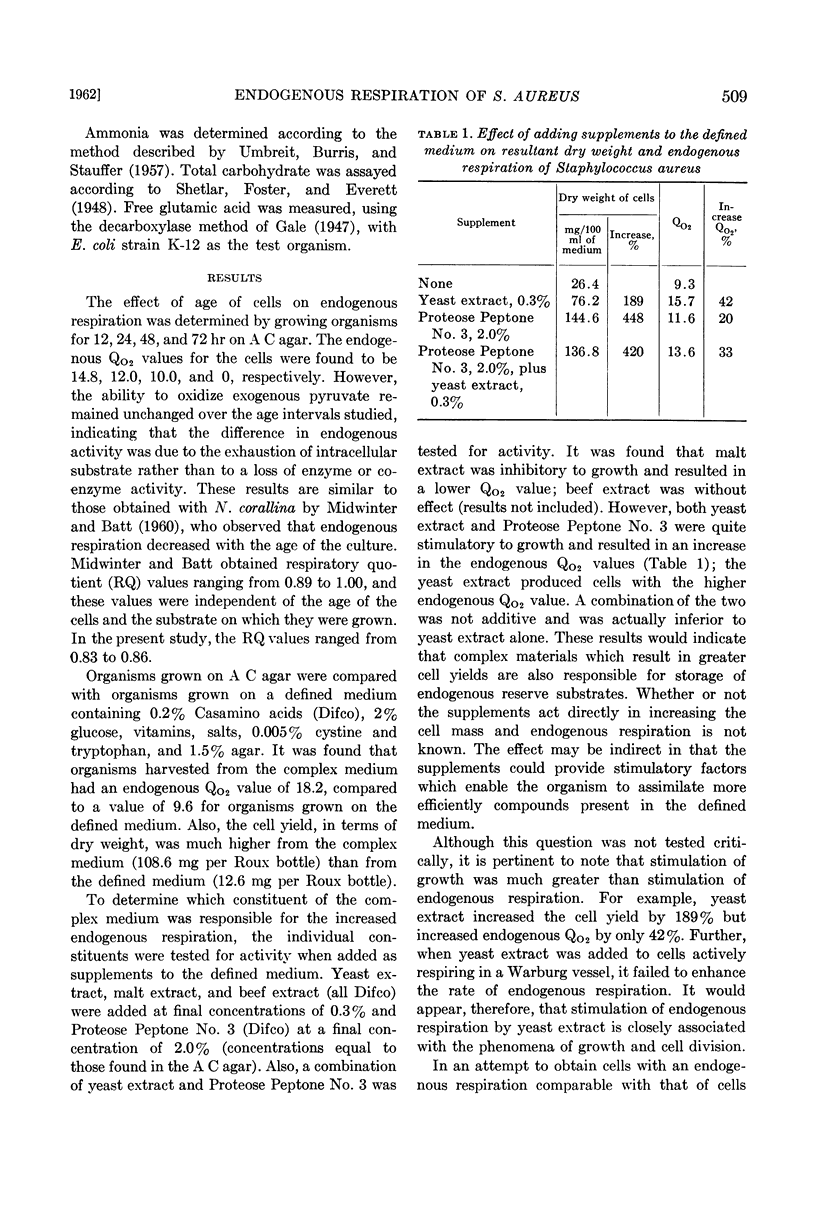
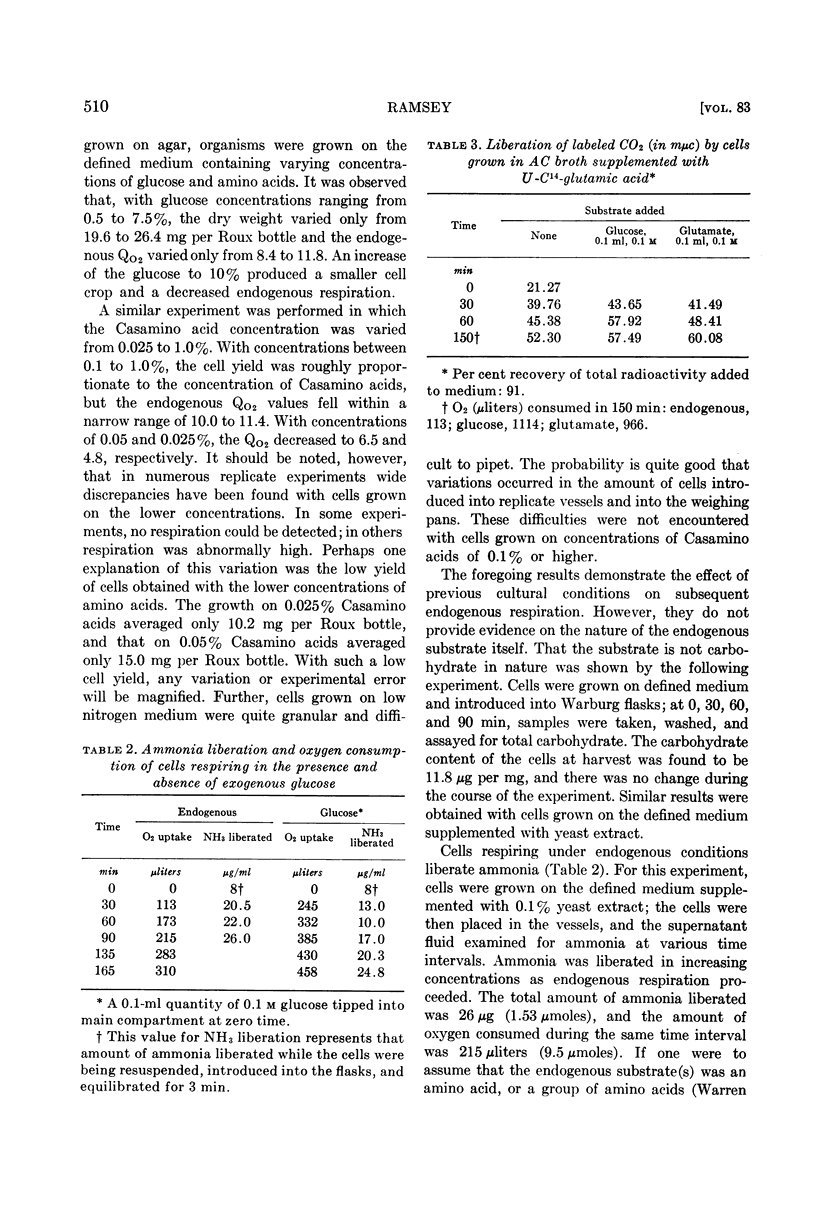
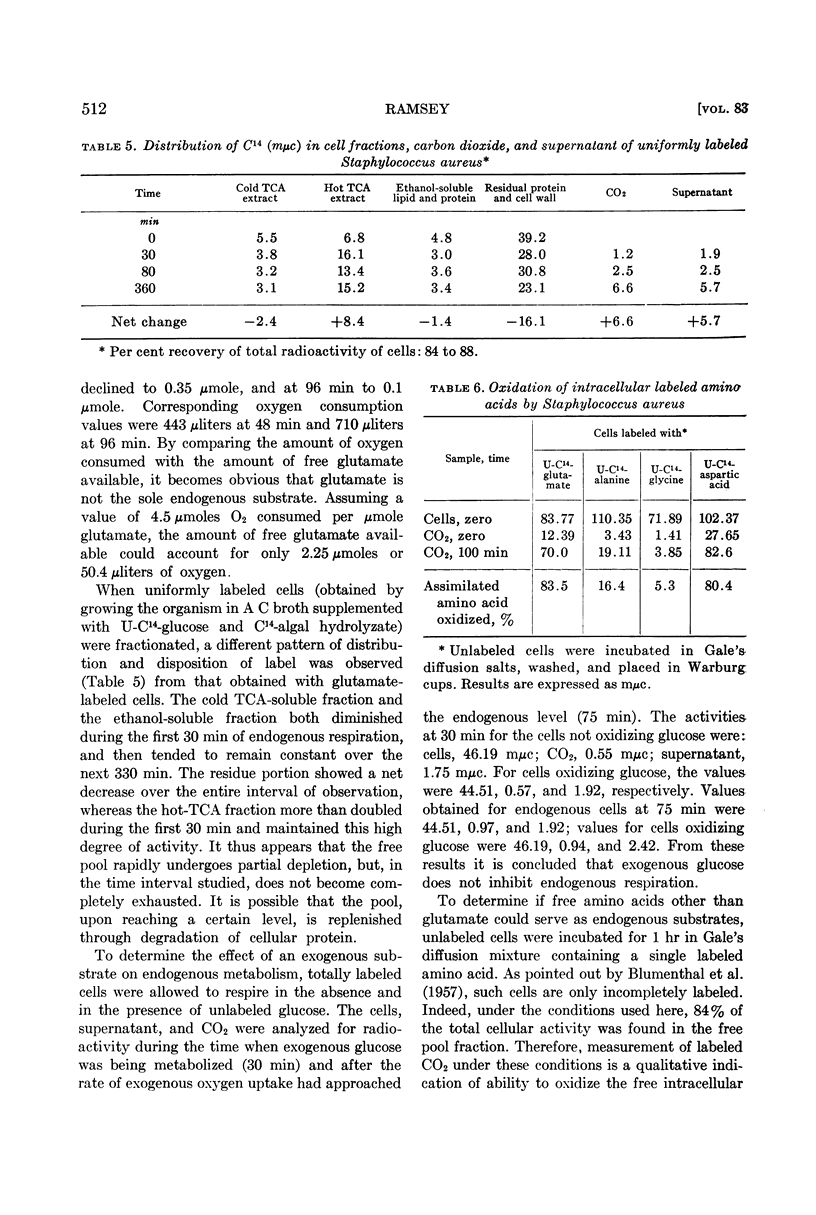
Abstract
Ramsey, H. H. (Stanford University, Palo Alto, Calif.). Endogenous respiration of Staphylococcus aureus. J. Bacteriol. 83:507–514. 1962.—The endogenous respiration of Staphylococcus aureus is dependent upon the medium used to grow the cell suspension. Within wide ranges, the concentration of glucose in the medium has no effect upon subsequent endogenous respiration of the cells, but the concentration of amino acids in the medium, within certain limits, has a very marked effect. The total carbohydrate content of the cells does not decrease during endogenous respiration. As endogenous respiration proceeds, ammonia appears in the supernatant, and the concentration of glutamic acid in the free amino acid pool decreases. Organisms grown in the presence of labeled glutamic acid liberate labeled CO2 when allowed to respire without added substrate. The principal source of this CO2 is the free glutamate in the metabolic pool; its liberation is not suppressed by exogenous glucose or glutamate. With totally labeled cells, the free pool undergoes a rapid, but not total, depletion and remains at a low level for a long time. Activity of the protein fraction declines with time and shows the largest net decrease of all fractions. Exogenous glucose does not inhibit the release of labeled CO2 by totally labeled cells. Other amino acids in the free pool which can serve as endogenous substrates are aspartic acid and, to much lesser extents, glycine and alanine. The results indicate that both free amino acids and cellular protein may serve as endogenous substrates of S. aureus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIM F. Effect of azide and cyanide on the respiration of a species of Mycobacterium. Science. 1954 Sep 10;120(3115):430–431. doi: 10.1126/science.120.3115.430. [DOI] [PubMed] [Google Scholar]
- BLUMENTHAL H. J., KOFFLER H., HEATH E. C. Biochemistry of filamentous fungi. V. Endogenous respiration during concurrent metabolism of exogenous substrates. J Cell Physiol. 1957 Dec;50(3):471–497. doi: 10.1002/jcp.1030500310. [DOI] [PubMed] [Google Scholar]
- COCHRANE V. W., GIBBS M. The metabolism of species of Streptomyces. IV. The effect of substrate on the endogenous respiration of Streptomyces coelicolor. J Bacteriol. 1951 Mar;61(3):305–307. doi: 10.1128/jb.61.3.305-307.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. III. Endogenous metabolism. Biochim Biophys Acta. 1958 Nov;30(2):278–293. doi: 10.1016/0006-3002(58)90052-0. [DOI] [PubMed] [Google Scholar]
- EATON N. R. Endogenous respiration of yeast. I. The endogenous substrate. Arch Biochem Biophys. 1960 May;88:17–25. doi: 10.1016/0003-9861(60)90192-2. [DOI] [PubMed] [Google Scholar]
- GRONLUND A. F., CAMPBELL J. J. Nitrogenous compounds as substrates for endogenous respiration in microorganisms. J Bacteriol. 1961 May;81:721–724. doi: 10.1128/jb.81.5.721-724.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINO R. J., CLIFTON C. E. Oxidative assimilation in suspensions and cultures of Hydrogenomonas facilis. J Bacteriol. 1955 Feb;69(2):188–192. doi: 10.1128/jb.69.2.188-192.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwinter G. G., Batt R. D. ENDOGENOUS RESPIRATION AND OXIDATIVE ASSIMILATION IN NOCARDIA CORALLINA. J Bacteriol. 1960 Jan;79(1):9–17. doi: 10.1128/jb.79.1.9-17.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Powelson D. M., Wilson P. W., Burris R. H. Oxidation of glucose, glycerol and acetate by Staphylococcus aureus. Biochem J. 1947;41(4):486–491. doi: 10.1042/bj0410486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Whetham M. D. The Equilibria existing between Succinic, Fumaric, and Malic Acids in the presence of Resting Bacteria. Biochem J. 1924;18(3-4):519–534. doi: 10.1042/bj0180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAGAKI G., HIRANO S., TSUKADA Y. Endogenous respiration and ammonia formation in brain slices. Arch Biochem Biophys. 1957 May;68(1):196–205. doi: 10.1016/0003-9861(57)90340-5. [DOI] [PubMed] [Google Scholar]
- WARREN R. A., ELLS A. F., CAMPBELL J. J. Endogenous respiration of Pseudomonas aeruginosa. J Bacteriol. 1960 Jun;79:875–879. doi: 10.1128/jb.79.6.875-879.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIAME J. M., DOUDOROFF M. Oxidative assimilation by Pseudomonas saccharophilia with C14-labeled substrates. J Bacteriol. 1951 Aug;62(2):187–193. doi: 10.1128/jb.62.2.187-193.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILNER B., CLIFTON C. E. Oxidative assimilation by Bacillus subtilis. J Bacteriol. 1954 May;67(5):571–575. doi: 10.1128/jb.67.5.571-575.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. J., Gunsalus I. C. The Production of Active Resting Cells of Streptococci. J Bacteriol. 1942 Sep;44(3):333–341. doi: 10.1128/jb.44.3.333-341.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]


