Abstract
The brain controls energy homeostasis and body weight by integrating various metabolic signals. Leptin, an adipose-derived hormone, conveys critical information about peripheral energy storage and availability to the brain. Leptin decreases body weight by both suppressing appetite and promoting energy expenditure. Leptin directly targets hypothalamic neurons, including AgRP and POMC neurons. These leptin-responsive neurons widely connect to other neurons in the brain, forming a sophisticated neurocircuitry that controls energy intake and expenditure. The anorexigenic actions of leptin are mediated by LEPRb, the long form of the leptin receptor, in the hypothalamus. LEPRb activates both JAK2-dependent and -independent pathways, including the STAT3, PI 3-kinase, MAPK, AMPK, and mTOR pathways. These pathways act coordinately to form a network that fully mediates leptin response. LEPRb signaling is regulated by both positive (e.g., SH2B1) and negative (e.g., SOCS3 and PTP1B) regulators and by endoplasmic reticulum stress. Leptin resistance, a primary risk factor for obesity, likely results from impairment in leptin transport, LEPRb signaling, and/or the neurocircuitry of energy balance.
the prevalence of obesity continues to increase rapidly around the globe. Body weight is normally maintained within a narrow range by a balance between energy intake (food intake) and energy expenditure. When energy intake exceeds energy expenditure, excess energy is stored as triglyceride in adipose tissue, resulting in overweight or obesity. A sophisticated neuroendocrine system has evolved in mammals to control energy balance by constantly monitoring energy storage, availability, and consumption. Adipose tissue and the brain are two key components of this neuroendocrine system.
Leptin Controls Energy Balance and Body Weight by Regulating Neuronal Activity in the Hypothalamus
In addition to storing triglyceride, adipose tissue secretes a variety of signaling molecules, including lipids and numerous polypeptides, that regulate systemic glucose and lipid metabolism. Leptin is the primary adipose hormone that conveys an adiposity signal to the brain (Fig. 1). The brain, particularly the hypothalamus, integrates leptin and various other metabolic signals to regulate energy homeostasis and body weight by controlling both behavior and metabolic responses. Leptin decreases body weight both by suppressing appetite and by increasing energy expenditure (25, 76, 158, 185). Leptin deficiency results in morbid obesity in both animals and humans (138, 186, 224). Similarly, genetic deficiency of functional leptin receptors (LEPR) also results in obesity and obesity-associated metabolic diseases (35, 192, 224). In addition to controlling energy balance and body weight, leptin also plays an important role in the regulation of immune responses, bone homeostasis, reproduction, and mood and emotion (22, 96, 112, 123).
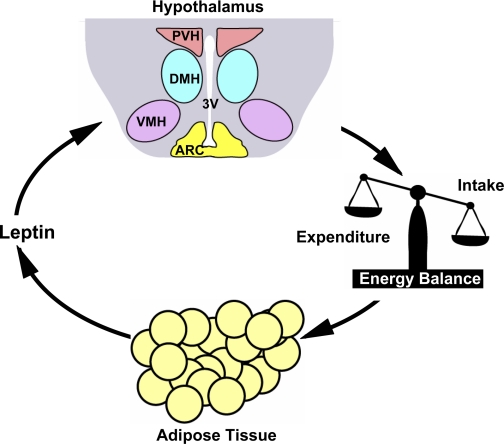
Fig. 1.
A model of leptin regulation of energy balance and body weight. Leptin is secreted by adipose tissue in proportion to adipose mass and relays information about peripheral energy storage and availability to the brain. Leptin regulates neuronal activity in multiple regions of the hypothalamus, including the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and paraventricular hypothalamus (PVH). Leptin suppresses appetite (energy intake) and promotes energy expenditure primarily by regulating these neuronal activities. Leptin resistance causes an imbalance between energy intake and expenditure, resulting in obesity. 3V, 3rd ventricle.
Leptin-targeted neurons.
LEPR mRNA is highly expressed in the arcuate nucleus (ARC), the ventromedial hypothalamus (VMH), the dorsomedial hypothalamus (DMH), the lateral hypothalamic area (LHA), and the ventral premammillary nucleus (PMV) (31, 46, 74, 116, 117, 133, 177–179). Central administration of recombinant leptin reduces food intake and body weight (25, 75, 185). Neuron-specific deletion of LEPR results in energy imbalance and obesity (36); conversely, neuron-specific restoration of functional LEPR rescues the obese phenotypes in LEPR-null (db/db) mice (41). Moreover, restoration of leptin signaling specifically in the ARC corrects hyperphagia and obesity in LEPR-null rats, suggesting that the ARC is a key leptin target (177). In particular, two subpopulations of ARC neurons [proopriomelanocortin (POMC) neurons and agouti-related protein (AgRP) neurons] have emerged as critical mediators for leptin action.
POMC neurons are anorexigenic neurons that coexpress anorexigenic POMC and cocaine- and amphetamine-regulated transcript (CART) (49, 177). Leptin stimulates both POMC neuronal excitability and expression of POMC and CART (39, 49, 108, 137, 178). POMC is proteolytically cleaved to generate α-melanocyte-stimulating hormone (α-MSH), which activates melanocortin-3 and melanocortin-4 receptors (MC3R and MC4R) (28, 55, 63, 89, 170). MC3R and MC4R are G protein-coupled receptors that are highly expressed in the hypothalamus, especially in the paraventricular hypothalamus (PVH) (145). Deletion of either MC3R or MC4R results in leptin resistance and obesity in mice (28, 89). Deletion of both MC3R and MC4R causes more severe obesity than deletion of either MC3R or MC4R alone, suggesting that MC3R and MC4R act together to mediate POMC's anorexigenic effect (28). Genetic defects in either the POMC or the MC4R genes are linked to obesity in humans, suggesting that the melanocortin system is an evolutionally conserved leptin target in mammals (35, 109).
AgRP neurons are orexigenic neurons that coexpress orexigenic AgRP and neuropeptide Y (NPY) (73, 177). NPY potently stimulates feeding behaviors (184). AgRP is a potent antagonist of both MC3R and MC4R and opposes the anorexigenic action of POMC (154). AgRP neurons also innervate POMC neurons and inhibit POMC neuronal activity by releasing inhibitory γ-aminobutyric acid (GABA) (39). Genetic ablation of AgRP neurons in adult mice leads to starvation, indicating that these neurons are required for feeding (71, 125). Leptin inhibits not only AgRP/NPY expression but also AgRP neuronal excitability (177, 185, 188). Additionally, leptin inhibits GABA release from AgRP neurons, thus reducing GABAergic inhibition of POMC neurons (39). Blocking presynaptic release of GABA from AgRP neurons increases POMC neuronal excitability, which contributes to increased energy expenditure and decreased adiposity in mice with AgRP neuron-specific deletion of vesicular GABA transporter (195).
Surprisingly, selective deletion of functional LEPR in either AgRP or POMC neurons results in mild obesity (3, 200). Deletion of LEPR in both AgRP and POMC neurons has an additive effect on energy balance and body weight; however, the metabolic phenotypes in these mutant mice are still milder than in systemic LEPR-deficient db/db mice (200). These observations suggest that leptin controls energy balance and body weight by regulating both arcuate and extra-arcuate neurons. Consistent with this idea, LEPR mRNA is expressed in multiple areas in the brain, including ARC, VMH, DMH, the lateral hypothalamic area (LHA), ventral tegmental area (VTA), the ventral premammillary nucleus, hippocampus, and the brainstem (46, 50, 62, 83, 117, 132). Deletion of LEPR in the VMH results in mild obesity (13, 44). Activation of LHA LEPRb-neurons suppresses feeding and weight gain (116). Leptin also stimulates LEPR-expressing neurons in the VTA, thereby inhibiting motivated food-seeking behaviors (62, 83). Therefore, these distinct leptin-responsive neurons may act redundantly, in parallel, and/or synergistically to fully mediate the physiological responses to leptin.
Leptin-targeted neural circuitry.
The neural circuitry that integrates leptin and other metabolic signals to control energy homeostasis and body weight remains largely undefined. Various components of this circuitry have been described. Both AgRP and POMC neurons (first-order neurons) extensively innervate second-order neurons in the PVH (Fig. 1) (60). The PVH mediates leptin stimulation of the sympathetic nervous system, contributing to the ability of leptin to promote energy expenditure (180). The PVH also contains neuroendocrine cells that secrete numerous anorexigenic factors, including thyrotropin-releasing hormone (TRH) and corticotropin-releasing hormone (CRH), and leptin stimulates both TRH and CRH secretion (77, 87, 151). α-MSH stimulates TRH biosynthesis and secretion, whereas NPY and AgRP suppress TRH secretion (77, 103, 151). TRH stimulates the pituitary-thyroid axis, thus increasing metabolic rate and energy expenditure. Both AgRP and POMC neurons synapse on VMH neurons that express brain-derived neurotrophic factor (BDNF) (215). Leptin stimulates the expression of anorexigenic BDNF in the VMH, presumably by stimulating MC4R signaling (107, 215). Deletion of BDNF in the VMH/DMH of adult mice results in hyperphagia and obesity (197). Additionally, TrkB (BDNF receptor) deficiency results in hyperphagia and obesity in both mice and humans (215, 217). Moreover, AgRP neurons innervate neurons in the parabrachial nucleus (PBN), a relay center for taste and gastric distension signals from the nucleus tractus solitaries (NTS) to the forebrain (211, 212). AgRP neurons inhibit PBN neurons by releasing GABA, and this inhibition is required for feeding behaviors (211, 212). These findings indicate that the neural circuits that control energy intake and expenditure are extremely complex, and leptin acts on multiple nodes in these circuits.
Leptin-targeted peripheral tissues.
In addition to the brain, leptin also acts directly on multiple peripheral tissues, including pancreatic islets, adipose tissue, skeletal muscle, and liver. LEPRb mRNA is expressed in islets, and leptin directly inhibits insulin expression and secretion (52, 111). Deletion of LEPR in the pancreas enhances first-phase insulin secretion and modestly improves glucose tolerance in mice (142). Leptin also directly stimulates fatty acid oxidation in isolated islets, thereby decreasing islet lipid levels (182). In isolated adipocytes, leptin inhibits lipogenesis but stimulates lipolysis and fatty acid oxidation (204). Adipocyte-specific overexpression of LEPRb prevents diet-induced obesity in mice (205). Leptin directly promotes fatty acid oxidation in isolated skeletal muscles, presumably by activating AMPK (136, 148). Leptin decreases lipid levels in isolated livers, and liver-specific overexpression of LEPRb prevents hepatic steatosis in LEPRb-deficient Zucker diabetic fatty (fa/fa) rats (88, 115). Surprisingly, genetic deletion of LEPR in these peripheral tissues does not alter energy balance, body weight, or glucose homeostasis in mice (72). Therefore, under normal conditions, leptin controls energy balance, body weight, and systemic metabolism primarily by activating LEPRb in the brain.
Leptin Stimulates Multiple Signal Transduction Pathways
The LEPR gene produces multiple leptin receptor isoforms (a, b, c, d, e, and f) via alternative mRNA splicing (18, 51, 59, 133, 206). All isoforms have an extracellular leptin-binding domain, but only the longest form, LEPRb, contains a full-length intracellular domain required for cell signaling (61). Genetic deficiency of LEPRb results in profound leptin resistance and morbid obesity in animals, indicating that LEPRb is required for leptin action (7, 29, 33, 68, 113). LEPRb belongs to the gp130 family of cytokine receptors (7, 29, 68). It constitutively binds to JAK2, a member of the Janus kinase (JAK) family of tyrosine kinases (Fig. 2). Leptin stimulates LEPRb dimerization, resulting in JAK2 activation and autophosphorylation (4, 42, 54, 209). JAK2 also phosphorylates LEPRb and various downstream signaling molecules on tyrosines (16, 67, 99). JAK2 phosphorylates Tyr985, Tyr1077, and Tyr1138 in the cytoplasmic domain of LEPRb, which then act as docking sites for downstream signaling molecules (4, 79, 118). The metabolic phenotypes of mice with deletion of key leptin-signaling molecules are listed in Table 1. Replacement of these three tyrosines in LEPRb with phenylalanines induces marked leptin resistance and obesity in mutant mice, indicating that phospho-Tyr985, -Tyr1077, and/or -Tyr1138 mediate the activation of key downstream pathways. However, the mutant mice are less obese and less hyperglycemic than LEPRb-deficient db/db mice, indicating that LEPRb can mediate some of the leptin signaling and action independently of phosphorylation on these tyrosines (93).
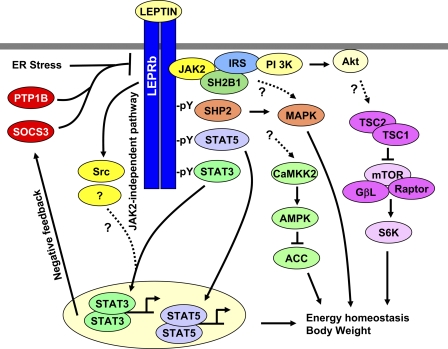
Fig. 2.
A model of leptin signaling and leptin resistance. Leptin binds to the long form of the leptin receptor (LEPRb) and activates LEPRb-associated JAK2. JAK2 phosphorylates LEPRb on Tyr985/1077/1138. SH2-containing protein tyrosine phosphatase 2 (SHP2) binds to phospho-Tyr985 and mediates the activation of the MAPK pathway. Suppressor of cytokine signaling-3 (SOCS3) also binds to phospho-Tyr985 and inhibits leptin signaling in a negative feedback manner. STAT5 and STAT3 bind to phospho-Tyr1077 and phospho-Tyr1138, respectively, and are subsequently phosphorylated and activated by JAK2. STAT3 and STAT5 activate their target genes, which mediate leptin's anorexigenic effect. JAK2 autophosphorylates on Tyr813, which binds to SH2B1. SH2B1 simultaneously binds to insulin receptor substrate (IRS)-1 and IRS-2 and recruits IRS proteins to the LEPRb/JAK2 complex, which results in JAK2-mediated tyrosine phosphorylation of IRS-1 and IRS-2 and subsequent activation of the phosphoinositide 3-kinase (PI3K) pathway. Leptin also stimulates a JAK2-independent pathway involving the Src tyrosine kinase family members. The JAK2-dependent and -independent pathways act coordinately and synergistically to promote STAT3 activation. Leptin also regulates the CaMKK2/AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) and the mammalian target of rapamycin (mTOR)/ribosomal S6 kinase (S6K) pathways; however, the molecular steps from the LEPRb to these 2 pathways are not clear. These diverse pathways act coordinately as a network to fully mediate leptin responses. LEPRb signaling is negatively regulated by SOCS3, protein tyrosine phosphatase 1B (PTP1B), and endoplasmic reticulum (ER) stress but positively regulated by SH2B1.
Table 1.
Metabolic phenotypes in mice with tissue-specific deletion of leptin signaling molecules
| Protein | Pathway(s) | Targeted Cells | Cre Used | Leptin Sensitivity | Food Intake | Energy Expenditure | Susceptibility to DIO | Ref No. |
|---|---|---|---|---|---|---|---|---|
| LEPR | All | Whole body | ↓ | ↑↑↑ | ↓↓↓ | ND | 192 | |
| Brain | Synapsin I | ↓ | ND | ND | ND | 36 | ||
| AgRP neurons | AgRP | ND | ↔ | ↓ | ND | 200 | ||
| POMC neurons | POMC | ND | ↔ | ↔ | ND | 3, 200 | ||
| AgRP and POMC neurons | AgRP and POMC | ↓ | ↔ | ↓ | ND | 200 | ||
| VMH neurons | SF-1 | ND | ↔ | ↔ | ↑ | 13 | ||
| SH2B1 | JAK2/STAT3 | Whole body | ↓ | ↑ | ↑ | ND | 120, 166 | |
| IRS/PI 3-kinase | ||||||||
| STAT3 | JAK2/STAT3 | Brain | Nestin | ↓ | ↑↑↑ | ↓↓↓ | ND | 64 |
| Hypothalamus | RIP | ↓ | ↑↑ | ND | ND | 40 | ||
| LEPR neurons | LEPRb | ND | ↑↑↑ | ND | ND | 161 | ||
| POMC neurons | POMC | ↔ | ↑ | ND | ↔ | 214 | ||
| AgRP neurons | AgRP | ↓ | ↔ | ↔ | ↔ | 69 | ||
| STAT5 | JAK2/STAT5 | Brain | Nestin | ND | ↑ | ↓ | ND | 114 |
| IRS2 | IRS/PI 3-kinase | Whole body | ↓ | ↑↑ | ND | ND | 24 | |
| Brain | Nestin | ↔ | ↑↑ | ↓ | ND | 32, 187 | ||
| Hypothalamus | RIP | ↔ or ↓ | ↑↑ | ND | ND | 32, 110, 122 | ||
| POMC neurons | POMC | ↔ | ↔ | ND | ND | 32 | ||
| p85α/β | IRS/PI 3-kinase | POMC neurons | POMC | ↓ | ↔ | ↔ | ND | 82 |
| PDK1 | IRS/PI 3-kinase | POMC neurons | POMC | ND | ↑ | ND | ↑ | 8 |
| PTEN | IRS/PI 3-kinase | LEPR neurons | LEPRb | ND | ↔ | ↑ | ND | 163 |
| POMC neurons | POMC | ND | ↑ | ↔ | ↑ | 162 | ||
| FOXO1 | IRS/PI 3-kinase | Whole body | ↑ | ND | ND | ND | 105 | |
| TSC1 | mTOR/S6K | Hypothalamus | RIP | ↓ | ↑↑ | ND | ND | 141 |
| POMC neurons | POMC | ↓ | ↑↑ | ND | ND | 141 | ||
| SHP2 | SHP2/MAPK | Brain | CaMKIIa | ↓ | ↔ | ND | ND | 221 |
| AMPKα2 | AMPK/ACC | POMC neurons | POMC | ↔ | ↑ | ↓ | ↑ | 34 |
| AgRP neurons | AgRP | ND | ↔ | ↔ | ↔ | 34 | ||
| CaMKK2 | AMPK/ACC | Whole body | ND | ↓ | ND | ↓ | 1 | |
| SOCS3 | All | Whole body | ↑ | ↔ | ND | ↓ | 86 | |
| Brain | Nestin | ↑ | ↓ | ND | ↓ | 140 | ||
| Brain | Synapsin I | ↑ | ↓ | ND | ↓ | 140 | ||
| POMC neurons | POMC | ↑ | ↔ | ND | ↓ | 101 | ||
| VMH neurons | SF-1 | ↑ | ↓ | ↓ | ↔ | 222 | ||
| PTP1B | All | Brain | Nestin | ↑ | ↓ | ↑ | ↓ | 11 |
LEPR, leptin receptor; LEPRb, long form of the leptin receptor; AgRP, agouti-related protein; POMC, proopriomelanocortin; VMH, ventromedial hypothalamus; IRS, insulin receptor substrate; PI, phosphoinositide; PDK1, phosphoinositide-dependent protein kinase-1; PTEN, phosphatase and tensin homolog deleted on chromosome 10; FOXO1, forkhead box O1; TSC1, tuberous sclerosis complex 1; mTOR, mammalian target of rapamycin; SHP2, SH2-containing protein tyrosine phosphatase 2; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; SOCS3, suppressor of cytokine signaling-3; PTP1B, protein tyrosine phosphatase 1B; ↑increased; ↓decreased; ↔no change compared with control mice; ND, not determined; DIO, diet-induced obesity; RIP, rat insulin II promoter; SF-1, steroidogenic factor-1.
The JAK2/STAT3/STAT5 pathways.
The STAT family members are SH2 domain-containing transcription factors located in the cytoplasm in quiescent cells. Cytokine-stimulated tyrosine phosphorylation of STATs induces homo- or heterodimerization, nuclear translocation, and transcriptional activation (84). Leptin stimulates tyrosine phosphorylation of STAT1, -3, -5, and -6 in cultured cells (7, 68, 171, 190); however, leptin primarily stimulates STAT3 and STAT5 phosphorylation in the hypothalamus in animals (70, 131, 199). Tyr1138 in LEPRb is within a YXXQ motif, a consensus-binding motif for STAT3 (183). In response to leptin, STAT3 binds to phospho-Tyr1138, allowing JAK2 to phosphorylate and activate STAT3 (Fig. 2). Mutation of Tyr1138 abolishes the ability of leptin to activate the STAT3 but not other leptin pathways in both cultured cells and mice (4, 6, 209). Nonetheless, disruption of the STAT3 binding site in LEPRb, or deletion of neuronal STAT3, results in severe hyperphagia and morbid obesity, indicating that the LEPRb/JAK2/STAT3 pathway in the brain is required for the antiobesity actions of leptin (6, 40, 64, 93).
In cultured cells, leptin stimulates STAT3-dependent activation of the POMC promoter (105, 147). In mice, deletion of STAT3 in POMC neurons decreases hypothalamic POMC expression (214). STAT3 also binds directly to the AgRP promoter and mediates leptin suppression of the AgRP promoter in cultured cells (105). However, deletion of STAT3 in AgRP neurons does not alter hypothalamic AgRP mRNA expression, suggesting that STAT3 signaling does not mediate leptin suppression of AgRP in vivo (94). Surprisingly, deletion of STAT3 in either POMC or AgRP neurons only slightly increases food intake and adiposity in mice (69, 214). In contrast, deletion of STAT3 in the entire population of LEPR-expressing neurons results in profound leptin resistance, hyperphagia, and obesity (161). These observations suggest that STAT3 signaling in various subpopulations of LEPR neurons acts coordinately to fully mediate leptin's anorexigenic effect.
Leptin stimulates phosphorylation of LEPRb on Tyr1077, which binds to STAT5 and subsequently mediates STAT5 phosphorylation (70, 79, 149). Deletion of both STAT5A and STAT5B in the brain causes leptin resistance, hyperphagia, and obesity, but to a lesser extent than STAT3 deletion, indicating that the JAK2/STAT5 pathway may also contribute to leptin regulation of energy balance and body weight (114).
The insulin receptor substrate/phosphoinositide 3-kinase pathway.
Leptin stimulates phosphoinositide (PI) 3-kinase both in cultured cells and in the hypothalamus (12, 78, 104, 207, 213, 225, 226), and insulin receptor substrate (IRS) proteins, particularly IRS-2, act downstream of LEPRb to activate the PI 3-kinase pathway (24, 110, 122, 128). Leptin-stimulated activation of hypothalamic PI 3-kinase pathway is impaired in diet-induced obesity (134). Inhibition of the PI 3-kinase pathway in the brain blocks the ability of leptin to reduce food intake and weight gain (153, 225). Additionally, inhibition of the hypothalamic PI 3-kinase pathway, but not the STAT3 pathway, blocks the ability of central leptin to suppress lipogenesis in white adipose tissue (23). Forkhead box O1 (FOXO1), a transcriptional factor that is phosphorylated and inactivated by Akt, appears to be an important downstream mediator of the PI 3-kinase pathway (102, 105, 129, 191). Leptin inhibits both the activity and expression of hypothalamic FOXO1 through the PI 3-kinase pathway (102). Adenoviral-mediated overexpression of a constitutively active FOXO1 mutant in the ARC decreases leptin sensitivity in mice; as expected, food intake and body weight are increased, whereas energy expenditure is decreased by hypothalamic FOXO1 activation (102, 105). Conversely, small interfering RNA-mediated knockdown of FOXO1 in ARC or FOXO1 haploinsufficiency increases leptin sensitivity and decreases food intake and body weight (102, 105). Additionally, POMC neuron-specific deletion of phosphoinositide-dependent kinase 1, a key activator of Akt, induces modest hyperphagia and obesity in a FOXO1-dependent manner (8). FOXO1 binds directly to the NPY, AgRP, and POMC promoters; it stimulates the expression of NPY and AgRP but inhibits POMC expression (102, 105). FOXO1 appears to antagonize STAT3 action in both AgRP and POMC neurons (102, 105, 216). Collectively, these findings indicate that the hypothalamic PI 3-kinase pathway is required for leptin's anorexigenic action.
Leptin appears to differentially activate the PI 3-kinase pathway in discrete hypothalamic neurons. Leptin activates the PI 3-kinase pathway in POMC neurons in hypothalamic slices; in contrast, leptin withdrawn activates PI 3-kinase in AgRP neurons in a synaptic transmission-dependent manner (213). Leptin stimulates POMC neuron depolarization and electrical activity by activating nonselective cation channels (39). Pharmacological or genetic inactivation of PI 3-kinase in POMC neurons abolishes the ability of leptin to excite POMC neurons, indicating that the PI 3-kinase pathway is required for leptin-stimulated POMC neuron activation (39, 82). Surprisingly, POMC neuron-specific inactivation of PI 3-kinase only attenuates acute suppression of food intake by leptin but does not impair long-term regulation of energy balance and body weight (82). Interestingly, deletion of IRS-2 in the brain results in leptin resistance and obesity in mice; in contrast, targeted deletion of IRS-2 in POMC neurons does not alter energy balance and body weight (32, 110, 122, 187). Chronic activation of the hypothalamic PI 3-kinase pathway by LEPR neuron-targeted deletion of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a PI 3-kinase specific inhibitor, increases leptin sensitivity and decreases adiposity (163); in contrast, deletion of PTEN in POMC neurons results in leptin resistance and hyperphagia (162). Taken together, these studies indicate that the PI 3-kinase pathway in non-POMC hypothalamic neurons appears to mediate the long-term anorexigenic effects of leptin.
The SH2-containing protein tyrosine phosphatase 2/MAPK pathway.
Leptin stimulates ERK1/2 activation via SH2-containing protein tyrosine phosphatase 2 (SHP2) in cultured cells and the hypothalamus (14, 16, 104, 165, 189, 221). Leptin stimulates phosphorylation of LEPRb on Tyr985, which is required for maximal activation of the MAPK pathway in response to leptin (4, 27, 118). SHP2 is a ubiquitously expressed cytoplasmic protein-tyrosine phosphatase that contains two NH2-terminal SH2 domains and one COOH-terminal phosphatase domain. SHP2 binds via its SH2 domain to phosphorylated Tyr985 in LEPRb and acts as an upstream activator of the MAPK pathway in leptin-treated cells (Fig. 2) (4, 14, 27, 118). Leptin also stimulates the activation of the MAPK pathway by a Tyr985 phosphorylation-independent mechanism, but to a lesser extent (14). Neuron-specific deletion of SHP2 results in leptin resistance and obesity (221). Pharmacological inhibition of ERK1/2 in the hypothalamus also abrogates the ability of leptin to inhibit food intake and weight gain (165). These observations suggest that the SHP2/MAPK pathway is involved in mediating leptin's anorexigenic action.
The AMPK/ACC pathway.
5′-AMP-activated protein kinase (AMPK) is activated by an increase in AMP/ATP ratio and functions as an energy sensor in multiple cell types (95). AMPK phosphorylates and inactivates acetyl-CoA carboxylase (ACC), a key enzyme in fatty acid biosynthesis (95). Leptin inhibits AMPK in multiple regions of the hypothalamus, including the ARC and PVH (2, 135). As expected, leptin stimulates hypothalamic ACC via inhibition of AMPK (65). Inhibition of hypothalamic AMPK is sufficient to reduce food intake and weight gain; in contrast, constitutive activation of hypothalamic AMPK attenuates leptin's anorexigenic response (2, 135). Inhibition of ACC also blocks leptin's anorexigenic action, suggesting that ACC is a downstream mediator of AMPK (65). The Ca2+/calmodulin (CaM)-dependent protein kinase kinase (CaMKK2) is an upstream activator of AMPK in the hypothalamus, and inhibition of CaMKK2 reduces appetite and body weight (1). These findings suggest that the hypothalamic CaMKK2/AMPK/ACC pathway also mediates leptin's anorexigenic action. Consistent with this idea, dietary fat inhibits the ability of leptin to suppress the AMPK pathway in the hypothalamus (127). However, it is unclear whether leptin directly or indirectly regulates this pathway in hypothalamic neurons. Moreover, deletion of AMPK in either POMC neurons or AgRP neurons does not alter leptin sensitivity (34), suggesting that the CaMKK2/AMPK/ACC pathway in extra-arcuate sites mediates leptin's metabolic action.
The mammalian target of rapamycin/S6 kinase pathway.
Leptin stimulates phosphorylation of ribosomal S6 kinase (S6K), a major physiological substrate of the mammalian target of rapamycin (mTOR) kinase in the hypothalamus (38). Rapamycin inhibits hypothalamic mTOR and attenuates leptin's anorexigenic effects (38). Systemic deletion of S6K1, or selective inhibition of S6K in the ARC by a dominant negative S6K mutant, also abolishes leptin's acute anorexigenic action in mice (21, 37). Conversely, activation of S6K in the ARC enhances leptin sensitivity (21). The molecular steps of leptin activation of the mTOR/S6K pathway remain unknown. mTOR binds to raptor and GβL to form the mTOR complex 1 (mTORC1), which directly phosphorylates and activates S6K (172). mTORC1 is inhibited by the TSC1/TSC2 complex (66, 90, 193). Akt phosphorylates TSC2 and inactivates the TSC1/TSC2 complex (90). Therefore, the mTOR/S6K pathway is likely to be a downstream target of the PI 3-kinase/Akt pathway in leptin-stimulated neurons. Surprisingly, in mice with POMC neuron-specific deletion of TSC1, the chronic activation of the mTOR/S6K pathway in POMC neurons results in leptin resistance, hyperphagia, and obesity, presumably due to an alteration of the hypothalamic neurocircuitry of energy balance (141). Interestingly, chronic activation of the PI 3-kinase pathway in POMC neurons similarly causes leptin resistance and hyperphagia in mice with POMC neuron-specific deletion of PTEN (162). These observations support the idea that the PI 3-kinase/Akt pathway stimulates the mTOR/S6K pathway at least in POMC neurons; additionally, chronic activation of the PI 3-kinase/Akt/mTOR/S6K pathway in POMC neurons may alter synaptic transmission and/or neural wiring in the hypothalamus, resulting in leptin resistance. It would be interesting to determine whether the mTOR/S6K pathway in POMC neurons is constitutively activated in mice with POMC neuron-specific PTEN and whether POMC neuron-specific inactivation of the mTOR/S6K pathway rescues leptin-resistant and hyperphagic phenotypes observed in mice with POMC neuron-specific deletion of PTEN.
The JAK2-independent pathway.
We observed that leptin still stimulates the STAT3 and the MAPK pathways in cultured cells that are genetically deficient of JAK2 (92). The Src tyrosine kinase family members appear to be involved in mediating JAK2-independent leptin signaling (Fig. 2) (10, 92, 139). Interestingly, overexpression of kinase-inactive JAK2 enhances leptin signaling in JAK2-deficient cells, suggesting that JAK2 functions both as a tyrosine kinase and as an adaptor to transduce leptin signals (92). The JAK2-dependent and JAK2-independent pathways appear to act synergistically to mediate leptin responses (92). However, the JAK2-independent pathway and its physiological importance have not been verified in animals.
SH2B1 Is an Important Positive Regulator of Leptin Sensitivity
SH2B1 is a SH2 and PH domain-containing adaptor involved in cell signaling in response to a variety of hormones, growth factors, and cytokines (174). We observed that genetic deletion of SH2B1 results in severe leptin resistance, hyperphagia, morbid obesity, hepatic steatosis, and type 2 diabetes in mice (120, 166). Neuron-specific restoration of SH2B1 restores leptin sensitivity and reverses the obesity phenotype in SH2B1-null mice (167). Additionally, neuron-specific overexpression of SH2B1 protects against diet-induced leptin resistance and obesity (167). These data indicate that SH2B1 in the brain is a key regulator of leptin sensitivity, energy balance, and body weight. Interestingly, single nucleotide polymorphisms within the SH2B1 loci have been linked to leptin resistance and obesity in humans (91, 168, 194, 210), suggesting that the metabolic functions of SH2B1 are conserved in mammals.
SH2B1 enhances leptin signaling by at least two mechanisms. We reported that leptin stimulates JAK2 autophosphorylation on Tyr813 (121). SH2B1 binds via its SH2 domain to phospho-Tyr813 and markedly enhances JAK2 activity, thereby promoting the activation of the leptin-signaling pathways downstream of JAK2 (121, 152, 173). Additionally, SH2B1 also binds directly to IRS-1 and IRS-2 (47). Leptin stimulates the formation of JAK2/SH2B1/IRS protein complexes, thereby specifically promoting JAK2-mediated phosphorylation of IRS proteins and subsequent activation of the PI 3-kinase pathway (47, 121, 166) (Fig. 2). SH2B1 forms homodimers via its NH2-terminal regions (43, 152), and this likely provides a platform to initiate the formation of JAK2/SH2B1/IRS protein complexes and/or to stabilize these complexes in response to leptin. Our recent data show that the SH2B1-IRS-1/2 interaction inhibits tyrosine dephosphorylation of IRS-1/2, thus increasing and/or prolonging the activation of the PI 3-kinase pathway (143).
The SH2B family consists of three members (SH2B1, SH2B2, and SH2B3) (130). In contrast to SH2B1, SH2B2 and SH2B3 do not appear to be required for the maintenance of normal leptin sensitivity, energy balance, and body weight (120, 201). Interestingly, SH2B2β, an alternative splicing variant of SH2B2 that lacks the COOH-terminal SH2 domain, binds directly to SH2B1 and antagonizes SH2B1 action (119). These findings suggest that the ability of SH2B1 to enhance leptin sensitivity can be modulated by other members of the SH2B family.
Leptin Resistance Is Induced by Multiple Mechanisms
Leptin resistance, referring to the reduced ability of circulating leptin to suppress appetite and weight gain and to promote energy expenditure, is a primary risk factor for the development of obesity. Leptin resistance likely results from defects in leptin transport into the brain, leptin signaling, and/or the hypothalamic neural circuitry that regulates energy homeostasis. The onset of leptin resistance may vary among discrete LEPRb-expressing neurons. For instance, diet-induced leptin resistance is developed initially in the ARC and later in the VMH, DMH, and PMV (134, 146). Additionally, the impairment in the PI 3-kinase pathway precedes that of the STAT3 pathway (134).
Impaired leptin transport.
Obesity is associated with a reduction in leptin transport into the brain, suggesting that impaired leptin transport contributes to leptin resistance (26, 48, 176). Leptin is actively transported across the blood-brain barrier by a saturable transport mechanism (5). LEPRa, a short form of the leptin receptor that lacks the entire cytoplasmic domain, mediates leptin transport across the blood-brain barrier (80, 81, 97). LEPRe, a soluble form of the leptin receptor, inhibits leptin transport by antagonizing LEPRa's action (196). However, a subpopulation of LEPR-expressing neurons in the ARC sends projections into the median eminence (ME), a circumventricular organ that lacks tight junctions and is permeable to blood-borne hormones (56, 175). These neurons are directly activated by circulating leptin via their projections into the ME (56). Thus, additional studies are needed to clarify the contribution of impaired leptin transport to the pathogenesis of leptin resistance in obese subjects.
Impaired LEPRb trafficking.
Most LEPRb is located in the trans-Golgi network and small vesicles, and only a small portion of LEPRb is present on the plasma membrane to mediate leptin signaling (9, 45). Bardet-Biedl syndrome proteins mediate/promote LEPRb trafficking to the plasma membrane (181). Deletion of Bardet-Biedl syndrome proteins impairs cell surface LEPRb expression, resulting in leptin resistance and obesity (164, 181). However, the contribution of defects in LEPRb trafficking as well as in LEPRb expression to leptin resistance remains to be determined in obese animals and humans.
Impaired LEPRb signaling.
LEPRb signaling is regulated by both negative [e.g., suppressor of cytokine signaling-3 (SOCS3) and protein tyrosine phosphatase 1B (PTP1B)] and positive (e.g., SH2B1) regulators. Leptin stimulates the expression SOCS3, which provides a critical negative feedback mechanism to prevent overactivation of leptin-signaling pathways (Fig. 2) (17, 18). SOCS3 binds to JAK2 and inhibits JAK2 activity (17, 18). SOCS3 also binds to phospho-Tyr985 in LEPRb and inhibits leptin signaling in cultured cells (19, 54). Replacement of Tyr985 with Leu increases leptin sensitivity in mice (20). Additionally, SOCS3 haploinsufficiency, or neuron-specific deletion of SOCS3, enhances leptin sensitivity and attenuates diet-induced leptin resistance and obesity (86, 140). POMC neuron-specific deletion of SOCS3 slightly increases leptin sensitivity and protects against diet-induced obesity (101). Deletion of SOCS3 in the VMH increases leptin sensitivity; however, long-term body weight is not altered due to a reduction in both food intake and energy expenditure (222). Therefore, SOCS3 appears to negatively regulate leptin sensitivity in multiple hypothalamic sites. SOCS3 expression is significantly increased in the hypothalamus in leptin-resistant animals, suggesting that increased SOCS3 expression contributes to leptin resistance (15, 53, 146, 160).
PTP1B binds to and dephosphorylates JAK2, thereby inhibiting leptin signaling (98, 150, 220). PTP1B is expressed in the ARC, VMH, and DMH, and both systemic and neuron-specific deletion of PTP1B improves leptin sensitivity and reduces adiposity in mice (11, 30, 220). The expression of hypothalamic PTP1B is increased in leptin-resistant animals, suggesting that PTP1B also contributes to leptin resistance (144, 208).
We showed that SH2B1 functions as an endogenous leptin sensitizer to enhance leptin sensitivity (120, 121, 166, 167). Interestingly, overexpression of SH2B1 counteracts PTP1B-mediated inhibition of leptin signaling in cultured cells (120, 166). Therefore, cellular leptin sensitivity may be determined, at least in part, by a balance between positive (e.g., SH2B1) and negative (e.g., SOCS3 and PTP1B) regulators.
Endoplasmic reticulum stress.
Secreted and transmembrane proteins are synthesized by the endoplasmic reticulum (ER) and folded into biologically active forms within the ER lumen. Unfolded and misfolded proteins are removed by proteasome-mediated degradation (202). ER homeostasis is maintained by balancing ER loading of nascent proteins with ER capacity to fold these proteins (202). An imbalance results in an accumulation of unfolded/misfolded proteins in the ER lumen, generating ER stress (169, 202). ER stress induces the unfolded protein response (UPR), which involves the activation of multiple intracellular signaling pathways (169). The inositol-requiring protein-1 pathway, the activating transcription factor-6 pathway, and the protein kinase RNA-like ER kinase pathway are well-characterized UPR-signaling pathways that play key roles in maintaining ER homeostasis (169). In response to short-term ER stress, the UPR restores ER homeostasis by reducing protein synthesis and increasing both ER folding capacity and degradation of unfolded/misfolded proteins (169). However, excessive or long-term ER stress induces apoptosis (169).
ER stress is increased in multiple tissues in leptin-resistant and obese animals, including the liver, adipose tissue, and the brain (155, 156, 223). Overnutrition stimulates the mTOR pathway, which promotes ER stress (157). Interestingly, ER stress is sufficient to inhibit leptin signaling in cultured cells, whereas pharmacological inhibition of ER stress improves leptin signaling (85, 157). Moreover, inhibition of ER stress in the hypothalamus by either genetic or pharmacological means markedly improves leptin sensitivity and decreases food intake and body weight in mice (157, 223). These findings suggest that chronic ER stress, presumably by activating various UPR-signaling pathways, contributes to leptin resistance and obesity. However, the cross-talk between UPR and leptin-signaling pathways remains unclear.
Defects in leptin-targeted neural circuitry.
MC4R- and TrkB-expressing hypothalamic neurons are key components of the neural circuitry that mediate leptin's anorexigenic action, as discussed above. Impairment in MC4R signaling in the PVH or TrkB signaling in the VMH has been well documented to induce leptin resistance, hyperphagia, and obesity (57, 58, 100, 124, 126, 159, 197, 198, 203, 215, 217–219). Genetic as well as environmental factors may modulate ongoing synaptic remodeling and neural circuitry rewiring of the leptin neural circuitry, thus altering leptin sensitivity and energy metabolism. In further support of this idea, ciliary neurotrophic factor, which also induces weight loss, stimulates hypothalamic neurogenesis in obese adult mice, and pharmacological inhibition of hypothalamic proliferation blocks the long-term anorexigenic effects of ciliary neurotrophic factor (106).
Concluding Remarks and Future Perspectives
A large body of evidence suggests that leptin relays a critical adiposity signal to the brain, and leptin resistance is a primary risk factor for obesity. Leptin directly targets multiple chemically defined neurons (e.g., AgRP and POMC neurons) located in different hypothalamic regions (e.g., ARC, VMH, DMH, LHA, and PMV). These leptin-responsive neurons broadly connect to other neurons in the brain, thus forming a sophisticated neurocircuitry that also integrates other forms of metabolic signals to control the balance between energy intake and expenditure. Genetic and environmental factors may modulate the synaptic remodeling and rewiring of this circuitry, thus regulating leptin sensitivity and body weight. It is extremely important to anatomically, chemically, and electrically characterize this neurocircuity in the future. Leptin controls energy homeostasis and body weight primarily by activating LEPRb in the hypothalamus. LEPRb activates numerous JAK2-dependent and -independent signaling pathways that act coordinately as a network to fully mediate leptin's action. The activation of individual pathways in the leptin signaling network appears to be differentially regulated in discrete subpopulations of LEPRb- expressing neurons. These pathways are also likely to be regulated by various hormonal, neuronal, and metabolic signals that cross-talk with leptin. It is important to determine whether and how positive (e.g., SH2B1) and negative (e.g., SOCS3 and PTP1B) regulators of LEPRb signaling, ER stress, metabolic state, and/or neuronal activity regulate the activation of the leptin signaling networks in a cell type-specific manner. Additionally, leptin resistance appears to be caused by multiple mechanisms (e.g., impairment in leptin transport, LEPRb signaling, and the hypothalamic neurocircuitry) that may vary considerably among different obese patients. It will be challenging to develop diagnostic approaches for the different forms of leptin resistance and design personalized healthcare programs to treat obesity.
GRANTS
This study was supported by American Diabetes Association Grant 1-09-RA-156 and National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK-065122 and RO1-DK-073601.
REFERENCES
- 1.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 7: 377–388, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279: 12005–12008, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA 93: 8374–8378, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 7: 291–301, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Belouzard S, Delcroix D, Rouille Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem 279: 28499–28508, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Bełtowski J, Wójcicka G, Trzeciak J, Marciniak A. H2O2 and Src-dependent transactivation of the EGF receptor mediates the stimulatory effect of leptin on renal ERK and Na+, K+-ATPase. Peptides 27: 3234–3244, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Berti L, Kellerer M, Capp E, Haring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia 40: 606–609, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149: 2138–2148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276: 4747–4755, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1: 619–625, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Bjørbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272: 32686–32695, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274: 30059–30065, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Bjørbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139: 3485–3491, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 275: 40649–40657, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117: 1354–1360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab 8: 459–467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blüher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes 14: 458–464, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burks DJ, de Mora JF, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407: 377–382, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA 95: 6061–6066, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26: 97–102, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2: 497–503, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest 115: 940–950, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994–996, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24: 258–269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115: 3484–3493, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devos R, Guisez Y, Van der Heyden J, White DW, Kalai M, Fountoulakis M, Plaetinck G. Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J Biol Chem 272: 18304–18310, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Dhe-Paganon S, Werner ED, Nishi M, Hansen L, Chi YI, Shoelson SE. A phenylalanine zipper mediates APS dimerization. Nat Struct Mol Biol 11: 968–974, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Diano S, Kalra SP, Horvath TL. Leptin receptor immunoreactivity is associated with the Golgi apparatus of hypothalamic neurons and glial cells. J Neuroendocrinol 10: 647–650, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 29: 5240–5250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 279: 43684–43691, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138: 839–842, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998 [PubMed] [Google Scholar]
- 52.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46: 313–316, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett 486: 33–37, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165–168, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 148: 5414–5423, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348: 1085–1095, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106: 271–279, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 94: 7001–7005, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20: 1550–1558, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem 268: 15174–15179, 1993 [PubMed] [Google Scholar]
- 64.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101: 4661–4666, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci USA 104: 17358–17363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 4: 699–704, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol 11: 393–399, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93: 6231–6235, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong L, Yao F, Hockman K, Heng HH, Morton GJ, Takeda K, Akira S, Low MJ, Rubinstein M, MacKenzie RG. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology 149: 3346–3354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 282: 31019–31027, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8: 1289–1291, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua S, Jr, Zhang Y. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148: 3987–3997, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1: 271–272, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Hakansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus—relationship with NPY neurones. Neuroreport 7: 3087–3092, 1996 [DOI] [PubMed] [Google Scholar]
- 75.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107: 111–120, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford ML. Essential role of phosphoinositide 3-kinase in leptin-induced K(ATP) channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem 275: 4660–4669, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J 272: 109–119, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Hileman SM, Pierroz DD, Masuzaki H, Bjørbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 143: 775–783, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Hileman SM, Tornøe J, Flier JS, Bjørbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology 141: 1955–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118: 1796–1805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Horvath CM. The Jak-STAT pathway stimulated by interferon gamma. Sci STKE 2004: tr8, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, Yoshii M, Ozawa K. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol 74: 1610–1619, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Huang Q, Rivest R, Richard D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology 139: 1524–1532, 1998 [DOI] [PubMed] [Google Scholar]
- 88.Huang W, Dedousis N, Bhatt BA, O'Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem 279: 21695–21700, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 90.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Jamshidi Y, Snieder H, Ge D, Spector TD, O'Dell SD. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity (Silver Spring) 15: 5–9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang L, Li Z, Rui L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem 283: 28066–28073, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang L, You J, Yu X, Gonzalez L, Yu Y, Wang Q, Yang G, Li W, Li C, Liu Y. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci USA 105: 18619–18624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaelin CB, Gong L, Xu AW, Yao F, Hockman K, Morton GJ, Schwartz MW, Barsh GS, MacKenzie RG. Signal transducer and activator of transcription (stat) binding sites but not stat3 are required for fasting-induced transcription of agouti-related protein messenger ribonucleic acid. Mol Endocrinol 20: 2591–2602, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4: 341–348, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides 20: 1449–1453, 1999 [DOI] [PubMed] [Google Scholar]
- 98.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol 195: 109–118, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40: 1358–1362, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19: 1290–1300, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci 9: 901–906, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest 105: 1005–1011, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 141: 2328–2339, 2000 [DOI] [PubMed] [Google Scholar]
- 105.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310: 679–683, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 139: 1107–1115, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393: 72–76, 1998 [DOI] [PubMed] [Google Scholar]
- 109.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19: 155–157, 1998 [DOI] [PubMed] [Google Scholar]
- 110.Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest 114: 917–927, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100: 2729–2736, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol 4: 1–13, 2007 [PubMed] [Google Scholar]
- 113.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 114.Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, Louis GW, Leinninger GM, Bertuzzi S, Seeley RJ, Robinson GW, Myers MG, Hennighausen L. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS ONE 3: e1639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem 276: 5629–5635, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10: 89–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29: 3138–3147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci USA 96: 9677–9682, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li M, Li Z, Morris DL, Rui L. Identification of SH2B2beta as an inhibitor for SH2B1- and SH2B2alpha-promoted Janus kinase-2 activation and insulin signaling. Endocrinology 148: 1615–1621, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li M, Ren D, Iseki M, Takaki S, Rui L. Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology 147: 2163–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 121.Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21: 2270–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest 114: 908–916, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 7: 648–652, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P, Vaisse C. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet 12: 145–153, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005 [DOI] [PubMed] [Google Scholar]
- 126.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21: 119–122, 1999 [DOI] [PubMed] [Google Scholar]
- 127.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281: 18933–18941, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Masaki T, Chiba S, Noguchi H, Yasuda T, Tobe K, Suzuki R, Kadowaki T, Yoshimatsu H. Obesity in insulin receptor substrate-2-deficient mice: disrupted control of arcuate nucleus neuropeptides. Obes Res 12: 878–885, 2004 [DOI] [PubMed] [Google Scholar]
- 129.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100: 11285–11290, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18: 38–45, 2007 [DOI] [PubMed] [Google Scholar]
- 131.McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology 139: 4442–4447, 1998 [DOI] [PubMed] [Google Scholar]
- 132.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141: 4442–4448, 2000 [DOI] [PubMed] [Google Scholar]
- 133.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett 387: 113–116, 1996 [DOI] [PubMed] [Google Scholar]
- 134.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology 149: 1121–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002 [DOI] [PubMed] [Google Scholar]
- 137.Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 47: 294–297, 1998 [DOI] [PubMed] [Google Scholar]
- 138.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997 [DOI] [PubMed] [Google Scholar]
- 139.Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann NY Acad Sci 1069: 463–471, 2006 [DOI] [PubMed] [Google Scholar]
- 140.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 141.Mori H, Inoki K, Münzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG, Jr, Guan KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9: 362–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 117: 2860–2868, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morris DL, Cho KW, Zhou Y, Rui L. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes 58: 2039–2047, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148: 433–440, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308, 1994 [DOI] [PubMed] [Google Scholar]
- 146.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 147.Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144: 2121–2131, 2003 [DOI] [PubMed] [Google Scholar]
- 148.Muoio DM, Dohm GL, Fiedorek FT, Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46: 1360–1363, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Mutze J, Roth J, Gerstberger R, Hubschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett 417: 286–291, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 276: 47771–47774, 2001 [DOI] [PubMed] [Google Scholar]
- 151.Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjørbak C, Flier JS. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. J Biol Chem 275: 36124–36133, 2000 [DOI] [PubMed] [Google Scholar]
- 152.Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE. Kinase activation through dimerization by human SH2-B. Mol Cell Biol 25: 2607–2621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 154.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278: 135–138, 1997 [DOI] [PubMed] [Google Scholar]
- 155.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29: 541–551, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 159.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol 131: 229–238, 1995 [DOI] [PubMed] [Google Scholar]
- 160.Peralta S, Carrascosa JM, Gallardo N, Ros M, Arribas C. Ageing increases SOCS-3 expression in rat hypothalamus: effects of food restriction. Biochem Biophys Res Commun 296: 425–428, 2002 [DOI] [PubMed] [Google Scholar]
- 161.Piper ML, Unger EK, Myers MG, Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 22: 751–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Brüning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116: 1886–1901, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Plum L, Rother E, Münzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Könner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Brüning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6: 431–445, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118: 1458–1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 58: 536–542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2: 95–104, 2005 [DOI] [PubMed] [Google Scholar]
- 167.Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest 117: 397–406, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Renstrom F, Payne F, Nordstrom A, Brito EC, Rolandsson O, Hallmans G, Barroso I, Nordstrom P, Franks PW. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet 18: 1489–1496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90: 8856–8860, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosenblum CI, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess JF, Phillips MS, Hey PJ, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler C, Van der Ploeg LH. Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology 137: 5178–5181, 1996 [DOI] [PubMed] [Google Scholar]
- 172.Rui L. A link between protein translation and body weight. J Clin Invest 117: 310–313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rui L, Carter-Su C. Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA 96: 7172–7177, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C. Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol 17: 6633–6644, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, I. Adult brain. Clin Exp Pharmacol Physiol 26: 11–19, 1999 [DOI] [PubMed] [Google Scholar]
- 176.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2: 589–593, 1996 [DOI] [PubMed] [Google Scholar]
- 177.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98: 1101–1106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46: 2119–2123, 1997 [DOI] [PubMed] [Google Scholar]
- 179.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes 53: 276–284, 2004 [DOI] [PubMed] [Google Scholar]
- 181.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet 18: 1323–1331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA 94: 4637–4641, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 267: 1349–1353, 1995 [DOI] [PubMed] [Google Scholar]
- 184.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 82: 3940–3943, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck PR, Jr, Schoner B, Smith D, Tinsley FC, Zhang ZY, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377: 530–532, 1995 [DOI] [PubMed] [Google Scholar]
- 186.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215, 1998 [DOI] [PubMed] [Google Scholar]
- 187.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317: 369–372, 2007 [DOI] [PubMed] [Google Scholar]
- 188.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146: 1043–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 189.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem 272: 12897–12900, 1997 [DOI] [PubMed] [Google Scholar]
- 190.Takahashi Y, Okimura Y, Mizuno I, Takahashi T, Kaji H, Uchiyama T, Abe H, Chihara K. Leptin induces tyrosine phosphorylation of cellular proteins including STAT-1 in human renal adenocarcinoma cells, ACHN. Biochem Biophys Res Commun 228: 859–864, 1996 [DOI] [PubMed] [Google Scholar]
- 191.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274: 16741–16746, 1999 [DOI] [PubMed] [Google Scholar]
- 192.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995 [DOI] [PubMed] [Google Scholar]
- 193.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 99: 13571–13576, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41: 18–24, 2009 [DOI] [PubMed] [Google Scholar]
- 195.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol 214: 301–305, 2008 [DOI] [PubMed] [Google Scholar]
- 197.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci 27: 14265–14274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20: 113–114, 1998 [DOI] [PubMed] [Google Scholar]
- 199.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996 [DOI] [PubMed] [Google Scholar]
- 200.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149: 1773–1785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 195: 1599–1611, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9: 944–957, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1037–R1045, 2007 [DOI] [PubMed] [Google Scholar]
- 204.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem 274: 17541–17544, 1999 [DOI] [PubMed] [Google Scholar]
- 205.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA 102: 18011–18016, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor isoform in rat. FEBS Lett 392: 87–90, 1996 [DOI] [PubMed] [Google Scholar]
- 207.Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H. Leptin receptor action in hepatic cells. J Biol Chem 272: 16216–16223, 1997 [DOI] [PubMed] [Google Scholar]
- 208.White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab 296: E291–E299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem 272: 4065–4071, 1997 [DOI] [PubMed] [Google Scholar]
- 210.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O'Rahilly S, Purmann C, Rees MG, Ridderstråle M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Wellcome Trust Case Control Consortium. Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN, Genetic Investigation of ANthropometric Traits Consortium Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41: 25–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137: 1225–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci 28: 9218–9226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115: 951–958, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148: 72–80, 2007 [DOI] [PubMed] [Google Scholar]
- 215.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6: 736–742, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang G, Lim CY, Li C, Xiao X, Radda GK, Cao X, Han W. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J Biol Chem 284: 3719–3727, 2009 [DOI] [PubMed] [Google Scholar]
- 217.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O'Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 7: 1187–1189, 2004 [DOI] [PubMed] [Google Scholar]
- 218.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20: 111–112, 1998 [DOI] [PubMed] [Google Scholar]
- 219.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12: 561–574, 2003 [DOI] [PubMed] [Google Scholar]
- 220.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell 2: 489–495, 2002 [DOI] [PubMed] [Google Scholar]
- 221.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA 101: 16064–16069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149: 5654–5661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]
- 225.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci 5: 727–728, 2002 [DOI] [PubMed] [Google Scholar]
- 226.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem 275: 11348–11354, 2000 [DOI] [PubMed] [Google Scholar]