts per event.
Serum GM-CSF Pharmacokinetics
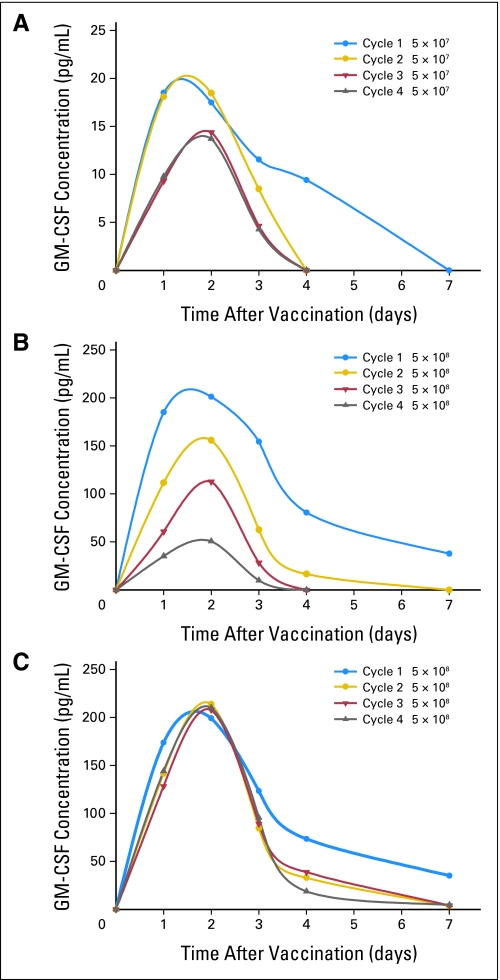
Serum GM-CSF levels were measured as an indicator of the vaccine's life span following injection. With vaccination alone, GM-CSF levels peaked by 48 hours regardless of cell dose or cycle; for the high vaccine cell dose, the peak amplitude decreased with each subsequent cycle (P = .0001) (Figs 2A and 2B). The addition of low-dose chemotherapy to the vaccine did not alter the timing of peak GM-CSF levels, but the peak GM-CSF level did not decline with subsequent vaccination (P = .99; Fig 2C). There was no statistically significant difference in peak GM-CSF level with time across the doses of CY and DOX tested (data not shown).
Fig 2.
Mean serum granulocyte-macrophage colony-stimulating factor (GM-CSF) levels across each vaccine cycle alone and with chemotherapy. The mean/standard error of peak GM-CSF levels is (A) 16.8 ± 29.4 for 5 × 107 cells alone, (B) 153.6 ± 36.3 for 5 × 108 cells alone, and (C) 224.7 ± 13 across all chemotherapy-modulated vaccination cycles.
HER2-Specific CD4+ T-Cell–Dependent Immunity
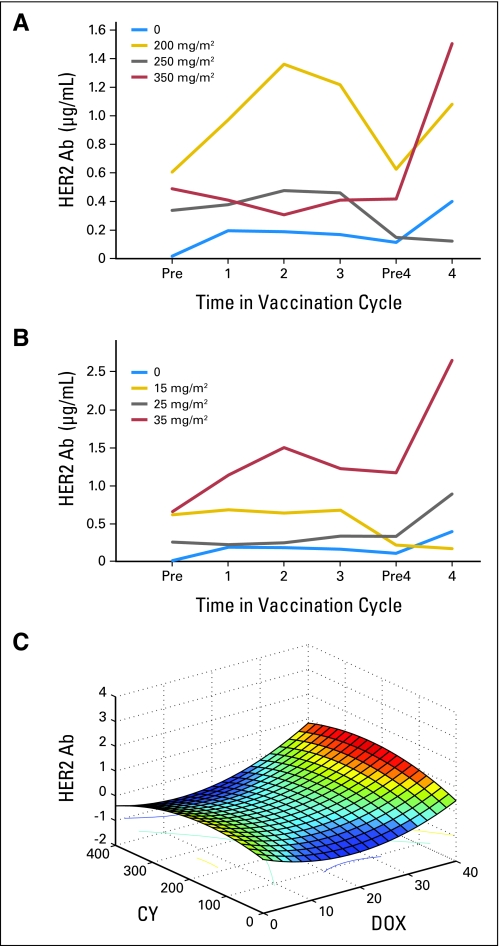
De novo HER2-specific DTH was observed in five (83%) of six patients receiving vaccine alone, and in seven (32%) of 22 patients receiving vaccine with chemotherapy (P = .034; Table 3). The addition of 200 mg/m2 CY had no impact on the rate of DTH development (P = .336), but CY doses higher than 200 mg/m2 suppressed vaccine-induced DTH compared with vaccine alone (P = .007) or combined with 200 mg/m2 CY (P = .03). The addition of 15 mg/m2 DOX suppressed the rate of DTH development (P = .016), whereas higher doses of DOX preserved the vaccine-induced DTH response (P = .15 to .18). Significant HER2-specific humoral immunity (≥ 1.13 μg/mL) developed in one (17%) of six patients who received vaccine alone and in seven (32%) of 22 patients who received vaccine with any dose of chemotherapy (P = .329). The induction of HER2-specific humoral immunity was optimally enhanced by the addition of 200 mg/m2 CY or 35 mg/m2 DOX to vaccination (Figs 3A and 3B); antibody levels declined after the third vaccination but were restored with the fourth cycle.
Table 3.
Impact of CY and DOX Dose on De Novo HER2-Specific DTH
| Dose Comparison | De Novo HER2-Specific DTH |
Fisher's Exact P | |
|---|---|---|---|
| No. of Patients | % | ||
| 0 v any chemotherapy | 5 v 7 | 83 v 32 | .034 |
| 0 v 200 CY | 5 v 5 | 83 v 63 | .336 |
| 0 v > 200 CY | 5 v 2 | 83 v 14 | .007 |
| 0 + 200 v > 200 CY | 10 v 2 | 71 v 14 | .003 |
| 200 v > 200 CY | 5 v 2 | 63 v 14 | .030 |
| 0 v 15 DOX | 5 v 1 | 83 v 13 | .016 |
| 0 v 25 DOX | 5 v 4 | 83 v 44 | .15 |
| 0 v 35 DOX | 5 v 2 | 83 v 40 | .18 |
Abbreviations: CY, cyclophosphamide; DOX, doxorubicin; DTH, delayed-type hypersensitivity.
Fig 3.
(A) Mean HER2 antibody (Ab) levels by cyclophosphamide (CY) dose. (B) Mean HER2 Ab levels by doxorubicin (DOX) dose. (C) Three-dimensional immune response surface of predicted median postvaccination HER2-specific Ab responses as a function of CY and DOX dose combinations.
Response Surface Analysis
The relationship between chemotherapy dose and antibody level is illustrated in a three-dimensional response surface generated by the model (Fig 3C). Canonical surface analysis showed eigenvalues of 0.68 and −0.46, indicating the stationary point is a saddle point. Ridge analysis estimated that the maximum HER2-specific antibody response is 0.739 μg/mL ± 0.37 μg/mL at 193 mg/m2 CY and 35 mg/m2 DOX. The closest dose combination formally tested is 200 mg/m2 CY and 35 mg/m2 DOX. A similar trend was observed for CY and DOX independently when antibody levels were analyzed as binary values by Fisher's exact test. The agreement between the experimental and predicted dose values supports the suitability of the model.
DISCUSSION
This phase I factorial study of an allogeneic HER2-positive GM-CSF-secreting breast tumor vaccine with low-dose CY and DOX supports the following five conclusions. First, up to four sequential vaccine treatments is safe and well-tolerated in metastatic breast cancer patients. Second, vaccine alone or sequenced with low-dose chemotherapy can induce de novo HER2-specific T-helper–dependent immunity. Third, low-dose CY can augment the magnitude of vaccine-induced humoral immunity, but the therapeutic window for enhancing immune responses is narrow. Specifically, 200 mg/m2 CY augments the magnitude of HER2-specific humoral immunity, whereas doses above 200 mg/m2 are more likely to suppress both DTH and antibody responses. Fourth, 35 mg/m2 DOX augments the level of vaccine-induced HER2-specific antibody. Finally, factorial arrangement of doses is a feasible and effective design for identifying the most active combination of interacting drugs for further testing in patients. Response surface analysis, a standard statistical method based on familiar linear regression models, revealed the most active chemotherapy dose combination tested to be 200 mg/m2 CY and 35 mg/m2 DOX. There are few clinical studies that use factorial design to identify the drug dose combination that maximizes a biologic or clinical outcome. Compared with traditional dose-finding schemes, factorial design is efficient and can identify productive drug interactions that might be essential for success.
Most vaccine-related adverse effects were grade 1 to 2 injection site reactions, with occasional enlargement and/or discomfort in vaccine-draining lymph nodes. These expected reactions are consistent with previous studies of GM-CSF–secreting tumor vaccines.5–13 They are the earliest manifestation of the self-limited locoregional inflammatory response that initiates the signal cascade resulting in tumor immunity. Notably, the addition of CY and DOX did not potentiate vaccine-related toxicity.
Pharmacokinetic analysis of serum GM-CSF levels demonstrated a peak level by 48 hours for both vaccine doses across all vaccination cycles. This pattern of clearance resembles those previously reported for GM-CSF–secreting vaccines.4–6,33,34 At the high vaccine cell dose alone, peak GM-CSF amplitude progressively diminished with subsequent treatment cycles. In contrast, sequencing low-dose chemotherapy with the vaccine preserved GM-CSF levels across all cycles. These data suggest that chemotherapy may preserve the initial inflammatory stimulus, maintaining vaccine bioactivity in the setting of previous immunization. Repetitive vaccination with an allogeneic vaccine might induce an allogeneic immune response that more rapidly clears the vaccine cells. Low-dose chemotherapy may inhibit this allogeneic response. It is also possible that low-dose chemotherapy inhibits effective T-cell responses against breast cancer. However, the fact that we observed vaccine-induced DTH and antibody responses in the context of preserved GM-CSF levels makes this possibility less likely.
A major difficulty in optimizing tumor cell vaccine-based therapies is the lack of biomarkers for assessing multidrug interactions. This study demonstrates the feasibility of using HER2-specific DTH and antibody responses pre- and postvaccination as immune response biomarkers. On the basis of these parameters, we found that 200 mg/m2 CY best preserves DTH development and augments the level of HER2-specific humoral immunity. In addition, 35 mg/m2 DOX increases the magnitude of HER2-specific humoral immunity. It is not clear whether these augmented levels of HER2 antibody also reflect humoral immunity with enhanced function.
The mechanism by which chemotherapy enhances vaccine-induced humoral immunity remains unknown. In preclinical models, DOX can augment tumor immunity by facilitating the vaccine-induced CD8+ T-cell response and by rendering tumor cells themselves more vulnerable to immune-mediated attack.35 CY augments tumor immunity in preclinical models by a variety of mechanisms.35 It upregulates type I interferons, facilitating the evolution of a CD44hi memory T-cell response.36 It also reverses immunologic skew, promoting the T-helper type I response.16 Several groups have shown that depletion of Tregs in animal models augments vaccine-induced antitumor immune responses.17,37–39 In tolerant neu-N transgenic mice, Treg depletion with low-dose CY enables the recruitment of latent, high-avidity CD8+ T cells to the antitumor immune response, resulting in tumor rejection in some mice.16,17 We are currently characterizing changes in peripheral Tregs and HER2-specific CD8+ T-cell responses in vaccinated patients.
Historically, clinical trials have used 250 to 300 mg/m2 CY 3 days before vaccination to alleviate immune suppression.6,40–45 One melanoma vaccine study46 tested CY doses of 75, 150, and 300 mg/m2. CY 300 mg/m2 most effectively depleted CD8+ suppressor T cells; antigen-specific immunity was not evaluated.46 Here we report the first study to optimize CY dose on the basis of the antigen-specific immune response. Our finding that CY doses higher than 200 mg/m2 were detrimental to the immune response suggests that previous phase III vaccine trials incorporating 300 mg/m2 CY could have used immunosuppressive doses.46–48 However, our study tested CY given 1 day before vaccination and also included DOX in the vaccination sequence. The additional drug and distinct CY schedule are alternative explanations for the differences in our results compared with those previously reported.
In conclusion, this allogeneic GM-CSF–secreting breast tumor vaccine is safe and bioactive given alone or sequenced with low-dose CY and DOX. Further, it can induce HER2-specific immunity in breast cancer patients, and this can be augmented by low-dose CY and DOX. Finally, factorial design is an efficient, effective method for identifying the most active dose combination of interacting drugs in patients. This small study examining vaccine safety and immune activation in patients with stable metastatic breast cancer was not designed to determine whether these promising effects on immune activation translate into a clinical benefit. A vaccine safety and efficacy trial of the optimal chemotherapy dose combination is currently being designed.
Acknowledgment
Presented in part at the 44th Annual Meeting of the Society of Clinical Oncology, May 31-June 3, 2008, Chicago, IL.
Footnotes
Supported by Grants No. 1K23CA098498-01 (L.E.), 1RO1CA93714-01 (E.J.), the Specialized Programs in Research Excellence (SPORE) in Breast Cancer P50CA88843 (N.D., E.J., and L.E.), the Rapid Access to Investigational Drugs (RAID) Program of the National Cancer Institute/National Institutes of Health (L.E.), DAMD17-01-1-0281 from the Department of Defense Breast Cancer Research Program (L.E.), BCTR0707297 from Susan G. Komen for the Cure (L.E.), the Breast Cancer Research Foundation (N.D.), the Dana and Albert “Cubby” Broccoli Foundation (L.E. and V.S.), the Avon Foundation (E.J. and L.E.), and in part by the Johns Hopkins University Institute for Clinical and Translational Research.
Under a licensing agreement between Cell Genesys and Johns Hopkins University, the University is entitled to milestone payments and royalties on sales of the vaccine product described in this article. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00093834.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Elizabeth M. Jaffee, Amplimmune (C), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Leisha A. Emens, Genentech, Roche Research Funding: Leisha A. Emens, Genentech; Mary L. Disis, GlaxoSmithKline; Elizabeth M. Jaffee, Cell Genesys Expert Testimony: None Other Remuneration: Mary L. Disis, University of Washington, holds a patent on HER2 immunogenicity on which she is an inventor. Dr. Disis has received compensation on this patent.
AUTHOR CONTRIBUTIONS
Conception and design: Leisha A. Emens, Steven Piantadosi, Nancy E. Davidson, Elizabeth M. Jaffee
Financial support: Leisha A. Emens, Nancy E. Davidson, Elizabeth M. Jaffee
Administrative support: Leisha A. Emens
Provision of study materials or patients: Leisha A. Emens, Barry J. Kobrin, Antonio C. Wolff, Vered Stearns, John H. Fetting, Nancy E. Davidson
Collection and assembly of data: Leisha A. Emens, Justin M. Asquith, James M. Leatherman, Barry J. Kobrin, Silvia Petrik, Marina Laiko, Joy Levi, Maithili M. Daphtary, Barbara Biedrzycki, Mary L. Disis, Nancy E. Davidson
Data analysis and interpretation: Leisha A. Emens, Barry J. Kobrin, Mary L. Disis, Xiaobu Ye, Steven Piantadosi, Elizabeth M. Jaffee
Manuscript writing: Leisha A. Emens, Mary L. Disis, Xiaobu Ye, Steven Piantadosi, Elizabeth M. Jaffee
Final approval of manuscript: Leisha A. Emens, Barbara Biedrzycki, Antonio C. Wolff, Vered Stearns, Mary L. Disis, Xiaobu Ye, Steven Piantadosi, Nancy E. Davidson, Elizabeth M. Jaffee
REFERENCES
- 1.Atlanta, GA: ACS; 2005. American Cancer Society: Breast Cancer Facts and Figures 2005-2006. [Google Scholar]
- 2.Emens LA, Jaffee EM. Toward a breast cancer vaccine: Work in progress. Oncology (Williston Park) 2003;17:1200–1211. [PubMed] [Google Scholar]
- 3.Emens LA, Jaffee EM. Cancer vaccines: An old idea comes of age. Cancer Biol Ther. 2003;2:S161–S168. [PubMed] [Google Scholar]
- 4.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: A Phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 8.Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 9.Simons JW, Jaffee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 10.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: Results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 11.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 12.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: Maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 15.Reilly RT, Gottlieb MB, Ercolini AM, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 16.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 17.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with breast or pancreas adenocarcinomas. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 20.Viguier M, Lemaître F, Verola O, et al. FoxP3-expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 22.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 23.Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 24.Piantadosi S. Clinical Trials: A Methodologic Perspective. ed 2. New York, NY: Wiley Series in Probability and Statistics; 2005. [Google Scholar]
- 25.Emens LA, Armstrong D, Biedrzycki B, et al. A phase I vaccine safety and chemotherapy dose-finding trial of an allogeneic GM-CSF-secreting breast cancer vaccine given in a specifically timed sequence with immunomodulatory doses of cyclophosphamide and doxorubicin. Hum Gene Ther. 2004;15:313–337. doi: 10.1089/104303404322886165. [DOI] [PubMed] [Google Scholar]
- 26.Davis-Sproul JM, Harris MP, Davidson NE, et al. Cost-effective manufacture of an allogeneic GM-CSF-secreting breast tumor vaccine in an academic cGMP facility. Cytotherapy. 2005;7:46–56. doi: 10.1080/14653240510018082. [DOI] [PubMed] [Google Scholar]
- 27.Disis ML, Schiffman K, Gooley TA, et al. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin Cancer Res. 2000;6:1347–1350. [PubMed] [Google Scholar]
- 28.Goodell V, Disis ML. Human tumor cell lysates as a protein source for the detection of cancer antigen-specific humoral immunity. J Immunol Methods. 2005;299:129–138. doi: 10.1016/j.jim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Hung HMJ, Chi GYH, Lipicky RJ. On some statistical methods for analysis of combination drug study. Commun Statist-Theory Meth. 1994;23:361–376. [Google Scholar]
- 30.Myers RH, Khuri AI, Carter WH., Jr Response surface methodology: 1966–1988. Technometrics. 1989;31:137–157. [Google Scholar]
- 31.Myers RH, Montgomery DC. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. New York, NY: Wiley; 2002. [Google Scholar]
- 32.Cary, NC: SAS Institute; 1999. SAS/STAT User's Guide, Version 8. [Google Scholar]
- 33.Jaffee EM, Thomas MC, Huang AY, et al. Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J Immunother Emphasis Tumor Immunol. 1996;19:176–183. doi: 10.1097/00002371-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MC, Greten TF, Pardoll DM, et al. Enhanced tumor protection by granulocyte-macrophage colony-stimulating factor expression at the site of an allogeneic vaccine. Hum Gene Ther. 1998;9:835–843. doi: 10.1089/hum.1998.9.6-835. [DOI] [PubMed] [Google Scholar]
- 35.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 36.Schiavoni G, Mattei F, Di Puchio T, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: Implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 38.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)CD25+ T regulatory cell function implicated in enhanced immune response by low dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 39.Litzinger MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: A Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases äafter treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–2577. [PubMed] [Google Scholar]
- 42.Berd D, Maguire HC, Jr, McCue P, et al. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: Clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8:1858–1867. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 43.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: Depletion of CD4+, 2H4+ suppressor-inducer T cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 44.Elias EG, Suter CM, Fabian DS. Adjuvant immunotherapy in melanoma with irradiated autologous tumor cells and low dose cyclophosphamide. J Surg Oncol. 1997;64:17–22. doi: 10.1002/(sici)1096-9098(199701)64:1<17::aid-jso4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.MacLean GD, Miles DW, Rubens RD, et al. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Hoon DS, Foshaq LJ, Nizze AS, et al. Suppressor cell activity in a randomized trial of patients receiving active specific immunotherapy with melanoma cell vaccine and low dosages of cyclophosphamide. Cancer Res. 1990;50:5358–5364. [PubMed] [Google Scholar]
- 47.Mayordomo J, Tres A, Miles D, et al. Long-term follow-up of patients concomitantly treated with hormone therapy in a prospective controlled randomized multicenter clinical study comparing STn-KLH vaccine with KLH control in stage IV breast cancer following first-line chemotherapy. J Clin Oncol. 2004;22:188s. abstr 2603. [Google Scholar]
- 48.Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3:S134–S138. doi: 10.3816/cbc.2003.s.002. [DOI] [PubMed] [Google Scholar]