Abstract
Glutamine (GLN) plays a key role in cellular protection following injury via enhancement of heat shock protein 70 (HSP70). The pathway by which GLN enhances HSP70 is unknown. GLN is a key substrate for the hexosamine biosynthetic pathway (HBP), which has been shown to induce HSP70. We sought to explore the role of the HBP in GLN-mediated HSP70 expression. Both chemical inhibitors and small interfering (si)RNA knockdown of key HBP enzymes were used in mouse embryonic fibroblast cells to determine the effects of the HBP on HSP70 expression. The O-glycosylation, nuclear translocation, and transcriptional activation of heat shock factor-1 (HSF-1) and Sp1 were evaluated using immunoprecipitation, Western blotting, and luciferase assays. HSP70 expression levels were evaluated via ELISA and Western blotting. GLN augmented HBP activity before and after heat stress (HS). Chemical inhibition of HBP enzymes reduced GLN-mediated HSP70 expression. Specific siRNA targeting of the key HBP enzyme UDP-N-acetylglucosamine (GlcNAc): polypeptide-O-β-acetylglucosaminyltransferase (OGT) blocked GLN-mediated HSP70 expression and attenuated GLN-mediated cellular protection post-HS. Chemical and siRNA attenuation of the HBP blocked GLN-induced nuclear translocation of Sp1 and HSF-1, which are key to maximal HSP70 expression. Finally, immunoprecipitation revealed HSF-1 was O-glycosylated, and GLN enhanced this effect. These results suggest that metabolism of GLN via the HBP enhances HSP70 expression. This effect appears to be mediated via O-glycosylation, nuclear translocation, and transcriptional activation of Sp1 and HSF-1. This is an important mechanistic description of a pathway that appears responsible for GLN-mediated HSP70 expression.
Keywords: heat shock proteins, O-GlcNAc, O-glycosylation, heat shock factor-1, cell protection, cell survival, cell injury
heat shock proteins (HSPs) are highly conserved proteins involved in the most basic mechanisms of cellular protection. HSP expression following a sublethal insult can induce “stress tolerance” and provide protection from subsequent stress that would otherwise be lethal (8, 13, 19, 20). Previous data indicate that the induction of HSPs (particularly HSP70) can provide significant protection against many forms of injury (8, 13, 19, 20). However, these findings have yet to be applied in a clinical setting because common laboratory inducers of HSPs are not safe for human administration. Our laboratory has shown that glutamine (GLN) can safely enhance HSP expression in in vitro and in vivo settings (9, 16, 18, 23, 24). Using genetic knockout models, we have also established that HSP induction is necessary for GLN's beneficial effect following injury (9, 18). Animals and cells that lack the HSP70 gene or the heat shock factor-1 (HSF-1) gene show no survival benefit with GLN administration (9, 18). However, the pathway by which GLN induces HSP expression is currently unknown.
Recent data have revealed that activation of the hexosamine biosynthetic pathway (HBP) can induce HSP70 expression (29). Many key cellular proteins and transcription factors are glycosylated by the HBP, particularly following stress or injury (26). This pathway appears to be part of an early cellular protective response to stress because this protein modification can occur rapidly (29). Known transcription factors modified by the HBP include Sp1, a key transcription factor required for optimal HSP expression following stress (4, 7). The O-glycosylation of Sp1 causes its translocation to the nucleus, at which point it can then become active and induce gene promoter activity (7). As previously mentioned, this finding is vital to HSP expression because expression of heat HSF-1, HSP70, and HSP27 all rely on Sp1 binding in their promoter sequences for optimal transcriptional activity (1, 10, 14).
GLN is a key substrate required for optimal activity of the HBP (2, 28). Furthermore, recent evidence reveals that GLN stimulates expression of the argininosuccinate synthetase (ASS) gene via O-glycosylation of Sp1 (2). Thus, precedent exists for GLN utilizing the HBP to modify gene expression via Sp1. Because HSF-1 promoter binding is also vital to optimal HSP70 expression (12), we hypothesize that HSF-1 may also be O-glycosylated and translocated into the nucleus following GLN administration. On the basis of the aforementioned preliminary data and literature, we investigated the possible link between GLN administration and activation of HSP70 via the HBP in vitro.
Our results reveal that the molecular pathway responsible for GLN-mediated HSP70 induction appears to depend on GLN's metabolism via the HBP. These data also demonstrate that HSF-1, a key factor in HSP expression, can be O-glycosylated.
EXPERIMENTAL PROCEDURES
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified.
Cells.
Murine embryonic fibroblasts (MEF) cells were grown in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids solution (Cellgro Mediatech, Herndon, VA), 2 mM l-GLN (Sigma), and 10 ml/l of antibiotic solution containing penicillin G (10,000 U/ml) and streptomycin (10,000 μg/ml) (Cellgro Mediatech). Cultured cells were maintained in a humidified 37°C incubator with 5% CO2. Four main study groups were run for every experiment: 0 mM GLN control cells (CT), 8 mM GLN-treated cells (GLN), heat-stressed 0 mM GLN control cells (HS CT), and heat-stressed 8 mM GLN-treated cells (HS GLN).
Heat-stress injury.
Before heat stress, standard media were replaced with either 0 or 8 mM GLN DMEM (no FBS) for 15 min. For cell viability, 96-well plates were submerged in a 44°C water bath for 50 min and allowed to recover at 37°C for 24 h. For protein expression and microscopy experiments, cells were subjected to a nonlethal heat stress at 43°C for 45 min and then allowed to recover for either 15–30 min (for measurement of global protein O-glycosylation) or 4 h [for HSP70 and O-β-acetylglucosaminyltransferase (OGT) expression]. For HSF-1 and SP1 reporter plasmid luciferase assays, cells were allowed to recover for 24 h.
Western blot analysis.
Cells were washed and harvested in ice-cold PBS and lysed in a solution containing 10 mM Tris (pH 7.2–8), 5 mM MgSO4, DNAse, and RNAse, per manufacturer's instructions (New England Biolabs, Ipswich, MA), plus protease inhibitors (Roche, Indianapolis, IN). For HSF-1 levels and localization, cells were lysed with the Pierce Nuclear/Cytoplasmic fractionation kit (catalog no. 78833, Pierce, Rockford, IL). Protein was determined with BCA protein assay (Pierce). Twenty micrograms of each sample were added to a 1× treatment buffer (0.125 M Tris, pH 6.8, 4% sodium dodecylsulfate, 20% glycerol, and 10% β-mercaptoethanol), boiled for 3 min, and then loaded into a NuPAGE 4–12% Bis-TrisGel (Invitrogen, Carlsbad, CA). Proteins were electrophoretically separated with a mini-gel system and transferred to polyvinylidine difluoride membranes (Millipore, Billerica, MA), using the semi-dry transfer system (Invitrogen). Membranes were blocked with either 5% nonfat milk in PBS-Tween for HSP70 HSF-1 and OGT antibodies or 5% bovine serum albumin (BSA) in PBS-Tween for O-linked N-acetylglucosamine (O-GlcNAc) antibody for 2 h at room temperature. Primary antibodies against HSP70 (1:25,000 dilution) (catalog no. SPA812; StressGen, Victoria, BC, Canada), HSF-1 (1:1,000) (catalog no. 4356; Cell Signaling Technology, Danvers, MA), OGT (1:1,000) (catalog no. 06264; Sigma-Aldrich), or O-GlcNAc (1:1,000) (catalog no. MMS248R; Covance, Princeton, NJ) were added to antibody buffer (blocking solution) and incubated overnight at 4°C. After washing three times with PBS-Tween over 30 min, secondary antibodies, peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG (Pierce), were applied at a 1:3,000 dilution for 2 h. Blots were washed three times with PBS-Tween over 30 min, incubated in commercial enhanced chemiluminescence reagents (Pierce), and exposed with utilizing a UVP chemiluminescent darkroom system (UVP, Upland, CA). Densitometry was normalized against β-actin (1:50,000) (Sigma-Aldrich).
Chemical inhibitors and small interfering RNA OGT silencing.
Known chemical inhibitors and/or small interfering (si)RNA against the three main enzymes in this pathway were utilized to determine the effects of the HBP in GLN-mediated cellular protection and HSP expression (Fig. 1). Groups were added to include cells pretreated with 40 μM 6-diazo-5-oxo-l-norleucine (DON) for 18 h to inhibit the enzyme glutamine fructose-6-amidotransferase (GFAT) or 1 mM alloxan for 1 h to inhibit UDP-GlcNAc:polypeptide-O-β-acetylglucosaminyltransferase (OGT). Since these inhibitors have limitations, potentially causing other “off target” effects on the cells, siRNA was utilized to bolster these data and to prove the specific role of the OGT enzyme in GLN-mediated cellular protection and HSP expression. For siRNA experiments, cells were seeded in either 96-well plates (for survival studies) or 10-cm dishes (for protein expression) and allowed to grow for 24 h (50–60% confluence) in full media. Media were changed to DMEM (with 2 mM GLN) plus 10% FBS only, and cells were transfected for 48 h using SilentFect (Bio-Rad, Hercules, CA) with either no RNA, OGT siRNA (20 nM), or control noncoding (NC) oligos (20 nM) with a comparable GC content to the OGT siRNA (Invitrogen). Cells were pretreated with DMEM (with 0 mM GLN) plus 10% FBS only 24 h before heat stress (transfection reagents still present). Cells were then treated with 0 or 8 mM GLN and subjected to heat stress, as previously described.
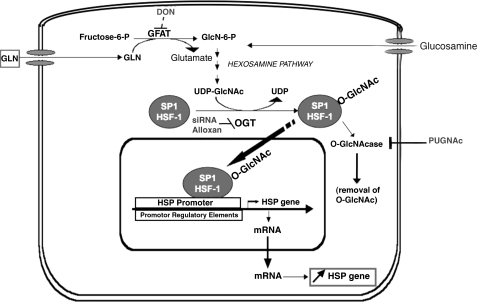
Fig. 1.
Proposed mechanism for glutamine (GLN)-mediated induction of heat shock protein 70 (HSP70). GLN is metabolized via the hexosamine biosynthetic pathway (HBP) to UDP-N-acetylglucosamine (GlcNAc). Key enzymes in hexosamine pathway are glutamine fructose-6-amidotransferase (GFAT), polypeptide-O-β-acetylglucosaminyltransferase (OGT), and O-N-acetylglucosaminidase (O-GlcNAcase). Glucosamine can enter pathway distal to the GFAT enzyme. The inhibitors utilized to inhibit GFAT [6-diazo-5-oxonorleucine (DON)], OGT [alloxan and targeted small interfering (si)RNA], and O-GlcNAcase [O-(2-acetamido-2-deoxy-D-glucopyranosylidene)-amino-N-phenylcarbamate (PUGNAc)] are shown. HSF-1, heat shock factor 1.
N-acetylglucosaminidase (O-GlcNAcase) inhibition.
To further evaluate the role of enhanced O-glycosylation on HSP70 expression, cells were treated with an inhibitor that prevents the removal of O-GlcNAc from intracellular proteins (Fig. 1). One-hundred micromolar O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino N-phenyl carbamate (PUGNAc) (Toronto Research Chemicals, Toronto, ON, Canada) was added to cells 1 h before GLN treatment, and heat stress was carried out as previously described. Western blots were run to analyze differences in HSP70 expression.
HSP70 ELISA.
HSP70 expression was also evaluated via HSP70 ELISA (catalog no. EKS700, StressGen). Cells were treated with chemical inhibitors DON or alloxan, as previously described, with either 0 or 8 mM GLN, heat stressed, and allowed to recover for 4 h. Cells were collected, lysed, and assayed for total protein (as specified in the Western blot analysis section above). Ten micrograms of protein was used per well, and the ELISA was performed via manufacturer's instructions.
MTS cell proliferation assay.
Cells were grown in 96-well plates, and cell viability was measured using the CellTiter96 MTS assay (catalog no. G5421, Promega, Madison, WI) according to the manufacturer's recommendations. Briefly, 1 part PMS was added to 20 parts MTS immediately before the solution was diluted 1:5 in phenol red-free DMEM and was then added to PBS-washed cells. MTS was bioreduced by cells into a colored, soluble formazan product. Absorbance values were read after 2.5 h at 490 nm using an ELISA plate reader (Thermo Electro, San Jose, CA); references included readings at 650 nm and no-cell blank wells. Higher absorbance values reflect greater cell proliferation/viability. Control plates that were not subjected to heat stress were run in parallel to assess growth rates and survival effects of transfection reagents, treatments, and OGT silencing. All heat-stressed groups were normalized to their non-heat-stressed controls to account for these differences. The values for the same six wells for each treatment group were averaged per experiment, and the whole procedure was repeated six times (n = 6).
Digital fluorescence microscope.
Cells were seeded on glass four-well-chamber slides and allowed to grow for 48 h. Cells were then treated with 0 or 8 mM GLN in the presence or absence of chemical inhibitors, DON (40 μM) or alloxan (1 mM), and subjected to nonlethal heat stress. A subset of cells were treated with glucosamine and DON or glucosamine and alloxan. This was to determine whether glucosamine could bypass the inhibition of O-glycosylation by DON. Slides were fixed in a 4% paraformaldehyde-PBS solution for 15 min at room temperature. Cells were washed three times (10 min each) with PBS. Cell membranes were permeabilized in ice-cold acetone methanol (70% and 30%, respectively) for 10 min and then allowed to air dry. Slides were blocked in 10% normal donkey serum-PBS for 1 h at room temperature. Blocking solution was removed, and a primary antibody solution containing mouse anti-O-GlcNAc (catalog no. MA1-076, Affinity Bioreagents, Golden, CO), goat anti-SP1 (Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-HSF-1 (StressGen) in 1% BSA-PBS was added and incubated overnight at 4°C. Normal mouse, goat, and rabbit IgGs (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) were used as negative controls (at the same concentrations as the primaries) to assess nonspecific binding of the secondary antibodies for each species of primary antibody. Slides were again washed three times in PBS, and then Cy3-conjugated donkey anti-mouse, Cy5 donkey anti-goat (Jackson ImmunoResearch), and Alexa Fluor 488-conjugated donkey anti-rabbit (Molecular Probes/Invitrogen, Carlsbad, CA) were diluted in 1% BSA-PBS and incubated for 1 h at room temperature. Nuclei were stained with a 1 mg/100 ml bis benzimide (Hoeschst no. 33342, Sigma-Aldrich) solution for 30 s and then washed again in PBS three more times for 10 min each. Slides were mounted in antiquenching media and examined for nuclear translocation of HSF-1, SP1, and total cellular O-GlcNAc levels. Fifty cells per experimental group were “masked,” and mean fluorescence intensity (MFI) was measured in the cytoplasm and nucleus for each stain. Images were acquired with a Zeiss Axiovert digital confocal microscope (Carl Zeiss, Thornwood, New York), fitted with a Cooke charge-coupled device SensiCam (Cooke, Eugene, OR) camera, and evaluated using Intelligent Imaging Innovations, (Denver, CO), SlideBook software.
Immunoprecipitation of O-glycosylated HSF-1.
Cells were treated and heat stressed as previously described. Cells were harvested at 15 min after heat shock, lysed, and measured for protein content via BCA protein assay kit (catalog no. 23227, Pierce). Protein (200 μg) was loaded into a new microcentrifuge tube containing 3 μl (= 9–15 μg) of anti-O-GlcNAc monoclonal primary antibody (catalog no. MMS-248R, Covance, Berkeley, CA). Lysis buffer was used to make equal volumes for each group, and samples were incubated at room temperature for 1 h. Then, 16 μl A/G PLUS-Agarose immunoprecipitation (IP) reagent (catalog no. SC-2003, Santa Cruz Biotechnology) were added, and samples were incubated overnight at 4°C on a shaking apparatus. Controls were run with the absence of the primary antibody or the absence of the IP reagent for validation purposes. The IP protocol was followed as per manufacturer's instructions. Then, 20 μl of treatment buffer were added to the pellets, and samples were boiled for 3 min. Samples were loaded into a 10% Bis/Tris gel and were run, as above, with the Invitrogen system. Western blots were probed with rabbit anti-HSF-1 primary antibody (1:1,000 dilution) (catalog no. 4356, Cell Signaling Technology) and donkey anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000) (Santa Cruz Biotechnology). The same membranes were stripped and reprobed for total O-GlcNAc levels to visualize total O-glycosylated proteins in each lane, using primary antibody (catalog no. MA1-076, Affinity Bioreagents). Identical separate experiments were run for the reciprocal immunoprecipitation (this time with the HSF-1 primary antibody and then probing for O-GlcNAc on the membrane) to prove that O-GlcNAc and HSF-1 were bound. This was to validate and strengthen the previous IP results. Total HSF-1 levels for each experimental group were evaluated via Western blot to determine whether it was upregulated.
Reporter plasmids for HSP70 and Sp1 binding.
To investigate the effect of GLN on HSP-70 promoter activation, we utilized a 1,500-bp HSP70 promoter plasmid that is known to be dependent on HSF-1 binding (gift from Dr. Eugene Chang, originally from R. Morimoto). For the HSP70 promoter plasmid, a previously published protocol was employed (18). The HSP70 promoter was subcloned into the multiple cloning site of the pGL3 basic firefly luciferase reporter vector (Promega). Luciferase activity was adjusted to the protein content of the cell lysate after initial optimization. The Mercury HSE-TA luciferase minimal reporter and corresponding empty vector control were obtained from BD Biosciences Clontech (Palo Alto, CA). For Sp1 transcription factor binding, we utilized a commercially available Sp1 reporter plasmid (catalog no. ER0117; Panomics, Fremont, CA) and followed the manufacturer's instructions. For both sets of plasmid experiments, cells were transfected at 70–80% confluence using FuGENE 6 transfection reagent (catalog no. 1815019, Roche) with either no plasmids, control, HSP70, or Sp1 luciferase reporter plasmids for 24 h. Cells were treated with or without GLN, as previously described, and subjected to a nonlethal HS. After a 24-h recovery, media were removed, and luciferase assay was performed as per the manufacturer's instructions (catalog no. E1501, Promega) on a Fluoroskan Ascent FL from Thermo-Fisher Scientific (Waltham, MA). Each group was normalized to total cellular protein.
Data analysis and statistics.
Immunoprecipitation, Western blotting, ELISA, and cell survival were compared using Student's t-test, two-tailed, or ANOVA followed by the Student-Newman-Keul test where applicable. For reporter plasmid activity, the Bonferroni multiple-comparisons test was utilized to compare fold-change in promoter activity. Results are presented as means ± SE.
RESULTS
GLN and heat stress increase global protein O-glycosylation.
Levels of O-glycosylated proteins were measured at 30 min post-heat stress by Western blot (all data are presented as relative densitometry units normalized to β-actin control). O-glycosylation increased in cells treated with GLN even in the absence of heat stress (Fig. 2). Levels of O-glycosylated proteins in non-heat-stressed cells increased from 3.2 ± 0.07 to 6.9 ± 0.7 (P < 0.01) following GLN treatment. Heat stress causes an increase in O-glycosylation vs. non-HS controls (HS CT: 8.9 ± 0.31 vs. CT: 3.2 ± 0.07, P < 0.01), and GLN treatment enhanced this effect even further (HS CT 8.9 ± 0.31 vs. HS GLN 11.2 ± 1.15, P < 0.05).
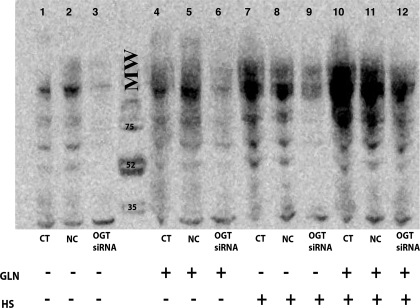
Fig. 2.
GLN enhances global O-glycosylation pre-/post-heat shock (HS), and silencing of OGT expression attenuates this effect. Lanes 1, 2, and 3 are control cells transfected with either no siRNA, noncoding (NC) siRNA, or OGT siRNA, respectively. OGT siRNA decreases basal levels of O-glycosylated intracellular proteins. Lanes 4, 5, and 6 are non-HS GLN-treated cells (transfected similarly). Cells showed increased O-glycosylation levels with GLN treatment even in the absence of stress. OGT silencing attenuates this GLN mediated O-glycosylation (lane 6). Lanes 7, 8, and 9 are HS control cells (HS CT) and lanes 10, 11, and 12 are HS with GLN (with the same transfections). HS increases O-glycosylation, and GLN treatment again enhances this effect. OGT silencing attenuates GLN-induced O-glycosylation, whereas NC oligos do not. Western blot is representative of 3 separate experiments. MW, molecular weight.
Inhibition of OGT activity via siRNA reduces global O-glycosylation of proteins.
Silencing of the OGT enzyme via siRNA decreased O-glycosylated protein levels in all treatment groups studied (Fig. 2). GLN enhancement of O-glycosylation in heat-stressed cells decreased back to non-heat-stressed control levels following OGT silencing (P = 0.02). Noncoding (NC) oligos had no effect on O-GlcNAc levels (n = 3). To confirm that the OGT siRNA was reducing OGT levels, Western blot analyses were performed which confirmed a decrease in OGT protein expression in the silenced groups (data not shown). An OGT knockdown of 86% was achieved in the OGT-silenced groups compared with nonsilenced groups (silenced: 0.311 ± 0.13 vs. nonsilenced: 2.31 ± 0.37, P < 0.01).
Chemical inhibition the HBP affects GLN-mediated increases in HSP70 expression.
To determine the effect of chemical inhibitors directed against key enzymes in the HBP, DON (an inhibitor of GFAT) and alloxan (an inhibitor of OGT) were utilized. Figure 3A shows GLN-mediated HSP70 expression decreased in groups treated with these chemical inhibitors. DON or alloxan alone did not alter HSP70 production (data not shown). HS GLN increased HSP70 10-fold compared with HS CT (P < 0.02). DON significantly decreased GLN induction of HSP70 (P < 0.04 vs. HS GLN without DON), and alloxan further inhibited GLN-mediated HSP70 induction (P < 0.01 vs. HS GLN without alloxan).
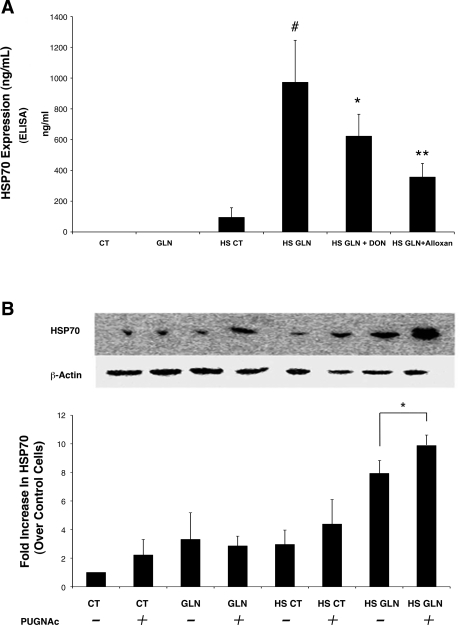
Fig. 3.
Chemical inhibition of HBP enzymes affects GLN-mediated HSP-70 expression. A: HSP70 measured by ELISA is decreased in cells receiving GLN following treatment with DON and alloxan. HS GLN increased HSP70 10-fold compared with HS CT (#P < 0.02 vs. HS CT). DON significantly decreased GLN-mediated enhancement of HSP70 (*P < 0.04 vs. HS GLN). Alloxan treatment also significantly decreased GLN-mediated HSP70 expression (**P < 0.01 vs. HS GLN) (n = 3). B: PUGNAc prevents the removal of O-GlcNAc from intracellular proteins by inhibiting O-GlcNAcase. Cells treated with PUGNAc showed an increase in HSP70 both with and without GLN treatment following heat stress injury. Heat-stressed cells treated with both GLN and PUGNAc showed the highest HSP70 expression. Following heat stress, PUGNAc treatment further increased HSP70 expression in GLN-treated cells (*P < 0.04 vs. HS GLN alone). Western blot is representative of three separate experiments.
N-acetylglucosaminidase (O-GlcNAcase)I inhibition further increases GLN-mediated HSP70 expression.
To further investigate the link between O-glycosylation and HSP70 expression, cells were treated with the chemical inhibitor PUGNAc, which increases O-glycosylation levels by preventing the removal of O-GlcNAc from proteins. Inhibition of O-GlcNAcase with 100 μM PUGNAc significantly increased HSP70 by 50% in HS GLN-treated cells versus HS GLN cells not treated with PUGNAc (Fig. 3B). GLN treatment increased HSP70 expression by 1.6-fold in HS cells (P = 0.005 vs. HS CT), and adding PUGNAc increased this effect even further to 2.3-fold (P = 0.032 vs. HS GLN alone). No other statistically significant effects of PUGNAc treatment were observed in the other groups.
Inhibition of OGT via siRNA completely attenuates GLN-mediated HSP70 increases.
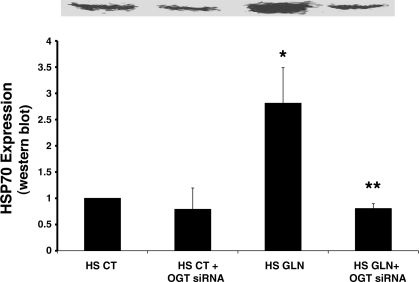
To determine the effect of targeted siRNA silencing of OGT on GLN-mediated HSP70 expression, we examined the expression of HSP70 before and after heat stress in OGT-silenced cells. As shown in Fig. 4, cells treated with GLN had a threefold increase in HSP70 production vs. control cells following HS (P < 0.04). GLN-mediated increase in HSP70 expression was completely blocked by OGT silencing (P < 0.02). The NC siRNA (with equal G-C content to the OGT siRNA) did not show an effect on HSP70 expression (data not shown). These data demonstrate that the HBP is vital to GLN's mechanism of HSP70 enhancement. Without the enzyme OGT, GLN cannot enhance HSP70.
Fig. 4.
OGT silencing via siRNA attenuates GLN-mediated HSP-70 expression. OGT gene silencing inhibits GLN-mediated HSP-70 expression. HS GLN groups showed a threefold increase in HSP70 production by Western blot (*P = 0.04 vs. HS CT). These increases were completely attenuated by OGT silencing (**P = 0.02 vs. HS GLN), suggesting that the HBP is essential for GLN-mediated HSP70 expression. GLN-NC siRNA groups did not show a decrease in HSP70 production (data not shown). Densitometry is normalized to β-actin and represents four separate experiments.
GLN-mediated cellular protection is decreased with OGT silencing.
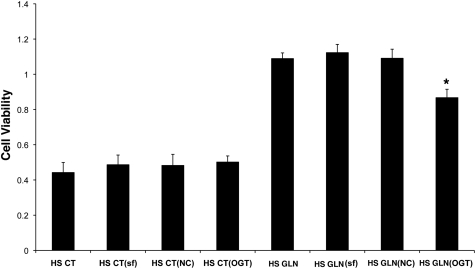
The effect of OGT siRNA on GLN-mediated cellular protection following lethal heat stress was evaluated via MTS assay. Control groups were added to measure any possible toxicity effects of the transfection reagent. As shown in Fig. 5, the transfection reagent (SilentFect, SF) alone did not affect cell viability. GLN treatment (in the nonsilenced groups) showed a 2.5-fold increase in cell viability (HS GLN: 1.123 ± 0.047 vs. HS CT: 0.486 ± 0.055 P < 0.05). OGT silencing decreased GLN-mediated protection by 43% (P < 0.05 vs. HS GLN). NC oligos had no effect on cell viability in either group (n = 6).
Fig. 5.
GLN-mediated cellular protection is attenuated by OGT silencing. Cells were treated with either no transfection reagent, SilentFect transfection reagent only (SF), noncoding RNAs (NC), or OGT siRNA. Cell survival following lethal heat stress measured via MTS assay demonstrates a 2.5-fold increase in all GLN-treated groups except the cells receiving OGT siRNA (P < 0.05 vs. CT cells). OGT silencing decreased GLN's protection by 43% versus HS GLN alone (*P < 0.05). CT and GLN groups containing the transfection reagent only (SF) were added to show that the reagent alone is not toxic to the cells. All groups were normalized to their non-HS controls to account for differences in cell growth. (Data are from n = 6 experiments.)
GLN increases total cell O-GlcNAc content and nuclear translocation of HSF-1 and SP1 via HBP by microscopy.
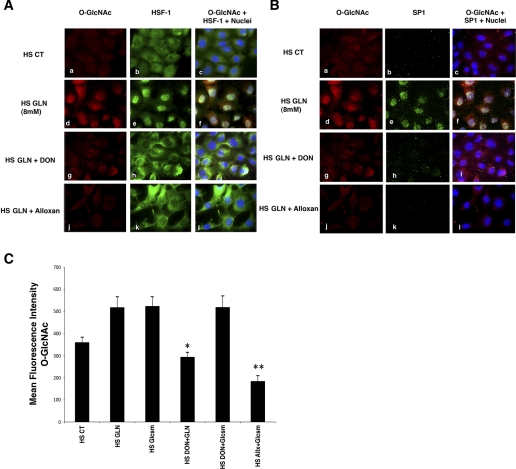
To directly visualize the cellular content and subcellular distribution of O-glycosylated HSF-1 and SP1, cells were cultured on glass slides. At 30 min post-heat-stress injury, cells were analyzed for total O-GlcNAc, HSF-1, and Sp1. A subset of cells were treated with glucosamine (see Fig. 6C) and/or HBP inhibitors (DON or alloxan). Cells were masked for total cell area and nuclei, and mean intensities were calculated (50 cells per experimental group). GLN treatment increased total cellular O-GlcNAc MFI (HS CT: 360 ± 24 vs. HS GLN:518 ± 51, P < 0.05) (Fig. 6A, a and d). Figure 6, A and B, demonstrates that GLN leads to a significant increase in nuclear HSF-1 and nuclear SP1. GLN treatment increased nuclear HSF-1 MFI (HS CT: 1005 ± 146 vs. HS GLN:1,403 ± 102, P < 0.05) and SP1 (HS CT: 214 ± 14 vs. HS GLN:330 ± 13, P < 0.05). Total cellular HSF-1 and total cellular SP1 levels did not change significantly. Chemical inhibitors decreased GLN-induced translocation of SP1 in HS GLN + DON (276 ± 16, P < 0.05)-treated cells and in HS GLN + alloxan (268 ± 24, P < 0.05)-treated cells. GLN-mediated increases in O-GlcNAc were also blocked by both chemical inhibitors. GLN-induced increases in total O-GlcNAc were inhibited by DON (294 ± 21; P < 0.05) and inhibited with alloxan (401 ± 33; P < 0.05). Interestingly, HSF-1 (green) and O-GlcNAc (red) appear to colocalize in Fig. 6Af, following glutamine treatment. SP1 nuclear translocation and colocalization show the same pattern in Fig. 6B. Microscopy images were all normalized identically (based on the negative control slides) for qualitative visual analysis. Since DON is a glutamine analog and may have other nonspecific inhibitory effects on HBP activity (distal to GFAT), we utilized glucosamine to bypass its inhibitory effects and ensure that HBP activity could be restored. Cells treated with DON and glucosamine showed a significant increase in O-glycosylation levels (see Fig. 6C). Thus, DON did not appear to affect HPB activity beyond the inhibition of the GFAT enzyme, because O-GlcNAc levels were restored when the HBP was rescued by glucosamine. DON decreased GLN-mediated O-glycosylation (P < 0.02 vs. HS GLN), but not glucosamine-mediated O-glycosylation. Alloxan (an inhibitor of OGT), decreased both GLN and glucosamine-mediated enhancement of the pathway (P < 0.05 vs. HS glucosamine).
Fig. 6.
Accumulation of nuclear O-GlcNAc modified HSF-1 and Sp1 after GLN treatment in mouse embryonic fibroblast (MEF) cells following heat stress. Cells treated with 0 or 8 mM GLN underwent heat stress as described in experimental procedures. Cy3-labeled O-GlcNAc (shown in red), Alexa Fluor 488-labeled HSF-1 (green; A) and Cy5-labeled SP1 (green; B) were visualized for cellular localization and levels. A: HSF-1 nuclear accumulation. Control cells show minimal O-GlcNAc staining (top, a) and predominantly cytoplasmic HSF-1 (top, b) following heat stress. GLN-treated cells show an increase in cellular O-GlcNAc-modified proteins (middle, d) and increased nuclear translocation of the transcription factor HSF-1 (middle, e). Nuclear colocalization of HSF-1 and O-GlcNAc residues appear yellow (middle, c, f, i, l). Both DON and alloxan, inhibitors of key enzymes in the HBP, attenuate GLN-mediated increase in O-GlcNAc-modified protein (middle, g and j) and nuclear HSF-1 translocation (middle, h and k). B: Sp1 nuclear accumulation. Control cells show minimal O-GlcNAc staining (top, a) and minimal Sp1 staining (top, b) following heat stress. GLN-treated cells show an increase in cellular O-GlcNAc-modified proteins (middle, d) and increased nuclear Sp1 (middle, e). Sp1 and O-GlcNAc appear to colocalize in the nucleus and again appear yellow (middle, c, f, i, l). Both DON and alloxan, inhibitors of key enzymes in the HBP, attenuate GLN-mediated increase in O-GlcNAc-modified protein (middle, g and j) (as above) and nuclear Sp1 content (middle, h and k). C: glucosamine (Glcsm) restores HBP activity in cells treated with DON. Microscopy showed that glucosamine (which activates HBP downstream of GFAT) enhanced intracellular O-GlcNAc levels as well as GLN (and also in the presence of the GFAT inhibitor, DON). DON decreased GLN-mediated O-glycosylation (*P < 0.02 vs. HS GLN), but not glucosamine-mediated O-glycosylation. Alloxan (an inhibitor of OGT) decreased both GLN and glucosamine mediated enhancement of the pathway (**P < 0.05 vs. HS glucosamine).
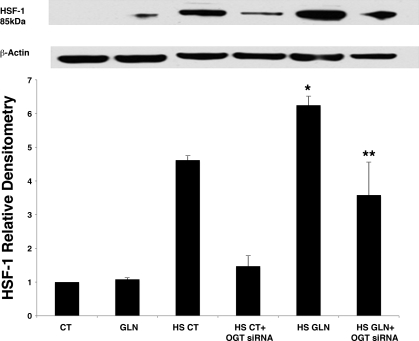
OGT-silencing significantly reduces nuclear HSF-1 nuclear translocation.
HSF-1 was predominantly cytoplasmic (82%) with only 18% in the nucleus in non-HS cells (P < 0.05). Figure 7 shows nuclear content of HSF-1 increased in HS CT cells (P = 0.0005 vs. CT) via Western blot. GLN treatment significantly increased nuclear HSF-1 content further in HS cells (P = 0.01 vs. HS CT). OGT silencing significantly attenuated HS-induced HSF-1 nuclear translocation in HS cells (P = 0.005 vs. non-OGT silenced HS CT cells). OGT silencing also significantly reduced GLN-mediated HSF-1 nuclear translocation versus non-OGT-silenced HS GLN cells (P < 0.05).
Fig. 7.
GLN treatment increases nuclear HSF-1 content by Western blot, which is decreased by siRNA knockdown of OGT. To confirm results obtained in Fig. 4A, cells were treated with 0 or 8 mM GLN and underwent heat stress as described in experimental procedures. Cell lysates were separated into nuclear and cytoplasmic fractions and analyzed by Western blot. Membranes were probed for HSF-1 using a rabbit primary antibody from Cell Signaling. Heat stress alone causes nuclear HSF-1 levels to increase, and GLN treatment increases this effect further. HS GLN showed an increase in nuclear HSF-1 content by 35% (*P = 0.017 vs. HS CT). OGT silencing completely attenuated nuclear translocation of HSF-1 in HS CT and significantly decreased nuclear HSF-1 in HS GLN groups (**P < 0.05).
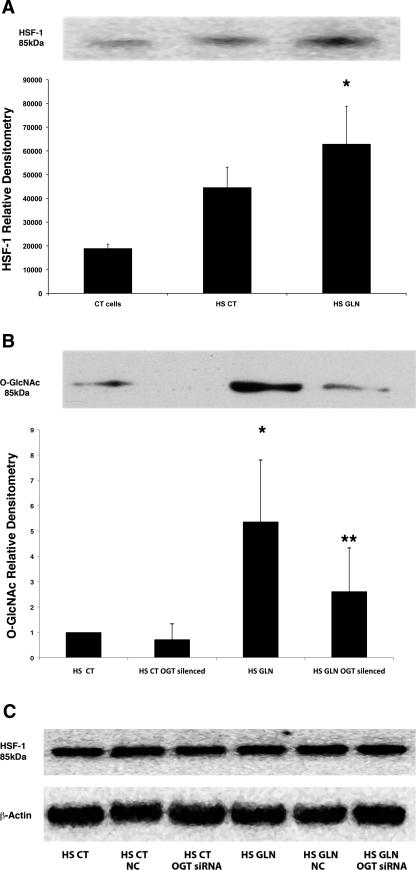
HSF-1 can be modified by O-glycosylation.
To confirm the O-GlcNAc modification of HSF-1, we performed an immunoprecipitation for O-GlcNAc using an anti-O-GlcNAc monoclonal primary antibody (Fig. 8A). We then performed Western blot analysis for HSF-1. Heat stress alone caused a 2.5-fold increase in the O-glycosylation of HSF-1 (P < 0.02 vs. CT cells alone). GLN treatment further increased the O-glycosylation of HSF-1, a 3.3-fold increase vs. HS CT cells (P < 0.02 vs. HS CT). Membranes were stripped and reprobed for total O-GlcNAc levels to verify our previous findings (that both heat stress and GLN treatment increase total cellular O-glycosylated proteins; data not shown). The reciprocal immunoprecipitation was performed to confirm the preceding experiments and, additionally, to investigate the effects of OGT silencing on O-glycosylated HSF-1. In this set of experiments, cell lysates were immunoprecipitated for HSF-1 and probed for O-GlcNAc (see Fig. 8B). GLN treatment again significantly increased (5-fold) O-glycosylated HSF-1 in HS GLN cells vs. HS CT cells, (P < 0.05). OGT silencing attenuated GLN-mediated O-glycosylation of HSF-1 in HS cells (P = 0.025 vs. non-OGT-silenced cells). Since the HS and recovery period was ∼1 h, we did not anticipate an increase in HSF-1 protein expression; however, Western blot analyses were run for total HSF-1 to verify that levels did not change with each treatment group (see Fig. 8C).
Fig. 8.
GLN enhances O-glycosylation of HSF-1. MEF cells were treated with 0 or 8 mM GLN, heat stressed, and harvested after a 15-min recovery. A: O-GlcNAc immunoprecipitation. Equal amounts of protein were incubated with monoclonal O-GlcNAc primary antibody and immunoprecipitated. Western blots were run and membranes were probed for HSF-1. Heat stress increased O-GlcNAc-bound HSF-1 versus control (CT) cells (P < 0.02). GLN treatment (HS GLN) led to a significant increase in O-glycosylated HSF-1 vs. HS CT cells (*P < 0.02). Western blot is representative of 3 separate experiments. B: HSF-1 immunoprecipitation. Cells were silenced for OGT and then treated (as above). Equal amounts of protein were incubated with a rabbit HSF-1 primary antibody and immunoprecipitated. Western blots were run and membranes probed for O-GlcNAc. GLN treatment in HS cells led to a significant increase in O-glycosylated HSF-1 vs. HS CT cells (*P = 0.04), and OGT silencing decreased O-glycosylated HSF-1 by about half (**P = 0.02). Western blot is representative of 3 separate experiments. C: total HSF-1 levels. Western blots were performed on whole cell lysates to evaluate total HSF-1 levels among the experimental groups. Cells treated with SilentFect only (CT), SilentFect with NC oligos (NC), and SilentFect with OGT siRNA (OGT siRNA) showed the same levels of HSF-1. GLN treatment did not alter HSF-1 levels.
GLN enhances HSP70 promoter activation and SP-1 binding/gene activation.
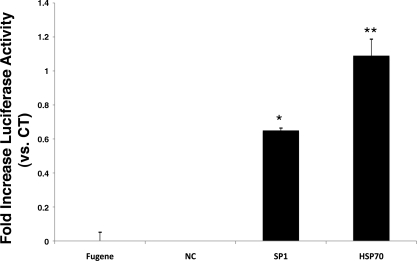
The literature shows that GLN leads to enhanced binding of HSF-1 to the HSP70 promoter (9). To confirm the effect of GLN on HSP70 promoter activation, we utilized a 1,500-bp HSP70 promoter plasmid that is known to be dependent on HSF-1 binding (gift from Dr. Eugene Chang, originally from R. Morimoto). Furthermore, to confirm GLN's effect on Sp1 gene binding, we utilized a commercially available Sp1 reporter plasmid. Figure 9 shows that GLN treatment led to a 100% increase in HSF-1-mediated HSP70 gene activation following heat stress (P < 0.05 vs. HS CT). Sp1 binding following heat stress injury increased (by ∼70%) with GLN treatment as well (P < 0.05 vs. 0 mM GLN). Cells transfected with the control plasmid or no plasmid showed no luciferase activity.
Fig. 9.
GLN increases HSP70 and Sp1 promoter-reporter activity. Cells were transfected with either an HSP70 or an Sp1 reporter construct. Presence of 8 mM GLN during heat shock led to significant increase in both HSP70 and Sp1 promoter-driven heat-inducible luciferase activity, compared with HS CT (*P < 0.05 by Bonferroni multiple-comparisons test; n = 3). FuGENE represents cells not transfected with plasmid but treated with FuGENE transfection reagent alone, and NC represents luciferase activity in control plasmid-transfected cells (all post-heat stress). All groups were normalized to total protein content.
DISCUSSION
To this point, the molecular pathway by which GLN leads to HSP70 induction has remained a mystery. These data demonstrate that GLN-mediated induction of HSP70 expression appears to depend on the metabolism of GLN by the HBP. GLN's effect on the HBP appears to lead to the O-glycosylation, nuclear translocation, and transcriptional activation of HSF-1 and Sp1, both of which are key transcription factors required for maximal HSP70 expression. GLN's metabolism via the HBP and how this pathway may lead to enhanced HSP70 expression are illustrated in Fig. 1.
Recent data indicate that enhanced HBP activity results in induction of HSP expression and cellular protection against stress (27, 29). These data demonstrated that a wide variety of cell stresses (including oxidants, ethanol, heat stress, and ultraviolet light) led to rapid increases in O-glycosylation of intracellular proteins (29). These data also revealed that increased O-glycosylation was associated with enhanced HSP70 expression (29). Our data confirm that GLN drives enhanced HBP activity, which also leads to enhanced HSP70 expression and cellular protection. Previous data also indicate that DON, an inhibitor of the GFAT enzyme, attenuates HSP70 expression following thermal stress (29). We also show that inhibition of GFAT by DON inhibits GLN-mediated expression of HSP70. Moreover, we show that inhibition of OGT by alloxan further inhibits HSP70 expression following GLN administration. However, alloxan is a chemical inhibitor and may lead to other nontarget effects on other cellular pathways. Thus, to specifically inhibit OGT activity, we applied a silencing RNA against the OGT enzyme. Earlier studies showed that selective deletion of OGT via CRE-LOX recombination led to cells that had attenuated HSP70 expression and decreased survival following injury (29). We achieved the same effect on HSP70 expression using siRNA targeted to OGT. This siRNA targeting of OGT also reduced the survival advantage that GLN confers following heat stress injury.
O-glycosylation alters the activity and cellular localization of specific transcription factors by modulating 1) DNA binding, 2) subcellular localization, and 3) half-life and proteolytic processing of transcription factors (2, 4, 7, 26). Specifically, O-glycosylation can direct modified transcription factors to the nucleus, where they can be subsequently activated (2, 4, 7, 26). It appears that GLN has significant effects on the O-glycosylation, nuclear translocation, and transcriptional activation of two key transcription factors required for HSP70 expression. We have previously shown that cells that contain a deletion of the HSF-1 gene are unable to induce HSP70 expression following GLN treatment (9). Thus, HSF-1 is clearly required for GLN-mediated induction of HSP70 expression. On the basis of this finding, it is not surprising that our data reveal that HSF-1 is O-glycosylated. Our laboratory has previously shown in an in vivo model that HSF-1 may be O-glycosylated in lung tissue following experimental sepsis (17). These data also revealed that GLN administration may drive this process. However, these data were only shown by immunoprecipitation in one direction. For the first time, the data presented in our current study confirm that HSF-1 is O-glycosylated by two separate reciprocal immunoprecipitation experiments. These data have particular relevance to our fundamental understanding of how HSF-1 may be guided to the nucleus following stress because inhibition of the O-glycosylation pathway attenuates HSF-1 nuclear translocation following stress. Sp1 has also been shown to be key for maximal HSP expression (1, 10, 14). Our data reveal that GLN-mediated O-glycosylation, nuclear translocation, and promoter binding of Sp1 may also be vital to maximal GLN-induction of HSP70. To our knowledge, this is the second gene that can be transcriptionally activated by GLN-mediated increases in HBP activity. The ASS gene is also activated via GLN-mediated Sp1 O-glycosylation, nuclear translocation, and promoter binding (2). In addition to GLN, glucose is known to be a key substrate for the HBP. However, previous studies on GLN's ability to drive ASS gene expression via enhanced HBP activity show that glucose is not necessary for GLN to exert its effect on ASS activity (2). Conversely, glucose administration to a GLN-deprived media had very little effect on gene expression in cultured adipocytes (15). Thus, it appears that GLN is the key substrate required to drive gene expression mediated by enhanced HBP activity.
In in vivo models, previous data indicate that enhanced O-GlcNAc activity via in vivo glucosamine supplementation can protect against myocardial injury after ischemia-reperfusion (I/R) injury and organ dysfunction following trauma-hemorrhagic shock (6, 25). This same lab has shown that GLN-mediated protection of the isolated rat heart from I/R injury is mediated by enhanced O-GlcNAc levels and increased hexosamine pathway activity (5). These data are very similar to data from our laboratory for in vivo GLN administration providing protection against myocardial I/R injury (22). These authors correlate their protection with increased O-GlcNAc activity but have not explored HSP expression as a possible mechanism of protection.
One limitation of our data is that siRNA knockdown of OGT expression did not lead to complete loss of GLN-mediated cellular protection following heat stress. This may be due to a few factors. First, the knockdown of OGT by the targeted siRNA was found to be an average of 86%. It is possible that the residual HBP activity led to some degree of GLN-mediated cellular protection. Also, it is possible that GLN's effect on other protective pathways, such as enhancement of cellular glutathione content, could be playing a role in GLN's protection independent of the stress pathways modulated by enhanced HBP activity. Another limitation of this study is the need for a thorough understanding of OGT enzyme regulation following stress and in the presence of GLN. Future studies should help to elucidate a more complete understanding of this enzyme.
We believe that our study raises a new and clinically important hypothesis. Recent data have shown that both local and systemic inflammatory injury leads to a deficit in HSP expression (3, 16, 21) which may impair recovery and/or survival from these injuries. Furthermore, many conditions of injury and stress, including the aforementioned inflammatory injuries, lead to rapid GLN deficiency (11). One hypothesis to explain this defect in HSP expression may be systemic and/or local GLN deficiency. GLN deficiency could lead to a loss of HBP activity and HBP-mediated HSP expression. Our data showing a sharp decrease in O-glycosylated proteins in non-GLN-treated cells versus GLN-supplemented cells following heat stress support this hypothesis. If this hypothesis is correct, these data have particular relevance to the clinical care of human disease because of the potential to optimize HSP70 expression, protect tissues/organs, and ultimately improve clinical outcome by exploiting GLN and other clinically relevant activators of the HBP [such as glucosamine (25)]. This concept of GLN being utilized to enhance HSP70 in clinical illness was recently demonstrated in a trial of critically ill patients in which GLN administration was correlated with enhanced HSP70 levels. The enhanced HSP70 levels were subsequently correlated with improved clinical outcome (30).
In summary, we report the first mechanistic description of a pathway by which GLN can enhance HSP70 expression (Fig. 1). It appears GLN enhances HBP activity leading to the O-glycosylation, nuclear translocation, and transcriptional activation of HSF-1 and Sp1, both of which are key transcription factors required for maximal HSP70 expression. Inhibition of the key enzymes of this pathway attenuated GLN-mediated HSP70 expression and cellular protection. Furthermore, we show that inhibition of O-glycosylation, via siRNA targeted to the key enzyme of the O-glycosylation pathway, limits nuclear accumulation of HSF-1 following stress. We believe that this is the first description that the O-glycosylation of HSF-1, the vital transcription factor required for HSP expression, may be vital for its translocation to the nucleus following stress.
GRANTS
This work was supported by National Institutes of Health National Institute of General Medical Sciences Grant RO1-GM-078312 (to P. E. Wischmeyer).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Sean Colgan, Ph.D, in The Mucosal Inflammation Program at the University of Colorado Health Sciences Center, for assistance in planning these experiments. We also extend thanks to Anirban Banerjee, Ph.D, and his research laboratory at the University of Colorado Health Sciences Center, Department of Surgery, for use of their Zeiss Axiovert microscope system. The authors thank Mary Abigail Westapher for proofreading the manuscript.
REFERENCES
- 1.Bevilacqua A, Fiorenza MT, Mangia F. A developmentally regulated GAGA box-binding factor and Sp1 are required for transcription of the hsp70.1 gene at the onset of mouse zygotic genome activation. Development 127: 1541– 1551, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Brasse-Lagnel C, Fairand A, Lavoinne A, Husson A. Glutamine stimulates argininosuccinate synthetase gene expression through cytosolic O-glycosylation of Sp1 in Caco-2 cells. J Biol Chem 278: 52504– 52510, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology 133: 1893– 1904, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudlow JE. Post-translational modification by O-GlcNAc: another way to change protein function. J Cell Biochem 98: 1062– 1075, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42: 177– 185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303– 312, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Majumdar G, Harrington A, Hungerford J, Martinez-Hernandez A, Gerling IC, Raghow R, Solomon S. Insulin dynamically regulates calmodulin gene expression by sequential o-glycosylation and phosphorylation of sp1 and its subcellular compartmentalization in liver cells. J Biol Chem 281: 3642– 3650, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Mestril R, Chi SH, Sayen MR, O'Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest 93: 759– 767, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison AL, Dinges M, Singleton KD, Odoms K, Wong HR, Wischmeyer PE. Glutamine's protection against cellular injury is dependent on heat shock factor-1. Am J Physiol Cell Physiol 290: C1625– C1632, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Oesterreich S, Hickey E, Weber LA, Fuqua SA. Basal regulatory promoter elements of the hsp27 gene in human breast cancer cells. Biochem Biophys Res Commun 222: 155– 163, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet 336: 523– 525, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15: 1118– 1131, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest 95: 1854– 1860, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter W, Wang F, Wang W, Duan R, Safe S. Role of estrogen receptor/Sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol Endocrinol 10: 1371– 1378, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Rumberger JM, Wu T, Hering MA, Marshall S. Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels: effects of glucose, glutamine, and glucosamine on glycerophosphate dehydrogenase, fatty acid synthase, and acetyl-CoA carboxylase mRNA levels. J Biol Chem 278: 28547– 28552, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med 33: 1206– 1213, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Singleton KD, Wischmeyer PE. Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr 32: 371– 376, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Singleton KD, Wischmeyer PE. Glutamine's protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol 292: R1839– R1845, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Edelson JD, Post M, Mullen JB, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis 147: 177– 181, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med 22: 914– 921, 1994 [PubMed] [Google Scholar]
- 21.Weiss YG, Bouwman A, Gehan B, Schears G, Raj N, Deutschman CS. Cecal ligation and double puncture impairs heat shock protein 70 (HSP-70) expression in the lungs of rats. Shock 13: 19– 23, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Wischmeyer PE, Jayakar D, Williams U, Singleton KD, Riehm J, Bacha EA, Jeevanandam V, Christians U, Serkova N. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr 27: 396– 403, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol 90: 2403– 2410, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol Gastrointest Liver Physiol 272: G879– G884, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock 25: 600– 607, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761: 599– 617, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta 1673: 13– 28, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Zachara NE, Hart GW. O-GlcNAc modification: a nutritional sensor that modulates proteasome function. Trends Cell Biol 14: 218– 221, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133– 30142, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med 31: 1079– 1086, 2005 [DOI] [PubMed] [Google Scholar]