the kidneys filter 120 ml/min at the renal glomeruli, and two thirds of this ultrafiltrate is reabsorbed in the proximal tubule. To accomplish this high rate of reabsorption, the proximal tubule has a tall brush border formed by densely packed microvilli each with a core of actin filaments bundled with cytoskeletal proteins (Fig. 1) (6). This microvillar specialization is estimated to increase the surface area of the cell >30-fold (10). Transporters in the apical microvilli include the Na+/H+ exchanger isoform 3 (NHE3) and anion transporters to reabsorb most of the sodium chloride and bicarbonate, the Na+-phosphate cotransporter 2 (NaPi2) to reabsorb phosphate, Na+-glucose and Na+-amino acid transporters to reabsorb substrates, and water channels (aquaporin-1) to reabsorb water.
Fig. 1.
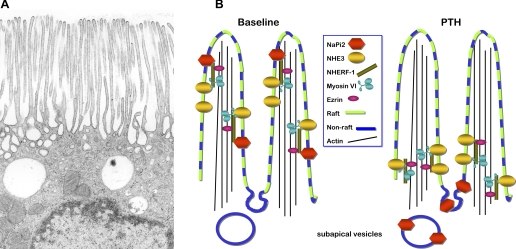
A: apical region of a proximal tubule cell illustrating dense apical microvilli. Micrograph provided by Arvid B. Maunsbach, University of Aarhus, Denmark. B: schematic representing response of renal proximal tubule sodium transporters NaPi2 and Na+/H+ exchanger 3 (NHE3) to parathyroid hormone (PTH). At baseline (left), both NaPi2 and NHE3 are located in the body of the microvilli: NaPi2 is enriched in non-lipid rafts along with NHE regulatory factor 1 (NHERF-1) and ezrin while NHE3 and myosin VI are enriched in lipid rafts (7). Both NaPi2 and NHE3 have been reported to be associated with the PDZ adaptor protein NHERF-1 and are presumably tethered to the cytoskeleton via ezrin (9). How myosin VI is tethered to the transporters and the actin core remains to be determined. After PTH treatment (right), NHERF-1 is phosphorylated and dissociates from NaPi2 (8), presumably allowing the cotransporter to move into the intermicrovillar cleft where it is endocytosed to subapical dense apical tubules and endosomes (1, 11). In contrast, NHE3 redistributes to a domain at the base of the microvilli (11) along with myosin VI. Both transporters are believed to move from the body to the base of the microvilli in the plane of the membrane. The study of Blaine et al. (3) uses total internal reflection fluorescence microscopy focused on the microvilli of cultured opossum kidney cells to provide evidence that the redistribution of NaPi2 in response to PTH requires a dynamic actin cytoskeleton and a functioning myosin VI.
Fractional proximal tubule reabsorption is increased by angiotensin II and catecholamines and is decreased by a variety of natriuretic and diuretic stimuli including elevation of blood pressure, high-salt diet, and parathyroid hormone (PTH) that is both natriuretic and phosphaturic. Interestingly, these stimuli all decrease both NHE3 and NaPi2 abundance in the microvilli (11, 13), suggesting a coordinated molecular mechanism of regulation. Immuno-electron microscopy studies have shown that these transporters traffic to the base of the microvilli in response to either PTH or elevated blood pressure and are then sorted to distinct destinations: NaPi2 to subapical dense apical tubules and endosomes and NHE3 remaining at the base of the microvilli above the coated pits (11). Both NHE3 and NaPi2 have been shown to associate with the PDZ domain protein NHE regulatory factor 1 (NHERF-1), which can tether to multiple proteins as well as to the actin filament core via the actin-binding protein ezrin (9).
In the study by Blaine and colleagues in this issue of American Journal of Physiology-Cell Physiology (3), the roles of myosin VI and the actin cytoskeleton were addressed using a proximal tubule cell line that has proven very useful for PTH studies: the OK cells derived originally from opossum kidney. These epithelial cells maintain significant number of microvilli and sensitivity to PTH and can be engineered to express fluorescent microvillar NaPi2. The study is notable for applying total internal reflection fluorescence microscopy (TIR-FM), a technique that specifically illuminates fluorophores in a 100-nm optical section above the interface between two media. This method has been applied successfully in many studies of exocytosis of secretory granules (4). Blaine et al. extend the TIR-FM method by placing the OK cells apical side down on coverslips to view trafficking of fluorescently tagged NaPi2 within the microvilli of the cells. With this approach they were able to view the kinetics of NaPi2 retraction from the microvilli in response to PTH and demonstrate that a dynamic actin cytoskeleton is required for the retraction.
A potential molecular mechanism for driving transporters from the body to the base of the microvilli was suggested in an earlier study that detected the atypical molecular motor myosin VI in proximal tubules (2). Myosin VI, unlike other myosins, moves cargo from the periphery to the center of the cell. However, this earlier study localized myosin VI only to the base (rather than the body) of the microvilli. Perfusion fixation before analysis likely led to this distribution of myosin VI. Indeed, subsequent studies in surface-fixed (not perfusion fixed) kidneys demonstrated that 50% of the myosin VI was localized to the microvilli, and the remaining 50% was found in the intermicrovillar and apical cytoplasmic zones (12). In addition, a significant fraction of the myosin VI redistributed from the villi to the base, along with NHE3 and NaPi2, during elevated blood pressure, high-salt diet, and after treatment with inhibitors of angiotensin II production (5, 12, 13). Perfusion fixation, if it elevates renal perfusion pressure, likely mimics the effect of hypertension on myosin VI activating its redistribution to the base.
These studies of coordinated redistribution of NHE3, NaPi2, and myosin VI, however, do not prove that myosin VI drives the redistribution. This issue has proven difficult to further explore with in vivo models. Using TIR-FM, Blaine and colleagues (3) addressed the role of myosin VI using a myosin VI motor dominant negative tail mutant that would interfere with the action of the native normal myosin VI. They found that the mutant myosin VI inhibits the PTH-stimulated NaPi2 retraction (3). Taken together, the results support the idea that the myosin VI motor drives NaPi2 down the microvilli tethered to the active actin filaments in the core of the microvilli.
Like many studies that apply a new approach to a long-standing question, these results provoke as many questions as they answer. How does PTH stimulation activate the redistribution? PTH is known to phosphorylate NHERF-1, which is thought to release it from association with NaPi2 (8); perhaps this opens up a site for binding to myosin VI directly or to a myosin VI-associated protein. Would the myosin dominant negative tail mutant also prevent NHE3 retraction from the microvilli in response to PTH? A related question arises from recent in vivo studies of the membrane domain properties of renal cortical NHE3 and NaPi2 that calculate that ∼70% of the NaPi2, NHERF-1, and ezrin partition into non-lipid raft domains of the membrane while 80% of the NHE3, the NHE3 regulator dipeptidyl peptidase IV, and myosin VI partition into lipid raft domains (7). If myosin VI is associated almost exclusively with lipid rafts in vivo, how does it physically connect with NaPi2 localized primarily to non-raft domains? Perhaps there are linker proteins that can bind to proteins in both domains. Finally, do NHE3 and NaPi2 require conventional myosin motors to traffic into the body of the microvilli? These questions are all well suited to further analysis of transporter redistribution in OK cells by TIR-FM.
GRANTS
This work was supported by National Institutes of Health Grant DK34316 (to A. A. McDonough).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
Arvid B. Maunsbach (University of Aarhus, Denmark) provided Fig. 1A, and Patrick K. K. Leong provided the original draft of Fig. 1B.
REFERENCES
References
- 1.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch 458: 39– 52, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Biemesderfer D, Mentone SA, Mooseker M, Hasson T. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am J Physiol Renal Physiol 282: F785– F794, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Blaine J, Okamura K, Arnal H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M. PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Cell Physiol (September 23, 2009). doi:10.1152/ajpcell.00260.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holz RW, Axelrod D. Secretory granule behaviour adjacent to the plasma membrane before and during exocytosis: total internal reflection fluorescence microscopy studies. Acta Physiol (Oxf) 192: 303– 307, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol 290: F854– F863, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Maunsbach AB. Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res 16: 239– 258, 1966 [DOI] [PubMed] [Google Scholar]
- 7.Riquier AD, Lee DH, McDonough AA. Renal NHE3 and NaPi2 partition into distinct membrane domains. Am J Physiol Cell Physiol 296: C900– C910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, EX, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412– 3420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Weinman EJ, Cunningham R, Wade JB, Shenolikar S. The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J Physiol 567: 27– 32, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welling LW, Welling DJ. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int 8: 343– 348, 1975 [DOI] [PubMed] [Google Scholar]
- 11.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896– F906, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890– 2896, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol 295: F1003– F1016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]