Abstract
The synthetic retinoid Fenretinide (FEN) increases insulin sensitivity in obese rodents and is in early clinical trials for treatment of insulin resistance in obese humans with hepatic steatosis (46). We aimed to determine the physiological mechanisms for the insulin-sensitizing effects of FEN. Wild-type mice were fed a high-fat diet (HFD) with or without FEN from 4–5 wk to 36–37 wk of age (preventive study) or following 22 wk of HF diet-induced obesity (12 wk intervention study). Retinol-binding protein-4 (RBP4) knockout mice were also fed the HFD with or without FEN in a preventive study. FEN had minimal effects on HFD-induced body weight gain but markedly reduced HFD-induced adiposity and hyperleptinemia in both studies. FEN-HFD mice gained epididymal fat but not subcutaneous or visceral fat mass in contrast to HFD mice without FEN. FEN did not have a measurable effect on energy expenditure, food intake, physical activity, or stool lipid content. Glucose infusion rate during hyperinsulinemic-euglycemic clamp was reduced 86% in HFD mice compared with controls and was improved 3.6-fold in FEN-HFD compared with HFD mice. FEN improved insulin action on glucose uptake and glycogen levels in muscle, insulin-stimulated suppression of hepatic glucose production, and suppression of serum FFA levels in HFD mice. Remarkably, FEN also reduced hepatic steatosis. In RBP4 knockout mice, FEN reduced the HFD-induced increase in adiposity and hyperleptinemia. In conclusion, long-term therapy with FEN partially prevents or reverses obesity, insulin resistance, and hepatic steatosis in mice on HFD. The anti-adiposity effects are independent of the RBP4 lowering effect.
Keywords: retinol-binding protein-4, type 2 diabetes, hyperleptinemia, retinoids
with the worldwide epidemic of obesity and type 2 diabetes, new and safe treatments for these metabolic disorders are increasingly needed. Obesity is a major risk factor for insulin resistance, which is a major pathogenic factor in type 2 diabetes. Adipose tissue plays an important role in regulating systemic insulin sensitivity and glucose homeostasis by serving as a storage depot for lipids and thereby preventing ectopic lipid deposition in other tissues (45) and by secreting hormones and cytokines that influence whole body metabolism (36). These secreted molecules and their signaling pathways represent potential therapeutic targets for prevention or treatment of obesity and/or type 2 diabetes. One such secreted molecule is retinol-binding protein-4 (RBP4).
Serum RBP4 levels are elevated in insulin-resistant humans and in many mouse models of obesity and insulin resistance including high-fat diet (HFD) (4, 12, 14, 20, 49), although not all studies show this effect (19, 28). In many studies, the level of elevation correlates highly with the degree of insulin resistance in both mice and humans, and serum RBP4 levels are highly predictive of metabolic syndrome risk in a large population-based study (34). Furthermore, chronic administration of RBP4 to normal mice is sufficient to cause insulin resistance, and RBP4 knockout (KO) mice have enhanced insulin sensitivity (49). The possibility that elevated RBP4 plays a causative role in type 2 diabetes is also supported by the fact that people with a single nucleotide polymorphism in the RBP4 promoter that increases RBP4 expression and serum levels have increased risk for type 2 diabetes (17, 33, 45a). Many therapeutic interventions that improve insulin sensitivity are associated with lowering of serum RBP4 levels (14, 25, 37), and efforts are underway to develop RBP4-lowering agents to treat diabetes. While several approaches that reduce serum RBP4 levels confer insulin sensitivity (49, 51), short-term treatment with one nonretinoid small molecule, an RBP4-lowering agent, failed to do so (31). The synthetic retinoid, Fenretinide [N-(4-hydroxyphenyl)retinamide (FEN)] reduces serum RBP4 levels and improves insulin sensitivity (21, 49). Currently, there is a phase II trial of Fenretinide for treatment of insulin resistance in obese humans with hepatic steatosis (46). Thus, the mechanisms by which lowering RBP4 can result in insulin sensitivity are of great interest.
Fenretinide lowers serum RBP4 levels in rodents and humans by disrupting the ternary complex of retinol-RBP4-transthyretin and thereby promoting renal clearance of RBP4 (3, 10, 49). We found that up to 16 wk of Fenretinide treatment of mice on HFD prevented elevations of serum RBP4 levels that are usually seen with HFD-induced obesity, ameliorated insulin resistance, and normalized glucose tolerance without altering food intake or HFD-induced body weight gain (49). It appeared that Fenretinide prevents insulin resistance, at least in part, by lowering serum RBP4 levels (49).
Fenretinide was originally developed as a chemotherapeutic agent (30) because of its ability to attenuate cancer cell growth and its relatively low toxicity. It inhibits cell growth through the induction of apoptosis by mechanisms that are not well defined and appear to differ in different tissues (reviewed in Refs. 9 and 15). Fenretinide is now the most widely studied retinoid in clinical trials of breast cancer chemoprevention due to its selective accumulation in breast tissue and to its favorable toxicological profile (21, 29, 38, 52). Since our initial mouse studies indicated that Fenretinide could be a promising therapeutic agent to treat or prevent insulin resistance and glucose intolerance, and since human cancer trials demonstrated few side effects, we sought to further investigate the mechanisms by which Fenretinide improves glucose homeostasis and insulin action. We aimed to determine whether Fenretinide has more global effects on energy balance and lipid homeostasis and whether the metabolic effects of Fenretinide are mediated entirely by lowering circulating RBP4 levels or also by other mechanisms.
METHODS
Animals.
FVB male mice (3–4 wk old) were obtained from Taconic. Mice with disruption of the RBP4 gene (RBP4KO) on a mixed background (C57BL/6J × 129Sv) were generously provided by Drs. William Blaner, Max Gottesman, and Loredana Quadro, (Columbia University) (35). Experimental cohorts were generated from breeding pairs of RBP4KO or wild-type (WT) littermates that were first-generation offspring from mice that were heterozygous for the RBP4 gene. Studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the IACUC at Beth Israel Deaconess Medical Center.
Diets.
Mice were housed individually in a temperature-controlled room, on a 14:10-h light-dark cycle and with ad libitum access to food and water. In prevention studies, cohorts of either FVB mice or RBP4KO and WT controls on the C57BL/6J × 129Sv mixed background were placed into weight-matched diet groups at 4–5 wk of age and fed standard chow (Labdiet 5008), HFD (55% fat calories, Harlan-Teklad TD-093075), or HF diet supplemented with Fenretinide (0.1% wt/wt) as an admixture by the manufacturer (Harlan-Teklad) (49). In intervention studies, some FVB mice (fed HF for 22 wk) were then placed on HFD+FEN. During breeding, RBP4KO C57BL/6J × 129Sv mice were fed Labdiet 5053, a standard chow diet. All diets contained 15–25 IU/g vitamin A, which is more than the minimum dietary retinol required to maintain normal vitamin A status in mice (2.5 IU/g diet) (42).
Body weight and food intake.
Body weights were measured weekly. Food intake was measured weekly over the period 0–3 wk and later at 9–11 wk of FEN treatment in the FVB strain prevention study and 0–9 wk of FEN treatment in the intervention study, when body weights were similar, and 17–20 wk of FEN treatment in the C57BL/6J × 129Sv strain (RBP4KO and WT) study, when body weights had begun to diverge.
Body composition.
Body composition was measured in anesthetized mice by dual-energy X-ray absorptiometry (Lunar PIXIMUS Densitometer, GE Medical Systems) at 8 and 19 wk of FEN treatment in the FVB strain prevention study; 0, 3, 6, and 9 wk of FEN treatment in the intervention study; and 20 wk FEN treatment in the C57BL/6J × 129Sv strain (RBP4KO and WT) study. Individual tissue masses were dissected and analyzed as follows after 34 wk of HFD ± FEN treatment. Epididymal white adipose tissues (WAT) and livers were weighed, and total subcutaneous and visceral (intra-abdominal) WAT mass was determined by chemical analysis of dissected carcasses. In addition, a small piece of subcutaneous adipose tissue was minced and fixed with osmic acid. Cells were counted in a Coulter counter (6, 16). Cell size was calculated as previously described (6).
Indirect calorimetry.
Metabolic rate of singly housed mice was measured at 17 wk FEN treatment in the FVB strain prevention study by indirect, open-circuit calorimetry (CLAMS; Columbus Instruments, Columbus, OH). Food was removed during the day (light period) to measure metabolic rate in the fasted state. All mice were acclimatized to monitoring cages for 48 h prior to 24-h measurements of O2 consumption.
Insulin tolerance tests.
Food was removed at 8:00 AM for 5 h, and blood glucose was measured as indicated after intraperitoneal injection of human insulin (Humulin, Lilly; 1.2 mU/g in FVB mice and 1.0 mU/g in C57BL/6J × 129Sv mixed-background mice). Tests were performed after 12–16 wk FEN treatment in all studies.
Hyperinsulinemic-euglycemic clamp with 2-deoxyglucose uptake.
A dual tracer clamp ([3-3H]glucose infusion and 2-deoxy-d-[1-14C]glucose bolus) was performed as previously described (23), with the following changes. After 16–18 wk of HFD ± FEN treatment, mice received an indwelling silicone catheter in the femoral vein and were allowed to recover for 4–7 days. Following a 5-h fast, a 10 mU·kg−1·min−1 insulin, euglycemic clamp was conducted for 120 min in awake, free-moving mice. Rates of basal and insulin-stimulated glucose turnover and hepatic glucose production were determined by the [3-3H]glucose dilution method. Glucose uptake in individual tissues was calculated (22). The glycogen content of muscle and liver was determined as described (23).
Statistical analysis.
ANOVA followed by Bonferroni or Fisher post hoc tests were performed using the Statview 4.0 software (Abacus, Baltimore, MD).
RESULTS
Long-term Fenretinide treatment protects against HFD-induced obesity: a prevention study.
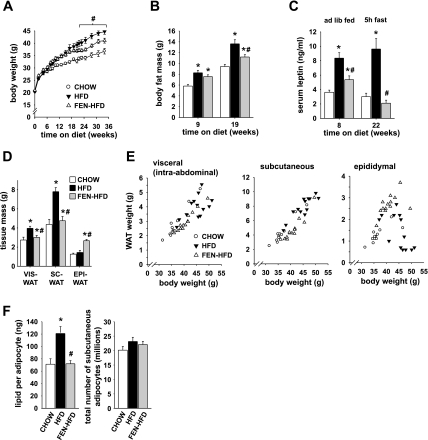
By 8 wk of diet treatment and thereafter, HFD mice were heavier than CHOW mice (P < 0.05; Fig. 1A). Accordingly, body fat content in HFD mice was 42% higher than CHOW after 9 wk of diet and increased further by 19 wk (Fig. 1B). As we previously showed (49), FEN-HFD treatment for 16 wk did not affect body weight compared with HFD controls. However, by 22 wk, FEN-HFD mice were lighter than HFD (P < 0.05; Fig. 1A), as a result of reduced fat mass (Fig. 1B) without a change in lean mass (data not shown). At 19 wk, body fat content was increased 45% in HFD mice but only 19% in FEN-HFD compared with CHOW mice (Fig. 1B). Fenretinide treatment strongly reduced fed hyperleptinemia after 8 wk and totally prevented 5-h-fasted hyperleptinemia at 22 wk of treatment (Fig. 1C). As expected, serum leptin levels in chow and HFD mice strongly correlated with body fat mass (see Supplemental data; supplemental materials are found in the online version of this paper). Interestingly, FEN-HFD mice had lower-than-expected serum leptin levels for their given fat mass compared with CHOW or HFD mice (Fig. 1, B and C and Supplemental data).
Fig. 1.
Fenretinide partially prevents high-fat diet (HFD)-induced obesity. A: body weight curves of male FVB mice on standard CHOW (○), HFD (▴), or HFD plus 0.1% wt/wt Fenretinide (FEN-HFD, ▵) from 4–5 wk of age. B: body fat content at 9 and 19 wk on diet (CHOW, open bar; HFD, filled bar, or FEN-HFD, gray bar). C: serum leptin levels at 8 and 22 wk on diet. D: visceral (VIS), subcutaneous (SC), and epididymal (EPI) white adipose tissue (WAT) depot weights dissected at 34 wk diet. E: association of dissected WAT depot weights with body weight at 34 wk diet. F: average size of adipocytes in SC-WAT expressed as amount of lipid per adipocyte (left), number of adipocytes in SC-WAT depot per mouse (right). Results are means ± SE from 12–14 mice per dietary group. *P < 0.05 vs. CHOW, #P < 0.05 vs. HFD.
By 34 wk of diet treatment, Fenretinide strikingly prevented the HFD feeding-induced expansion of both visceral and subcutaneous fat masses (Fig. 1D). In contrast, epididymal fat mass was comparable in HFD and CHOW mice and it was about twofold greater in FEN-HFD. As expected, visceral and subcutaneous fat masses showed a linear relationship to body weight in all mice (Fig. 1E, left and middle). Interestingly, epididymal fat mass had a bell-shaped relationship to body weight (Fig. 1E, right). Fenretinide completely prevented the HFD-induced overaccumulation of lipid in subcutaneous adipocytes (Fig. 1F, left). Adipocyte number in subcutaneous WAT did not differ between diet groups (Fig. 1F, right).
In parallel with lower-than-expected serum leptin levels (Fig. 1C and Supplemental data), subcutaneous fat mass was lower than expected for a given body weight in FEN-HFD mice (Fig. 1E, middle). Interestingly, serum leptin levels correlated better with subcutaneous fat (Supplemental data) than with visceral or epididymal fat masses (not shown).
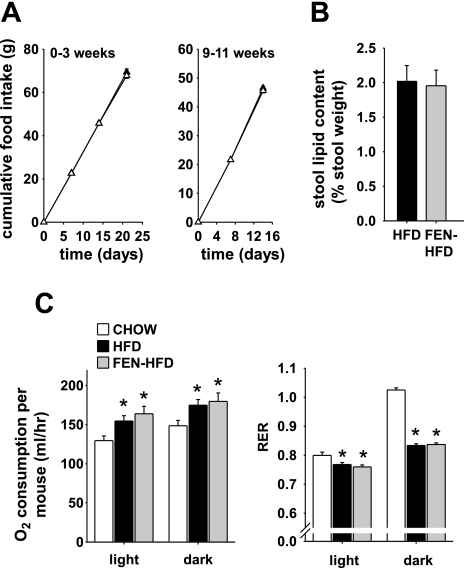
Leptin regulates body fat mass by decreasing food intake and increasing energy expenditure. In obese hyperleptinemic animals, leptin action is impaired, which may account, at least partly, for hyperleptinemia. Fenretinide treatment improved HFD-induced hyperleptinemia even in mice with increased adiposity, suggesting improved leptin sensitivity. Fenretinide did not alter food intake compared with HFD alone, measured during the first 3 wk of treatment (Fig. 2A, left) or over wk 9–11 (Fig. 2A, right) when body weights were similar between HFD and FEN-HFD mice. Similar data were obtained at the onset of body weight divergence between FEN-HFD and HFD mice (see below). Thus, Fenretinide treatment neither decreased food intake nor induced food aversion. Fenretinide also did not alter stool lipid content in HFD-fed mice (Fig. 2B), suggesting normal intestinal lipid absorption. Thus, Fenretinide does not alter energy intake and may instead preserve leanness by increasing energy expenditure. Indirect calorimetry performed at 17 wk on diet (when HFD and FEN-HFD body weights were similar) did not reveal any difference in oxygen consumption during the light or dark period expressed either per mouse (Fig. 2C, left) or per gram of body weight (not shown). Resting metabolic rate and locomotor activity were also not altered (data not shown). In addition, FEN-HFD mice did not show altered substrate metabolism compared with HFD alone as measured by respiratory exchange ratio (RER; Fig. 2C, right). Both FEN-HFD and HFD mice had similar decreases in RER compared with CHOW. However, changes in body mass can develop with very small mismatches of energy intake and expenditure especially over prolonged periods of time (see discussion).
Fig. 2.
Fenretinide does not affect food intake or energy expenditure. A: cumulative food intake during first 3 wk (left) and 9–11 wk (right) of diet in mice put on HFD (▴) or FEN-HFD (▵) diet from 4–5 wk of age. B: stool lipid content at 30 wk of diet. C: energy expenditure expressed as O2 consumption of individual mice (left) and respiratory exchange ratio (RER; right) at 17 wk of diet. Results are means ± SE from 12–14 mice per dietary group. *P < 0.05 vs. CHOW, #P < 0.05 vs. HFD.
Fenretinide partially protected mice from HFD-induced insulin resistance.
We previously showed (49) that Fenretinide limits HFD-induced insulin resistance. To determine in which tissues Fenretinide improves insulin action, we performed euglycemic-hyperinsulinemic clamp studies in a second cohort of male FVB mice fed CHOW, HFD, or FEN-HFD. Body weight progression of HFD and FEN-HFD mice over 16 wk of diet (Table 1 and data not shown) was comparable to that in previous cohorts (49). After 8 wk on diet, plasma insulin levels were elevated ∼2.5-fold in both FEN-HFD and HFD mice compared with CHOW mice (Table 2). After 13–14 wk on diet, HFD mice displayed severe hyperinsulinemia (15-fold elevated) and hyperglycemia (Table 2) and insulin resistance upon insulin tolerance testing (Fig. 3A) compared with CHOW controls. Strikingly, Fenretinide treatment strongly prevented overt hyperinsulinemia (Table 2) and markedly improved insulin sensitivity (Fig. 3A). Thus, euglycemic-hyperinsulinemic clamp studies were performed at 16 wk of FEN treatment.
Table 1.
Euglycemic-hyperinsulinemic clamp parameters
| CHOW | HFD | FEN+HFD | |
|---|---|---|---|
| Body weight, g | 32.1±1.2 | 40.3±1.1** | 38.0±1.4* |
| Plasma insulin, ng/ml | |||
| Basal | 1.5±0.2 | 6.7±1.2*** | 2.9±0.6†† |
| Clamp | 6.1±0.5 | 10.0±1.2** | 7.4±0.7 |
| Plasma glucose, mg/dl | |||
| Basal | 157.9±8.9 | 181.2±8.9 | 166.7±10.2 |
| Clamp | 130.2±3.2 | 136.2±4.9 | 121.4±3.8*† |
| Hepatic glucose production, mg·kg−1·min−1 | |||
| Basal | 15.3±0.5 | 17.2±0.7 | 17.9±1.8 |
| Clamp | 2.0±0.6 | 14.4±1.1*** | 4.1±1.7††† |
| Liver glycogen, μg/mg | 47.7±15.6 | 146.5±25.3* | 129.8±19.3* |
| Serum free fatty acid, mM | |||
| Basal | 1.16±0.06 | 1.04±0.11 | 1.35±0.10 |
| Clamp | 0.47±0.1 | 0.90±0.10*** | 0.71±0.1 |
| Plasma AST activity, arbitrary units | 29.1±4.1 | 47.0±29.7 | 36.8±23.0 |
| Plasma ALT activity, arbitrary units | 26.4±13.8 | 28.0±13.5 | 34.0±17.6 |
| No. of mice | 13 | 14 | 11 |
Male FVB mice were placed on high-fat diet (HFD) or HFD with Fenretinide (FEN+HFD) at 4–5 wk old, and a euglycemic-hyperinsulinemic (10mU·kg−1·min−1 insulin) clamp was performed at 16 wk on diet. AST, aspartate amino transferase: ALT, alanine aminotransferase. Data are means ± SE. *Significantly different vs. CHOW (*P < 0.05, **P < 0.001, ***P < 0.0001); †significantly different vs. HFD (†P < 0.05, ††P < 0.005, †††P < 0.0001).
Table 2.
Serum parameters: prevention study
| CHOW | HFD | FEN+HFD | |
|---|---|---|---|
| Insulin, (ng/ml) | |||
| 8 wk | 2.1±0.2 | 5.4±0.7* | 5.1±1.0**† |
| 13 wk | 2.9±0.4 | 44.3±10.4*** | 18.6±5.3† |
| Glucose, (mg/dl) | |||
| 8 wk | 199±4.9 | 226.8±6.8* | 228.8±8.1*** |
| 13 wk | 194±7.4 | 238±13.6* | 190.5±4.6† |
| Resistin, (ng/ml) | 5.6±0.2 | 13±0.5*** | 14.3±0.9*** |
| Adiponectin, (μg/ml) | 0.66±0.03 | 0.59±0.02 | 0.72±0.05† |
| Free fatty acids, (meq/l) | 0.93±0.04 | 1.06±0.01 | 0.94±0.04 |
| Triglycerides, (mg/dl) | 203±12 | 263±21 | 240±23 |
| Glycerol, (mg/dl) | 196±18 | 330±30* | 258±21† |
| No. of mice | 15 | 18 | 18 |
Serum parameters in the ad libitum-fed state in male FVB mice. Mice were placed on HFD or FEN+HFD at 4–5 wk old and bled at 13 wk on diet (except where specified). Data are means ± SE. *Significantly different vs. CHOW (*P < 0.05), **P < 0.01, ***P < 0.005;
significantly different vs. HFD (P < 0.05).
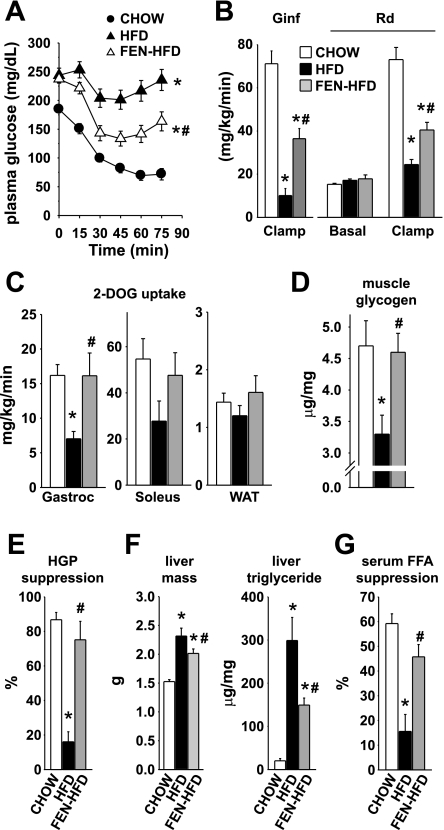
Fig. 3.
Fenretinide partially prevents HFD-induced insulin resistance in skeletal muscle and liver. A: insulin tolerance test at 14 wk of diet (CHOW, ○; HFD, ▴; FEN-HFD, ▵) in male FVB mice put on diet from 4–5 wk of age. B–F: euglycemic-hyperinsulinemic (10 mU·kg−1·min−1 insulin) clamp at 16–18 wk diet (CHOW, open bar; HFD, filled bar; FEN-HFD, gray bar). B: insulin-stimulated glucose infusion rate (Ginf) and basal and insulin-stimulated whole body glucose uptake (Rd). C: insulin-stimulated glucose uptake into skeletal muscle (gastrocnemius and soleus) and WAT. D: glycogen content in tibialis anterior muscle at the end of the clamp. E: insulin-stimulated suppression of hepatic glucose production. F: liver mass (left) and liver triglyceride levels (right) at 16–18 wk of diet post-euglycemic-hyperinsulinemic clamp. G: insulin-stimulated suppression of serum FFA levels (%suppression relative to basal) at 16–18 wk of diet during euglycemic-hyperinsulinemic clamp. Results are means ± SE from 11–14 mice per dietary group. *P < 0.05 vs. CHOW, #P < 0.05 vs. HFD.
In the 5-h-fasted “basal” state, plasma insulin in HFD mice was elevated 4.5-fold over CHOW values (Table 1). In FEN-HFD mice, plasma insulin was 57% lower than in HFD mice and not significantly different from that of CHOW mice. Under euglycemic-hyperinsulinemic conditions, HFD mice displayed severe insulin resistance, with an 86% reduction in glucose infusion rate (GINF) compared with chow mice (Fig. 3B). Fenretinide treatment improved GINF 3.6-fold. Clamp data closely mirrored other indexes of insulin sensitivity at 13–14 wk on HFD (Table 2 and Fig. 3A). Thus, overall, Fenretinide's insulin-sensitizing actions in FVB mice started by 13 wk on HFD treatment and thus preceded Fenretinide's action to reduce weight gain in obese FVB mice.
Fenretinide preserved insulin action on glucose metabolism in liver and skeletal muscle in HFD-induced obese FVB mice.
Basal (5-h-fasted) whole body glucose uptake (Rd) was similar in all three groups of mice (Fig. 3B). In CHOW mice, insulin infusion stimulated whole body glucose uptake 4.8-fold over basal (Fig. 3B). In HFD mice, insulin-stimulated whole body glucose uptake was impaired 66%, and Fenretinide partially prevented this, in agreement with GINF data. Insulin-stimulated 2-deoxyglucose uptake into gastrocnemius was reduced 57% in HFD mice, and this effect was prevented by Fenretinide treatment (Fig. 3C). Similar trends were observed in soleus and WAT. Glycogen content in skeletal muscle at the end of the clamp was reduced (30%) in HFD mice compared with CHOW animals, and this effect was also prevented by Fenretinide treatment (Fig. 3D).
In the basal state, endogenous glucose production (mainly hepatic, HGP) was similar in all diet groups (Table 1). Insulin infusion suppressed HGP 87% in CHOW mice but only 16% in HFD mice, indicating marked hepatic insulin resistance (Table 1 and Fig. 3E). Remarkably, Fenretinide completely prevented this defect despite the obese phenotype of FEN-HFD mice (Table 1 and Fig. 3E). Livers were ∼60% heavier in HFD mice but only 40% heavier in FEN-HFD compared with CHOW (Fig. 3F, left). Accordingly, triglyceride accumulation in liver of HFD mice was increased 15-fold compared with CHOW controls. This severe hepatic steatosis was reduced by 50% with Fenretinide treatment (Fig. 3F, right). Thus, Fenretinide reduces HFD-induced hepatic steatosis and nearly normalizes insulin action on HGP even when hepatic lipid content is increased sevenfold above levels in CHOW mice.
Some retinoids used for cancer or acne treatment cause hypertriglyceridemia (7, 13). In this study, Fenretinide did not increase circulating triglycerides, FFAs, or glycerol (Table 2). FFA levels in FEN-HFD and HFD mice were comparable to CHOW values in the fed and 5-h-fasted states, (Tables 1 and 2). During the clamp, insulin infusion suppressed serum FFA levels 59% in CHOW mice but only 12% in HFD mice (Fig. 3G and Table 1). Strikingly, Fenretinide completely prevented this defect (Table 1 and Fig. 3G). Thus, Fenretinide does not cause hypertriglyceridemia as other retinoids do, but instead preserves normal insulin action on lipid homeostasis in obese mice.
Many retinoids, including retinoic acid, cause hepatotoxicity (32, 40). In contrast, Fenretinide treatment in HFD mice did not increase plasma levels of aspartate amino transferase and alanine aminotransferase, two hepatic enzymes that are used as indexes of hepatocyte damage (Table 1).
Fenretinide reversed obesity and insulin resistance in HFD-fed mice: an intervention study.
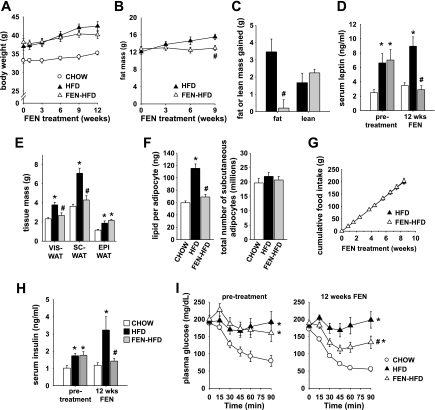
Fenretinide's potential therapeutic value would be increased if, in addition to its preventive actions, Fenretinide could reverse or slow down the progression of existing obesity and insulin resistance. Thus, we performed an interventional study in FVB mice with established HFD-induced obesity and insulin resistance. After 32 wk on HFD, obese mice were randomized into groups matched for body weight, adiposity, plasma insulin levels, and insulin tolerance and either continued on HFD alone or given FEN-HFD. Remarkably, Fenretinide treatment of obese mice for 12 wk stopped the progression of obesity (Fig. 4, A–C). Body weight gain tended to be slightly less in mice switched to FEN-HFD compared with HFD alone (Fig. 4A). Fenretinide treatment totally suppressed gain of fat mass (Fig. 4, B and C), whereas gain of lean mass was unchanged (Fig. 4C). Leptin levels in HFD mice prior to Fenretinide intervention were increased more than twofold compared with CHOW mice (Fig. 4D). Strikingly, Fenretinide intervention in FEN-HFD mice for 12 wk normalized to CHOW values not only hyperleptinemia (Fig. 4D) but also both visceral and subcutaneous fat pad masses (Fig. 4E). In contrast, epididymal WAT mass in both HFD and FEN-HFD mice was approximately twofold greater than in CHOW mice. Fenretinide specifically suppressed HFD-induced accumulation of lipid in subcutaneous adipocytes (Fig. 4F, left), whereas adipocyte number in subcutaneous WAT was similar in all diet groups (Fig. 4F, right). Fenretinide treatment did not alter cumulative food intake over the whole intervention period (Fig. 4G) or stool lipid content (data not shown).
Fig. 4.
Fenretinide treatment ameliorates obesity and insulin resistance in diet-induced obese mice. A: body weight curves of HFD-induced obese male FVB mice treated with Fenretinide (0.1% wt/wt HFD, FEN-HFD, ▵), untreated HFD-induced obese mice (HFD, ▴), and CHOW-fed controls (○) from 26–27 wk of age. B: fat mass during the course of Fenretinide treatment. (HFD, ▴; FEN-HFD, ▵). C: total fat mass gained and lean mass gained at 9 wk of Fenretinide treatment (HFD, filled bar; FEN-HFD, gray bar). D: serum leptin levels before and at 12 wk of Fenretinide treatment (CHOW, open bar; HFD, filled bar; FEN-HFD, gray bar). E: VIS-, SC-, and EPI-WAT weights at 12 wk of Fenretinide treatment. F: average size of adipocytes in SC-WAT expressed as amount of lipid per adipocyte (left) and number of adipocytes in SC-WAT depot per mouse (right). G: cumulative food intake during first 9 wk of Fenretinide treatment. H: serum insulin levels before and at 12 wk of Fenretinide treatment (5-h-fasted levels). I: insulin tolerance test pretreatment and at 12 wk of Fenretinide treatment (CHOW, ○; HFD, ▴; FEN-HFD, ▵). Results are means ± SE from 8–10 mice per dietary group. *P < 0.05 vs. CHOW, #P < 0.05 vs. HFD. Statistical symbols indicate a difference for the curve.
Serum insulin was 75% higher in HFD mice than in CHOW pretreatment and increased to threefold higher over the next 12 wk of diet. Fenretinide not only prevented that progression, but normalized HFD-induced hyperinsulinemia to CHOW values (Fig. 4H), indicating improved insulin sensitivity even though adiposity was still somewhat increased in FEN-HFD mice compared with CHOW. Accordingly, Fenretinide treatment improved insulin tolerance at 12 wk of treatment (Fig. 4I). Thus, Fenretinide can both prevent and reverse obesity and insulin resistance in HFD mice.
Fenretinide partially prevented HFD-induced obesity in RBP4KO mice.
We recently showed (49) that elevated serum RBP4 levels contribute to insulin resistance and that lowering serum RBP4 levels genetically or pharmacologically (with Fenretinide) improves insulin sensitivity independently of changes in body weight. However, since longer treatment with Fenretinide dramatically reduces adipose mass and serum leptin levels, we hypothesized that Fenretinide has other mechanisms for its metabolic actions in addition to lowering serum RBP4 levels. Therefore, we investigated whether Fenretinide can prevent HFD-induced obesity in RBP4KO mice and in their WT controls on the C57BL/6J × 129Sv mixed background.
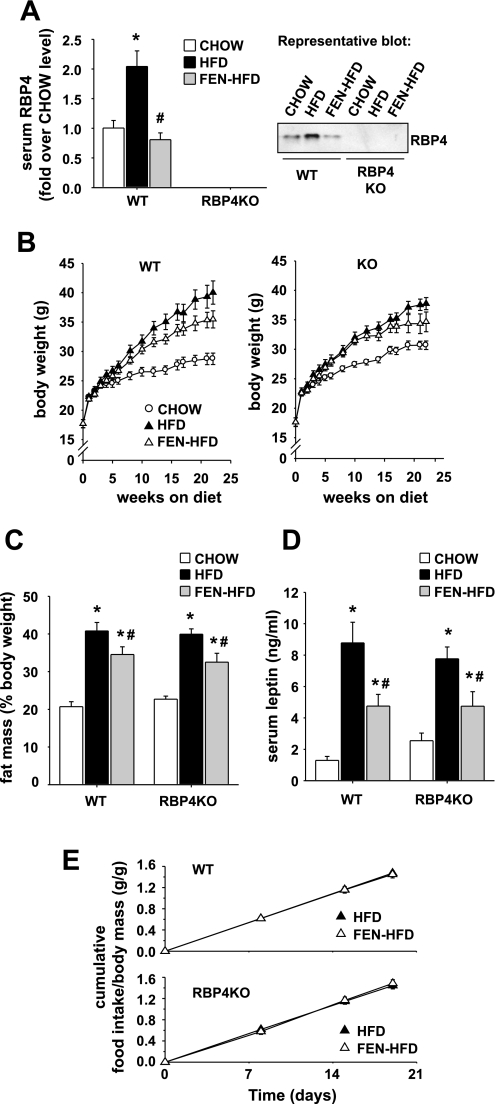
Twenty-two weeks of HFD treatment in WT mice elevated serum RBP4 levels twofold, whereas Fenretinide treatment prevented this (Fig. 5A). RBP4 was expectedly absent in sera from all RBP4KO groups (Fig. 5A). WT mice on FEN-HFD diet gained less weight than on HFD without Fenretinide over the diet treatment period (Fig. 5B, left, P < 3 × 10e−8, repeated-measures ANOVA), and body weights were significantly lower after 21 wk of Fenretinide treatment (Fig. 5B, left, P < 0.05, ANOVA with Bonferroni post hoc test). Similarly, RBP4KO mice on FEN-HFD diet gained less weight than on HFD without Fenretinide [Fig. 5B, right, over the diet treatment period (P < 2 × 10e−7, repeated-measures ANOVA)]. Furthermore, Fenretinide treatment in WT mice reduced fat mass gain induced by HFD (Fig. 5C) without a change in lean mass (data not shown). In RBP4KO mice, Fenretinide similarly limited the increase of fat mass elicited by HFD diet treatment (Fig. 5C). After 22 wk of HFD, fed serum leptin levels were substantially increased in both WT HFD and RBP4KO HFD mice compared with their respective CHOW controls. This effect was strongly attenuated by Fenretinide treatment in both genotypes (Fig. 5D). Fenretinide treatment did not alter cumulative food intake in either WT or RBP4KO HFD mice (measured at 17–20 wk on the diet when body weights had begun to diverge) even when expressed per gram of body weight (Fig. 5E).
Fig. 5.
Fenretinide partially prevents HFD-induced obesity in mice lacking serum RBP4. A: serum RBP4 levels in WT and retinol-binding protein-4 knockout (RBP4KO) male mice on C57BL/6J × 129Sv mixed background after 22 wk of either standard CHOW (open bar), HFD (filled bar), or HFD + Fenretinide (0.1% wt/wt; FEN-HFD, gray bar) from 4–5 wk of age. Right: representative Western blot. B: body weight progression of WT and RBP4KO mice on either CHOW (○), HFD (▴), or FEN-HFD (▵) from 4–5 wk of age. In both WT and RBP4KO genotypes, body weight progression was significantly different among all 3 treatment groups (repeated-measures ANOVA, P < 2 × 10e−7). Body weights in WT FEN-HFD were significantly different from WT HFD at weeks 21 and 22 of diet treatment (P < 0.05, ANOVA with Bonferroni post hoc test). C: body fat content at 20 wk on diet. (CHOW, open bar; HFD, filled bar; FEN-HFD, gray bar). D: ad libitum-fed serum leptin levels at 22 wk on diet. E: cumulative food intake during 17–20 wk of diet. Results are means ± SE from 10–15 mice per dietary group. *P < 0.05 vs. CHOW, #P < 0.05 vs. HFD.
DISCUSSION
Insulin resistance is closely associated with weight gain and obesity. We (49) and others (51) recently showed that altering RBP4 levels genetically or pharmacologically results in alterations in insulin sensitivity without changes in adiposity. Treatment with the synthetic retinoid Fenretinide inhibits the severity of insulin resistance in mice fed an HFD for 16 wk without affecting weight gain (49). Here, we now show that, with prolonged exposure to HFD (34 wk), Fenretinide prevents the severity of obesity. The antiobesity effects progress over time and do not involve a measurable alteration in either caloric intake or energy expenditure. There are a number of examples of genetically modified mice for which the obese phenotype could not be accounted for by measurable alterations in either energy intake or energy expenditure (1). Accordingly, a mismatch in energy balance of only a few percent over months can lead to a difference in adipose tissue accretion of several grams.
Fenretinide's total inhibition of visceral and subcutaneous WAT expansion occurred in both the 34-wk prevention study and the 12-wk intervention study and is probably a major contributor to the improved insulin sensitivity and markedly lower leptin levels in FEN-HFD mice. Reduction of visceral WAT mass is highly correlated with improved insulin sensitivity in numerous clinical studies (50). The antiobesity action of Fenretinide appears to be independent of its ability to lower circulating RBP4, since Fenretinide reduces obesity development in mice lacking RBP4 (Fig. 5). Furthermore, altering RBP4 levels alone does not affect adiposity, since RBP4KO mice are not leaner on CHOW (Fig. 5C and Ref. 49) or resistant to fat gain on HFD (Fig. 5C). The effects of RBP4 on insulin action are not dependent on alterations in adiposity, since transgenic or pharmacological elevations in serum RBP4 levels result in insulin resistance without accompanying changes in body weight (49). Since RBP4KO mice have enhanced insulin sensitivity with normal adiposity compared with WT controls, lowering RBP4 improves insulin sensitivity independently of changes in adiposity (49), while data in this study show that, in RBP4KO HFD mice, Fenretinide also has an RBP4-independent effect on adiposity per se (Fig. 5).
Whether the mechanism of Fenretinide action on adiposity involves altering retinoid transport or metabolism is unclear. In livers from vitamin A-deficient rats, Fenretinide increases the activity of lecithin:retinol acyltransferase (LRAT) (27), which catalyzes the conversion of retinol to retinyl ester for storage. However, retinyl ester stores in liver were not changed with Fenretinide treatment in WT mice on a vitamin A-sufficient diet in two relatively short-term studies (26, 30), and in vitro studies reported that Fenretinide inhibited the esterification of retinol (2, 8). Preliminary results in our mice indicate that prolonged Fenretinide treatment reduces retinyl ester storage in liver in mice on HFD (B. B. Kahn, N. Mody, F. Preitner, T. E. Graham, and W. S. Blaner, unpublished observation; not shown). It seems possible that alterations in enzymes affecting retinol esterification might also indirectly affect esterification and storage of other lipids in liver, or Fenretinide may directly inhibit enzymes involved in hepatic triglyceride formation, thereby contributing to the reduced hepatic steatosis that we observe in FEN-HFD mice.
The fact that Fenretinide's antiadiposity effects are largely independent of its RBP4-lowering effect does not exclude the possibility that the antiadiposity effects involve alterations in retinoid metabolism. Fenretinide competition with retinoic acid for CYP26A1 (or other members of the family of cytochrome P-450 enzymes that degrade retinoids) may lead to increases in tissue retinoic acid levels (44, 47). Retinoic acid can inhibit or promote adipogenesis depending on the stage at which preadipocytes are exposed (41, 48). With early exposure, retinoic acid can completely inhibit adipogenesis by inhibiting CCAAT-enhancer-binding protein-β (39). Since Fenretinide preferentially accumulates in adipose and mammary tissue (18, 29, 43), it could inhibit adipose tissue expansion via increases in retinoic acid signaling in adipocytes. Alternatively, it could induce apoptosis, as is seen with Fenretinide treatment of cancer cells. However, we found that the total number of adipocytes per fat depot was not changed in our experiments, suggesting that the Fenretinide effects to reduce adiposity are not due to inhibition of adipogenesis or apoptosis of adipocytes in vivo.
Our euglycemic-hyperinsulinemic clamp studies showed that Fenretinide markedly prevented hepatic insulin resistance and improved glucose uptake in muscle but only partly prevented whole body insulin resistance (Rd and GINF). The mechanism of Fenretinide's effects on hepatic insulin sensitivity may involve partial reduction in hepatic steatosis and lowering RBP4, since elevated RBP4 induces PEPCK expression and impairs insulin action to suppress glucose production in cultured hepatocytes (49). Fenretinide's near normalization of insulin action on HGP in HFD-fed mice is particularly impressive, since it occurs even with a considerable residual increase in hepatic steatosis compared with CHOW-fed mice. The effects on insulin action in muscle may be due to improved insulin signaling with Fenretinide treatment (49). We previously published (49) that insulin stimulated phosphorylation of IRS-1 threefold on Tyr612 in skeletal muscle of chow-fed FVB mice; this response was reduced by more than 50% in HFD mice, but Fenretinide completely prevented this impairment in IRS-1 phosphorylation. We did not observe differences in the expression of IRS-1 or the expression or tyrosine phosphorylation of the insulin receptor (data not shown). Thus, improved post-receptor insulin signaling in muscle may contribute to the improved insulin sensitivity parameters seen in Fenretinide-treated mice (e.g., glucose infusion rate and 2-deoxyglucose uptake into muscle during the hyperinsulinemic-euglycemic clamp).
There is an urgent need for efficient and non toxic drugs to treat obesity and insulin resistance. Unlike other retinoids, Fenretinide is relatively nontoxic and has been tested in phase III clinical trials for cancer for periods of up to 5 years (11, 38). Moreover, a recent trial found that prolonged Fenretinide treatment in overweight premenopausal women improved insulin sensitivity and decreased serum leptin levels, suggestive of a decrease in fat stores (21). In addition, in normal-weight women who showed a decrease in insulin sensitivity, Fenretinide prevented an increase in serum triglyceride levels (21). Thus, Fenretinide could be a novel and safe drug not only for treatment of type 2 diabetes but also for prevention and treatment of obesity and dyslipidemia. The antiobesity effects appear to be independent of Fenretinide's RBP4-lowering effects.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37 DK-43051, P01 DK-56116 and P30 DK-57521 and a research grant from Takeda Pharmaceutical (to B. B. Kahn), postdoctoral fellowships from the Swiss National Foundation, no. PAOOA-101447 (to F. Preitner), and American Heart Association and American Diabetes Association-European Association for the Study of Diabetes (to N. Mody), and Grants K08 DK-69624 and R03 DK-080195 and the Smith Family New Investigator Award (to T. E. Graham).
DISCLOSURES
B. B. Kahn, T. E. Graham, and O. D. Peroni are inventors on a patent related to RBP4. B. B. Kahn has a research grant from Takeda Pharmaceutical Co.
ACKNOWLEDGMENTS
We thank Simon J. Fisher for invaluable discussions about the euglycemic clamp technique, Anna Lee for expert technical help, and Bill Blaner, Loredana Quadro, and Max Gottesman for the RBP4 knockout mice and for helpful discussions about retinoid biology.
Current address of F. Preitner: Cardiomet Mouse Metabolic Facility, University of Lausanne, Lausanne, Switzerland.
Current address of N. Mody: Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen, Scotland, UK.
REFERENCES
- 1.Arch JR. Lessons in obesity from transgenic animals. J Endocrinol Invest 25: 867–875, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ball MD, Furr HC, Olson JA. Enhancement of acyl coenzyme A:retinol acyltransferase in rat liver and mammary tumor tissue by retinyl acetate and its competitive inhibition by N-(4-hydroxyphenyl) retinamide. Biochem Biophys Res Commun 128: 7–11, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Berni R, Formelli F. In vitro interaction of fenretinide with plasma retinol-binding protein and its functional consequences. FEBS Lett 308 1: 43–45, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 11: 2457–2461, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cushman SW, Salans LB. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res 19: 269–273, 1978 [PubMed] [Google Scholar]
- 7.Davies PJ, Berry SA, Shipley GL, Eckel RH, Hennuyer N, Crombie DL, Ogilvie KM, Peinado-Onsurbe J, Fievet C, Leibowitz MD, Heyman RA, Auwerx J. Metabolic effects of rexinoids: tissue-specific regulation of lipoprotein lipase activity. Mol Pharmacol 59: 170–176, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Dew SE, Wardlaw SA, Ong DE. Effects of pharmacological retinoids on several vitamin A-metabolizing enzymes. Cancer Res 53: 2965–2969, 1993 [PubMed] [Google Scholar]
- 9.Fontana JA, Rishi AK. Classical and novel retinoids: their targets in cancer therapy. Leukemia 16: 463–472, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M. Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res 49: 6149–6152, 1989 [PubMed] [Google Scholar]
- 11.Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, Veronesi U. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol 11: 2036–2042, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab 92: 1886–1890, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gerber LE, Erdman JW., Jr Retinoic acid and hypertriglyceridemia. Ann NY Acad Sci 359: 391–392, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354: 2552–2563, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis 11: 1677–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res 9: 110–119, 1968 [PubMed] [Google Scholar]
- 17.Hu C, Jia W, Zhang R, Wang C, Lu J, Wu H, Fang Q, Ma X, Xiang K. Effect of RBP4 gene variants on circulating RBP4 concentration and type 2 diabetes in a Chinese population. Diabet Med 25: 11–18, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hultin TA, May CM, Moon RC. N-(4-hydroxyphenyl)-all-trans-retinamide pharmacokinetics in female rats and mice. Drug Metab Dispos 14: 714–717, 1986 [PubMed] [Google Scholar]
- 19.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes 55: 2805–2810, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K. Association of serum retinol binding protein 4 and visceral adiposity in chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab 92: 3224–3229, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Johansson H, Gandini S, Guerrieri-Gonzaga A, Iodice S, Ruscica M, Bonanni B, Gulisano M, Magni P, Formelli F, Decensi A. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res 68: 9512–9518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, Zong H, Dong J, Kahn CR, Kahn BB, Shulman GI. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest 108: 153–160, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest 114: 1666–1675, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JW, Lee HR, Shim JY, Im JA, Lee DC. Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocr J 55: 811–818, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lewis KC, Zech LA, Phang JM. Effects of N-(4-hydroxyphenyl)retinamide supplementation on vitamin A metabolism. Cancer Res 54: 4112–4117, 1994 [PubMed] [Google Scholar]
- 27.Matsuura T, Zhao Z, Ross AC. N-(4-hydroxyphenyl)-retinamide increases lecithin:retinol acyltransferase activity in rat liver. J Nutr 126: 2474–2480, 1996 [DOI] [PubMed] [Google Scholar]
- 28.McTernan PG, Kumar S. Editorial: Retinol binding protein 4 and pathogenesis of diabetes. J Clin Endocrinol Metab 92: 2430–2432, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mehta RG, Moon RC, Hawthorne M, Formelli F, Costa A. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur J Cancer 27: 138–141, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Moon RC, Thompson HJ, Becci PJ, Grubbs CJ, Gander RJ, Newton DL, Smith JM, Phillips SL, Henderson WR, Mullen LT, Brown CC, Sporn MB. N-(4-hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res 39: 1339–1346, 1979 [PubMed] [Google Scholar]
- 31.Motani A, Wang Z, Conn M, Siegler K, Zhang Y, Liu Q, Johnstone S, Xu H, Thibault S, Wang Y, Fan P, Connors R, Le H, Xu G, Walker N, Shan B, Coward P. Identification and characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo. J Biol Chem 284: 7673–7680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muenter MD, Perry HO, Ludwig J. Chronic vitamin A intoxication in adults. Hepatic, neurologic and dermatologic complications. Am J Med 50: 129–136, 1971 [DOI] [PubMed] [Google Scholar]
- 33.Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Erdenebulgan B, Zolzaya K, Lkhagvasuren T, Iwamoto S. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet 120: 879–888, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab 92: 4827–4834, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 18: 4633–4644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93, Suppl 1: S64–S73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab 93: 2287–2293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotmensz N, De Palo G, Formelli F, Costa A, Marubini E, Campa T, Crippa A, Danesini GM, Delle Grottaglie M, Di Mauro MG. Long-term tolerability of fenretinide (4-HPR) in breast cancer patients. Eur J Cancer 27: 1127–1131, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol 17: 1552–1561, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med 294: 805–808, 1976 [DOI] [PubMed] [Google Scholar]
- 41.Stone RL, Bernlohr DA. The molecular basis for inhibition of adipose conversion of murine 3T3-L1 cells by retinoic acid. Differentiation 45: 119–127, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Subcommittee on Laboratory Animal Nutrition. Committee on Animal Nutrition. Board on Agriculture, National Research Council Nutrient Requirements of Laboratory Animals Washington, DC: National Academy Press, 1995 [Google Scholar]
- 43.Swanson BN, Zaharevitz DW, Sporn MB. Pharmacokinetics of N-(4-hydroxyphenyl)-all-trans-retinamide in rats. Drug Metab Dispos 8: 168–172, 1980 [PubMed] [Google Scholar]
- 44.Taimi M, Breitman TR. N-4-hydroxyphenylretinamide enhances retinoic acid-induced differentiation and retinoylation of proteins in the human acute promyelocytic leukemia cell line, NB4, by a mechanism that may involve inhibition of retinoic acid catabolism. Biochem Biophys Res Commun 232: 432–436, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie 87: 57–64, 2005 [DOI] [PubMed] [Google Scholar]
- 45a.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 51: 1423–1428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veterans Medical Research Foundation, University of California, San Diego A Randomized, Double-Blind Study of the Effects of Fenretinide Administered in Subjects With Obesity. ClinicalTrials.gov Identifier: NCT00546455 [Online] http://clinicaltrials.gov/ct2/show/study/NCT00546455.
- 47.Villani MG, Appierto V, Cavadini E, Valsecchi M, Sonnino S, Curley RW, Formelli F. Identification of the fenretinide metabolite 4-oxo-fenretinide present in human plasma and formed in human ovarian carcinoma cells through induction of cytochrome P450 26A1. Clin Cancer Res 10: 6265–6275, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Xue JC, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARgamma. Mol Cell Biol 16: 1567–1575, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436 (7049): 356–362, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia 50: 1127–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Yu XX, Watts LM, Manchem P, Monia BP, Bhanot S. Antisense reduction of retinol-binding protein 4 expression in liver and adipose tissues causes robust improvements in insulin sensitivity in diabetic and obese mice. American Diabetes Association 68th Scientific Sessions 59-LB, 2008 [Google Scholar]
- 52.Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A. Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer 13: 51–68, 2006 [DOI] [PubMed] [Google Scholar]