Abstract
Overactive bladder syndrome (OAB) is an idiopathic condition characterized by urinary urgency and urge incontinence. Detrusor overactivity has been traditionally described as the physiologic mechanism for OAB. However, the bladder urothelium (BU) may also be involved in the pathophysiology. This study measured polyamine signaling and its downstream effects on membrane conductivity in bladder urothelial cells (BUC) obtained from asymptomatic and OAB subjects. Immunohistofluorescence was used to measure ornithine decarboxylase (ODC) expression in BU. BUC, cultured from BU biopsies, were used for electrophysiologic studies. dl-α-Difluoromethylornithine (DFMO), spermine, or spermidine was used to modulate polyamine signaling in BUC. Results showed ODC overexpression in OAB BU. In OAB BUC, whole cell and cell-attached configuration showed significantly decreased currents. Using inside-out patches, outward currents increased significantly, suggesting a cytoplasmic source of the outward current block in OAB BUC. In control BUC, outward currents were mediated by the large-conductance calcium-activated potassium (BK) channel due to calcium dose-dependence and block by iberiotoxin. Spermidine and spermine blocked the outward current in normal BUC in dose-dependent fashion. Conversely, DFMO significantly increased (P < 0.01) outward currents in OAB BUC both in cell-attached and in whole cell configuration. The outward currents in DFMO-treated-OAB BUC could be significantly reduced (P < 0.05) by adding back spermidine and spermine. These data suggest that polyamine signaling is upregulated in OAB urothelium and OAB BUC. Furthermore, polyamines in BUC block the BK channel. Targeting of bladder urothelial polyamine signaling may represent a novel approach for OAB treatment based on pathophysiologic mechanisms.
Keywords: urothelial cell, urothelium, electrophysiology
overactive bladder syndrome (OAB) is characterized by bladder symptoms such as urinary frequency, urgency, urge incontinence, and nocturia in the absence of other identifiable etiologies (e.g., bacterial cystitis, bladder cancer, urethral diverticulum, radiation cystitis, and bladder outlet obstruction). OAB symptoms are common, with a prevalence estimate of 33 million in the United States (18) and with the overall cost being estimated at $12.6 billion (7). This makes OAB both a common and a costly problem.
The diagnosis and management of OAB syndrome is, unfortunately, almost entirely based on patient symptoms rather than a precise pathophysiologic basis. There are several possible OAB pathophysiologic mechanisms, including bladder (detrusor) smooth muscle, bladder neural afferent, central nervous system, and immune function abnormalities. All of these mechanisms can even coexist in the same patient. Recently, the contribution of bladder urothelial cells (BUC) and bladder urothelium to bladder function has been reviewed (2). The BUC can provide a “sensor transducer” role in bladder physiology (3). BUC can release substances that can affect nerve, smooth muscle, and interstitial and immune cells. Therefore, the role of BUC in OAB pathogenesis should be investigated, and furthermore, BUC can be a potential target area in treatment of OAB if a specific pathophysiologic BUC abnormality is discovered.
Polyamines are highly charged aliphatic cationic compounds that include putrescine, spermidine, and spermine. The role of polyamines in bladder function has been sparsely studied. Recently, spermine has been shown to mediate bladder smooth muscle relaxation (14). The role of polyamines on urothelial carcinoma cells has also been described (12), although there have been no further follow-up studies on utility of polyamines in bladder cancer treatment. The functions of polyamines in other epithelial cells, such as intestinal epithelial cells (IEC), are well established. These functions include regulation of IEC permeability (6), apoptosis (23), restitution (healing) (17), and RNA stability (24).
Furthermore, polyamines have been shown to block the calcium-activated large-conductance potassium (BK) channel (21). The BK channel has been shown to have a central role in mediating detrusor overactivity and resultant increased voiding frequency in animal models (11, 19), suggesting that BK channel may have a role in human OAB. While the implication from these animal models is that decreased BK channel expression in detrusor smooth muscle underlies detrusor overactivity, these animal models are constitutive BK knockouts, and the specific role of urothelial BK (and not detrusor smooth muscle) channel activation cannot be ascertained from these studies. Because of the data demonstrating a link between BK channel and detrusor activity/urinary incontinence, the electrophysiologic experiments in this study focused on BK channel activity.
Using both bladder urothelium from human bladder biopsies and in vitro cultured BUC derived from these biopsies, we explored the role of polyamines in OAB BUC. Furthermore, we also explored the link between polyamines and BK channel activity using whole cell and single-channel electrophysiologic experiments. This work represents the initial description associating polyamine signaling with OAB urothelial pathophysiology.
MATERIALS AND METHODS
Inclusion and exclusion criteria for study subject selection.
This study was approved by our Institutional Review Board. Inclusion criteria for OAB syndrome subjects included the following: 1) >18 yr of age; 2) female; 3) at least one urge incontinent episode per day based on history; 4) no clinical suspicion for bacterial cystitis based on urinalysis and urine culture, if obtained; and 5) no complaints of bladder pain (0 on a 9-point Likert scale). Exclusion criteria for OAB syndrome subjects included the following: 1) history of bladder or current active ureteral calculi; 2) genital herpes within past 12 mo; 3) history of uterine, cervical, vaginal, or urethral cancer; 4) urethral diverticulum; 5) history of cyclophosphamide or chemical cystitis; 6) history of tuberculous cystitis; 7) history of pelvic radiation; 8) history of malignant bladder tumors; 9) pregnancy; and 10) active vaginitis. Inclusion criteria for control subjects included the following: 1) >18 yr of age; 2) female; 3) no clinical suspicion for bacterial cystitis based on urinalysis and urine culture, if obtained; 4) planned concomitant cystoscopy during other pelvic surgery; and 5) denial of any urinary incontinence based on history and American Urological Association symptom score of <7. Exclusion criteria for control subjects included the same criteria as for the OAB syndrome subjects. All study patients were counseled and gave informed consent for this IRB-approved research study.
Bladder urothelial cell culture.
Bladder biopsies were obtained using the cystoscopic cold-cup biopsy technique. Four random areas within the floor of the bladder posterior to the trigone were biopsied. Two of the biopsies were immediately placed in 10% buffered neutral formalin for later immunohistofluorescence studies and the other two biopsies in sterile saline, and both were transferred to the laboratory within 30 min. The samples in formalin were refrigerated at 4°C. The samples in saline were minced manually into 0.5-mm pieces. These minced samples were placed in uncoated plastic tissue culture plates, urothelial side down against the plate surface, with buffer containing complete cell medium composed of MEM plus l-glutamine supplemented with 1 U/ml insulin, 10% heat-inactivated FBS, 1.25 μg/ml amphotericin B, 100 U/ml penicillin, and 100 μg/ml streptomycin. Samples were anchored to the bottom of the well with sterile glass coverslips, and incubated in 95% air-5% CO2 at 37°C. Once cell growth could be seen and confluent monolayers were present, cells were transferred using 0.25% trypsin. After dissociation, the cell suspension was centrifuged at 2,000 g and resuspended in cell medium. Cell counts were obtained, and the suspension was titrated with cell medium to give a cell count of 2,000 cells/ml; 250 μl of suspension was then pipetted onto individual glass coverslips arranged in a sterile Petri dish and incubated in 95% air-5% CO2 at 37°C overnight. This technique has been described previously and the cells derived from this technique have been shown to be epithelial in origin (20). Furthermore, immunofluorescence staining of the cultured BUC were positive for pan-cytokeratin and negative for smooth muscle α-actin.
Immunohistofluorescence staining of bladder urothelium for ornithine decarboxylase.
Bladder urothelial biopsies from normal control and OAB subjects were removed from formalin, paraffin embedded, and sectioned at 10 μm. Sections were deparaffinized according to standard procedure and were incubated in 1% H2O2 for 20 min at room temperature. After incubation in blocking solution, the slides were incubated overnight at 4°C with mouse anti-ornithine decarboxylase (DOC) antibody (1:200; Sigma, St. Louis, MO). The slides were then incubated with Cy3-conjugated secondary antibody goat anti- mouse IgG (1:200; Chemicon, Temecula, CA) for 60 min at room temperature. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; 1 μg/ml, Sigma). The slides were then analyzed using Zeiss LSM510 META confocal microscope.
Electrophysiology recording.
Experiments were carried out on cultured BUC serum starved at least 12 h before the electrophysiological recording. Membrane currents were amplified (Axopatch 200A, Axon Instruments, Foster City, CA) and sampled online at 5 kHz using a microcomputer equipped with a digitizing board (Digidata 1200A, Axon Instruments) and running Clampex software (version 8.0, Axon Instruments). Macroscopic currents were recorded in intact cells using the cell-attached, inside-out single-channel or conventional whole cell configuration. Patch-clamp pipettes, pulled from borosilicate glass, had resistances of 4–5 MΩ for single-channel recording and 2–3 MΩ for whole cell technique. Cells with seal resistances of <3 GΩ and access resistances of >50 MΩ were discarded. Membrane currents were measured during ramp pulses (0.38 mV/ms) from a holding potential of −67.5 mV. We used a bath solution containing (in mM) 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4. The pipette solution contained (in mM) 140 KCl, 5 NaCl, 1 MgCl2, 0.05 EGTA, and 10 HEPES, pH 7.2. The solution served as bath solution, and pipette solution in inside-out patch measurements was composed of (in mM) 138 KCl, 5 NaCl, 1 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose, pH 7.4. The measured osmolarity of the pipette solutions was ∼295 mosM, and that of the extracellular solution was ∼300 mosM. Single-channel current amplitude and open probability (Po) were estimated from all-point current amplitude histograms and fitted to Gaussian densities using Origin software (OriginLab, Northampton, MA). For current-voltage (I-V) relationships, the amplitudes were determined by fitting a Gaussian curve to an all-point histogram. Po was determined using unedited segments of data, which were typically 1–5 min long. For multiple-channel patches, the mean Po was measured as nPo, where n is the number of channels in the patch.
To determine the effect of polyamines to the outward current, spermidine and spermine (Sigma) were applied in the bath solution in inside-out single-channel patch-clamp configuration or in the pipette solution in whole cell patch-clamp configuration. The cultured urothelial cells were exposed to the specific ODC blocker dl-α-difluoromethylornithine (DFMO; Sigma) at 5 mM for 24 h to deplete the intracellular polyamines significantly in the culture medium without the serum and ornithine.
Statistical analyses.
The data are presented as means ± SE. Data were analyzed using unpaired Student's t-tests followed by Bonferroni post hoc test to identify specific mean differences. The α-level of difference was set at 0.05.
RESULTS
Immunohistofluorescence for ODC expression in human bladder urothelial biopsies.
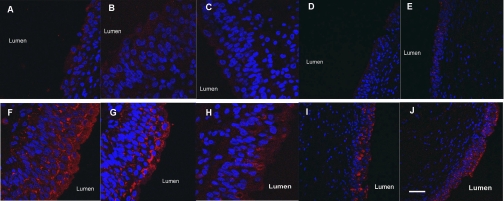
To characterize the expression of polyamines in human bladder urothelium, we performed immunohistofluorescence for ODC in bladder biopsies from OAB and control subjects. Figure 1, A–E, is bladder urothelium from five different control subjects. Orientation of the biopsies is provided by label identifying the lumen side of the bladder biopsies. There is faint ODC expression (red) in these five different normal subjects. However, in OAB subjects, there is a much stronger expression of ODC (Fig. 1, F–J). Most of the ODC appears to be in the apical urothelium rather than the basilar compartment.
Fig. 1.
Immunohistofluorescence for ornithine decarboxylase (ODC) (Cy3, red) and nuclei (DAPI, blue). A–E: urothelium from five different normal control subjects. F–J: urothelium from five different subjects with overactive bladder syndrome (OAB). Staining conditions were identical for all 10 patients. There is higher expression of ODC in OAB urothelium compared with control. Scale bar: 100 μm.
Single-channel electrophysiologic characteristics of OAB BUC.
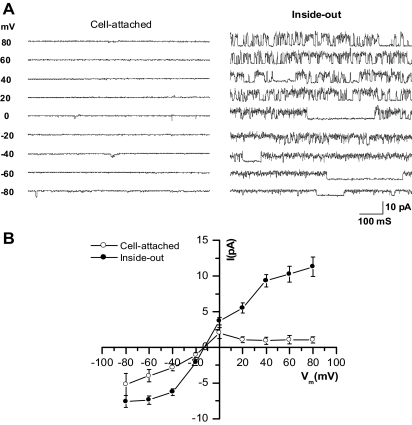
Figure 2A shows single-channel recordings of an OAB BUC with holding potentials from −80 to +80 mV using two types of patch-clamp configurations, cell-attached and inside-out excised patch. There is very little current measured in the cell-attached configuration (Fig. 2A, left set of tracings). However, using the inside-out excised patch configuration (Fig. 2A, right set of tracings), when any cytoplasmic blockers are removed because the patch is detached from the cytoplasm, measureable currents appeared. An I-V plot for both cell-attached and inside-out recordings is shown in Fig. 2B showing significant increases in currents from 0 mV to +80 mV in the inside-out patches compared with cell-attached configuration. There are also increased inward currents in the inside-out excised patch configuration at potentials negative of −20 mV.
Fig. 2.
Single-channel electrophysiologic characteristics of OAB bladder urothelial cells (BUC). A: representative single-channel currents recorded in cell-attached and inside-out patches in human BUC from OAB patients. B: current-voltage (I-V) relationships for single-channel currents recorded from cell-attached patches (○) and inside-out patches (•). Vm, membrane potential; n = 16 from five different subjects.
BK channels mediated the outward current in human BUC.
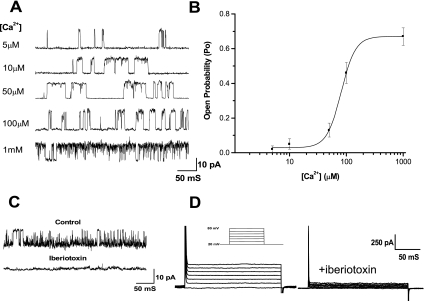
To characterize the outward current in human BUC, the dependence of the outward current open probability on the intracellular Ca2+ concentration was observed in inside-out single-channel configuration. Channel open probability exhibited clear Ca2+ dependence. Figure 3A shows representative records of outward current activity in an inside-out patch at +60 mV membrane potential when different concentrations of calcium were applied to the cytoplasmic side of the cell membrane. Figure 3B plots the mean open probability as a function of intracellular Ca2+ at a membrane potential at +60 mV. There was no channel activity when Ca2+ was below 1 μM and the maximal open probability was 0.72, achieved above 100 μM Ca2+. The data were fitted to the Hill function, and the value of the dissociation constant (K1/2) was 54.2 ± 7.3 μM (n = 6). The outward current was largely blocked by Ba2+ (100 nM, data not shown) and iberiotoxin (100 nM, Fig. 3, C and D) in single-channel and whole cell recordings. These electrophysiologic and pharmacological properties suggest that the outward current is primarily due to BK channels.
Fig. 3.
Large-conductance calcium-activated potassium (BK) channels mediated the outward current in human BUC. A: representative single-channel currents recorded at +60 mV in symmetric K+ at different intracellular Ca2+ concentrations ([Ca2+]i) in an inside-out patch. Single-channel I-V relationship showed that the outward current had a conductance of 176.7 pS at +60 mV. B: dose-response curve showing the effect of [Ca2+]i on open probability (Po) of BK channels at +60 mV. The data are fitted to the Hill function, and value of K1/2 was 54.2 ± 7.3 μM (n = 6). C: outward currents at +60 mV recorded in inside-out patches are completely blocked by 100 nM iberiotoxin in the pipette solution. D: outward currents measured during step pulses (600 ms from a test potential of 20 to 80 mV at 10 mV intervals) could be largely blocked by iberiotoxin.
Effect of exogenous spermidine and spermine on electrophysiologic properties of normal BUC: relationship to BK-channel.
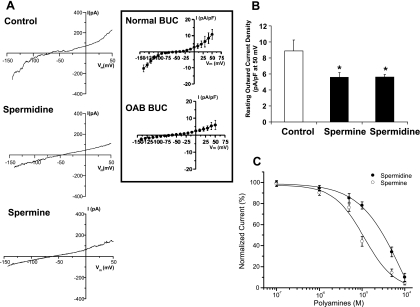
These series of experiments were performed to determine whether the electrophysiologic phenotype of normal BUC can be altered to resemble OAB BUC phenotype by addition of the polyamines spermidine and spermine to normal BUC. In the whole cell I-V configuration, 100 μM spermidine and 100 μM spermine qualitatively reduced the inward and outward currents (Fig. 4A). The whole cell I-V configurations of OAB and normal BUC are shown in the inset of Fig. 4 (these measurements were derived from cells obtained from 3 OAB and 3 control subjects). When quantitated, spermine and spermidine significantly reduced normalized outward current measured at +50 mV (Fig. 4B). Polyamine was applied to the cytosolic side of inside-out patches. The spermidine and its metabolite spermine reduced the amplitude of the outward single-channel current in a dose-dependent curve at +60 mV (Fig. 4C). These data show that polyamine effectively blocks the BK channel, with spermine being more potent in its block of the BK channel compared with spermidine.
Fig. 4.
Effect of exogenous spermidine and spermine on electrophysiologic properties of normal BUC. A: representative whole cell I-V relationships recorded without (control) or with 100 μM spermidine or spermine in pipette solution in human BUC from normal control patients. Inset: normal (n = 3 subjects) and OAB (n = 3 subjects) whole cell I-V currents. B: resting outward current density measured at +50 mV potential. Spermine and spermidine inhibit the outward current density significantly, P < 0.05, n = 9. C: dose-response curves for spermidine (•) and spermine (○) obtained at +60 mV from inside-out patches. The polyamines were applied to the cytosolic side; n = 12–16 from five different subjects.
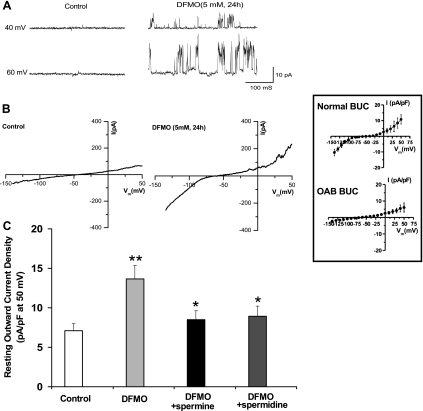
Effect of DFMO (block of polyamine synthesis) on electrophysiologic properties of OAB BUC.
These series of experiments were performed to determine whether blocking the synthesis of polyamines (by inhibiting the rate limiting enzyme ODC with DFMO) in OAB BUC would convert the OAB phenotype to a normal phenotype. Again, the whole cell I-V configurations of OAB and normal BUC are shown in the inset of Fig. 5 . In Fig. 5A, single-channel measurements (cell-attached configuration) in OAB BUC revealed no outward current at holding potential of +40 and +60 mV. However, after OAB BUC were treated for 24 h with 5 mM DFMO, there was the appearance of an outward current. We observed similar changes in whole cell I-V measurements (Fig. 5B). Treatment of OAB BUC with DFMO resulted in I-V tracing similar to normal BUC. We could make DFMO-treated OAB BUC I-V tracing revert back to OAB phenotype by adding 100 μM spermidine and spermine. Quantification of normalized outward currents in OAB, DFMO-treated OAB, and DFMO-treated OAB with spermine or spermidine added is shown in Fig. 5C. DFMO significantly increased outward current (P < 0.01, n = 18 cells from 6 different OAB subjects), and spermine or spermidine significantly reduced the outward current (P < 0.05, n = 15 from 6 different OAB subjects).
Fig. 5.
Effect of dl-α-difluoromethylornithine (DFMO) on electrophysiologic properties of OAB BUC. A: more outward currents were recorded in human OAB BUC incubation with ODC inhibitor DFMO (5 mM) for 24 h from cell-attached patches at membrane potentials 40 mV and 60 mV. B: representative I-V relationship recording in human OAB BUC and human OAB BUC incubated with DFMO (5 mM) for 24 h. Inset: normal (n = 3 subjects) and OAB (n = 3 subjects) whole cell I-V currents. C: whole cell outward current measured in membrane potential at 50 mV in OAB BUC. DFMO increased the outward current significantly compared with control, **P < 0.01, while 100 μM spermidine and spermine in pipette solution further blocked the DFMO-increased outward current. *P < 0.05, n = 15–19 from six different subjects.
DISCUSSION
The pathophysiology of OAB syndrome is unknown. Previous investigators have primarily focused on bladder smooth muscle dysfunction as the cause of OAB syndrome. The concept of augmented muscarinic receptor activity and resultant detrusor contraction overactivity underlies the current basis of treating OAB syndrome with antimuscarinic agents (1). However, recent evidence also points to the importance of the BK channel (11, 19) and β3-adrenergic receptor activity (22) in detrusor smooth muscle physiology and possible linkage to OAB pathophysiology.
The theory that bladder urothelium actively contributes to bladder function is a new paradigm. Most of the research into bladder urothelial physiology has been related to bladder urothelial cancer. However, the role of the bladder urothelium to bladder storage and emptying function, specifically in humans, remains unknown. A few reviews of bladder urothelium and how it regulates bladder function have been published (2, 4). The role of the bladder urothelium in OAB has only been investigated in the context of the vanilloid receptor TRPV1 and muscarinic receptor expression (9, 10). Various animal models purported to mimic OAB syndrome have been developed and are used to determine the contribution of urothelium to OAB (5, 8, 13). While no perfect animal model for OAB exists, investigators have used acetic acid infusion into bladder, bladder outlet obstruction, and cyclophosphamide-induced inflammation to explore how different physiologic mechanisms regulate bladder function. While each of these animal models is useful, the relevance to human OAB syndrome is questioned. OAB syndrome is excluded in patients with a history of exposure to cyclophosphamide or if bladder outlet obstruction is present. Bladder exposure to exogenous acid, such as acetic acid, is not a problem encountered clinically.
In this study, we found evidence of altered polyamines expression in OAB urothelium derived from human subjects. The in vitro data were obtained from dissociated BUC in culture. The method of dissociating these cells in culture from cystoscopic biopsies has been previously published, and these cells are epithelial in origin (20). One drawback of the cell culture system is that the polarity of the urothelium (in which there is a spatial orientation with apical cell layer on the luminal side and a basal cell layer on the stromal side) is lost in a monolayer. Therefore, we cannot correlate the microarchitecture inherent in the urothelium with a cell culture system. Nevertheless, single-channel electrophysiology cannot be done at the tissue level but requires dissociated single cells.
We detected an increased polyamine signaling in OAB BUC/urothelium, both at the single cell and tissue levels. This was manifested by decreased outward currents which could be reversed when polyamine synthesis was inhibited by DFMO. When spermidine or spermine was added back to DFMO-treated OAB BUC, the cells then behaved similarly to untreated OAB BUC. The increased polyamine expression was detected in urothelium using immunofluorescence of human bladder biopsies from control and OAB subjects (Fig. 1). There was an obvious visual increase in ODC expression in OAB compared with control urothelium. ODC is the rate limiting enzyme in polyamine synthesis, and levels of ODC expression parallel polyamine signaling. Furthermore, this increased activity appears to be in the apical rather than the basal layer. Expression of ODC in the human OAB and normal control BUC was also confirmed by Western blot analysis (data not shown).
We could make normal BUC behave as OAB BUC by the addition of the exogenous polyamines spermidine and spermine (Fig. 4). Spermidine and spermine inhibited open probability of channels conducting the outward current. This effect was likely due to blockage of polyamines on the BK channel. This is based on the observation that iberiotoxin, a BK channel-specific blocker, independently blocked the outward current in normal BUC (Fig. 3). Polyamines have been shown to block BK channels (21), so these results were not unexpected.
The mechanistic link between OAB syndrome and BK channel activity has been supported from transgenic BK-knockout animal models (11, 19). In these constitutive knockout animals, both in vivo and in vitro studies reveal hyperactivity or overactivity of bladder smooth muscle. It was theorized that these animals have urinary incontinence (although it is impossible to know because urinary incontinence and urgency are symptoms which are not elicitable from a rodent). Furthermore, it is not possible to know the contribution of BK channel from non-detrusor muscle compartments, such as urothelial or neural compartments, to bladder function because the BK knockout is constitutive. The data presented strongly suggest that there is increased block of BK channel in the OAB urothelial cell due to increased intracellular polyamines. Whether there is increased polyamine leading to block of BK channel in the OAB detrusor smooth muscle is unknown, although this could be a possibility. There is an ethical issue with obtaining bladder smooth muscle from OAB syndrome subjects. The reason is that human bladder smooth muscle biopsies have more associated severe risks compared with urothelial biopsies. Furthermore, there are no known clinical reasons to perform bladder smooth bladder biopsies in OAB syndrome.
Extracellular polyamines have been shown to induce bladder smooth muscle relaxation (14). Polyamines also relax large intestine smooth muscle (15). However, differential effects on vascular smooth muscle have been demonstrated, depending on whether the polyamines was located extracellularly (in which case there was decreased contractility) or intracellularly (in which case there was increased contractility) (16). We do not know the level of ODC expression/polyamine signaling in OAB bladder smooth muscle. Therefore the effects of polyamines on bladder function can occur at different cellular levels (smooth muscle and urothelial) and could have different effects. The EC50 values of extracellular polyamines which block detrusor smooth muscle contractions are between 0.1 mM and 1 mM (14). Our study focused on the effects of polyamine only on the intracellular side. Polyamines, because they are highly positively charged, do not easily cross the lipid cell membrane. Cytoplasmic polyamines presumably mediate the block of OAB BUC currents (Fig. 2A, left tracings) because once an inside-out patch configuration is created, the cytoplasmic polyamines are washed away, thus unblocking BK channels with subsequent measurable currents (Fig. 2B, right tracings). Intracellular spermidine and spermine, however, can also block outward currents in BUC, with EC50 values between 0.01 mM for spermine and 0.1 mM for spermidine (Fig. 4C). Total cytoplasmic concentrations of polyamines vary in different tissue types and can be in the micromolar range. However, most of the intracellular polyamines are bound to negatively charged macromolecules such as RNA, DNA, and phospholipids. Cytoplasmic polyamines can exert a variety of actions including apoptosis (8) and RNA stability (24). Quantitative measurements of intracellular polyamine levels of spermine, spermidine, and putrescine can be performed with high performance liquid chromatography, and this is currently being pursued in OAB and normal BUC.
In summary, this work represents the initial description of human urothelial polyamines pathophysiology from human OAB subjects. This pathway is unique and not related to the previously described abnormalities in TRPV1 and muscarinic signaling in urothelium (9, 10). Whether urothelium can be targeted to successfully treat OAB syndrome remains to be seen; however, pathophysiologic advances would help to establish the rationale for development of new pharmacologic agents for specific urothelial targets. Furthermore, it might be possible to base treatment or prognosis based on urothelial biopsy tests. Further confirmatory experiments remain to be done.
GRANTS
This work is supported by National Institutes of Health Grant RO1-DK075728.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Vladimir Gerzanich and Alexander Ivanov for the electrophysiologic technical assistance.
REFERENCES
- 1.Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int 100: 987– 1006, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057– 64, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Birder LA. Role of the urothelium in urinary bladder dysfunction following spinal cord injury. Prog Brain Res 152: 135– 146, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4: 46– 54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol 562: 859– 871, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174– C1187, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology 63: 461– 465, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kim JC, Yoo JS, Park EY, Hong SH, Seo SI, Hwang TK. Muscarinic and purinergic receptor expression in the urothelium of rats with detrusor overactivity induced by bladder outlet obstruction. BJU Int 101: 371– 375, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Mansfield KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis of urgency: regional difference of vanilloid receptor expression in the human urinary bladder. Neurourol Urodyn 26: 433– 438, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Mansfield KJ, Liu L, Moore KH, Vaux KJ, Millard RJ, Burcher E. Molecular characterization of M2 and M3 muscarinic receptor expression in bladder from women with refractory idiopathic detrusor overactivity. BJU Int 99: 1433– 1438, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746– 36752, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Messing EM, Hanson P, Reznikoff CA. Normal and malignant human urothelium: in vitro response to blockade of polyamine synthesis and interconversion. Cancer Res 48: 357– 361, 1988 [PubMed] [Google Scholar]
- 13.Mitobe M, Inoue H, Westfall TD, Higashiyama H, Mizuyachi K, Kushida H, Kinoshita M. A new method for producing urinary bladder hyperactivity using a non-invasive transient intravesical infusion of acetic acid in conscious rats. J Pharmacol Toxicol Methods 57: 188– 193, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Myung SC, Oh SY, Kim KD, Kim SC, Lee MY. Effects of spermine on the relaxation response of rat detrusor smooth muscles. Eur J Pharmacol 573: 196– 200, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Nilsson BO, Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand 148: 37– 43, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Nilsson BO, Gomez M, Santiago Carrilho R, Nordström I, Hellstrand P. Differential actions of exogenous and intracellular spermine on contractile activity in smooth muscle of rat portal vein. Acta Physiol Scand 154: 355– 365, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-gamma1 expression promoting intestinal epithelial restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335– G343, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327– 336, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604– F610, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Trifillis AL, Cui X, Jacobs S, Warren JW. Culture of bladder epithelium from cystoscopic biopsies of patients with interstitial cystitis. J Urol 153: 243– 248, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Weiger T, Hermann A. Polyamines block Ca(2+)-activated K+ channels in pituitary tumor cells (GH3). J Membr Biol 140: 133– 142, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn 26: 752– 756, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem 279: 22539– 22547, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Zou T, Liu L, Rao JN, Marasa BS, Chen J, Xiao L, Zhou H, Gorospe M, Wang JY. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1. Biochem J 409: 389– 398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]