Abstract
We previously reported that circulating levels of pigment epithelium-derived factor (PEDF), a newly identified adipokine, are increased in patients with type 2 diabetes, correlating with body mass index. However, the role of PEDF in adipogenesis remains elusive. In the present study, we have investigated the effects and mechanisms of PEDF on adipocyte differentiation in 3T3-L1 preadipocytes. Differentiation of 3T3-L1 preadipocytes was induced in the presence or absence of human recombinant PEDF protein. The effects of PEDF on adipogenic gene expression, mitotic clonal expansion (MCE), and MAPK activation were investigated. Physiological concentrations of human PEDF protein inhibited adipocyte differentiation, evidenced by decreased lipid accumulation, downregulation of adipocyte markers, and inhibition of master adipogenic transcription factors such as C/EBP-α and PPARγ. The antiadipogenic effects of PEDF were observed only when PEDF was added to the cells on day 0, but not on day 3 during differentiation, suggesting that PEDF targets some early adipogenic events. Similarly, overexpression of PEDF by adenovirus attenuated adipocyte differentiation. Further studies revealed that PEDF, or U-0126, a specific MAPK/ERK inhibitor, sequentially inhibited the early activation of ERK and MCE. Moreover, PEDF attenuated expression and the phosphorylation of C/EBP-β at Thr188, an essential step for transcriptional activation of C/EBP-β. In addition, PEDF expression was decreased significantly in the first 24 h during adipocyte differentiation, suggesting that downregulation of PEDF may be essential for the initiation of MCE and adipogenesis. We conclude that PEDF inhibits adipogenesis in 3T3-L1 preadipocytes partially because of inhibition of the MAPK/ERK signaling pathway and MCE.
Keywords: mitogen-activated protein kinase, extracellular signal-regulated kinase
obesity, characterized by excess fat (adipose) tissue, has been recognized as a pivotal risk factor in a broad range of diseases, such as metabolic syndrome, insulin resistance, diabetes, hypertension, cardiovascular diseases, and cancer (2, 16, 20, 21, 34). Adipose tissue is not only the major site for triglyceride storage but also an important endocrine organ in the body (1). It is estimated that 20–30% of genes expressed in adipose tissue encode secreted proteins, including proinflammatory cytokines (e.g., tumor necrosis factor-α and interleukin-6), complementary and related proteins (e.g., adiponectin), fibrinolytic proteins, and other hormone-like proteins (e.g., leptin and resistin) (15). These bioactive molecules, collectively termed as adipokines, regulate the biological functions of adipocytes via autocrine, paracrine, and endocrine mechanisms, and thus adipokines play an important role in inflammation, energy metabolism, and insulin sensitivity (11).
Pigment epithelium-derived factor (PEDF) is a 50-kDa monomeric glycoprotein, which has recently been identified as an adipokine (14, 43). PEDF is a member of the serine proteinase inhibitor (Serpin) family but lacks a serine-reactive loop and thus has no function on protease inhibition (5). However, PEDF has demonstrated multiple diverse and cell type-specific functions, including the regulation of cell apoptosis, proliferation, and differentiation. Consequently, PEDF has been found to play a role in many important biological processes, such as angiogenesis, neuronal development, inflammation, and oxidative stress (10, 28). In endothelial cells, PEDF inhibits cell proliferation, migration, and tube formation and induces endothelial cell apoptosis (10). In renal mesangial cells, PEDF does not affect cell growth but attenuates mesangial expansion and fibrogenic factor expression (31, 32). In macrophages, PEDF induces cell apoptosis and attenuates macrophage activation (37). In addition, PEDF promotes the survival and differentiation of developing spinal motor neurons and accelerates neurite outgrowth of retinal neurons (28). Moreover, PEDF inhibits tumor cell growth and promotes tumor cell differentiation (9). Thus, PEDF appears to be an important endogenous protein with pleotropic effects on cell growth and function. However, to the best of our knowledge, the effect of PEDF on preadipocyte growth and differentiation has not previously been studied.
Recently, we reported that circulating levels of PEDF are increased significantly in patients with type 2 diabetes, correlating with body mass index (38). Significant correlation between serum/plasma concentration of PEDF and body habitus has also been observed independently in patients with the metabolic syndrome (35) and type 1 diabetes (39) as well as in healthy subjects (38). However, the pathophysiological significance of elevated PEDF levels in these obesity-related diseases remains elusive. Therefore, studying the function of PEDF in adipogenesis and adipocyte biology may provide pivotal information for understanding the mechanisms underlying the association of PEDF and obesity. In the present study, we studied the effects of PEDF on adipocyte differentiation using a well-established cell model, mouse 3T3-L1 preadipocytes.
MATERIALS AND METHODS
Cell culture and induction of differentiation in 3T3-L1 cells.
Mouse embryo fibroblast 3T3-L1 cells were obtained from American Type Culture Collection (Manassas, VA) and grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum and 1% antibiotics (Cellgro, Manassas, VA) until confluence and induced to differentiation, as described previously (14). Briefly, 2 days postconfluence (defined as day 0), cells were exposed to differentiation medium containing 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, 1.67 μM insulin (MDI; Sigma, St. Louis, MO), and 10% fetal bovine serum (FBS) for 3 days. Then, cells were transferred to DMEM with 1.67 μM insulin and 10% FBS and refed every 2 days. Maturation of adipocytes was confirmed by Oil Red O staining of lipid droplet on day 7.
Construction of the human PEDF expression vectors and establishment of PEDF stable cell line.
Human PEDF (hPEDF) cDNA was amplified from a human liver cDNA library by PCR reaction, purified, and cloned into the pGEM-T vector (Promega, Madison, WI). The target gene was subcloned into a mammalian expression vector pLV-CIG (kind gift from Dr. Takao Hashimoto) between the XbaI and PstI sites following the enhanced green fluorescent protein (eGFP) driven by internal ribosomal entry site. Human embryonic kidney-293T cells were used to generate the PEDF stable cell line. Briefly, 5 μg of the pLV-CIG-hPEDF vector was transfected into 0.5 × 106 cells in 35-mm dishes using the Fugene 6 transfection reagent (Roche, Indianapolis, IN). Single cells were selected on the basis of eGFP expression and seeded in a 96-well plate for 48 h. Colonies expressing PEDF were picked and expanded. PEDF expression was confirmed by Western blot analysis.
Recombinant hPEDF purification using ion exchange chromatography.
Conditional medium (serum free) from the cultured PEDF stable cell line was collected for purification using ion exchange chromatography with an NaCl gradient. Briefly, the proteins in the medium were precipitated with 80% ammonium sulfate, and the pellet was resuspended in 30 ml of 20 mM Na-phosphate buffer A containing 150 mM NaCl. The resuspended material was dialyzed overnight against 50 mM Na-phosphate buffer B (50 mM NaCl, 1 mM DTT, and 10% glycerol, pH 6.2), and the insoluble materials were removed by centrifugation and filtration through a 0.45-μM membrane. The filtered solution was loaded onto the S Sepharose column (50-ml bed volume; GE Healthcare BioSciences, Piscataway, NJ) preequilibrated in the 50-mM, pH 6.2, Na-phosphate buffer B. Unbound materials were washed out with 20-column volumes of the equilibrating buffer. The hPEDF protein was eluted with a linear gradient from 50 to 500 mM NaCl. The purified protein was concentrated using ultrafree filter unit (Millipore, Bedford, MA) and dialyzed against 1× PBS using the Slide-A-Lyzer 3K MWCO Dialysis Cassettes (Pierce, Rockford, IL). The purity of hPEDF was determined by SDS-PAGE using Coomassie staining. The biological activity of hPEDF was confirmed by inhibition of retinal capillary endothelial cell proliferation, as described previously (12).
Preparation of adenovirus expressing hPEDF.
To construct adenovirus expression hPEDF (Ad-PEDF), the full-length coding region of the hPEDF cDNA was cloned into the pShuttle vector (Qbiogene, Carlsbad, CA). The purified plasmid DNA was digested with PmeI and cotransformed with pAdEasy-1 vector (Qbiogene) into BJ5183-competent cells. The recombinant adenovirus DNA was digested with PacI, purified, and transfected into QBI-293A cells to generate virus particles. The construct sequence was confirmed by DNA sequencing (OMRF, Oklahoma City, OK). The recombinant virus was amplified, purified, and tittered using Adeno-X Maxi Purification kit (Clontech Laboratories, Mountain View, CA), as described previously (32).
Subconfluent 3T3-L1 cells were grown in DMEM supplemented with 10% FBS and transfected by Ad-PEDF or control adenovirus expressing GFP (Ad-GFP) at a multiplicity of infection of 5–10. Forty-eight hours after transfection, cells were subjected to differentiation medium to induce differentiation, as described above.
Oil Red O staining and quantification of lipid accumulation in adipocytes.
Lipid droplets in mature adipocytes or preadipocytes (as control) were stained with Oil Red O, as described previously (13). Briefly, cells were fixed overnight with 10% formalin and incubated with 60% (wt/wt) filtered Oil Red O (Sigma, St., Louis, MO) in 100% isopropanol for 1 h at 60°C. Then, the cells were washed twice with distilled water to remove excess dye and photographed under microscopy. To quantify intracellular lipid accumulation, the stained lipid droplets were dissolved with 100% isopropanol for 10 min. The optical density was measured at 500 nm by spectrophotometer.
Western blot analysis.
Cells were washed twice with prechilled PBS and lysed in RIPA buffer with protease inhibitor cocktail, PMSF, and sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA). Protein concentration was measured by Bradford method. Thirty micrograms of protein was resolved by SDS-PAGE and blotted with specific antibodies, including anti-adipocyte fatty acid-binding protein (aP2) and anti-adipose tissue triglyceride lipase (ATGL) antibodies (Cayman Chemical, Ann Arbor, MI), anti-hormone-sensitive lipase (HSL), anti-CCAAT/enhancer-binding protein-α (C/EBPα), anti-C/EBPβ, and anti-pref-1 antibodies (Santa Cruz Biotechnology), anti-peroxisome proliferator-activated receptor-γ (PPARγ), anti-phospho-p42/44 MAPK (ERK1/2) at Thr202/Tyr204, and total ERK antibodies, anti-phospho-p38 at Thr180/Tyr182 and total p38 antibodies, and anti-phospho-C/EBPβ at Thr235 (human)/Thr188 (mouse) antibody (Cell Signaling Technology, Danvers, MA).
Real-time RT-PCR analysis.
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Real-time RT-PCR was performed using the iScript cDNA Synthesis kit and SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA) as described (41). mRNA levels of target genes were normalized by 18s ribosomal RNA. Primers used in the study were Pref-1: forward 5′-gtccaacctgcgctacaa-3′, reverse 5′-agagcaaactccaccacaaa-3′; and PEDF: forward 5′-aagtcatatgggaccaggccc-3′, reverse 5-′ttacccactgccccttgaagt-3′.
Measurement of PEDF secretion from preadipocytes and adipocytes by ELISA.
PEDF secreted by preadipocytes or adipocytes into the medium was measured by a high-sensitivity PEDF ELISA kit (Bioproducts MD, Middletown, MD), as described previously (42). Briefly, conditional medium (serum free) was collected from preadipocytes or mature adipocytes after 24-h culture. Medium was diluted at 1:10 and applied to a plate precoated with polyclonal anti-PEDF antibody per the manufacturer's instruction. Results were normalized by total cell number and expressed as means ± SD.
Cell proliferation assay.
3T3-L1 cells (1 × 103 cells/well) were seeded in a gelatin-coated 96-well plate. Postconfluent cells were subjected to the differentiation medium in the presence or absence of PEDF (10–100 nM) or U-0126 (40 μM), as described above. Cell proliferation on day 3 was evaluated by measuring cellular DNA content using a fluorescent dye from CyQuant NF Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR), following manufacturer's protocol. Fluorescence intensity was measured by a fluorescence microplate reader, with excitation at 485 nm and emission at 530 nm.
Statistical analysis.
All experiments were repeated at least three times. Data are expressed as means ± SD. Statistical analysis was performed using Student's t-test when comparing two groups or ANOVA with Bonferroni's post hoc test when comparing three or more groups. Statistical difference was considered significant at a P value of <0.05.
RESULTS
PEDF inhibits differentiation and lipid accumulation in 3T3-L1 preadipocytes.
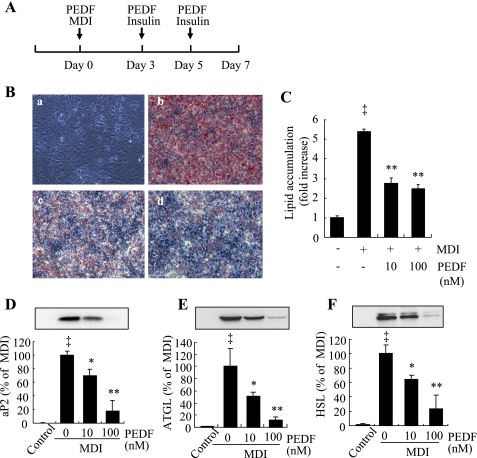
Bioactive recombinant hPEDF with purity of 95% or greater was used in the experiments (Supplemental Fig. S1; Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism website). Postconfluent 3T3-L1 preadipocytes were exposed to differentiation medium in the presence or absence of PEDF (Fig. 1A). Adipocyte formation, lipid accumulation, and adipocyte gene expression were evaluated on day 7. At physiological concentrations (10–100 nM), PEDF significantly inhibited 3T3-L1 cell differentiation in a dose-dependent manner (Fig. 1B). Lipid accumulation in differentiated adipocytes was also significantly reduced in the PEDF-treated cells (Fig. 1C). Moreover, several adipocyte marker proteins that are normally robustly expressed in mature adipocytes, including adipocyte-specific gene aP2 and genes involved in lipid metabolism, ATGL and HSL, were downregulated by PEDF in a dose-dependent manner (Fig. 1, D–F).
Fig. 1.
Inhibition of 3T3-L1 preadipocyte differentiation by pigment epithelium-derived factor (PEDF). Differentiation was induced by 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, and 1.67 μM insulin (MDI) in postconfluent 3T3-L1 preadipocytes, as described. A: schema indicating that PEDF (10–100 nM) was added on days 0, 3, and 5 during differentiation. B: intracellular lipid was stained by Oil Red O on day 7. B, a: nondifferentiated cells; B, b: MDI; B, c: MDI + 10 nM PEDF; B, d: MDI + 100 nM PEDF. C: quantification of lipid content by spectrophotometer. D–F: expression of adipocyte marker proteins, adipocyte fatty acid-binding protein (aP2; D), adipose tissue triglyceride lipase (ATGL; E), and hormone-sensitive lipase (HSL; F) on day 7 by Western blot analysis. D–F, top: representative blots. D–F, bottom: quantification by densitometry. Results represent 3 independent experiments. Values were expressed as means ± SD. ‡P < 0.01 vs. nondifferentiated cells; *P < 0.05, **P < 0.01 vs. MDI (ANOVA).
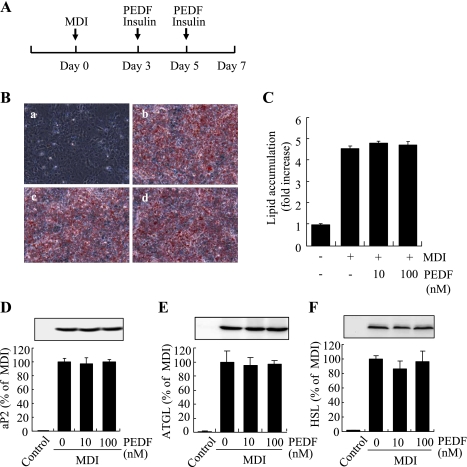
PEDF has no effect on late-stage adipocyte differentiation in 3T3-L1 cells.
To determine whether PEDF has an effect on terminal adipocyte differentiation, PEDF was added to differentiating cells on days 3 and 5, and mature adipocyte formation and lipid accumulation were evaluated on day 7 (Fig. 2A). The results show that PEDF (10–100 nM) did not affect mature adipocyte formation and lipid accumulation (Fig. 2, B and C). In addition, PEDF treatment during late stages of adipocyte differentiation did not alter expression of adipocyte markers, including aP2, ATGL, and HSL (Fig. 2, D–F), suggesting that PEDF had no effect on terminal adipocyte differentiation. To further confirm that PEDF acts during the initial stages of adipogensis to inhibit adipoctye differentiation, PEDF was added only on day 0 (hour 0) with MDI for 3 days, and adipocyte differentiation was analyzed on day 7, as described above (Supplemental Fig. S3A). The results show that PEDF significantly inhibited adipocyte differentiation, lipid accumulation, and adipocyte gene expression (Supplemental Fig. S3, B–E), indicating that PEDF inhibits some early events during adipocyte differentiation.
Fig. 2.
PEDF has no effect on late-stage differentiation in 3T3-L1 preadipocytes. A: schema indicating that PEDF (10–100 nM) was added on days 3 and 5 during differentiation. B: intracellular lipid was stained by Oil Red O on day 7. B, a: nondifferentiated cells; B, b: MDI; B, c: MDI + 10 nM PEDF; B, d: MDI + 100 nM PEDF. C: quantification of lipid content by spectrophotometer. D–F: expression of adipocyte marker proteins aP2 (D), ATGL (E), and HSL (F) on day 7 by Western blot analysis. D–F, top: representative blots. D–F, bottom: quantification by densitometry. Results represent 3 independent experiments (means ± SD).
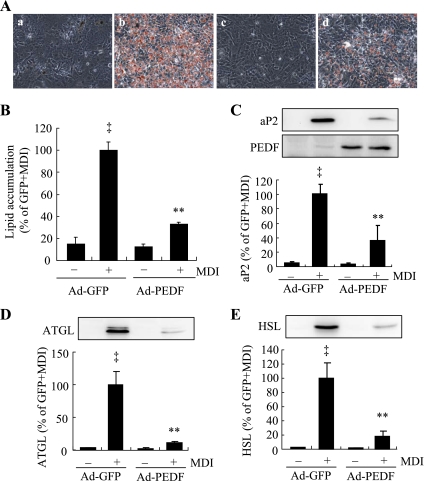
Overexpression of PEDF by adenovirus inhibits adipocyte differentiation.
To further confirm the effect of PEDF on adipocyte differentiation, 3T3-L1 cells were infected with adenovirus expressing PEDF (Ad-PEDF) or adenovirus expressing GFP (Ad-GFP) as control. Forty-eight hours after infection, cells were exposed to differentiation medium to induce differentiation. Adipocyte formation, lipid accumulation, and expression of adipocyte-specific genes were evaluated on day 7 (Fig. 3). Overexpression of PEDF in Ad-PEDF-treated cells was confirmed by Western blot analysis (Fig. 3C). Results show that Ad-PEDF inhibited adipocyte differentiation (Fig. 3A) and reduced lipid accumulation to 32% compared with Ad-GFP-treated cells (Fig. 3B). Consistently, the protein levels of adipocyte markers, including aP2, ATGL, and HSL, were decreased significantly in Ad-PEDF-treated cells (Fig. 3, C–E). These results corroborate our finding that exogenous PEDF inhibits adipocyte differentiation in 3T3-L1 cells.
Fig. 3.
Overexpression of PEDF attenuates adipocyte differention. Subconfluent 3T3-L1 cells were infected with adenovirus expression PEDF (Ad-PEDF) or adenovirus expressing green fluorescent protein (Ad-GFP) for 48 h, followed by induction of differentiation by MDI. A: intracellular lipid was stained by Oil Red O on day 7. A, a: Ad-GFP; A, b: Ad-GFP + MDI; A, c: Ad-PEDF; A, d: Ad-PEDF + MDI. B: quantification of lipid content by spectrophotometer. C–E: expression of adipocyte marker proteins aP2 (C), ATGL (D), and HSL (E) on day 7 by Western blot analysis. C–E, top: representative blots. C–E, bottom: quantification by densitometry. Results represent 3 independent experiments. Values were expressed as means ± SD. ‡P < 0.01 vs. Ad-GFP; **P < 0.01 vs. Ad-GFP + MDI (ANOVA).
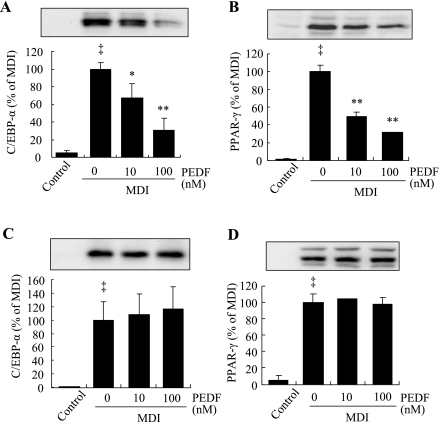
PEDF attenuates adipogenic transcription factor C/EBPα and PPARγ expression.
C/EBPα and PPARγ are the two master transcription factors controlling the common final process of adipogenesis (19). In differentiating 3T3-L1 cells, C/EBPα and PPARγ expression increases from undetectable levels in preadipocytes to detectable levels on day 2 and to full expression on day 5 after MDI stimulation (19). Thus, we determined the effect of PEDF on C/EBPα and PPARγ expression in differentiating 3T3-L1 cells on day 5. The results show that when PEDF was added on day 0, PEDF significantly inhibited C/EBPα (Fig. 4A) and PPARγ (Fig. 4B) expression in a dose-dependent manner. However, when PEDF was added to the cells after 3 days of differentiation, PEDF had no effect on C/EBPα (Fig. 4C) and PPARγ expression (Fig. 4D). These results suggest that PEDF acts during the early phase of adipogenesis to inhibit expression of C/EBPα and PPARγ but has no direct effect on C/EBPα and PPARγ expression after the early differentiation period.
Fig. 4.
Effect of PEDF on expression of CCAAT/enhancer-binding protein-α (C/EBPα) and peroxisome proliferator-activated receptor-γ (PPARγ). PEDF (10–100 nM) was added to postconfluent 3T3-L1 preadipocytes on day 0 (A and B) or to the differentiating cells on day 3 (C and D). Expression of C/EBPα (A and C) and PPARγ (B and D) was determined by Western blot analysis and quantified by densitometry. Results represent 3 independent experiments (means ± SD; n = 3). ‡P < 0.01 vs. nondifferentiated cells; *P < 0.05, **P < 0.01 vs. MDI (ANOVA).
PEDF inhibits mitotic clonal expansion.
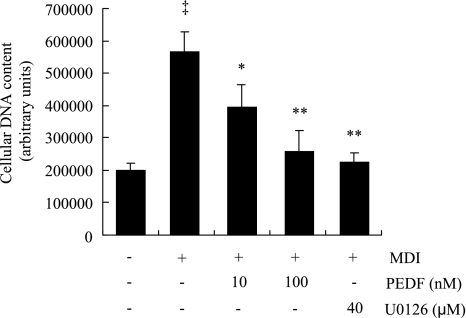
It is well established that, after hormonal induction, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle and undergo two to three cycles of mitosis, or mitotic clonal expansion (MCE), which is essential for expression of key adipogenic genes, including C/EBPα and PPARγ, and for terminal differentiation in 3T3-L1 cells (25, 27). Inhibition of MCE with either MAPK/ERK kinase (MEK) inhibitor U-0126 or the cyclin-dependent kinase inhibitor roscovitine, each of which inhibits the cell cycle at different points, suppresses MCE and adipogenesis (27). Thus, we investigated the effect of PEDF on MCE by measuring cellular DNA content. Three days after induction of differentiation with MDI, DNA content was increased 2.8-fold, indicating increased DNA synthesis in differentiating cells during MCE (Fig. 5). PEDF significantly decreased DNA content in a dose-dependent manner (Fig. 5). The addition of U-0126 (40 nM) almost completely blocked the increase in DNA content (Fig. 5). In addition, PEDF alone had no effect on cell proliferation in 3T3-L1 preadipocytes (Supplemental Fig. S4). These results suggest that PEDF is a potent inhibitor of MCE in 3T3-L1 cell differentiation.
Fig. 5.
PEDF inhibits mitotic clonal expansion in 3T3-L1 preadipocytes. Postconfluent preadipocytes were treated with MDI in the presence or absence of PEDF (10–100 nM) for 72 h. Cellular DNA content was measured by CyQuant NF Cell Proliferation Assay Kit. Results represent 3 independent experiments (means ± SD). ‡P < 0.01 vs. nondifferentiated cells; *P < 0.05, **P < 0.01 vs. MDI (ANOVA).
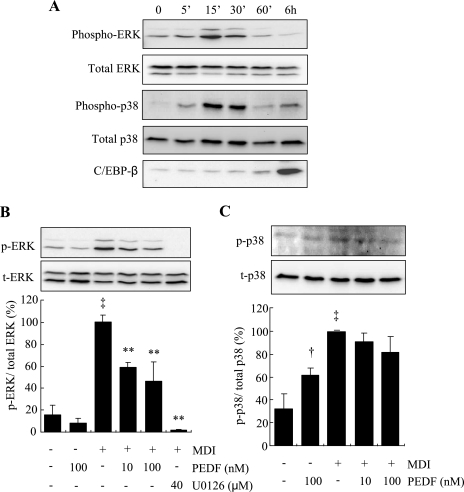
PEDF inhibits the MDI-induced phosphorylation of ERK but not p38 MAPK in 3T3-L1 cells during early differentiation.
Intracellular MAPK signaling pathways play a major role in the regulation of cell proliferation and differentiation (24, 27). There are three groups of kinases that belong to the MAPK family, ERKs, c-Jun NH2-terminal kinases (JNKs), and p38 MAPK, but only the activation of ERK has been shown to be essential for the induction of MCE and adipogenesis. In our previous studies, we have shown that PEDF inhibited ERK1/2 activation in endothelial cells, which is responsible for its inhibitory effect on angiogenesis (41). Here we determined whether PEDF inhibits MCE and adipogenesis via blockade of the MAPK/ERK pathway. In growth-arrested 3T3-L1 preadipocytes, ERK was expressed constitutively and was rapidly and transiently phosphorylated in the first hour, peaking at 15 min after the induction with MDI (Fig. 6A). The activation of ERK subsided to quiescent levels by 6 h, when expression of C/EBPβ was markedly increased (Fig. 6A). At 15 min after MDI treatment, PEDF significantly attenuated both ERK1 and ERK2 phosphorylation in a dose-dependent manner (Fig. 6B). The ERK-specific inhibitor U-0126 completely blocked the MDI-induced ERK activation and also decreased the endogenous phosphorylation of ERK (Fig. 6B). PEDF alone had no effect on ERK phospohrylation in 3T3-L1 cells (Fig. 6B).
Fig. 6.
Effect of PEDF on phosphorylation of ERK and p38 during early differentiation of 3T3-L1 preadipocytes. A: postconfluent preadipocytes were treated with MDI for indicated times (0–6 h). Phosphorylation of ERK1/2 at Thr202/Tyr204, total ERK1/2, phosphorylated p38 at Thr180/Tyr182, total p38, and C/EBPβ expression were determined by Western blot analysis. B and C: preadipocytes were pretreated with PEDF (10–100 nM) or U-0126 (40 μM) for 30 min, followed by treatment with MDI for 15 min. Phosphorylated (p-) and total (t)ERK1/2 (B) and phosphorylated and total p38 (C) were measured by Western blot analysis and quantified by densitometry (means ± SD; n = 3). †P < 0.05, ‡P < 0.01 vs. nondifferentiated cells; **P < 0.01 vs. MDI (ANOVA).
In addition to ERK, p38 MAPK has also been shown to be activated by MDI, and inhibition of p38 MAPK attenuated adipogenesis in 3T3-L1 preadipocytes, whereas the JNK pathway does not appear to be involved directly in adipocyte differentiation (3, 4). Thus, we determined the effect of PEDF on p38 MAPK phosphorylation. The results show that phosphorylation of p38 MAPK was rapidly induced at 5–30 min after MDI treatment, and phosphorylation subsided within 60 min (Fig. 6A). Interestingly, pretreatment with PEDF for 30 min induced a modest but significant increase in p38 MAPK phosphorylation but did not alter p38 MAPK phosphorylation induced by MDI at 15 min (Fig. 6B).
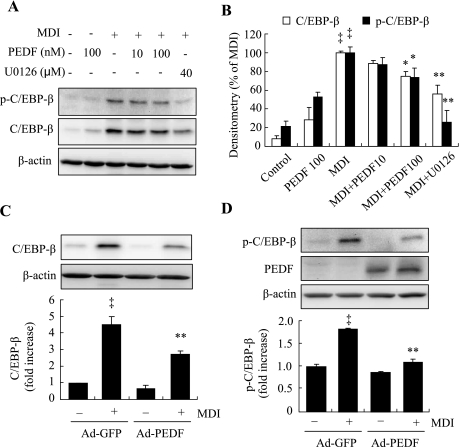
PEDF downregulates the MAPK-mediated C/EBPβ expression and phosphorylation in differentiating 3T3-L1 cells.
C/EBPβ is a transcription factor induced in the initial phase of adipocyte differentiation. C/EBPβ expression is essential for the induction of MCE (26). Mouse embryonic fibroblasts derived from C/EBPβ-deficient mice are unable to undergo MCE or differentiate into adipocytes (26). In addition, C/EBPβ binds to the C/EBP regulatory element, activating C/EBPα and PPARγ expression after MCE (33). Studies from Tang et al. (24) show that C/EBPβ is induced shortly after MDI treatment but has no transcriptional activity until being phosphorylated sequentially by MAPK and glycogen synthase kinase-3β (GSK-3β). Phosphorylation of C/EBPβ at Thr188 is essential for priming the phosphorylation of Thr179 and Ser184 by GSK-3β (24). Thus, we determined the effects of PEDF on C/EBPβ expression as well as phosphorylation of C/EBPβ at Thr188 by MAPK. The results show that expression of C/EBPβ was significantly increased 6 h after MDI treatment, but in the presence of exogenous PEDF or MAPK inhibitor U-0126, C/EBPβ expression was decreased in a dose-dependent manner (Fig. 7, A and B). Likewise, Ad-PEDF-treated cells had decreased C/EBPβ expression relative to Ad-GFP-treated cells (Fig. 7C). Moreover, MDI treatment significantly increased Thr188 phosphorylation of C/EBPβ, but MDI-induced phosphorylation of Thr188 was significantly attenuated in both Ad-PEDF-treated cells (Fig. 7D) and cells treated by addition of exogenous PEDF (Fig. 7, A and B). The addition of MAPK inhibitor U-0126 also completely blocked the phosphorylation of C/EBPβ (Fig. 7, A and B).
Fig. 7.
Effect of PEDF on expression and phosphorylation of C/EBPβ in differentiating 3T3-L1 preadipocytes. Postconfluent preadipocytes pretreated with PEDF (10–100 nM) or U-0126 (40 μM) for 30 min (A and B) or infected with Ad-PEDF or Ad-GFP (C and D) for 48 h followed by treatment with MDI for 6 h. Expression and phosphorylation of C/EBPβ at Thr188 were measured by Western blot analysis and quantified by densitometry (means ± SD; n = 3). B: ‡P < 0.01 vs. nondifferentiated cells; *P < 0.05, **P < 0.01 vs. MDI (ANOVA). C and D: ‡P < 0.01 vs. Ad-GFP; **P < 0.01 vs. Ad-GFP + MDI (ANOVA).
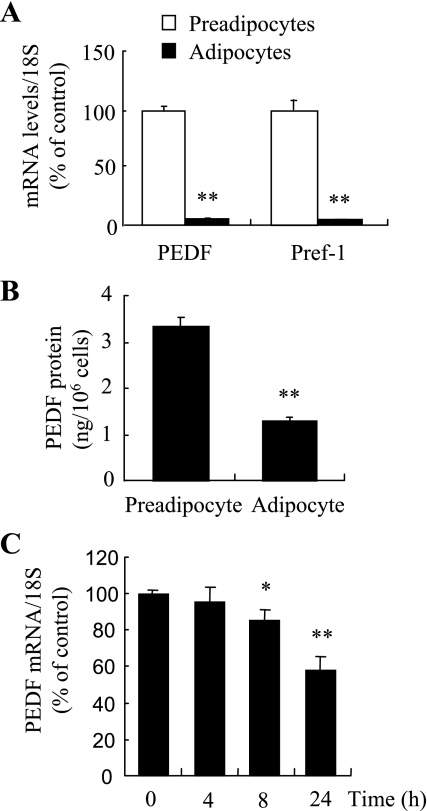
PEDF expression is decreased during early adipogenesis in 3T3-L1 cells.
PEDF has recently been reported as an adipokine (14). However, the changes in expression of PEDF in preadipocytes and adipocytes appear controversial. Studies from Kratchmarova et al. (14) and Vankoningsloo et al. (30) show that PEDF expression is decreased in mature adipocytes differentiated from 3T3-L1 preadipocytes, whereas in human adipose-derived stem cells, PEDF secretion is significantly increased in differentiated adipocytes (7, 43). Since we have shown that PEDF inhibits MCE and adipogenesis in 3T3-L1 adipocytes, we further evaluated the expression of PEDF in undifferentiated preadipocytes, mature adipocytes, and cells undergoing early adipocyte differentiation prior to MCE. Similarly to previous studies on 3T3-L1 cells, we found that PEDF is highly expressed in undifferentiated preadipocytes, and its expression decreases significantly in mature adipocytes (Fig. 8A). A comparable expression pattern was observed for Pref-1, a preadipocyte marker (Fig. 8A). Moreover, secretion of PEDF was also lower in mature adipocytes than in preadipocytes (Fig. 8B). We next determined the expression of endogenous PEDF in cells during the early stage of differentiation in 3T3-L1 cells. The results show that the mRNA expression of PEDF was significantly decreased as early as 8 h after MDI treatment (Fig. 8C). These results suggest that the decrease of endogenous PEDF level may be essential for the initiation of MCE in differentiating 3T3-L1 preadipocytes.
Fig. 8.
Expression of endogenous PEDF was downregulated during early adipogenesis in 3T3-L1 preadipocytes. A: mRNA levels of PEDF and Pref-1 in untreated or mature adipocytes (day 7) were determined by real-time RT-PCR and normalized by 18S RNA levels (means ± SD). **P < 0.01 (Student's t-test). B: PEDF secreted into the medium was measured by ELISA and normalized by cell number (means ± SD). **P < 0.01 (Student's t-test). C: PEDF mRNA expression was determined by real-time RT-PCR in differentiating cells during the first 24 h after MDI treatment. Results represent 3 independent experiments (means ± SD). *P < 0.05, **P < 0.01 (ANOVA).
DISCUSSION
In the present study, we demonstrated for the first time that PEDF, a newly identified adipokine, inhibits MCE and adipogenesis in 3T3-L1 preadipocytes. Furthermore, we show that one mechanism whereby PEDF inhibits adipogenesis is by inhibiting MAPK/ERK-mediated signaling and the subsequent induction of C/EBPβ expression and phosphorylation. These inhibitory effects of exogenous PEDF protein on adipogenesis occur at physiological concentrations (10–100 nM), whereas the same concentration range of PEDF has no effect on preadipocyte growth. In addition, overexpression of PEDF in 3T3-L1 cells attenuates adipocyte differentiation. Moreover, we have confirmed that PEDF expression is abundant in 3T3-L1 preadipocytes and decreased in early differentiated and mature adipocytes. These results collectively indicate that PEDF is a potent inhibitor of adipogenesis in 3T3-L1 preadipocytes.
PEDF was originally identified as a neuroprotective and neurotrophic factor in cultured retinal pigment epithelial cells, and it has also been shown to be a major angiogenic inhibitor in the retina (10). Systemic or local delivery of PEDF ameliorated aberrant new vessel growth in the retina, choroid, and cornea in a variety of animal models, including laser-induced choroidal neovascularization and oxygen-induced retinopathy (17, 22, 23). PEDF is expressed ubiquitously in various cell types and exhibits diverse functions in different tissues (29). Our previous studies show that PEDF is expressed at high levels in the kidney and that renal PEDF expression is decreased in diabetic nephropathy (31). Administration of adenovirus expressing PEDF to streptozotocin-induced diabetic rats ameliorates albuminuria, glomerular mesangial expression, and fibrogenesis (31, 32). Moreover, PEDF functions as an anti-inflammatory, antioxidant, and antipermeability factor inhibiting leukostasis and reducing retinal vascular leakage in diabetic animals (36, 40, 42). Interestingly, we previously found that PEDF concentration is increased significantly in the serum from patients with type 2 diabetes and correlated with body habitus (38, 39). A potential explanation could be that PEDF is produced by adipose tissue and/or functions as a regulator of adipogenesis and lipid metabolism. In the present study, we show that PEDF at physiological concentrations (10–100 nM) as well as overexpression of PEDF by adenovirus inhibited differentiation of 3T3-L1 preadipocytes, suggesting a direct inhibitory effect of PEDF on adipocyte differentiation. Although this effect should be tested in other in vitro and in vivo models of adipogenesis, it is notable that PEDF has been shown to be a potent angiogenic inhibitor and anti-inflammatory factor. Because angiogenesis and inflammation are well-known essential factors contributing to adipogenesis, PEDF may also suppress adipogenesis through inhibition of angiogenesis and inflammation. On the other hand, recent studies from Chung et al. (8) show that PEDF promotes triglyceride metabolism in hepatocytes. Furthermore, PEDF-null mice exhibit increased triglyceride content and steatosis in the liver, suggesting that PEDF is essential for lipolysis (8). Future studies are warranted to elucidate whether the antiadipogenic and lipolytic effects of PEDF contribute to the development of insulin resistance in type 2 diabetes.
In contrast to the extensive studies focusing on the functions of PEDF, the mechanisms underlying the diverse actions of PEDF are poorly understood. In the present study, we show that PEDF attenuated the early ERK1/2 activation induced by MDI during adipogenesis, which thereby inhibited C/EBPβ expression and activation and ultimately prevented MCE and adipogenic gene expression. However, the mechanism by which PEDF inhibits ERK1/2 activation remains unresolved. In our previous studies, we found that PEDF binds to VEGF receptor 2 and inhibits VEGF function in endothelial cells (41). Moreover, PEDF inhibits growth factor-induced ERK1/2 activation and subsequent expression of hypoxia-inducible factor-1α (41). PEDF has also been shown to inhibit growth factor-induced angiogenesis through cleavage and intracellular translocation of the transmembrane domain of the VEGF receptor 1 in endothelial cells (6). We found that PEDF binds to the cell membrane in 3T3-L1 preadipocytes (Supplemental Fig. S2); however, the cell surface receptor for PEDF remains elusive. Recently, Notari et al. (18) demonstrated that PEDF binds to a membrane protein that has structural homology with the ATGL in retinal pigment epithelium cells. Moreover, Chung et al. (8) confirmed that PEDF binds to ATGL in hepatic cells, but interestingly, it appears that PEDF needs to traffic to the adiposome to interact with ATGL. In our present study, we found that ATGL is expressed at low levels in preadipocytes, and its expression increased more than 50 times at both the mRNA (not shown) and the protein levels in mature adipocytes. These results do not support the notion that PEDF acts through ATGL in preadipocytes. Moreover, PEDF alone has no effect on MAPK/ERK activation or MCE. Therefore, elucidating the potential interactions between PEDF and individual components of the adipogenesis pathway may provide insight into the mechanisms of PEDF in adipogenesis.
GRANTS
This study was supported by Juvenile Diabetes Research Foundation Grants 5-2009-475 and 18-2007-860, National Institutes of Health (NIH) Grants EY-012231, EY-015650, and P20-RR-024215 from the National Center for Research Resources, and grants from the American Diabetes Association and Oklahoma Center for the Advancement of Science and Technology.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH.
ACKNOWLEDGMENTS
We thank Krysten Farjo for critical proofreading and editing of the manuscript.
REFERENCES
- 1.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14, Suppl5: 242S–249S, 2006 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2007. Diabetes Care 30: S4–S41, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 88: 1091–1098, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Aouadi M, Jager J, Laurent K, Gonzalez T, Cormont M, Binétruy B, Le Marchand-Brustel Y, Tanti JF, Bost F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett 581: 5591–5596, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem 270: 25992–25999, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Cameron ME. Histology of pterygium: an electron microscopic study. Br J Ophthalmol 67: 604–608, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiellini C, Cochet O, Negroni L, Samson M, Poggi M, Ailhaud G, Alessi MC, Dani C, Amri EZ. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol 9: 26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, Crawford SE. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL). J Hepatol 48: 471–478, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, De Vries GH, Abramson LP, Bouck N. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci 114: 4421–4428, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285: 245–258, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Drevon CA. Fatty acids and expression of adipokines. Biochim Biophys Acta 1740: 287–292, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gao G, Shao C, Zhang SX, Dudley A, Fant J, Ma JX. Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia 46: 689–698, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19–27, 1975 [DOI] [PubMed] [Google Scholar]
- 14.Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS, Fernandez MM, Bunkenborg J, Roepstorff P, Kristiansen K, Lodish HF, Mann M, Pandey A. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics 1: 213–222, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 580: 2917–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol 188: 253–263, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez-Sanchez F, Escribano J, Laborda J, Becerra SP. Identification of a lipase-linked cell-membrane receptor for pigment epithelium-derived factor (PEDF). J Biol Chem 281: 38022–38037, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr 130: 3122S–3126S, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49: 289–297, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MW, Porte JD. Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, Ma JX. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci 45: 1758–1762, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci USA 98: 2593–2597, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang QQ, Grønborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA 102: 9766–9771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev 13: 2231–2241, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA 100: 850–855, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 100: 44–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci 4: 628–636, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 15: 4992–5003, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vankoningsloo S, De Pauw A, Houbion A, Tejerina S, Demazy C, de Longueville F, Bertholet V, Renard P, Remacle J, Holvoet P, Raes M, Arnould T. CREB activation induced by mitochondrial dysfunction triggers triglyceride accumulation in 3T3-L1 preadipocytes. J Cell Sci 119: 1266–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wang JJ, Zhang SX, Lu K, Chen Y, Mott R, Sato S, Ma JX. Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes 54: 243–250, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Wang JJ, Zhang SX, Mott R, Knapp RR, Cao W, Lau K, Ma JX. Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes 55: 1678–1685, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev 9: 2350–2363, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, Hino A, Jinnouchi Y, Takenaka K, Matsui T, Nakamura K, Imaizumi T. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab 91: 2447–2450, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Yamagishi S, Matsui T, Nakamura K, Takeuchi M, Imaizumi T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res 72: 86–90, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Zamiri P, Masli S, Streilein JW, Taylor AW. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci 47: 3912–3918, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Jenkins A, Zhang SX, Gosmanova A, Aston A, Dashti A, Baker MZ, Lyons TJ, Ma JX. Increased serum pigment epithelium derived factor levels in type 2 diabetes patients. Diabet Res Clin Pract 82: e5–e8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, O'Neal DN, Januszewski AS, Croft KD, Mori TA, Dragicevic G, Harper CA, Best JD, Lyons TJ, Ma JX. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med 24: 1345–1351, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, Szweda L, Ma JX, Lyons TJ. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol 41: 135–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 37: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma Jx. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 20: 323–325, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X, Gimble JM. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics 6: 18–28, 2007 [DOI] [PubMed] [Google Scholar]