Abstract
Purpose
To determine 1-year outcomes of a four-component behavioral therapy (BT) sleep intervention (Individualized Sleep Promotion Plan [ISPP]) versus a healthy eating control (HEC) on cancer-related fatigue in women receiving breast cancer adjuvant chemotherapy treatment (CTX).
Patients and Methods
A total of 219 participants from 12 oncology clinics were randomly assigned in a clinical trial. Before CTX, research nurses coached intervention participants to develop a BT plan including stimulus control, modified sleep restriction, relaxation therapy, and sleep hygiene. BT plans were revised before each CTX and 30, 60, and 90 days after the last CTX and reinforced 7 to 9 days later. HEC participants received nutritional information and equal attention. Pittsburgh Sleep Quality Index (PSQI), Daily Diary, Wrist Actigraph, and Piper Fatigue Scale measures and Repeated Linear Mixed Model analysis following the Intent to Treat paradigm were used.
Results
Sleep quality differed over 1 years time (F [4,162] = 7.7, P < .001; by group, F [1,173] = 4.8, P = .029; and over time by group, F [4,162] = 3.3, P = .013). Pairwise comparisons revealed significant differences between groups at 90 days (P = .002) but not at 1 year (P = .052). Seven days of diary and actigraphy data did not corroborate with monthly reflections (PSQI). The night awakenings (Actigraph) pattern was significantly different by group over time (P = .046), with no differences between groups at 90 days or at 1 year. Fatigue was lower at 1 year than before CTX; no group effects were found.
Conclusion
The BT group, on average, experienced significant improvement on global sleep quality compared with the HEC group, but not on objective sleep or fatigue outcomes.
INTRODUCTION
Maximum physical recovery occurs by 1 year after a diagnosis of breast cancer,1 with cancer-related fatigue as the strongest predictor of overall quality of life (QOL).2 Approximately 30% to 40% of women report moderate to severe fatigue during the 5 years after breast cancer treatment and limitations in emotional, social, role, and cognitive functioning.3–5 Persistent fatigue and psychological distress have predicted continued distressing fatigue,6 recurrence-free periods, and overall survival outcomes.7
Review articles have concluded that fatigue is associated with poor sleep in all three phases (before,8–9 during,10 and after10–12) of breast cancer adjuvant chemotherapy treatment (CTX). Randomized clinical trials (RCTs) have reported positive effects on both sleep and fatigue after 4 to 5 weekly behavioral sleep sessions in breast and mixed-diagnosis cancer survivors with insomnia years after receiving CTX.13–15 The pilot study of this trial16,17 reported in all three phases of CTX results of a behavioral therapy (BT) sleep intervention designed to prevent or reduce known perpetuating factors likely to lead to fatigue and poor sleep. Fatigue was mild before CTX, moderate but decreasing during CTX, and mild 1 year after CTX. Total sleep time and sleep percent were normal, but number and duration of night awakenings were elevated.
This larger RCT identified a population experiencing an acute life stressor: breast cancer. Intervention occurred after diagnosis and surgery, before starting CTX, and continuing through all three phases of CTX. Our purpose was to determine the effects of a BT sleep intervention (Individualized Sleep Promotion Plan [ISPP]) on cancer-related fatigue in women receiving breast cancer adjuvant chemotherapy over 1 year. Outcomes 30 days after the last CTX were described previously.18
PATIENTS AND METHODS
Design
This RCT used a stratified random sampling procedure at each site, controlling for the number of planned anthracycline-based CTX (four or more than four [taxanes]) and history of sleep quality (good v poor).
Sample
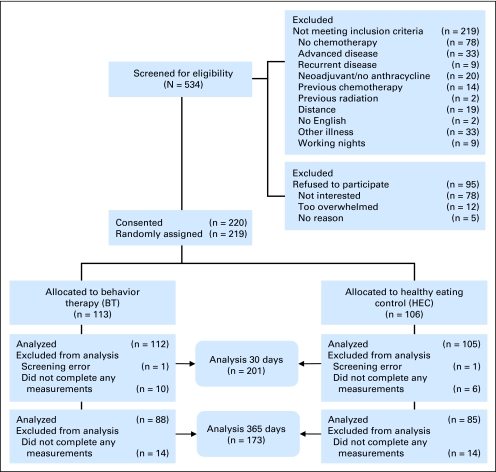
Recruitment occurred between April 2003 and May 2006 at two cancer centers and 10 community oncology clinics in the Midwestern United States. Participants were enrolled 48 hours before their first CTX. Inclusion criteria were as follows: (1) women ≥ 19 years of age, (2) first diagnosis of stages I to IIIA breast cancer, (3) postmodified radical mastectomy or lumpectomy, (4) scheduled for anthracycline-based intravenous CTX with or without additional taxanes (four or > four), and (5) Karnofsky performance scale score more than 60. Exclusion criteria included self-reported history of chronic insomnia; chronic fatigue syndrome; unstable heart; lung or neuromuscular disease; insulin-dependent diabetes; sleep apnea; chronic steroid therapy; and night-shift employment. Figure 1 shows rates of recruitment screening, exclusion, refusal, and drop-outs. The intervention was designed for patients with acute insomnia precipitated by the cancer experience; therefore preexisting chronic insomnia and chronic fatigue were excluded.
Fig 1.
Flow diagram of participants' progress through the study.
Procedures
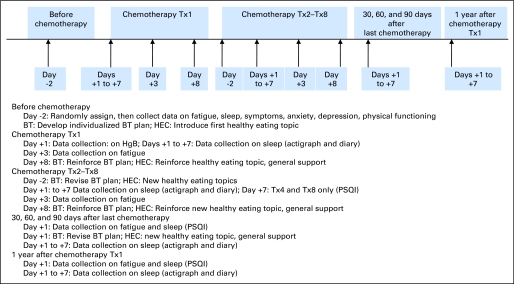
The study was approved by each site's institutional review board. The timeline, details of measurements, and participant visits are shown in Figure 2. Interested eligible women were visited by a nurse at home or a preferred location before their first CTX. After consenting to this study designed to modify fatigue, participants were randomized based on site, the number of planned CTX, and history of sleep quality.19 Baseline instruments were completed after the home visit but before the first CTX. All prescription, over-the-counter, and herbal medications for sleep and other conditions were recorded at each visit, but not restricted during the study.
Fig 2.
Timeline of measurements and development, reinforcement, and revisions to behavioral therapy (BT) plan. Tx, treatment; HEC, healthy eating control; HgB, hemoglobin; PSQI, Pittsburgh Sleep Quality Index.
Experimental Condition
After randomization, BT intervention group participants spent 90 minutes with the research nurse developing a 12-item plan [ISPP (Appendix)]18 using the coscientist model,20 Participants spent 30 minutes with the research nurse revising the BT plan 2 days before each later CTX (range, 4 to 8) and 30, 60 and 90 days after the last CTX. Revisions were based on sleep diary and BT plan adherence data. All BT plans were bolstered during 15 minute, in-person sessions 7 to 9 days after each revision. BT group participants spent an average of 327.3(SD = 86.6) minutes in an average of 6.3(1.9) sessions. Women were praised for adhering and coached to address problem areas. Each woman was provided with advice and information tailored to their specific needs (Appendix). The BT plan included four components common to BT therapy for insomnia: 1) stimulus control, 2) modified sleep restriction, 3) relaxation therapy, and 4) sleep hygiene counseling.21 Treatment fidelity was established by reviewing randomly selected BT plans which were determined to be consistent with the intended protocol. Each clinic managed symptoms that were related to CTX.
Control Condition
Healthy Eating Control (HEC) group participants received in-person sessions of equal time and attention before each CTX and 30, 60 and 90 days after the last CTX. HEC group participants spent an average of 316.7(85.5) minutes in an average of 6.0(1.9) sessions. A new healthy eating topic and general support were provided at each visit. Although the general public might expect healthy eating to impact fatigue, this content was selected to standardize time and attention across groups and was not expected to influence fatigue perceptions. If the topic of fatigue or sleep was raised, participants were coached to call the clinic.
Instruments
Measurements taken at baseline and at 1 year included demographic/medical and primary (sleep and fatigue) and secondary (symptoms, anxiety, depression, physical functioning) variables.18 Symptoms were measured using the Symptom Experience Scale (SES). Six CTX-related symptoms, nausea, pain, appetite, bowel pattern, concentration, and appearance were included with fatigue and sleep excluded.22 Anxiety and depression were screened using the Hospital Anxiety and Depression Scale (HADS).23 Physical functioning and physical and mental components of health were measured using the Medical Outcomes Study Short-Form General Health Survey (MOS SF-36-v2).24
Subjective sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI), a 19-item tool of subjective sleep quality during the previous month19 and the daily diary. Global scores can range from 0 to 21 and higher scores indicate poorer sleep quality. Global scores include seven equally weighted components: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleeping medication use, and daytime dysfunction. In this study, Cronbach's alpha for global PSQI was 0.74 to 0.83. A global PSQI score greater than 5 in the general population19 and greater than 8 in breast cancer patients has been associated with poor sleep.25 Sleep diary data have shown modest-to-poor correlations with objective findings but these perceptions represent a valid index of insomnia.26,27
Objective sleep variables were captured by the Wrist Actigraph. The octagonal Motionlogger actigraph (Ambulatory Monitoring, Inc., Ardsley NY) is a noninvasive method used to quantify continuous monitoring of body movement in 1 minute epochs (intervals) over time in the subject's usual environment. Actigraph measurements of sleep have been reported to agree with polysomnography (PSG) within ±10%.28 Women wore the actigraph on the nondominant wrist and usually followed instructions to push the event marker when turning out the lights at bedtime and getting up in the morning. The 7-day actigraphy recording was started on the day when CTX was administered. Variables29 were total sleep time after sleep onset (min), number of awakenings, minutes and percent awake after sleep onset (WASO-M and WASO-P), sleep percent after onset (percent time asleep after first falling asleep out of total time in bed), and mesor (24-hour rhythm adjusted mean of the activity counts). Sleep latency was excluded because of difficulties with this variable in uncontrolled settings.30
Fatigue was measured using the 22-item, 4-dimension Piper Fatigue Scale (PFS).31 Participants indicated their current fatigue on a 0 to 10 scale for each item: mild (0 to 3.99), moderate (4.0-6.99), or severe (7.0 or >). Internal consistency reliability of the PFS was 0.93 to 0.98.
Data Analysis
The statistical end points for the primary variables (sleep and fatigue) were 30 days after the last CTX18 and 1 year after the first CTX, compared to baseline values (before the first CTX). The effect size of 0.17 obtained from the feasibility study, combined with a 0.05 level of significance and a two-sided test, determined that 110 participants in each arm would provide 79% power.
Data from 219 participants were double-entered and inspected for artifacts, missing or out of range values, and normality. Actigraph data were retrieved using the Act Millennium and analyzed with Action4 software (Ambulatory Monitoring, Inc., Ardsley, NY). Procedures were followed rigorously for data editing, analysis, and entry into the master data files.32 Consistent with intent to treat analysis, all available data were included once a participant was randomized into a group. No data imputation strategies were used. Descriptive and inferential statistics were performed using SPSS 15.0 statistical software (SPSS, Chicago, IL). Baseline data were compared using t-tests and χ2 tests to ensure comparability between groups. Repeated measures, linear mixed model analysis following the Intent to Treat paradigm, was used to compare the primary outcomes of sleep quality and fatigue between groups and over time, controlling for baseline measures. Additional details regarding the trial methodology are included in the on-line Appendix of this article and were reported previously.18
RESULTS
Demographic/Medical and Study Variables at Baseline and 1 Year
At baseline, both groups reported similar values for demographic/medical data and for primary and secondary variables.18 One-year later, participants remaining in both groups reported similar demographic/medical data (Table 1). Values and unadjusted comparisons were similar for all secondary variables except symptoms (P = .04), which were lower in the intervention group (Table 2). Sleep aid use recorded on the daily diary did not differ between groups and aids were taken less than 10% of the nights before and during CTX and on less than 5% of the nights at 1 year.
Table 1.
Demographic and Medical Variables of Participants Providing Data at 1 Year
| Variable | Behavioral Therapy (n = 88) |
Healthy Eating Control (n = 85) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .33 | ||||
| Median | 51.57 | 52.86 | |||
| Range | 29-73 | 35-83 | |||
| BMI | .13 | ||||
| Median | 28.27 | 29.34 | |||
| Range | 16-53 | 19-48 | |||
| Ethnicity | |||||
| Hispanic | 2 | 2.3 | 4 | 4.7 | .33 |
| Non–Hispanic | 86 | 97.7 | 81 | 95.3 | |
| Race | |||||
| White | 86 | 97.7 | 81 | 95.3 | .24 |
| Non-White | 2 | 2.2 | 4 | 4.7 | |
| Marital status | |||||
| Partnered | 62 | 70.5 | 63 | 74.1 | .36 |
| Nonpartnered | 26 | 29.5 | 22 | 25.9 | |
| Employment | |||||
| Professional | 42 | 47.7 | 36 | 42.4 | .35 |
| Service | 10 | 11.4 | 18 | 21.2 | |
| Office | 16 | 18.2 | 12 | 14.1 | |
| Homemaker/retired | 20 | 22.7 | 19 | 22.4 | |
| Education | |||||
| Up to high school | 17 | 19.3 | 17 | 20.0 | .69 |
| Some college or more | 71 | 80.7 | 68 | 80.0 | |
| Household income, $ | |||||
| < 20,000 | 5 | 5.7 | 5 | 6.0 | .46 |
| 20,000-40,000 | 19 | 21.6 | 18 | 21.4 | |
| > 40,000 | 59 | 67 | 60 | 71.4 | |
| Does not wish to provide | 5 | 5.7 | 1 | 1.2 | |
| Surgical procedure | |||||
| Lumpectomy | 35 | 39.8 | 42 | 49.4 | .31 |
| MM | 28 | 31.8 | 19 | 22.4 | |
| MM with reconstruction | 25 | 28.4 | 24 | 28.2 | |
| Cancer stage | |||||
| I | 26 | 29.5 | 31 | 36.5 | .53 |
| II | 49 | 55.7 | 45 | 52.9 | |
| IIIA | 13 | 14.8 | 9 | 10.6 | |
| Menopause status | |||||
| Stopped 12 months ago | 42 | 48.8 | 48 | 57.8 | .24 |
| Stopped 6-12 months ago | 2 | 2.3 | 3 | 3.6 | |
| Irregular during past 6 months | 13 | 15.1 | 5 | 6.0 | |
| Regular | 29 | 33.7 | 27 | 32.5 | |
| Karnofsky performance score | |||||
| 60-80 | 12 | 13.6 | 13 | 15.3 | .95 |
| 90 | 29 | 33.0 | 27 | 31.8 | |
| 100 | 47 | 53.4 | 45 | 52.9 | |
| Chemotherapy regimen | |||||
| A/C | 35 | 39.8 | 40 | 47.1 | .46 |
| A/C followed by taxane | 47 | 53.4 | 38 | 44.7 | |
| A/C/T | 5 | 5.7 | 7 | 8.2 | |
| AT | 1 | 1.1 | 0 | 0 | |
| Frequency | |||||
| Dose dense (every 14 days) | 36 | 40.9 | 36 | 42.4 | .49 |
| Dose standard (every 21 days) | 52 | 59.1 | 49 | 57.6 | |
| No. of treatments | |||||
| 4 Tx (no taxanes) | 35 | 39.8 | 39 | 45.9 | .25 |
| > 4 Tx (with taxanes) | 53 | 60.2 | 46 | 54.1 | |
| Duration of treatments | |||||
| No. of weeks | 15.3 | 4.4 | 14.2 | 4.0 | .07 |
| No. of days | 204.5 | 33.0 | 196.6 | 30.3 | |
| Radiation therapy | |||||
| Yes | 53 | 60.2 | 54 | 63.5 | .39 |
| No | 35 | 39.8 | 31 | 36.5 | |
Abbreviations: BMI, body mass index; MM, modified mastectomy; A/C, doxorubicin/cyclophosphamide; A/C/T, doxorubicin/cyclophosphamide/docetaxel or paclitaxel; A/T, doxorubicin/docetaxel or paclitaxel; Tx, treatment.
Table 2.
Means and Standard Errors for Study Variables By Group 1 Year After First Treatment
| Variable | Behavioral Therapy |
Healthy Eating Control |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | Mean | SE | No. | Mean | SE | ||
| Fatigue* (0-10) | 88 | 2.16 | 0.204 | 85 | 2.31 | 0.223 | .59 |
| Sleep† (0-21) | 85 | 5.55 | 0.257 | 84 | 6.39 | 0.391 | .05‡ |
| Symptoms§ (0-4.0) | 88 | 0.34 | 0.034 | 85 | 0.45 | 0.053 | .04‡ |
| Anxiety‖ (0-21) | 88 | 4.38 | 0.370 | 85 | 4.96 | 0.397 | .16 |
| Depression‖ (0-21) | 88 | 2.26 | 0.263 | 85 | 2.78 | 0.316 | .12 |
| Physical Functioning¶ | 87 | 82.07 | 2.16 | 83 | 78.92 | 2.57 | .46 |
NOTE. Sample = 203; 16 patients consented but did not complete baseline data = 187; 14 patients did not provide data at 1 year = 173.
Piper Fatigue Scale.30
Pittsburgh Sleep Quality Index.19
P < .05.
Symptom Experience Scale (18 items).22
Hospital Anxiety and Depression Scale.23
Medical Outcomes Study Short-Form General Health Survey.24
Patterns of Sleep
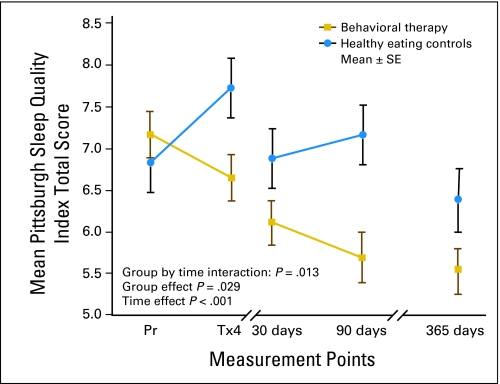
Both groups' mean global sleep quality scores (> 5 on PSQI) at each measurement indicated they were fairly poor sleepers (Fig 3 and Table A on-line only). However, these scores were lower than the cutoff of greater than 8 for poor sleep in breast cancer patients.25 PSQI scores greater than 8 were found in 22% of the BT group and 36% of the HEC group at 90 days (P < .004) and in 19% of the BT group and 28% of the HEC group at 1 year (ns).
Fig 3.
Patterns of perceived sleep quality by group at selected times during 1 year with higher scores indicating poorer sleep quality. Pr, prior to chemotherapy; Tx, treatment.
Potential covariates associated with global sleep quality were examined for inclusion in the mixed model analysis. Baseline variables correlated with PSQI at a significance level of P < .20 were included in the model following a model-building strategy outlined by Hosmer and Lemeshow.33 Variables examined were anxiety, education, fatigue, physical functioning, surgical procedure, symptoms, and physical and mental health status. Sleep quality differed over 1 year's time [F(4,162) = 7.7, P < .001], by group [F(1,173) = 4.8, P = .029], and over time by group [F(4,162) = 3.3, P = .013]. The BT group, on average, experienced significant improvement on sleep quality compared to the HEC group. Pair-wise comparisons revealed significant differences between the groups at 90 days (P = .002) but not at 1 year (P = .052). Baseline higher fatigue (P = .027), higher anxiety (P = .012), and more education were associated with poorer sleep at 1 year.
There was no group difference on most diary and objective sleep data at selected times over 1 year (Table B on-line only). Groups were similar on total sleep time, WASO-M, and sleep percent. Both groups' total sleep time and mean WASO-P were within normal ranges; values recorded in the diary were slightly higher than from actigraphs. However, perceptions of WASO-M differed based on measurement at selected times; diary entries averaged 22 to 32 minutes and actigraphs averaged 52 to 64 minutes per night. Similarly, the number of awakenings differed by measurement; diary entries averaged 1 to 2 and actigraph averaged 9 to 12 per night. The night awakenings (actigraph) pattern was significantly different by group over 1 year's time (P = .046), with no differences between groups at 90 days or at 1 year. All sleep variables obtained by diary and actigraph were significantly different over time for both groups (all P < .01).
Patterns of Fatigue
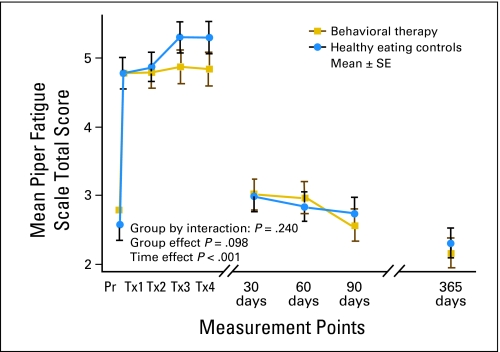
Fatigue reported by both groups was mild before CTX, moderate during CTX, and returned to mild after CTX ended (Fig 4 and Table C on-line only). Moderate to severe fatigue was reported at 1 year by 20% of women in the BT group and 24% in the HEC group. Potential covariates associated with fatigue (PFS) were examined for inclusion in the model, using the same model-building strategy.33 Baseline variables examined were age, anxiety, mesor, sleep quality, symptoms, physical functioning, and physical and mental health. Fatigue changed significantly over time (P < .001), but no group effects were found. Baseline higher symptoms and lower physical and mental health (P < .001) and higher anxiety (P = .004) were related to higher fatigue at 1 year.
Fig 4.
Patterns of fatigue by group at selected times over 1 year with higher scores indicating higher fatigue. Pr, prior to chemotherapy; Tx, treatment.
DISCUSSION
This study found a BT sleep intervention (ISPP©) significantly improved global sleep quality, but not fatigue, in women over 1 year after the first BT intervention and CTX. Seven days of diary and objective sleep data did not corroborate with this perception. To our knowledge, this is the first RCT designed to prevent disturbed sleep, and thereby to reduce fatigue, as women navigate through all three phases of CTX. Although no group effects were found, fatigue was mild at 1 year and lower than before CTX.
Previous cross-sectional studies have reported 30% to 50% insomnia prevalence rates in patients with various diagnoses during cancer treatment34 and 23% to 44% rates in breast cancer patients 2 to 5 years after cancer treatment.34,35 Both rates are double the 10% to 15% rates in healthy women.36 Savard et al35 reported that 58% of 300 breast cancer survivors who were approximately 4 years postdiagnosis indicated that cancer caused or aggravated their sleep difficulties and 33% stated insomnia began following diagnosis. This study reported poor sleep quality rates at 1 year that were one half as frequent as in Savard's sample. Our findings are similar to previous RCTs reporting positive results from BT sleep interventions in breast and mixed diagnoses cancer survivors with insomnia.13–15
Espie et al15 also reported positive subjective sleep results that were not corroborated by objective sleep data. Our results are also consistent with data from community-dwelling adults indicating that subjective sleep measures do not always correspond with PSG or actigraph data37,38 but are more closely associated with other symptom or psychological ratings.39,40 In addition, our results suggest perceptions of sleep quality during the past month were clearly impacted during this stressful life event and emphasize the value of measuring symptoms, and both global and daily subjective and objective sleep.
Similar to previous reports, our sample's 7 nights of actigraphy data identified increased number and duration of night awakenings.14–17 Actigraph values of more than 10 awakenings and more than 50 minutes WASO-M per night may be related to perceptions of nonrestorative sleep and higher fatigue. However, percent sleep was more than 85% and total sleep time was about 420 minutes, both within normal ranges. This sample slept longer and had fewer WASO-M than a large sample (n = 589) of healthy women at various menopausal stages who had PSG testing.41 The excess awakenings were likely not perceived by participants, who recorded a normal number of awakenings and WASO-M in the daily diary. The fluctuating pattern of awakenings (actigraph) over time is likely associated with reports of hot flash interruptions on sleep42 or related to dietary influences. Sleep aids use was low and similar in both groups in all three phases of CTX and does not explain study results.
Few studies have examined cancer-related fatigue in all three phases of CTX, but comparisons can be made at 1 year. In this study, fatigue levels at 1 year were comparable to, but lower than, before CTX.5,6,43–45 Also, prevalence of moderate to severe fatigue was lower than the 20% to 40% rates in descriptive reports.5,44–46 A potential explanation for the lack of group differences in fatigue is participants' expectation of the trial. The consent stated the study was designed to reduce fatigue, potentially impacting perceptions of fatigue modification in the HEC group. We found milder fatigue ratings than reported previously16,17,47; this finding appears to have restricted the range necessary to obtain significant differences between groups.
BT interventions have shown long-term sleep benefits in persons with chronic insomnia, but they take time and require changing daily habits.48–49 Women may have been more accepting and adherent to the BT plan after CTX ended if they felt less anxious and distressed. Similar to previous reports, we found that baseline anxiety was significantly associated with poor sleep and higher fatigue at 1 year.4,5,45–50 The BT plan may have provided a sense of control for participants and impacted results 90 days after CTX ended; a time referred to as “Lost in Transition.”51 The coscientist model20 afforded patients the freedom to design their own BT plan. One drawback to this method was that patients often chose strategies that were easier habits to change as opposed to more effective strategies.52
This study's strengths included the accrual and retention of a large sample, the use of well-validated subjective and objective measures, and the employment of an attention control group rather than a treatment as usual group. We intervened in a high-risk population before they developed sleep disturbances and severe fatigue. Nurses with a minimum of a Bachelor of Science degree were used to deliver the intervention, replicating previous research using advanced practice nurses.15,53 Limitations include the lack of racial/ethnic diversity and lack of measurement of participants' expectations. In retrospect, the study may have been hampered by inclusion criteria that did not require sufficient levels of poor sleep and/or moderate fatigue to detect group differences in outcome variables. The potential impact of the BT sleep intervention may have been reduced by the lack of variance due to better sleep and lower fatigue than expected. Trial effectiveness might have been improved by involving the bed partner or family.
Implications for practice include the need to identify and intervene with the BT intervention when women with breast cancer report moderate/severe insomnia. These findings can be added to the National Comprehensive Cancer Network and the Oncology Nursing Society-Putting Evidence into Practice guidelines.54–56 Future research needs to explore the impact of, and test methods to, reduce the number and length of night awakenings. Identifying factors influencing adherence and revising the intervention to enhance its acceptability and effectiveness is warranted. Testing the effectiveness of multidimensional interventions that promote daytime activity, night sleep, robust circadian rhythms, and lower anxiety to promote sleep and reduce fatigue during cancer treatment and survivorship is warranted.
Supplementary Material
Acknowledgment
We thank Kathryn Lee, PhD, RN, CBSM, FAAN, for her mentoring and the physicians, nurses, and staff at participating sites, especially the University of Nebraska Medical Center/Eppley Cancer Center & Oncology Hematology West PC, Omaha; and Southeast Nebraska Hematology and Oncology Consultants, Lincoln, NE.
Appendix
Table A1.
Mean Sleep* Quality Data by Group at Selected Times Over 1 Year
| Sleep* | No. | Behavioral Therapy |
No. | Healthy Eating Control |
||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Before | 106 | 7.16 | 0.325 | 100 | 6.84 | 0.376 |
| Tx 4 | 85 | 6.64 | 0.280 | 84 | 7.73 | 0.373 |
| 30 days after last treatment | 90 | 6.12 | 0.250 | 85 | 6.88 | 0.349 |
| 90 days after last treatment | 89 | 5.69 | 0.302 | 82 | 7.16 | 0.357 |
| 1 year after last treatment | 85 | 5.55 | 0.257 | 84 | 6.39 | 0.391 |
Abbreviation: Tx, treatment.
Pittsburgh Sleep Quality Index.19
Table A2.
Means, SEs, and RM-ANOVAs of Daily Diary* and Actigraph† Sleep Variables at Selected Times‡ Over 1 Year
| Time Point | Diary |
Actigraph |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral Therapy |
Healthy Eating Control |
Behavioral Therapy |
Healthy Eating Control |
|||||||||
| No. | Mean | SE | No. | Mean | SE | No. | Mean | SE | No. | Mean | SE | |
| Total sleep time§ | ||||||||||||
| Before | 96 | 432.2 | 6.6 | 89 | 425.3 | 8.2 | 93 | 416.4 | 8.4 | 84 | 395.3 | 9.9 |
| 30 days | 85 | 465.4 | 7.3 | 81 | 461.5 | 7.5 | 86 | 423.4 | 6.5 | 77 | 418.9 | 7.3 |
| 60 days | 84 | 464.4 | 6.9 | 78 | 459.8 | 8.1 | 83 | 419.4 | 7.2 | 76 | 406.1 | 9.8 |
| 90 days | 74 | 450.5 | 7.8 | 79 | 441.7 | 7.9 | 84 | 428.0 | 6.7 | 76 | 418.4 | 7.8 |
| 1 year | 78 | 452.3 | 7.1 | 79 | 436.5 | 5.3 | 83 | 431.4 | 7.1 | 72 | 415.0 | 8.3 |
| No. of awakenings‖ | ||||||||||||
| Before | 103 | 2.24 | 0.14 | 100 | 2.40 | 0.15 | 93 | 9.55 | 0.57 | 84 | 9.93 | 0.54 |
| 30 days | 95 | 1.64 | 0.11 | 87 | 1.87 | 0.12 | 86 | 11.8 | 0.51 | 77 | 10.6 | 0.48 |
| 60 days | 94 | 1.88 | 0.18 | 87 | 1.79 | 0.12 | 83 | 12.0 | 0.61 | 76 | 11.1 | 0.59 |
| 90 days | 91 | 1.67 | 0.14 | 86 | 1.87 | 0.12 | 84 | 11.3 | 0.69 | 76 | 10.8 | 0.60 |
| 1 year | 88 | 1.80 | 0.14 | 85 | 1.90 | 0.11 | 83 | 10.7 | 0.62 | 72 | 10.7 | 0.63 |
| Wake after sleep onset§ | ||||||||||||
| Before | 97 | 31.8 | 2.5 | 90 | 33.4 | 2.8 | 93 | 51.6 | 4.5 | 84 | 59.8 | 6.2 |
| 30 days | 87 | 23.6 | 3.2 | 81 | 26.1 | 2.6 | 86 | 59.1 | 4.4 | 77 | 59.0 | 4.8 |
| 60 days | 85 | 22.4 | 2.4 | 78 | 24.8 | 2.6 | 83 | 63.9 | 6.6 | 76 | 75.7 | 11.8 |
| 90 days | 76 | 23.2 | 2.8 | 80 | 24.1 | 2.3 | 84 | 53.8 | 4.9 | 76 | 56.3 | 5.4 |
| 1 year | 78 | 19.2 | 2.2 | 80 | 31.5 | 5.4 | 83 | 52.2 | 5.4 | 72 | 56.8 | 6.4 |
| Sleep percent after onset§ | ||||||||||||
| Before | 96 | 0.90 | 0.01 | 89 | 0.88 | 0.01 | 93 | 0.89 | 1.1 | 84 | 0.87 | 1.5 |
| 30 days | 85 | 0.93 | 0.01 | 81 | 0.92 | 0.01 | 86 | 0.88 | 0.93 | 77 | 0.88 | 1.1 |
| 60 days | 84 | 0.93 | 0.01 | 78 | 0.92 | 0.01 | 83 | 0.87 | 1.3 | 76 | 0.85 | 1.8 |
| 90 days | 74 | 0.92 | 0.01 | 79 | 0.91 | 0.01 | 84 | 0.89 | 1.0 | 76 | 0.88 | 1.2 |
| 1 year | 78 | 0.93 | 0.01 | 79 | 0.91 | 0.01 | 83 | 0.89 | 1.1 | 72 | 0.88 | 1.3 |
Daily diary.26
Actigraph (Ambulatory Monitoring, Ardsley, NY).
Selected time: before = mean of 2 nights; 30, 60, 90 days, and 1 year = mean of 7 nights.
Significant difference over time (P < .01 to .001).
Significant time by group interaction (P < .05; actigraphy).
Table A3.
Mean Fatigue Scores* by Group at Selected Times Over 1 Year
| Time Point | No. | Behavioral Therapy |
No. | Healthy Eating Control |
||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Before | 103 | 2.80 | 0.197 | 100 | 2.58 | 0.200 |
| Tx 1 | 102 | 4.78 | 0.237 | 100 | 4.78 | 0.240 |
| Tx 2 | 97 | 4.80 | 0.230 | 92 | 4.87 | 0.232 |
| Tx 3 | 96 | 4.88 | 0.245 | 93 | 5.30 | 0.233 |
| Tx 4 | 89 | 4.85 | 0.252 | 89 | 5.31 | 0.240 |
| 30 days after last treatment | 95 | 3.02 | 0.225 | 87 | 3.01 | 0.232 |
| 60 days after last treatment | 94 | 2.97 | 0.228 | 87 | 2.84 | 0.220 |
| 90 days after last treatment | 93 | 2.57 | 0.232 | 87 | 2.74 | 0.241 |
| 1 year after last treatment | 88 | 2.16 | 0.204 | 85 | 2.31 | 0.223 |
Abbreviation: Tx, treatment.
Piper Fatigue Scale30 (mild = 0 to 3.99; moderate = 4.0 to 6.99; severe = 7.0 to 10).
Footnotes
See accompanying editorial on page 5864
Supported by Grant No. 5R01NR007762-05 from the National Institute of Health and National Institute of Nursing Research.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00572416.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ann M. Berger, Brett R. Kuhn, Lynne A. Farr, Julie Chamberlain, James C. Lynch, Sangeeta Agrawal
Financial support: Ann M. Berger
Administrative support: Ann M. Berger
Provision of study materials or patients: Ann M. Berger, Brett R. Kuhn
Collection and assembly of data: Ann M. Berger, Brett R. Kuhn, Susanna G. Von Essen, James C. Lynch
Data analysis and interpretation: Ann M. Berger, Brett R. Kuhn, Lynne A. Farr, Susanna G. Von Essen, Julie Chamberlain, James C. Lynch, Sangeeta Agrawal
Manuscript writing: Ann M. Berger, Brett R. Kuhn, Lynne A. Farr, Susanna G. Von Essen, Julie Chamberlain, James C. Lynch
Final approval of manuscript: Ann M. Berger, Brett R. Kuhn, Lynne A. Farr, Susanna G. Von Essen, Julie Chamberlain, James C. Lynch, Sangeeta Agrawal
REFERENCES
- 1.Ganz PA, Coscarelli A, Fred C, et al. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 2.Arndt V, Stegmaier C, Ziegler H, et al. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107:2496–2503. doi: 10.1002/cncr.22274. [DOI] [PubMed] [Google Scholar]
- 3.Arndt V, Merx H, Sturmer T, et al. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer. 2004;40:673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE. Prevalence and causes of fatigue after cancer treatment: The next generation of research. J Clin Oncol. 2005;23:8280–8282. doi: 10.1200/JCO.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 6.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 7.Groenvold M, Petersen MA, Idler E, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger AM, Farr LA, Kuhn BR, et al. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw. 2008;6:3–13. doi: 10.6004/jnccn.2008.0002. [DOI] [PubMed] [Google Scholar]
- 11.de Jong N, Courtens AM, Abu-Saad HH, et al. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs. 2002;25:283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):S35–S42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 13.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61:664–675. doi: 10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 14.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34:E51–E59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 15.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavioral therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 16.Berger AM, VonEssen S, Kuhn BR, et al. Feasibility of a sleep intervention during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 2002;29:1431–1441. doi: 10.1188/02.ONF.1431-1441. [DOI] [PubMed] [Google Scholar]
- 17.Berger AM, VonEssen S, Kuhn BR, et al. Adherence, sleep, and fatigue outcomes after adjuvant breast cancer chemotherapy: Results of a feasibility intervention study. Oncol Nurs Forum. 2003;30:513–522. doi: 10.1188/03.ONF.513-522. [DOI] [PubMed] [Google Scholar]
- 18.Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality in cancer-related fatigue. Psycho-Oncology. 2009;18:634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Hauri PJ. Consulting about insomnia: A method and some preliminary data. Sleep. 1993;16:344–350. doi: 10.1093/sleep/16.4.344. [DOI] [PubMed] [Google Scholar]
- 21.Morin A, Jarvis C, Lynch A. Therapeutic Options for Sleep Maintenance and Sleep-Onset Insomnia. Pharmacotherapy. 2007;27:89–110. doi: 10.1592/phco.27.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Samarel N, Leddy SK, Greco K, et al. Development and testing of the symptom experience scale. J Pain Symptom Manage. 1996;12:221–228. doi: 10.1016/0885-3924(96)00150-9. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 25.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 26.Rogers AE, Caruso CC, Aldrich MS. Reliability of sleep diaries for assessment of sleep/wake patterns. Nurs Res. 1993;42:368–372. [PubMed] [Google Scholar]
- 27.Summers MO, Crisostomo MI, Stepanski EJ. Recent developments in the classification, evaluation, and treatment of insomnia. Chest. 2006;130:276–286. doi: 10.1378/chest.130.1.276. [DOI] [PubMed] [Google Scholar]
- 28.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Perlis ML, Jungquist C, Smith MT, et al. Cognitive Behavioral Treatment of Insomnia. New York NY: Springer; 2008. [Google Scholar]
- 30.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- 31.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 32.Berger AM, Wielgus K, Young-McCaughan S, et al. Methodological Challenges when Using Actigraphy in Research. J Pain Symptom Manage. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied Logistic Regression. ed 2. New York, NY: Wiley; 2000. [Google Scholar]
- 34.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 35.Savard J, Simard S, Blanchet J, et al. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 36.Sateia MJ, Piegeon WR. Identification and management of insomnia. In: Lee-Chiong TL, editor. Medical Clinics of North America. Vol. 88. Philadelphia, PA: Saunders; 2004. pp. 567–596. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- 38.Lauderdale DS, Knutson KL, Yan LL, et al. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bower J. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regestein QR, Friebely J, Shifren JL, et al. Self-reported sleep in postmenopausal women. Menopause. 2004;11:198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 42.Berger AM, Marunda HT, Agrawal S. Influence of menopausal status on sleep and hot flashes throughout breast cancer adjuvant chemotherapy. J Obstet Gynecol Neonatal Nurs. 2009;38:353–366. doi: 10.1111/j.1552-6909.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 43.Fan HGM, Houede-Tchen N, Yi Q-L, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen PB, Donovan KA, Small BJ, et al. Fatigue after treatment for early stage breast cancer: A controlled comparison. Cancer. 1997;110:1851–1859. doi: 10.1002/cncr.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: A longitudinal study. J Clin Oncol. 2005;23:8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 46.Andrykowski MA, Schmidt JE, Salsman JM, et al. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol. 2005;23:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo HH, Chiu MJ, Liao WC, et al. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105:64–69. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs GD, Pace-Schott EF, Stickgold R, et al. Cognitive behavior therapy and pharmacotherapy for insomnia: A randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 49.Sivertsen BR, Omvik S, Pallesen Sl, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. JAMA. 2006;295:2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 50.Von Ah DM, Kang D-H, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31:134–144. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- 51.Earle CC. Failing to plan is planning to fail: Improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24:5112–5116. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 52.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24:411–417. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 53.Espie CA, MacMahon KMA, Kelly H-L, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–584. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 54.Mock V, Atkinson A, Barsevick AM, et al. Cancer-related fatigue. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2007;5:1054–1078. doi: 10.6004/jnccn.2007.0088. [DOI] [PubMed] [Google Scholar]
- 55.Page MS, Berger AM, Johnson LB. Putting evidence into practice: Evidence-based interventions for sleep-wake disturbances. Clin J Oncol Nurs. 2006;10:753–767. doi: 10.1188/06.CJON.753-767. [DOI] [PubMed] [Google Scholar]
- 56.Page M, Berger A. Oncology Nursing Society putting evidence into practice: Resource on sleep-wake disturbances. In: Eaton L, Tipton J, editors. Oncology Nursing Society Putting Evidence into Practice: Improving Oncology Patient Outcomes. Pittsburgh, PA: Oncology Nursing Society; 2009. pp. 291–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.