Abstract
Platelet-activating factor (PAF) and lysophosphatidylcholine (LPC) are potent inflammatory lipids. Elevated levels of PAF and LPC are associated with the onset of diabetic retinopathy and neurodegeneration. However, the molecular mechanisms underlying such defects remain elusive. LPCAT1 is a newly reported lysophospholipid acyltransferase implicated in the anti-inflammatory response by its role in conversion of LPC to PC. Intriguingly, the LPCAT1 enzyme also catalyzes the synthesis of PAF from lyso-PAF with use of acetyl-CoA as a substrate. The present studies investigated regulatory roles of LPCAT1 in the synthesis of inflammatory lipids during the onset of diabetes. Our work shows that LPCAT1 plays an important role in the inactivation of PAF by catalyzing the synthesis of alkyl-PC, an inactivated form of PAF with use of acyl-CoA and lyso-PAF as substrates. In support of a role of LPCAT1 in anti-inflammatory responses in diabetic retinopathy, LPCAT1 is most abundantly expressed in the retina. Moreover, LPCAT1 mRNA levels and acyltransferase activity toward lyso-PAF and LPC were significantly downregulated in retina and brain tissues in response to the onset of diabetes in Ins2Akita and db/db mice, mouse models of type 1 and type 2 diabetes, respectively. Conversely, treatment of db/db mice with rosiglitazone, an antidiabetes compound, significantly upregulated LPCAT1 mRNA levels concurrently with increased acyltransferase activity in the retina and brain. Collectively, these findings identified a novel regulatory role of LPCAT1 in catalyzing the inactivation of inflammatory lipids in the retina of diabetic mice.
Keywords: lysophosphatidylcholine, lysophosphatidylcholine acyltransferase, diabetic retinopathy, platelet-activating factor
phosphatidylcholine (PC) is the major phospholipid of the brain and comprises almost half of vertebrate retinal phospholipids. Lyso-PC (LPC) is a bioactive proinflammatory lipid generated by the pathological metabolism of PC. Accumulation of LPC is associated with a host of diseases, including atherosclerosis, myocardial ischemia, neurodegeneration, inflammatory diseases, and diabetic complications (22). Plasma LPC concentrations were significantly elevated in insulin-dependent and non-insulin-dependent diabetes mellitus patients (25), diabetic retinopathy (15, 17), and experimental obesity (12). Elevated LPC levels cause postprandial hyperglycemia by inhibiting glucose uptake by liver, heart, and muscle tissues (20). LPC has also been shown to activate several signal transduction pathways implicated in insulin resistance, such as JNK and protein kinase C (11, 23). Platelet-activating factor (PAF) is an ether lipid of choline, and its presence has been demonstrated in a variety of eye tissues, including the retina, ciliary body, and iris (1, 8, 27). PAF and its metabolite lyso-PAF are potent inflammatory lipids, and PAF and lyso-PAF levels are elevated in serum of type 1 diabetic patients (4, 10), as well as in oxygen-induced retinopathy (2). Intravenous injection of PAF into rabbits induced an intense ischemia accompanied by a marked plasma leakage (26). Consistent with the elevated PAF levels, PAF acetyltransferase (PAF-AT) activities are significantly increased in type 1 and type 2 diabetic patients (19, 28, 29), and treatment of BB/Wor rats with recombinant PAF acetylhydrolase reduces the incidence of insulitis and the frequency of diabetes (21). Incubation of corneas with PAF delays epithelial healing rates. In contrast, treatment of diabetic rats with an antioxidant accelerates corneal wound healing (5, 13). Furthermore, treatment of diabetic rats with PAF receptor antagonists improves retinal functions, as measured by the percentage of retinal area occupied by horseradish peroxidase-labeled vessels (7, 9). Treatment of diabetic patients with Ginkgo biloba extract, which functions as a PAF receptor antagonist, reduces platelet aggregation (18).
Major progress has been made in recent years in identification and characterization of acyltransferases involved in the remodeling of PAF and PC, including the first PAF-AT and four isoforms of LPC acyltransferases (LPCAT1–4) (6, 14, 16, 24, 31, 35). However, little is known about the role of these LPCATs in regulating the remodeling of PAF and PC under physiological and pathophysiological conditions. For example, LPCAT1 has been shown to be involved in the inflammatory and anti-inflammatory responses by catalyzing the synthesis of PAF and PC (14, 24), respectively, but the underlying mechanism remains elusive. To clarify this issue, we investigated a novel function of LPCAT1 in anti-inflammatory responses in catalyzing the acylation of lyso-PAF to alkyl-PC, a key step involved in PAF inactivation. We also investigated a regulatory role of LPCAT1 in the remodeling of PC and PAF in the retina and brain during the onset of diabetes and after treatment with an antidiabetes drug. Our work identified, for the first time, an important role of LPCAT1 in the inactivation of lyso-PAF and LPC associated with diabetes.
MATERIALS AND METHODS
Materials.
[Oleoyl-1-14C]oleoyl-coenzyme A (55 mCi/mmol) and [dipalmitoyl-1-14C]phosphatidylcholine l-α-dipalmitoyl (170 mCi/mmol) were purchased from American Radiolabeled Chemicals; 1,2-dipalmitoleoyl-sn-glycerophosphoethanolamine, 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (18:1), 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine (18:1), 1-oleoyl-2-hydroxy-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt) (18:1), 1-oleoyl-2-hydroxy-sn-glycero-3-[phospho-l-serine] (sodium) (18:1), and l-α-lysophosphatidylinositol (liver, bovine-sodium salt) from Avanti Polar Lipids; and 1-O-hexadecyl-sn-glyceryl-3-phosphorylcholine (lyso-PAF C-16), 1-O-hexadecyl (7,7,8,8-d4)-sn-glycerophosphorylcholine (lyso-PAF C-16-d4), and 1-O-octadecyl-sn-glyceryl-3-phosphorylcholine (lyso-PAF C-18) from Cayman Chemical. Mouse anti-M2 was purchased from Sigma, rabbit anti-calnexin antibody from Stressgen Bioreagents, mouse anti-β-actin antibody from Santa Cruz Biotechnology, Mito-Tracker Red CMXRos from Invitrogen, and TRITC- and FITC-conjugated goat anti-mouse or goat anti-rabbit IgGs from Jackson Immunoresearch Laboratories. TLC plates were purchased from Whatman.
Cloning of full-length LPCAT1 cDNA.
The coding region of mouse LPCAT1 cDNA was cloned by PCR amplification using a PCR primer pair [5′-TCTACTCCTTGCTCAGGCGCG-3′ (forward) and 5′-CCTCCGGAACCTTCCATTCTA-3′ (reverse)] and Marathon-Ready cDNA prepared from the mouse lung (Clontech) as template. PCR amplification was performed using Pfu DNA polymerase (Stratagene) and 35 rounds of thermal cycling (94°C for 30 s, 60°C for 30 s, and 72°C for 2 min), resulting in a 1.7-kb cDNA product that was cloned into the Srf I site of pPCR-Script Amp SK(+) vector (Stratagene, La Jolla, CA) and sequenced.
Expression of FLAG-LPCAT1 in mammalian cells.
A mammalian expression vector for full-length human LPCAT1 was engineered by subcloning the 1.7-kb cDNA fragment of the mouse LPCAT1 from the pPCR-Script Amp SK(+) vector described above into the HindIII and NotI sites of the pcDNA3.1(+)/Neo mammalian expression vector (Invitrogen). A FLAG-tagged version of LPCAT1 was engineered by PCR amplification with Pfu DNA polymerase using a primer pair [5′-GCCACCATGGATTACAAGGATGACGACGATAAGAGGCTGCGGGGCCGCGGGC-3′ (forward) and 5′-CCTCCGGAACCTTCCATTCTA-3′ (reverse)] designed to add a FLAG tag to the NH2 terminus of LPCAT1 and 35 rounds of thermal cycling (94°C for 30 s, 60°C for 30 s, and 72°C for 2.5 min). The amplified DNA fragment was cloned into the pPCR-Script Amp SK(+) vector (Stratagene) and verified by sequencing. The insert was subcloned into the HindIII and NotI sites of the pcDNA3.1(+)/Neo vector for transient expression in COS-7 cells. COS-7 cells were maintained under the conditions recommended by American Tissue Culture Collection (Manassas, VA). At 1 day before transfection, 2 × 106 cells were subcultured onto a 100 × 20-mm plate, resulting in 70% confluence. Cells were transfected with 10 μg of DNA premixed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. At 48 h after transfection, cells were harvested in ice-cold PBS, pelleted by centrifugation, lysed, and assayed immediately or frozen at −80°C for later use.
Tissue and cell homogenate preparation.
Frozen mouse tissues were homogenized in 10 volumes of 50 mM Tris·HCl (pH 7.5) containing 0.1% Triton X-100 on ice. Aliquots of the crude homogenate were stored at −80°C or immediately processed for Western blot analysis or enzymatic assay. For cultured cells, 48 h after transfection, COS-7 cells were rinsed in cold PBS, scraped in cold PBS, and centrifuged for 5 min at 1,500 rpm at 4°C. The pellet was resuspended in 500 μl of cold buffer (50 mM Tris·HCl, pH 7.5, and 0.1% Triton X-100) and homogenized on ice. Aliquots of the crude homogenate were stored at −80°C or immediately processed for Western blot analysis or enzymatic assay. The protein concentrations in homogenates were determined by a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instruction.
Assay of LPCAT activity.
LPCAT activity was determined by measurement of the incorporation of radiolabeled acyl moieties of [14C]oleoyl-CoAs (acyl donors) into lyso-PAF or LPC (acyl acceptors) using the recombinant LPCAT1 expressed in COS-7 cells or homogenate from different mouse tissues. The reaction mixture contained 50 mM Tris·HCl, pH 7.0, 200 μM lysophospholipids, 25 μM [14C]acyl-CoA (50 mCi/mmol; American Radiolabeled Chemicals), and cell or tissue lysates (100 μg) in a total volume of 200 μl. The reaction was incubated at room temperature for 10 min. The enzymatic reaction was stopped by addition of 600 μl of a cold mixture of 2:1 (vol/vol) chloroform-methanol, and radiolabeled lipid was extracted by the organic phase, dried by vacuum, and dissolved in 40 μl of 2:1 (vol/vol) chloroform-methanol. For radiochromatographic assays, the dissolved samples were spotted on TLC plates and developed in 80:20:2 chloroform-methanol-28% NH4OH. After separation, TLC plates were exposed to a PhosphorImager screen for visualization of radioactivity on the plates with a scanner (Typhoon 9400; Molecular Dynamics, Sunnyvale, CA). For quantification of radiolabeled phospholipids on TLC plates, the samples were scraped into scintillation vials, and scintillation counting was performed using a scintillation system (model LS 6500; Beckman, Fullerton, CA). The specific activity of the enzyme was calculated on the basis of nanomoles of lipid product formed per minute of incubation time per milligram of protein. All quantitative data are expressed as means ± SE. Statistical analyses for differences between two groups were carried out using a Student's t-test.
RT-PCR.
Total mRNA of tissue samples was extracted by TRIzol reagent (Invitrogen). cDNA was prepared using the SuperScript reverse transcriptase kit (Invitrogen) according to the manufacturer's instruction. PCR was performed with 30 rounds of thermal cycling (94°C for 30 min, 64°C for 30 min, and 72°C for 45 min) using Taq DNA polymerase and the following primers: mouse LPCAT1 (163 bp) 5′-GTGCACGAGCTGCGACT-3′ (forward) and 5′-GCTGCTCTGGCTCCTTATCA-3′ (reverse) and mouse β-actin (188 bp) 5′-AGAGGGAAATCGTGCGTGAC (forward) and 5′-CAAGAAGGAAGGCTGGAAAA-3′ (reverse). The RT-PCR products were quantified by ImageQuaNT software (Molecular Dynamics) with β-actin cDNA as an internal control. All quantitative data are expressed as means ± SE. Statistical analyses for differences between two groups were carried out using a Student's t-test.
Northern blot analysis.
Multiple tissue poly(A)+ RNA Northern blots (OriGene Technologies, Rockville, MD) were hybridized with [32P]dCTP-labeled probes prepared from full-length cDNA of the mouse LPCAT1 gene. Labeling, hybridization, and Northern blot analysis were carried out essentially as described elsewhere (3), with β-actin cDNA as an internal control.
Experimental animals.
All procedures were carried out following guidelines of the Pennsylvania State University College of Medicine Animal Care and Use Committee. Animals used for the present studies include 7-wk-old male Ins2Akita diabetic mice and the nondiabetic controls, 10-wk old male db/db diabetic mice and the nondiabetic controls, and db/db mice treated with rosiglitazone (Avandia, GSK Pharmaceuticals) at 0.375 mg/day for 4 consecutive weeks. Rosiglitazone maleate tablets were delivered daily in emulsion in vegetable oil (1 ml) by oral gavage. Control (non-rosiglitazone-treated) animals were given vegetable oil (1 ml) only. The animals were housed (n = 4 per cage) under 12:12-h light-dark cycles with ad libitum access to food and water. Animals were euthanized at the end of the studies according to protocols approved by the Pennsylvania State University College of Medicine Animal Care and Use Committee, and tissue samples were processed as described above.
RESULTS
Analysis of LPCAT1 mRNA expression.
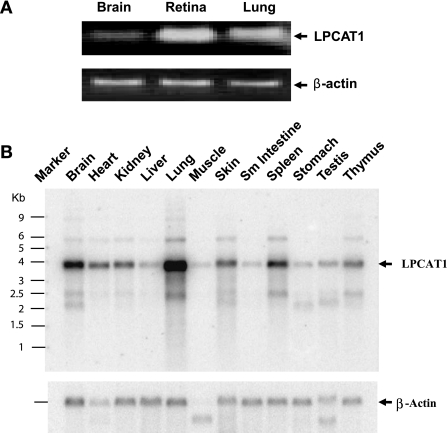
Northern blot and quantitative RT-PCR analysis were carried out to analyze the mRNA expression level in various mouse tissues. As shown in Fig. 1B, LPCAT1 mRNA was detected in all the tissues examined, with high expression levels in lung, followed by brain, spleen, and kidney. In support of a role of LPCAT1 in regulating retina function, RT-PCR analysis showed a higher LPCAT1 mRNA expression level in mouse retina (Fig. 1A) than lung.
Fig. 1.
RT-PCR and Northern blot analysis of lysophosphatidylcholine (LPC) acyltransferase (LPCAT1) mRNA expression in different mouse tissues. A: RT-PCR analysis using primer pairs designed from the coding sequence of lysophosphatidylcholine acyltransferase (LPCAT1) and β-actin genes. B: Northern blot analysis. For each tissue, 2 μg of poly(A)+ RNA for each tissue were hybridized with radiolabeled probes prepared from full-length cDNA of the mouse LPCAT1 gene or β-actin cDNA (internal control). Difference in the size of the β-actin transcript in skeletal muscle and testis is caused by expression of different isoforms of the gene in these tissues.
Catalytic properties of the recombinant LPCAT1 involved in PAF inactivation.
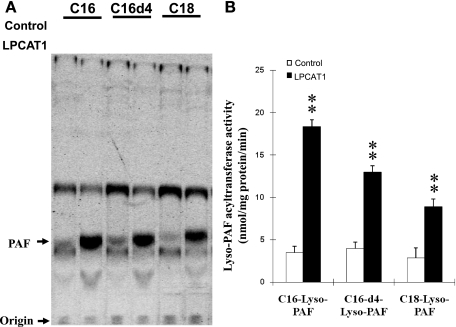
LPCAT1 has recently been identified as an enzyme that catalyzes the acylation of LPC to PC, a key step in PC remodeling. Intriguingly, the recombinant LPCAT1 has also been demonstrated to possess lyso-PAF-AT activity, suggesting a role of LPCAT1 in the synthesis of the inflammatory lipid PAF (24). However, neither its expression nor its enzyme activity was stimulated by inflammatory stimuli (14). To identify underlying molecular mechanisms, we analyzed acyl-CoA-dependent acyltransferase activity of the recombinant LPCAT1 toward various lyso-PAFs, a key step involved in PAF inactivation. As shown in Fig. 2A and quantified in Fig. 2B, the recombinant LPCAT1 expressed in COS-7 cells efficiently catalyzed the acylation of lyso-PAF to alkyl-PC, with oleoyl-CoA and various lyso-PAFs used as substrates. In addition to C-16 lyso-PAF and C-18 lyso-PAF, the recombinant LPCAT1 also demonstrated strong acyltransferase activity toward lyso-PAF C-16-d4, a lyso-PAF analog that contains four deuterium atoms at the 7, 7′, 8, and 8′ positions of the hexadecyl moiety. The results suggest that LPCAT1 has a regulatory role in the anti-inflammatory response by because of its role in catalysis of alkyl-PC synthesis from lyso-PAF, a key step in PAF inactivation.
Fig. 2.
Analysis of lyso-platelet-activating factor (PAF) acyltransferase (PAF-AT) activity of recombinant LPCAT1 expressed in COS-7 cells. Lyso-PAF-AT activity was analyzed at room temperature for 10 min using 100 μg of cell homogenate from COS-7 cells expressing LPCAT1 or empty vector in a 100-μl reaction mixture containing 50 mM Tris·HCl, pH 7.5, 0.1% Triton X-100, 20 mM EDTA, 20 μM [14C]oleoyl-CoA, and 200 μM lyso-PAF as acyl acceptors. Lyso-PAFs include 1-O-hexadecyl-sn-glyceryl-3-phosphorylcholine (lyso-PAF C-16), 1-O-hexadecyl-(7,7,8,8-d4)-sn-glyceryl-3-phosphorylcholine (lyso-PAF C-16-d4), and 1-O-octadecyl-sn-glyceryl-3-phosphorylcholine (lyso-PAF C-18). Radiolabeled lipids were extracted and separated by TLC using solvent containing 40:10:1 chloroform-methanol-NH4OH and detected by PhosphorImager analysis, and the radiolabeled alky-PC was quantified. A: image from a representative TLC plate. B: quantified data. Values are means ± SE from 3 independent experiments. **P < 0.01 vs. vector controls.
LPCAT1 mRNA expression and enzyme activity were significantly downregulated in the retina and brain in response to onset of type 1 and type 2 diabetes.
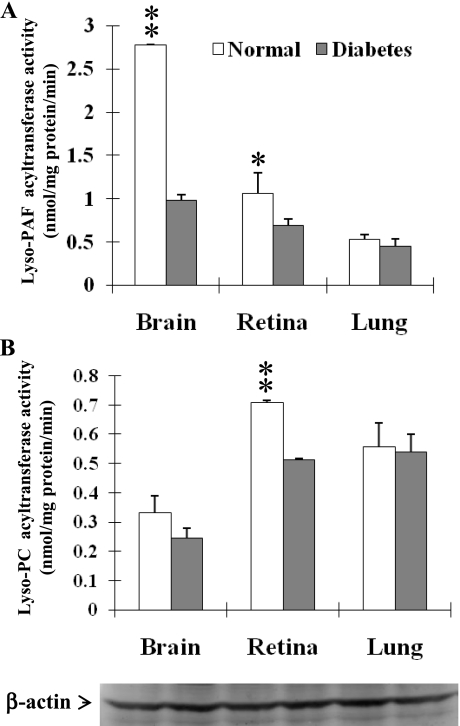
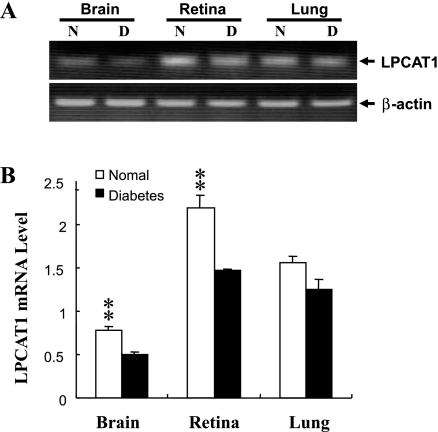
Elevated levels of PAF and LPC are associated with onset of diabetic retinopathy, but the underlying mechanisms remain elusive. We hypothesize that decreased lyso-PAF and LPCAT activities might be responsible for elevated levels of PAF and LPC in diabetic retinopathy. To test this hypothesis, we analyzed lyso-PAF and LPCAT activity from the retina, brain, and lung, the predominant sites for LPCAT1 expression in Ins2Akita mice, a mouse model of type 1 diabetes. As shown in Fig. 3, the total lyso-PAF and LPCAT activities in the retina were significantly downregulated by the onset of type 1 diabetes in Ins2Akita mice compared with the nondiabetic control mice. Similarly, the lyso-PAF-AT activities were also significantly downregulated in the brain by the onset of type 1 diabetes (Fig. 3), consistent with the concept that the retina is part of the central nervous system. In contrast, acyltransferase activities toward lyso-PAF or LPC in the lung were not significantly affected by the onset of type 1 diabetes, further implicating a role for LPCAT enzymes in diabetic retinopathy. In support of a role of LPCAT1 in the decreased acyltransferase activities associated with type 1 diabetes, LPCAT1 mRNA expression was significantly downregulated in the brain and retina, but not lung, of Ins2Akita diabetic mice (Fig. 4). Although the brain exhibited the highest LPCAT1 enzyme activity, the expression level of LPCAT1 was much lower than in the retina, consistent with multiple LPCAT isoforms in the brain. The results suggest a regulatory role of LPCAT1 in the elevated levels of PAF and LPC associated with diabetic retinopathy.
Fig. 3.
Lyso-PAF and LPCAT activities were downregulated in brain and retina at the onset of type 1 diabetes. Protein lysates were prepared from brain, retina, and lung of 7-wk-old Ins2Akita diabetic mice and nondiabetic controls. Lysates were used to analyze acyltransferase activity using oleoyl-CoA (acyl donor) and lyso-PAF C-16 (A) or LPC (B). Acetyltransferase assays were carried out as described in Fig. 2. Amount of protein lysate used in the experiments was independently verified by Western blot analysis using anti-β-actin antibodies (bottom). Values are means ± SE from 5 individual mice. *P < 0.05; **P < 0.01 vs. normal.
Fig. 4.
Effect of type 1 diabetes on LPCAT1 mRNA expression. LPCAT1 mRNA expression in brain, retina, and lung of 7-wk-old Ins2Akita diabetic mice (D) and nondiabetic controls (N) was analyzed by RT-PCR and normalized to levels of β-actin, which was used as an internal control. A: results from a representative RT-PCR analysis. B: quantified results. Values are means ± SE from 5 individual mice. **P < 0.01 vs. normal.
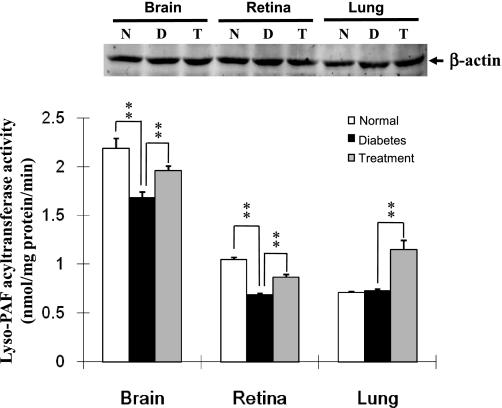
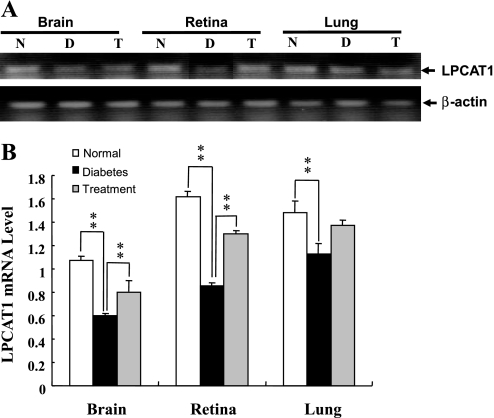
To further decipher a role of PAF remodeling in type 2 diabetes, we next investigated lyso-PAF-AT activity and LPCAT1 mRNA expression in the retina, lung, and brain of db/db diabetic mice, wild-type control mice, and db/db diabetic mice treated with an antidiabetes drug, rosiglitazone, for 4 consecutive weeks. The db/db mice develop diabetes at 4 wk of age as a result of obesity caused by genetic mutation of the leptin receptor gene. Compared with the wild-type control mice, the db/db mice demonstrated a significant reduction in LPCAT enzyme activity (Fig. 5) and LPCAT1 mRNA expression (Fig. 6) in the retina and brain, but not lung. Furthermore, such defects were partially corrected by treatment with rosiglitazone (Figs. 5 and 6). These data are consistent with previous reports that elevated levels of LPC and PAF are associated with the onset of diabetic retinopathy (7, 15, 17).
Fig. 5.
Downregulation of lyso-PAF-AT and LPCAT activities by type 2 diabetes and reversal by antidiabetes drug treatment. Protein lysates were prepared from brain, retina, and lung of 10-wk-old db/db diabetic mice (D), nondiabetic controls (N), and db/db mice treated with rosiglitazone (0.375 mg/day) for 4 consecutive weeks (T). Lyso-PAF-AT enzyme assays were carried out as described in Fig 2. Amount of protein lysate used in the experiments was independently verified by Western blot analysis using anti-β-actin antibodies (top). Values are means ± SE from 5 individual mice. **P < 0.01 vs. diabetes.
Fig. 6.
Effect of type 2 diabetes and antidiabetes drug treatment on LPCAT1 mRNA expression. LPCAT1 mRNA expression in brain, retina, and lung of 10-wk-old db/db diabetic mice, nondiabetic controls, and db/db mice treated with rosiglitazone (0.375 mg/day) for 4 consecutive weeks was analyzed by RT-PCR. Results were normalized to levels of β-actin, which was used as an internal control. A: results from a representative RT-PCR analysis. B: quantified data. Values are means ± SE from 5 individual mice. **P < 0.01 vs. diabetes.
DISCUSSION
LPC and PAF are potent inflammatory phospholipids implicated in diabetic complications. Plasma LPC concentrations were significantly elevated in insulin-dependent and non-insulin-dependent diabetes mellitus patients (25, 30), diabetic retinopathy (15), and experimental obesity (12). Elevated LPC levels cause postprandial hyperglycemia by inhibiting glucose uptake by liver, heart, and muscle tissues (20). LPC in LDL is an important atherogenic substance that contributes to diabetic complications (30). LPC concentration in LDL is significantly higher in patients with preproliferative or proliferative retinopathy and those with nephropathy than in controls (17). Treatment of diabetic mice with an antioxidant significantly decreased the level of LPC in LDL (34). LPC has been shown to activate several signal transduction pathways implicated in insulin resistance, such as JNK and protein kinase Cα (11, 23). PAF is an ether lipid of choline, and its presence has been demonstrated in a variety of eye tissues, including the retina, ciliary body, and iris (1, 8, 27). PAF and its metabolite lyso-PAF are potent inflammatory lipids; their levels are elevated in serum of type 1 diabetic patients (4, 10), as well as in oxygen-induced retinopathy (2). Despite their important roles in regulating the onset of diabetic complications, the molecular mechanisms underlying the elevated levels of LPC and PAF remain elusive.
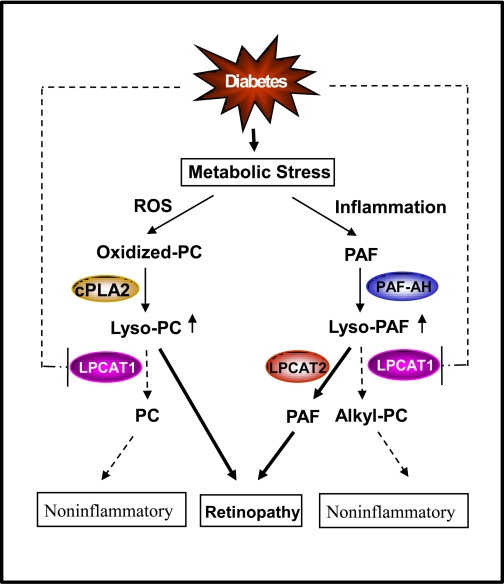
PAF can be generated by two biosynthetic pathways, the de novo pathway and the remodeling pathway. The remodeling pathway begins with hydrolysis of alkyl-PC at the sn-2 position by phospholipase A2, generating lyso-PAF, and is followed by acetylation by lyso-PAF-AT in response to inflammatory stimuli. Lyso-PAF can also be used to synthesize alkyl-PC, an inactive PAF analog, catalyzed by lyso-PAF-ATs under noninflammatory conditions (Fig. 7). It is generally believed that the remodeling pathway is mainly responsible for pathological conditions that generate PAF. Major progress has recently been achieved in identification and characterization of acyltransferase genes that catalyze acylation of LPC and lyso-PAF (6, 14, 16, 24, 31–33, 35). Four isoforms that encode LPCAT (LPCAT1–4) have been identified, and they differ significantly in tissue expression pattern and catalytic properties. Among the four isoforms of LPCAT, LPCAT1 and LPCAT2 have also been shown to catalyze the synthesis of PAF from lyso-PAF, in addition to acyltransferase activity toward LPC. Intriguingly, LPCAT2, but not LPCAT1, has been shown to play a role in the inflammatory response by catalyzing the synthesis of PAF from lyso-PAF (14, 31). The molecular mechanism underlying such a difference remains elusive.
Fig. 7.
A projected role of LPCAT1 in remodeling of phosphatidylcholine (PC) and PAF during the onset of diabetes. Diabetes is associated with increased levels of reactive oxygen species (ROS) and inflammation, which stimulate remodeling of PC and PAF. During remodeling, oxidized PC is hydrolyzed by cytoplasmic PLA2 (cPLA2), whereas PAF is hydrolyzed by PAF acetylhydrolase (PAF-AH), producing lyso-PC and lyso-PAF. Lyso-PC and lyso-PAF can be converted to PC and alkyl-PC, a noninflammatory PAF analog, which is catalyzed by LPCAT1. LPCAT1 expression and enzyme activity are significantly inhibited by the onset of diabetes, as highlighted by dashed lines, resulting in increased levels of lyso-PC and PAF, which contribute to the onset of diabetic retinopathy.
In this study, we analyzed a regulatory role of LPCAT1 in the anti-inflammatory response during the onset of diabetic retinopathy. Our data show that the recombinant LPCAT1 enzyme efficiently catalyzed the synthesis of alkyl-PC, an inactive analog of PAF, in addition to the previously identified acyltransferase activity toward LPC. This is a key step in the inactivation of two inflammatory mediators, LPC and PAF. In support of an anti-inflammatory role of LPCAT1, LPCAT1 mRNA expression and enzyme activity in the retina were significantly downregulated during the onset of type 1 and type 2 diabetes in rodents. Such defects were also identified in the brain, but not lung, where LPCAT1 was abundantly expressed, which is consistent with the concept that the retina is an integral part of the brain. The data suggest that decreased levels of LPCAT1 mRNA expression and activity contribute to the increased levels of PAF and LPC in diabetes and diabetic retinopathy (15). Furthermore, such a defect was partially reversed in type 2 diabetic mice treated with rosiglitazone, an antidiabetes drug that improves insulin sensitivity. However, it remains to be tested whether the effect of rosiglitazone on LPCAT1 expression is directly mediated by peroxisome proliferator-activated receptor-γ activation of the LPCAT1 gene or as a consequence of normoglycemia associated with the drug treatment. In further support of a protective role of LPCAT1 in diabetic retinopathy, LPCAT1 mRNA is the most abundantly expressed in the retina among all the tissues examined. These results are corroborated by a recent report that LPCAT1 plays a role in the anti-inflammatory response. In contrast to LPCAT2, neither LPCAT1 mRNA levels nor its lyso-PAF-AT activity was induced by inflammatory stimuli (14). Collectively, our data support an anti-inflammatory role of LPCAT1 in diabetes (Fig. 7). Accordingly, diabetes generates oxidative stress and inflammation, leading to increased remodeling of PC and PAF. Diabetes also suppresses mRNA expression and enzymatic activity of LPCAT1, resulting in increased levels of LPC and PAF, which contribute to the onset of diabetic retinopathy. However, it should be considered that the downregulation of LPCAT1 expression at the onset of diabetes in the retina and brain may not be the direct cause but, rather, the consequence of diabetes-related inflammation. Consequently, the role of LPCAT1 in regulating the onset of diabetic retinopathy remains to be elucidated.
GRANTS
This research project was partly sponsored by American Diabetes Association Grant 7-07-RA-148 (to Y. Shi) and National Institute of Diabetes and Digestive Diseases Grant 1R01 DK-076685.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Tom Gardner, Mark Kester, and John Flanagan for critically reading the manuscript.
REFERENCES
- 1.Bazan HE, Hurst JS, Bazan NG. Differences in the acyl composition of the platelet-activating factor (PAF) precursor and other choline phosphoglycerides of the rabbit retinal rod outer segments and neural retina. Curr Eye Res 13: 45–50, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp MH, Marrache AM, Hou X, Gobeil F, Jr, Bernier SG, Lachapelle P, Abran D, Quiniou C, Brault S, Peri KG, Roberts J, 2nd, Almazan G, Varma DR, Chemtob S. Platelet-activating factor in vasoobliteration of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 43: 3327–3337, 2002 [PubMed] [Google Scholar]
- 3.Cao J, Hawkins E, Brozinick J, Liu X, Zhang H, Burn P, Shi Y. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem 279: 18878–18886, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Cavallo-Perin P, Lupia E, Gruden G, Olivetti C, De Martino A, Cassader M, Furlani D, Servillo L, Quagliuolo L, Iorio E, Boccellino MR, Montrucchio G, Camussi G. Increased blood levels of platelet-activating factor in insulin-dependent diabetic patients with microalbuminuria. Nephrol Dial Transplant 15: 994–999, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekher G, Ma X, Lallier TE, Bazan HE. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet-activating factor. Invest Ophthalmol Vis Sci 43: 1422–1428, 2002 [PubMed] [Google Scholar]
- 6.Chen X, Hyatt BA, Mucenski ML, Mason RJ, Shannon JM. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci USA 103: 11724–11729, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Cruz JP, Moreno A, Ruiz-Ruiz MI, García-Campos J, Sánchez de la Cuesta F. Effect of WEB 2086-BS, an antagonist of platelet-activating factor receptors, on retinal vascularity in diabetic rats. Eur J Pharmacol 360: 37–42, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Dharma S, Bazan HE, Peyman GA, Atef MS. Production of platelet-activating factor in photocoagulated retinas. Curr Eye Res 10: 1031–1035, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Doly M, Cluzel J, Bonhomme B, Millerin M, Braquet P. Protective effect of a specific PAF antagonist on vincristine-induced experimental retinopathy. Acta Ophthalmol Scand 73: 155–157, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Ersoy B, Huseyinov A, Darcan S. The role of platelet-activating factor in pathogenesis of type 1 diabetes. Diabetes Care 28: 980, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Gibson S, Flowers M, Furui T, Bast RJ, Mills G. Lysophosphatidylcholine stimulates activator protein 1 and the c-Jun N-terminal kinase activity. J Biol Chem 272: 13683–13689, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Galili O, Versari D, Sattler K, Olson M, Mannheim D, McConnell J, Chade A, Lerman L, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol 292: H904–H911, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant Trolox. Res Commun Mol Pathol Pharmacol 93: 3–12, 1996 [PubMed] [Google Scholar]
- 14.Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem 283: 11097–11106, 2008 [DOI] [PubMed] [Google Scholar]
- 15.He J, Qiu Y, Yan Y, Niu Y. [Relationship between changes of phospholipid and lipid peroxide of erythrocyte membrane and diabetic retinopathy]. Zhonghua Yan Ke Za Zhi 34: 202–204, 1998 [PubMed] [Google Scholar]
- 16.Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc Natl Acad Sci USA 105: 2830–2835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwase M, Sonoki K, Sasaki N, Ohdo S, Higuchi S, Hattori H, Iida M. Lysophosphatidylcholine contents in plasma LDL in patients with type 2 diabetes mellitus: relation with lipoprotein-associated phospholipase A2 and effects of simvastatin treatment. Atherosclerosis 196: 931–936, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kudolo GB, Dorsey S, Blodgett J. Effect of the ingestion of Ginkgo biloba extract on platelet aggregation and urinary prostanoid excretion in healthy and type 2 diabetic subjects. Thromb Res 108: 151–160, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kujiraoka T, Iwasaki T, Ishihara M, Ito M, Nagano M, Kawaguchi A, Takahashi S, Ishi J, Tsuji M, Egashira T, Stepanova IP, Miller NE, Hattori H. Altered distribution of plasma PAF-AH between HDLs and other lipoproteins in hyperlipidemia and diabetes mellitus. J Lipid Res 44: 2006–2014, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Labonte E, Kirby R, Schildmeyer N, Cannon A, Huggins K, Hui D. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes 55: 935–941, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee ES, Jiang J, Sund GC, Simonson WT, Graham J, Dietsch G, Schimpf B, Bieg S, Peterman G, Lernmark A. Recombinant human platelet-activating factor acetylhydrolase reduces the frequency of diabetes in the diabetes-prone BB rat. Diabetes 48: 43–49, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem 14: 3209–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Motley E, Kabir S, Gardner C, Eguchi K, Frank G, Kuroki T, Ohba M, Yamakawa T, Eguchi S. Lysophosphatidylcholine inhibits insulin-induced Akt activation through protein kinase C-α in vascular smooth muscle cells. Hypertension 39: 508–512, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi H, Shindou H, Hishikawa D, Harayama T, Ogasawara R, Suwabe A, Taguchi R, Shimizu T. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J Biol Chem 281: 20140–20147, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Rabini R, Galassi R, Fumelli P, Dousset N, Solera M, Valdiguie P, Curatola G, Ferretti G, Taus M, Mazzanti L. Reduced Na+-K+-ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes 43: 915–919, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum JT, Angell E, Wilson D, Broquet C, Boney RS, Braquet P. Intravitreally injected platelet activating factor induces retinitis in experimental animals. Curr Eye Res 18: 342–348, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum JT, Boney RS, Samples JR, Valone FH. Synthesis of platelet activating factor by ocular tissue from inflamed eyes. Arch Ophthalmol 109: 410–413, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Quesada JL, Benítez S, Pérez A, Wagner AM, Rigla M, Carreras G, Vila L, Camacho M, Arcelus R, Ordóñez-Llanos J. The inflammatory properties of electronegative low-density lipoprotein from type 1 diabetic patients are related to increased platelet-activating factor acetylhydrolase activity. Diabetologia 48: 2162–2169, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Serban M, Tanaseanu C, Kosaka T, Vidulescu C, Stoian I, Marta DS, Tanaseanu S, Moldoveanu E. Significance of platelet-activating factor acetylhydrolase in patients with non-insulin-dependent (type 2) diabetes mellitus. J Cell Mol Med 6: 643–647, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi AH, Yoshinari M, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm Metab Res 31: 283–286, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem 282: 6532–6539, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem 284: 1–5, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Soupene E, Fyrst H, Kuypers FA. Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proc Natl Acad Sci USA 105: 88–93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshinari M, Shi AH, Yoshizumi H, Wakisaka M, Iwase M, Fujishima M. Probucol reduces lysophosphatidylcholines in low-density lipoprotein. Eur J Clin Pharmacol 55: 787–792, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Chen YQ, Bonacci TM, Bredt DS, Li S, Bensch WR, Moller DE, Kowala M, Konrad RJ, Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J Biol Chem 283: 8258–8265, 2008 [DOI] [PubMed] [Google Scholar]