Abstract
The present experiments show that IFNγ receptors are mainly localized to the basolateral membrane of human retinal pigment epithelium (RPE). Activation of these receptors in primary cultures of human fetal RPE inhibited cell proliferation and migration, decreased RPE mitochondrial membrane potential, altered transepithelial potential and resistance, and significantly increased transepithelial fluid absorption. These effects are mediated through JAK-STAT and p38 MAPK signaling pathways. Second messenger signaling through cAMP-PKA pathway- and interferon regulatory factor-1-dependent production of nitric oxide/cGMP stimulated the CFTR at the basolateral membrane and increased transepithelial fluid absorption. In vivo experiments using a rat model of retinal reattachment showed that IFNγ applied to the anterior surface of the eye can remove extra fluid deposited in the extracellular or subretinal space between the retinal photoreceptors and RPE. Removal of this extra fluid was blocked by a combination of PKA and JAK-STAT pathway inhibitors injected into the subretinal space. These results demonstrate a protective role for IFNγ in regulating retinal hydration across the outer blood-retinal barrier in inflammatory disease processes and provide the basis for possible therapeutic interventions.
Keywords: interferon, inflammation, CFTR , JAK-STAT, cAMP, cGMP, proliferation
interferon-γ (IFNγ) is intimately involved in the regulation of innate and adaptive immune responses, and its major biological activities are associated with antiviral and immunomodulatory activities, cell growth and differentiation, and control of apoptosis (56, 64, 71). The receptor for this pleiotropic cytokine is composed of two distinct subunits, IFNγ receptor subunits 1 and 2 (IFNGR1 and IFNGR2) (3, 25, 63, 64). Interaction of IFNγ with cell surface receptors activates receptor-associated JAK1 and JAK2, which phosphorylate and activate STAT1. Phosphorylated STAT1 dimers translocate into the nucleus, where they bind to a well-defined DNA sequence in the promoter of IFNγ-regulated genes. Genes coding for members of the interferon regulatory factor (IRF) family of transcription factors, such as IRF-1, IRF-2, and IRF-8 [IFN consensus sequence-binding protein (ICSBP)] are the major targets of STAT1 and help regulate cell growth through their effects on the cell cycle (16, 48, 67). IRF-1 is a tumor suppressor, activating a set of genes whose products are required for negative regulation of cell growth. IRF-2 shares significant sequence similarity to IRF-1 within the DNA-binding domain but represses IRF-1 regulable genes (66).
IFNγ can also activate several other signal transduction proteins, including MAPK, ERK, JNK, and p38 MAPK (11, 56, 71). Although the precise function of p38 MAPK remains unclear, it can mediate fast cellular responses and help sustain longer-lasting responses to IFN stimulation by regulating gene transcription (11, 35, 53). Recently, p38 MAPK was shown to mediate the flagellin-induced activation of CFTR-dependent Cl− secretion in Calu-3 airway epithelial cells (30, 76). In other experiments using lung epithelia, activation of inducible nitric oxide (NO) synthase (iNOS) by IRF-1 led to a subsequent NO-induced activation of CFTR (12, 36). IFNγ has been implicated in the pathogenesis of a number of inflammatory diseases of infectious or presumed autoimmune origin (13, 19, 74). In the eye, IFNγ has been detected in vitreous aspirates of patients with uveitis, proliferative vitreoretinopathy, and other inflammatory ocular diseases (21, 26, 40, 51).
The retinal pigment epithelium (RPE) is a single monolayer of epithelial cells in the back of the eye that forms the outer blood-retinal barrier and is fundamentally important for the health and integrity of the distal retina. It plays a critical role in maintaining the visual cycle and photoreceptor outer segment phagocytosis, and is the main transport pathway for the flow of nutrients, metabolic waste products, ions, and fluid between the distal retina and the choriocapillaris. Dysfunction of RPE cells has been implicated in inflammatory and degenerative diseases of the retina and choroid (10, 31, 39, 59, 72), but relatively little is known about the direct effects of inflammatory mediators on RPE physiology or pathophysiology. The present experiments using human RPE and an animal model of retinal reattachment show that IFNγ can be therapeutic in the removal of fluid from cystic spaces. The mechanism that underlies this result is a novel connection between the canonical JAK-STAT pathway and CFTR, mediated by the cAMP-PKA pathway and NO.
MATERIALS AND METHODS
Cell culture.
The research followed the tenets of the Declaration of Helsinki and the National Institutes of Health Institutional Review Board. Fetal eyes (16–18 wk gestation) were obtained from Advanced Bioscience Resources (Alameda, CA), and adult eyes were obtained from Analytical Biological Services (Wilmington, DE). Primary cell cultures of human fetal RPE (hfRPE) and cells from human fetal choroid (hfCHC) were prepared as described previously (42). For immunofluorescence localization or fluid transport experiments, cells were seeded in transwells (passage 2) and maintained for 6 wk before experiments.
Quantitative RT-PCR and immunoblot.
Quantitative RT-PCR (qRT-PCR) and immunoblots were performed as previously described (39). The blotted membrane was incubated with antibodies against human IFNGR1 (catalog no. MAB6731, R & D Systems, Minneapolis, MN), IFNGR2 (catalog no. sc-970, Santa Cruz Biotechnology, Santa Cruz, CA), IFNα receptor subunits 1 and 2 (IFNAR1 and IFNAR2, Abcam, Cambridge, MA), IRF-1, IRF-2, and ICSBP (Santa Cruz Biotechnology), and CFTR (CFTR antibody 217, Cystic Fibrosis Foundation Therapeutics, Bethesda, MD). Immunoblot signals were detected using the West Dura chemiluminescence system (Pierce Biotechnology) and imaged using the Autochemie system (UVP, Upland, CA). hfRPE cells cultured in flasks (6–8 wk) were homogenized in isolation buffer (250 mM sucrose, 10 mM Tris·HCl, 10 mM MgCl2, and 1 mM CaCl2, pH 7.4). Cell lysates were centrifuged at 900 g for 10 min, the supernatant was collected and centrifuged at 10,000 g for 5 min and 18,000 g for 20 min at 4°C, and the pellet was discarded. The supernatant was centrifuged at 100,000 g for 1 h at 4°C using a Sovall ultracentrifuge (Thermo Fisher Scientific, Waltham, MA), and plasma membrane fraction pellets were collected for CFTR immunoblot analysis. Membrane proteins from Calu-3 cells were used as positive control.
In the phosphorylation studies, cells were starved for 24 h in serum-free medium and then treated with IFNγ (5–50 ng/ml) at various times at 37°C before the immunoblot analysis. Antibodies against p38, phosphorylated (Thr180/Tyr182) p38, STAT1, and phosphorylated (Tyr701 and Ser727) STAT1 (Cell Signaling Technology, Danvers, MA) and GAPDH (the loading control; Abcam) were used. Cycloheximide (CHX), a protein synthesis inhibitor, was used at 62 μM.
Immunofluorescence localization and JC-1 staining.
Zenon technology (Invitrogen) was used to localize IFNGR1, IFNGR2, and CFTR on hfRPE cells, as described previously (39). Normal animal serum matched with the antibody species of the target proteins was labeled with fluorophore and used as the negative control. ZO-1 antibody was obtained from Zymed (South San Francisco, CA). Mitochondrial membrane potential change was assessed in live hfRPE cells using JC-1 following the manufacturer's instructions (Invitrogen) (73).
RPE proliferation and migration.
Bromodeoxyuridine incorporation and a wound-healing assay were used to determine the effects of inflammatory cytokines on hfRPE cell proliferation and migration (39). IFNγ, TNFα, and IL-1β were obtained from Peprotech (Rocky Hill, NJ), and universal type I interferon and human interferon-β 1a were obtained from PBL Biomedical Laboratories (New Brunswick, NJ). Cumulative effects were measured by addition of VEGF-165, EGF, and bFGF (Peprotech), and PDGF-BB (R & D Systems) with IFNγ. Anti-human IFNGR1 blocking antibody (R & D Systems), or JAK inhibitor I, AG490, or JAK3 inhibitor II (EMD Biosciences, Gibbstown, NJ) was added to the cells 1 h before the addition of IFNγ. Cell viability was confirmed using a Live/Dead Viability/Cytotoxicity kit (Invitrogen).
Fluid transport.
Confluent monolayers of hfRPE cells cultured on transwells were mounted in a modified Ussing chamber, and transepithelial water flow (JV) was measured with a capacitance probe technique, as described previously (18, 32, 42, 43, 59). Tissue viability was ascertained by recording transepithelial potential (TEP) and total tissue resistance (RT) (29). It should be noted that the solution composition changes in this chamber are relatively slow (∼1–2 chamber volumes per min). The data sampling rate is once per minute, more than two orders of magnitude slower than the sampling rate in the electrophysiology chamber; therefore, it is not possible to record fast (seconds or minutes) changes in JV, TEP, and RT. After addition of IFNγ to the apical or basal bath, steady-state JV, TEP, and RT were recorded for 20–30 min. To block IFNγ effects, RPE cells were incubated for 30–60 min with anti-IFNGR1 blocking antibody, JAK inhibitor I, PKA inhibitors (H-89, EMD Biosciences; Rp-8-Br-cAMPS, Sigma-Aldrich), NO synthase inhibitor (aminoguanidine hydrochloride, Sigma-Aldrich), p38 MAPK inhibitors (SB-203580 and SB-202190), or CHX. CFTRinh-172 (44) or DIDS (EMD Biosciences) was used to block cAMP- or Ca2+-activated Cl− channels. Intracellular levels of NO were elevated with 1-hydroxy-2-oxo-3-(3-aminopropyl)-3-isopropyl-1-triazene (NOC-5) (Enzo Life Sciences, Plymouth, PA).
Electrophysiology.
The equivalent circuit analysis and electrophysiological methods have been previously described (34, 42, 54). Briefly, confluent monolayers of cultured hfRPE cells are mounted on a nylon mesh support and clamped into a modified Ussing chamber, which allows the rapid exchange of Ringer solution (∼10 chamber volumes per minute) and the measurement of fast electrical changes (seconds). Intracellular potentials were recorded with conventional microelectrodes, backfilled with 150 mM KCl, with resistances of 80–200 MΩ. The apical and basolateral membrane potentials (VA and VB) are the voltage differences between the intracellular microelectrode and the apical and basal bath electrodes, respectively. RA and RB are the resistances of the apical and basolateral membranes, and TEP = VB − VA. Epithelial resistance parameters were obtained by passage of 4-μA bipolar current pulses (i) using Ag-AgCl electrodes, one located in the apical chamber and the other located in the basal chamber. RT is calculated from the current-induced changes in TEP (RT = ΔTEP/i); the apparent membrane resistance ratio (RA/RB) is calculated from the change in VA and VB (RA/RB = ΔVA/ΔVB). 8-Bromo-cAMP (8-Br-cAMP, or dibutyryl cAMP) and forskolin were obtained from Sigma-Aldrich.
In vivo retinal reattachment model.
All animal experiments were conducted in compliance with the Association for Research in Vision and Ophthalmology statement, and the protocol was approved by the Animal Care and Use Committee of the National Institutes of Health. The procedures for these in vivo experiments have been previously described in detail (43). In the present experiments, retinal detachments were created in rat eye by injection of 0.5–3 μl of modified PBS (MPBS)-Ringer solution alone or with a combination of JAK-STAT and PKA pathway inhibitors into the subretinal space (SRS), which separates the photoreceptor outer segments and the apical processes of the RPE apical membrane. In the control part of each experiment (0–70 min after creation of the retinal detachment), the rate of change of bleb volume was measured. The control period of “constant” volume was defined by JV ≤2 μl·h−2·cm−1 over ≥40 min. Any control injections that exceeded this threshold rate were rejected from subsequent analysis. IFNγ was dissolved in MPBS, mixed with Celluvisc solution, and applied as eye drops (0.4 ml). Optical coherence tomography (OCT) imaging (Institute of Applied Physics, Russian Academy of Science, Nizhniy Novgorod, Russia) was used to measure the time course of the volume change in SRS.
Statistics.
Values are means ± SE. Data were analyzed using the Student's t-test and were considered statistically significant if P < 0.05.
RESULTS
Localization of IFNγ receptor in human RPE.
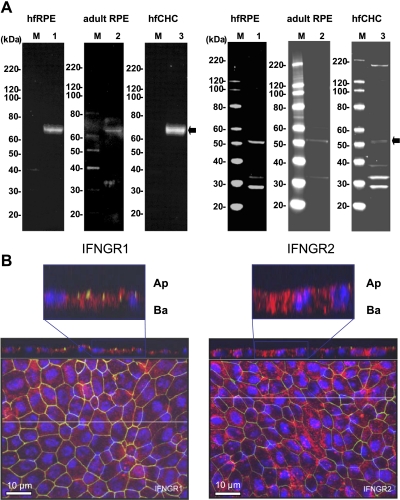
Evidence for the presence of IFNGR1 is summarized in Fig. 1A, which shows prominent antibody-specific bands at 65 kDa in hfRPE cells, as well as in adult tissue. Antibody-specific bands for IFNGR2 are also shown, at ∼50 kDa, but the expression level is much lower in native adult RPE and in hfCHC cells. In Fig. 1B, maximum-intensity projections show immunofluorescence of IFNGR1 and IFNGR2 in confluent monolayers of hfRPE primary cell cultures. The top of Fig. 1B, left and right, shows one of the z sections, part of which is shown at higher gain in the inset. Figure 1B, left, shows that IFNGR1 (red signal) is located mainly under the ZO-1 protein (green), indicating a mainly basolateral location, whereas IFNGR2 (Fig. 1B, right) is more evenly distributed on the apical and basolateral membranes.
Fig. 1.
Expression and localization of IFNγ receptors. A: immunoblots of IFNγ receptor subunits 1 and 2 (IFNGR1 and IFNGR2) in human fetal and adult retinal pigment epithelium (RPE) and fetal choroidal (hfCHC) cells. M, molecular weight marker lanes; lane 1, primary hfRPE cell culture; lane 2, native human adult RPE; lane 3, primary hfCHC cells. B: immunofluorescence localization of IFNγ receptor in hfRPE cells. For IFNGR1 and IFNGR2, cross section through the z plane is shown at top. In each case, x-y plane is shown as an en face view of the apical membrane (maximum-intensity projection through the z-axis). ZO-1 (green) stains tight junctions; 4,6-diamidino-2-phenylindole (DAPI, blue) labels nuclei. Inset at higher gain shows a z-section above each panel; note that IFNGR1 and IFNGR2 are mainly located on the basolateral membrane (Ba). Ap, apical membrane.
Activation and regulation of the JAK-STAT pathway in hfRPE cells.
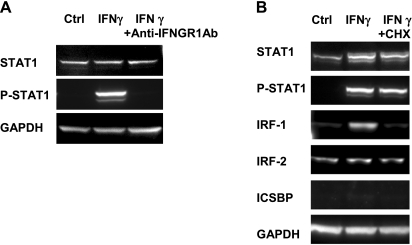
The immunoblot data in Fig. 2A show that STAT1 was tyrosine phosphorylated after addition of IFNγ (5 ng/ml) for 15 min, and this response was blocked by pretreatment with anti-IFNGR1 monoclonal antibody. Figure 2B shows that prolonged (4 h) treatment with IFNγ phosphorylated STAT1 and activated the nuclear transcription factor IRF-1. IRF-2 is constitutively expressed and unaffected by IFNγ, whereas ICSBP was below our detection limit. Protein synthesis inhibition by CHX (62 μM; Sigma-Aldrich) had no effect on IRF-2 but completely blocked the de novo synthesis of IRF-1. The top of Fig. 2B also shows a significant increase of the STAT1 protein signal after IFNγ treatment and partial inhibition of its synthesis by CHX. In native adult RPE, similar protein expression levels of IRF-1, IRF-2, and ICSBP were observed (n = 2; data not shown).
Fig. 2.
IFNγ activates JAK-STAT signaling pathway in hfRPE cells. A: IFNγ stimulated tyrosine phosphorylation of STAT1 in hfRPE cells (15 min) and can be blocked by anti-IFNGR1 blocking antibody. GAPDH was used as loading control. B: regulation of interferon regulatory factor (IRF) gene expression in hfRPE cells. Cells were treated with serum-free medium [SFM (Ctrl)], IFNγ, or cycloheximide (CHX) + IFNγ for 4 h. ICSBP, IFN consensus sequence-binding protein.
Similar to our immunoblot results, qRT-PCR (Table 1) shows that transcription of IRF-1 and STAT1 was significantly upregulated (>400- and >30-fold, respectively) by 24 h of treatment with 25 ng/ml IFNγ in hfRPE cells. IRF-2 mRNA was also upregulated; however, the change (∼3-fold) was much less than IRF-1. Constitutive expression of IRF-8 mRNA was not detected; however, it became detectable after 2 h of treatment with IFNγ, although there was no significant change in IRF-8 protein level. On the basis of previous studies in cystic fibrosis epithelia (36), we also determined the gene expression levels of IFNGR1, IFNGR2, suppressor of cytokine signaling 3 (SOCS3), protein inhibitor of activated STAT1 (PIAS1), iNOS (NOS2A), and CFTR after IFNγ treatment. There was no significant change in IFNGR1, but there was a nearly twofold increase in IFNGR2 after 6 h. IFNγ had no effect on PIAS1 gene expression, but it increased SOCS3. The constitutive level of NOS2A is undetectable; however, there was a significant upregulation after 6 h. CFTR levels are low and do not appreciably change over time.
Table 1.
IFNγ-induced alterations in gene expression by quantitative RT-PCR
| Threshold Cycle |
||||||
|---|---|---|---|---|---|---|
| Gene | 0 h | 0.5 h | 2 h | 6 h | 15 h | 24 h |
| STAT1 | 26.2 | 25.8 | 22.1 | 21.0 | 21.3 | 21.2 |
| IRF-1 | 31.1 | 27.4 | 22.2 | 22.3 | 22.3 | 22.2 |
| IRF-2 | 27.6 | 27.6 | 26.0 | 25.6 | 26.0 | 26.1 |
| IRF-8 | ND | ND | 30.9 | 31.1 | 32.0 | 31.9 |
| IFNGR1 | 26.4 | 26.4 | 26.4 | 26.3 | 26.5 | 26.3 |
| IFNGR2 | 26.0 | 26.0 | 25.1 | 24.7 | 25.1 | 25.2 |
| SOCS3 | 26.9 | 25.4 | 24.6 | 24.0 | 24.8 | 24.9 |
| PIAS1 | 27.0 | 26.9 | 27.2 | 27.1 | 26.8 | 26.7 |
| NOS2A | ND | 39.1 | 35.9 | 31.6 | 32.7 | 33.5 |
| CFTR | 38.0 | 36.9 | 37.3 | 38.5 | ND | 37.9 |
GAPDH was used for data normalization. IRF, interferon regulatory factor; IFNGR1 and IFNGR2, IFNγ receptor subunits 1 and 2; SOCS3, suppressor of cytokine signaling 3; PIAS1, protein inhibitor of activated STAT1; NOS2A, inducible nitric oxide synthase; ND, not determined.
IFNγ decreased RPE mitochondrial membrane potential.
JC-1 staining was used to evaluate mitochondrial membrane potentials in confluent hfRPE cell monolayers. Step increases of IFNγ (0.5, 5, and 50 ng/ml) caused a monotonic decrease in red J-aggregate fluorescence compared with control (see supplemental Fig. S1 in the online version of this article), reflecting a dose-dependent decrease in mitochondrial membrane potential. These effects were observed after 72 h of treatment with IFNγ; no significant differences were observed at 24 or 48 h.
IFNγ regulates hfRPE and hfCHC cell proliferation and migration.
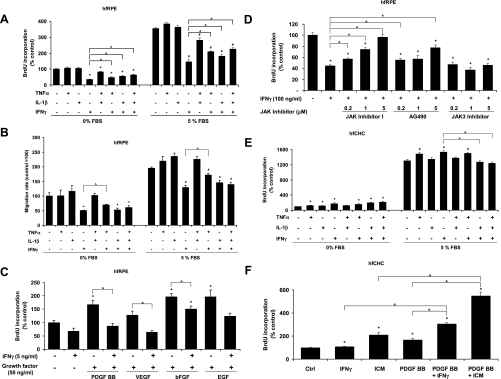
Previously, we demonstrated the strong inhibitory effect of a proinflammatory cytokine mixture (TNFα, IL-1β, and IFNγ) on hfRPE cell proliferation and migration (39). The dose-response curve of each cytokine was determined (see supplemental Fig. S2A). As shown in Fig. 3, A and B, IFNγ (5 ng/ml), but not TNFα or IL-1β, significantly inhibited hfRPE cell proliferation and migration in 5% or 0% FBS (n = 4, P < 0.001). Furthermore, IFNγ significantly inhibited hfRPE cell proliferation induced by bFGF, PDGF-BB, and VEGF (Fig. 3C). Pretreatment with anti-human IFNGR1 blocking antibodies abolished the inhibitory effect of IFNγ on hfRPE cell proliferation (see supplemental Fig. S2B), indicating the specificity of this effect for IFNγ. Figure 3D shows that JAK inhibitor I, a universal JAK inhibitor, and AG490 (a JAK2 inhibitor), but not JAK3 inhibitor, block the IFNγ-induced hfRPE cell proliferation in a dose-dependent way. Similar experiments were performed using hfCHC cells (Fig. 3E; see supplemental Fig. S3). In striking contrast to hfRPE cells, IFNγ, singly or in combination with TNFα, or IL-1β showed a stimulatory effect on hfCHC cell proliferation. IFNα and IFNβ (type I IFN) have no effect on hfRPE cell proliferation (data not shown). This conclusion was corroborated by immunoblot analysis, which shows that IFNAR1 is undetectable in human RPE cells, although IFNAR2 is expressed at intermediate levels (see supplemental Fig. S4).
Fig. 3.
Proinflammatory cytokines regulate hfRPE and human fetal choroidal (hfCHC) cell proliferation and migration. A: modulation of hfRPE cell proliferation by combinations of IFNγ, TNFα, and IL-1β (n = 4). *P < 0.05 vs. control in 0% FBS. #P < 0.05 vs. control in 5% FBS. ^P < 0.05, IFNγ vs. IFNγ + TNFα or IL-1β. B: modulation of hfRPE cell migration by combinations of IFNγ, TNFα, and IL-1β (n = 3). C: modulation of growth factor-induced hfRPE cell proliferation by IFNγ. *P < 0.05 vs. SFM control. ^P < 0.05, growth factor alone vs. growth factor + IFNγ. D: effects of JAK inhibitors on IFNγ-induced inhibition of hfRPE cell proliferation (n = 4). *P < 0.05 vs. control. ^P < 0.05, IFNγ alone vs. IFNγ + different JAK inhibitors. E: modulation of hfCHC proliferation by combinations of IFNγ, TNFα, and IL-1β (n = 4). *P < 0.05 vs. control in 0% FBS. #P < 0.05 vs. control in 5% FBS. ^P < 0.05, IFNγ vs. IFNγ + TNFα or all 3 components. F: modulation of PDGF-BB-induced hfCHC proliferation by IFNγ or proinflammatory cytokine mixture (ICM, i.e., TNFα, IL-1β, and IFNγ) (n = 4). *P < 0.05 vs. SFM control. ^P < 0.05, PDGF-BB + IFNγ or ICM-induced proliferation vs. single components.
Expression, localization, and function of CFTR in human RPE.
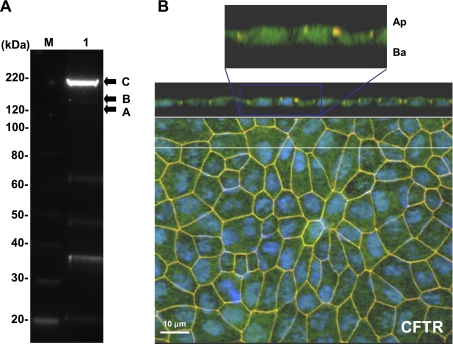
Figure 4A shows a representative immunoblot of CFTR in hfRPE cells at 190 kDa, as previously described (6, 33, 38). Immunofluorescence shows that CFTR is mainly localized at the basolateral membrane of confluent hfRPE cell monolayers grown on transwells (Fig. 4B).
Fig. 4.
Localization of CFTR in hfRPE cells. A: CFTR was detected in membrane-enriched extracts of hfRPE cells. M, molecular weight marker lane; lane 1, major mature (band C) and immature (bands A and B) bands of CFTR in primary hfRPE cell culture. B: immunofluorescence localization of CFTR (green label). Top: cross section through the z plane and below the x-y plane showing an en face view of the apical membrane (maximum-intensity projection through the z-axis). ZO-1 (labeled as red) serves as a tight junction marker delineating apical (Ap) and basolateral (Ba) sides of the cell, and DAPI (blue) labels the nuclei located close to the basement membrane. ZO-1 is seen as yellow/orange, since its red label overlaps green fluorescence from CFTR. In the inset at higher gain, DAPI (blue) channel was removed for more clear visualization of the basolateral localization of CFTR (green).
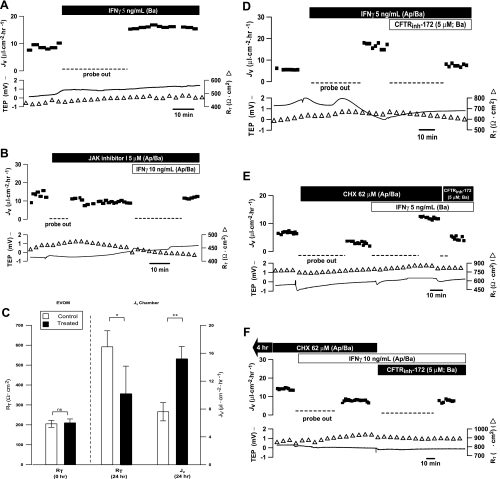
Figure 5A shows that addition of IFNγ (10 ng/ml) to the basal bath increased JV by ∼8.6 μl·cm−2·h−1, which reflects an increase in steady-state fluid absorption from the retinal to the choroidal side of the tissue. For technical reasons, rapid changes in TEP and RT were not recorded in these experiments. In 10 experiments, JV increased from 12.9 ± 1.6 to 20.5 ± 3.1 μl·cm−2·h−1 (P < 0.01), with little change in TEP or RT. In contrast, addition of IFNγ to the apical bath had no significant effect on JV, consistent with the low level of IFNGR1 detected at the apical membrane. In another set of experiments (see supplemental Fig. S5A), pretreatment with anti-IFNγR1 blocking antibody (2 μg/ml) for 30 min completely blocked IFNγ-induced JV changes. In the presence of blocking antibody, no significant JV changes were observed (n = 9). Furthermore, Fig. 5B represents another set of experiments in which JAK inhibitor I (5 μM) was added ∼0.5–1 h before the addition of IFNγ. JAK inhibitor I alone had no significant effect on baseline JV, but it significantly blocked the IFNγ-induced increase in JV (n = 3).
Fig. 5.
IFNγ-induced changes in hfRPE fluid transport. A: basal bath addition of IFNγ increased transepithelial fluid transport (JV) across monolayer of hfRPE cells. JV is plotted as a function of time in the top trace and net fluid absorption (apical to basal bath) is indicated by positive values; transepithelial potential (TEP, −) and total tissue resistance (RT, ▵) are plotted as a function of time in bottom traces. B: pretreatment with JAK inhibitor I (5 μM) in apical/basal baths inhibited IFNγ-stimulated JV increase. C: summary of JV and RT measurements after 24-h treatment with IFNγ. A statistically significant increase in JV and decrease in RT were observed in 24-h IFNγ-treated filters vs. control: *P < 0.05, **P < 0.01. NS, nonsignificant. D: IFNγ-stimulated JV increase was inhibited by addition of 5 μM CFTRinh-172 to basal bath. E and F: addition of CHX (62 μM) for ∼30 min and 4 h, respectively. Subsequent addition of IFNγ (Ap/Ba) increased JV acutely (E), whereas 4 h of incubation with CHX significantly inhibited IFNγ-stimulated JV increase (F).
Steady-state JV is determined by two basolateral membrane Cl− channels, CFTR and a DIDS-sensitive mechanism (see Fig. 10). Figure 6D shows that basal bath CFTRinh-172, a specific CFTR inhibitor, significantly inhibited baseline JV, and in five experiments, JV was decreased from 12.5 ± 3.1 to 3.2 ± 1.0 μl·cm−2·h−1 (P = 0.01). Basal DIDS also decreased steady-state JV from 20.3 ± 8.2 to 11.2 ± 6.0 μl·cm−2·h−1 (n = 9, P < 0.05) (1). As shown in Fig. 5D, the IFNγ-induced JV is inhibited by CFTRinh-172 (5 μM). In 11 experiments, JV was reversibly decreased from 16.6 ± 1.2 to 6.8 ± 1.0 μl·cm−2·h−1 (P < 0.001). However, the IFNγ-induced JV increase was not significantly affected by the addition of basal DIDS (n = 4, P = 0.3; see supplemental Fig. S5C). The subsequent addition of CFTRinh-172 significantly decreased JV from 17.8 ± 1.5 (in the presence of DIDS) to 8.7 ± 2.7 μl·cm−2·h−1 (n = 4, P < 0.05). Conversely, addition of CFTRinh-172 inhibited the IFNγ-induced JV increase, but subsequent basal bath addition of DIDS produced no further inhibition of JV (n = 2). Taken together, these experiments indicate that the IFNγ-induced increase in JV is predominantly mediated by CFTR.
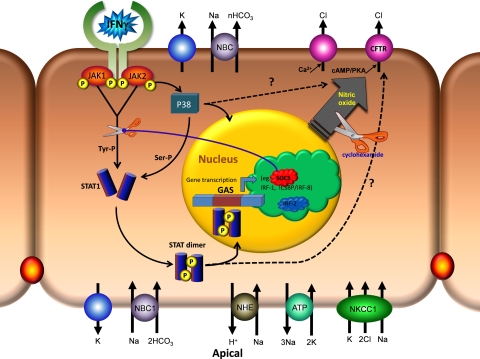
Fig. 10.
Schematic diagram of IFNγ signaling pathway in retinal pigment epithelium. Major plasma membrane transporters and channels mediate fluid absorption across the RPE (Ref. 1) after activation of the canonical JAK-STAT pathway. GAS, γ-interferon activation site.
Fig. 6.
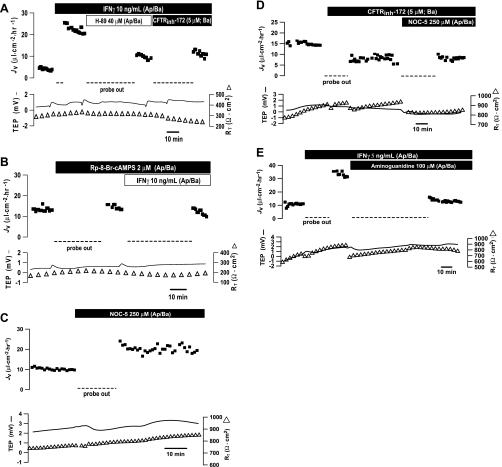
Role of second messengers in IFNγ-induced JV changes. A: PKA inhibitor H-89 inhibited IFNγ-stimulated JV across the hfRPE monolayer. A similar effect was produced by CFTRinh-172. B: pretreatment with Rp-8-Br-cAMPS blocked IFNγ-stimulated JV. C: NOC-5 [a nitric oxide (NO) donor] stimulated JV in hfRPE cells. D: pretreatment with 5 μM CFTRinh-172 blocked NOC-5-stimulated JV. E: IFNγ-induced JV increase was inhibited by 100 μM aminoguanidine hydrochloride, an inducible NO synthase (iNOS) inhibitor.
CHX, a protein synthesis inhibitor, was used to discriminate between effects of IFNγ that require de novo protein synthesis and those that do not. In acute experiments, CHX had no effect on the IFNγ-induced increase in JV (Fig. 5E). In contrast, the IFNγ-induced JV increase was completely blocked after 4 h of pretreatment with CHX, as shown in Fig. 5F and confirmed in two additional experiments. In 24-h incubation experiments, paired RPE tissues were treated with IFNγ (5 ng/ml; apical/basal) or IFNγ and CHX (62 μM, apical/basal). Compared with control, CHX treatment decreased JV from 15.9 to 8.8 μl·cm−2·h−1 across RPE (see supplemental Fig. S5D). A practically identical result was obtained in another experiment, in which JV decreased from 14.9 to 9.8 μl·cm−2·h−1.
In chronic exposure experiments, pairs of hfRPE inserts with matching RT were incubated with 5 ng/ml IFNγ in the apical/basal bath or left in control media for 24 h. Fluid transport and electrical parameters were then measured in each pair. These data, which are summarized in Fig. 5C, show no significant difference in mean RT between these two groups at time 0 (205 ± 17 vs. 210 ± 20 Ω·cm2, n = 5, P = 0.86). After 24 h of treatment, JV in the control monolayers was 7.6 ± 1.3 μl·cm−2·h−1; IFNγ treatment increased JV to 15.2 ± 1.8 μl·cm−2·h−1 (P < 0.01). Addition of CFTRinh-172 decreased JV from 15.1 to 4.8 μl·cm−2·h−1 (n = 2; see supplemental Fig. S5B). In these chronic experiments, IFNγ induced a significant decrease in RT from 592 ± 80 to 356 ± 139 Ω·cm2 (n = 5, P < 0.05).
Second messenger-induced changes in JV.
Elevation of cell cAMP leads to the activation of CFTR (15, 22, 23, 57, 58); therefore, we examined the possibility that CFTR is part of the IFNγ signaling pathway. Primary cultures of hfRPE cells were incubated in control media or in the presence of IFNγ (0.5, 5, or 50 ng/ml) or in forskolin-containing media (10 μM) for 5 min, 45 min, 12 h, and 24 h, and then total intracellular cAMP levels were measured using a cAMP ELISA system (Applied Biosystems, Foster City, CA). Control levels of cAMP (7.2 ± 2.8 pmol/mg) were elevated after 24 h to 96.5 ± 22.7 pmol/mg by forskolin (n = 3, P < 0.05). Compared with control, IFNγ had no apparent effect on total intracellular cAMP (3.0 ± 1.0 pmol/mg, P > 0.3). In a separate set of experiments (n = 4), fluorescence imaging was used to measure intracellular Ca2+ concentration with the addition of IFNγ (5 or 50 ng/ml) to both sides of the hfRPE cell monolayer; no change in total cell intracellular Ca2+ concentration was observed (data not shown).
Even though IFNγ did not elevate total cell cAMP in hfRPE cells, we measured the effect of H-89, a specific PKA inhibitor, on JV to examine the possibility that local changes in cAMP can mediate IFNγ-induced increase in fluid transport. As shown in Fig. 6A, H-89 (40 μM) significantly inhibited the IFNγ-stimulated JV increase. Addition of CFTRinh-172 had no further effect on IFNγ-induced fluid transport. In three experiments, IFNγ increased JV from 8.4 ± 1.6 to 20.1 ± 0.2 μl·cm−2·h−1 (P < 0.05), and addition of H-89 (40 μM, both sides) decreased JV to baseline (8.3 ± 1.3 μl·cm−2·h−1). In another set of experiments, pretreatment with H-89 (40 μM) for ≥30 min had no significant effect on baseline JV but completely blocked the effect of IFNγ. In four experiments, steady-state JV was 12.8 ± 5.2 μl·cm−2·h−1; this baseline JV was not significantly altered by H-89 (12.9 ± 3.7 μl·cm−2·h−1) and the subsequent addition of IFNγ (14.7 ± 4.7 μl·cm−2·h−1; data not shown). Rp-8-Br-cAMPS is a more specific PKA inhibitor than H-89 (41, 46), and incubation with this inhibitor (2 μM) for ∼40 min had no effect on baseline JV but completely blocked the effects of IFNγ (Fig. 6B). In six experiments, steady-state JV was 8.9 ± 1.2 μl·cm−2·h−1. This baseline JV was not significantly altered by Rp-8-Br-cAMPS (10.4 ± 2.0 μl·cm−2·h−1), and the subsequent addition of IFNγ produced no significant change in JV (8.4 ± 1.7 μl·cm−2·h−1) in the continued presence of this inhibitor (2 or 20 μM). These data indicate that PKA mediates the IFNγ-induced increase in fluid absorption.
The data in Table 1 show that IFNγ significantly increased the expression of iNOS. Therefore, we examined the effects of NO donors and inhibitors on the IFNγ-induced increase in JV. Figure 6C shows that the NO donor NOC-5 more than doubled JV, and in a total of four experiments, JV increased from 7.0 ± 3.4 to 17.4 ± 4.1 μl·cm−2·h−1 (P < 0.03). Addition of CFTRinh-172 to the basal bath significantly blocked NOC-5-induced changes in JV (Fig. 6D). In three experiments, the control JV was 19.0 ± 4.0 μl·cm−2·h−1. After the addition of CFTRinh-172, JV decreased to 10.6 ± 1.2 μl·cm−2·h−1, and the further addition of NOC-5 was without significant effect (7.9 ± 0.4 μl·cm−2·h−1, n = 3, P > 0.2). In addition, the iNOS inhibitor aminoguanidine hydrochloride decreased the IFNγ-induced JV increase from 31.3 to 13.2 μl·cm−2·h−1 (Fig. 6E), and similar results were obtained in another experiment. In another set of experiments, pretreatment with aminoguanidine hydrochloride completely blocked the IFNγ-induced increase in JV. In these experiments, the initial control JV (14.2 ± 0.5 μl·cm−2·h−1) was decreased by the iNOS inhibitor to 11.1 ± 1.9 μl·cm−2·h−1, and the addition of IFNγ produced no significant change (10.4 ± 0.3 μl·cm−2·h−1, n = 3, P > 0.7).
Since NO could be exerting its effects through cGMP-dependent or -independent pathways, we elevated cGMP using the membrane-permeable analog 8-bromo-cGMP (8-Br-cGMP). Elevation of cell cGMP significantly increased JV (see supplemental Fig. S5E), and this increase was significantly reduced by addition of CFTRinh-172 to the basal bath. In four experiments, 8-Br-cGMP increased JV from 7.4 ± 1.1 to 15.8 ± 1.6 μl·cm−2·h−1, and subsequent addition of CFTRinh-172 decreased JV to 8.9 ± 1.3 μl·cm−2·h−1 (P < 0.03). The 8-Br-cGMP-induced JV increase was also inhibited by basal DIDS from 21.5 ± 1.8 to 13.4 ± 0.9 μl·cm−2·h−1 (n = 4, P < 0.02; see supplemental Fig. S5F).
Role of p38 in IFNγ-induced JV increase.
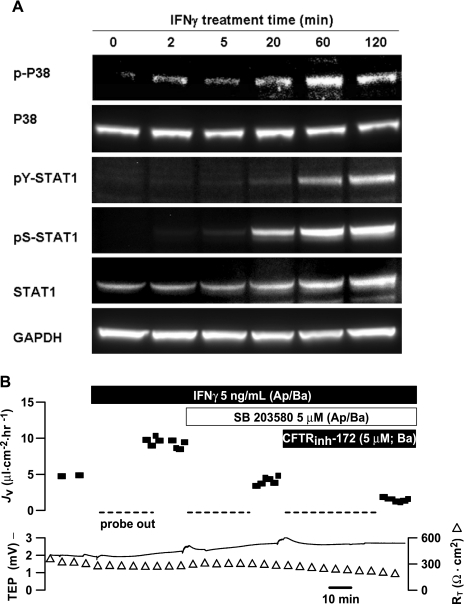
In Calu-3 airway epithelial cells, it was previously shown that p38 MAPK mediates the flagellin-induced activation of CFTR-dependent Cl− secretion (30, 76). Therefore, it seemed possible that one or more MAPKs could play a role in generating IFNγ-stimulated fluid transport. In hfRPE cells, IFNγ had no effect on JNK or p44/p42 phosphorylation (data not shown). However, IFNγ induced the phosphorylation of p38 MAPK within 2–5 min, and this response reached its peak at 1 h and started to decline at ∼2 h (Fig. 7A).
Fig. 7.
Role of p38 in IFNγ-induced JV increase. A: IFNγ stimulates phosphorylation of p38 MAPK. pY/pS-STAT1 antibodies were used to detect tyrosine or serine phosphorylation of STAT1. B: p38 MAPK-specific inhibitor blocks IFNγ-stimulated JV increase. Subsequent addition of CFTRinh-172 further decreased JV.
As shown in Fig. 7B, a specific inhibitor of p38 MAPK (SB-203580, 5 μM) decreased IFNγ-induced JV by ∼62%, and a further decrease in JV was produced by subsequent addition of CFTRinh-172 to the basal bath. IFNγ-stimulated fluid transport was also inhibited (∼46%) by another p38-specific inhibitor (SB-202190, 1–5 μM, n = 2). In a total of nine experiments, IFNγ increased JV from 7.5 ± 1.9 to 16.5 ± 2.2 μl·cm−2·h−1 (P < 0.05), and addition of p38 MAPK inhibitor (1–5 μM, both sides) decreased JV to control levels (6.7 ± 1.2 μl·cm−2·h−1, P < 0.001). Pretreatment with SB-203580 for ≥30 min had no significant effect on baseline JV: initial control levels of JV were 8.4 ± 1.1 and 9.1 ± 1.8 μl·cm−2·h−1 in the presence of the p38 inhibitor (n = 6, P = 0.8). The addition of CFTRinh-172 following SB-203580 inhibition of IFNγ-induced JV produced a further decrease from 3.6 to 1.4 μl·cm−2·h−1. In a separate set of experiments (n = 3), IFNγ increased JV from 10.0 ± 4 to 25.1 ± 4 μl·cm−2·h−1, and the subsequent addition of CFTRinh-172 decreased JV to 8.2 ± 4.5 μl·cm−2·h−1. Addition of SB-203580 after CFTRinh-172 had no effect (7.7 ± 4.8 μl·cm−2·h−1, P = 0.3).
IFNγ-induced changes mediated by cAMP and cGMP.
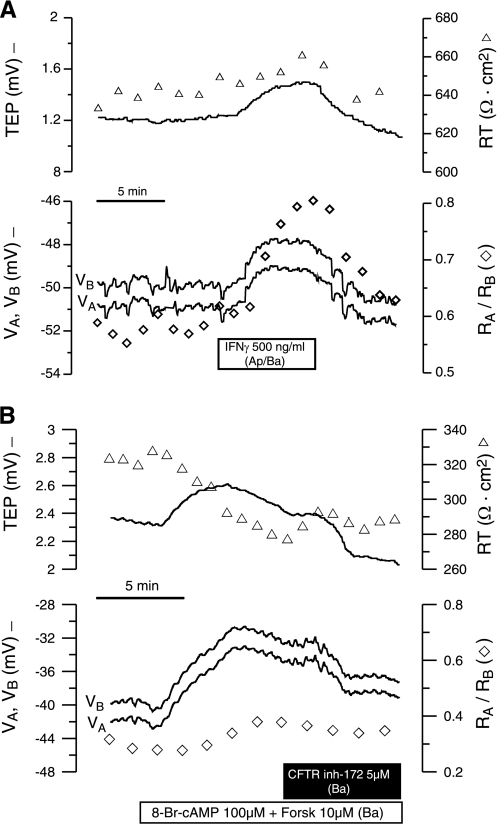
Perfusion of IFNγ (500 ng/ml) to both bathing solutions depolarized VB by 2.5 mV and VA by 2.2 mV (Fig. 8A). This difference indicates a greater rate of depolarization at the basolateral membrane, as shown by the increase in TEP (0.33 mV). In five experiments, the TEP increase was 0.23 ± 0.04 mV and the depolarization of VB was 1.5 ± 0.3 mV (P < 0.004 in both cases). The resistance changes (RT and RA/RB) were small and not statistically significant. Figure 8B shows that elevation of cell cAMP depolarized VA and VB by ∼9 mV and increased TEP (0.4 mV). During this time, RT decreased by ∼47 Ω·cm2 and RA/RB increased by ∼0.1. These electrical measurements are consistent with the activation of a basolateral membrane cAMP-dependent anion channel. Subsequent addition of CFTRinh-172 to the basal bath caused both membrane potentials to hyperpolarize, decreased TEP, increased RT, and slightly decreased RA/RB. In five experiments, the resting potential (VA) and TEP before the addition of exogenous cAMP were −44.2 ± 1.5 and 0.7 ± 0.4 mV, respectively. Addition of exogenous forskolin and 8-Br-cAMP significantly depolarized the basolateral membrane by 4.6 ± 1.1 mV, decreased RT by 40 ± 8.7 Ω·cm2 (P = 0.02), and increased RA/RB by 0.09 ± 0.03 (n = 5, P < 0.015). Together, these changes strongly suggest the presence of a basolateral membrane cAMP-dependent anion channel, probably CFTR, whose equilibrium potential is less negative than the basolateral membrane resting potential.
Fig. 8.
A: intracellular recording of IFNγ-induced changes in TEP and apical and basolateral membrane potential (VA and VB, respectively, −) in a modified Ussing chamber. ▵, RT; ◊, ratio of apical to basolateral resistance (RA/RB). B: intracellular recordings of forskolin- and 8-Br-cAMP-induced changes in TEP, RT, VA, VB, and RA/RB. Data represent results from 5 similar recordings for A and B.
Elevation of intracellular cGMP depolarized VB by ∼3.7 mV and increased TEP by ∼1.2 mV (see supplemental Fig. S6). Concomitantly, RT decreased by ∼20 Ω·cm2, and RA/RB increased by ∼0.1. These results are consistent with the activation of a basolateral membrane cGMP-dependent anion channel (n = 2). In another intracellular recording, basal DIDS (500 μM) hyperpolarized VB by ∼3.6 mV and decreased TEP by ∼2.3 mV, whereas RA/RB decreased by ∼0.16, with no change in RT. Subsequent addition of 8-Br-cGMP (100 μM) produced no further electrical changes. In additional experiments (n = 8), the microelectrode impalements could not be held, but we were able to record TEP and resistance changes. In these experiments, elevation of cell cGMP increased TEP by 1.5 ± 0.2 mV (P = 0.0001) and decreased RT by 16.6 ± 2.4 Ω·cm2 (P = 0.0002). In four of these experiments, subsequent addition of basal DIDS decreased TEP by 3.3 ± 0.2 mV (P = 0.0009) and increased RT by 6 and 22 Ω·cm2 (2 of 4 experiments). These electrophysiological results taken together strongly suggest that the RPE basolateral membrane also contains a DIDS-sensitive anion channel that is activated by cGMP and has an equilibrium potential that is less negative than the resting membrane potential.
In vivo studies.
We used a previously described in vivo rodent model of retinal reattachment (43) to measure the effect of IFNγ on RPE fluid transport in the intact eye. The initial detachment was created by injection of ∼1 μl of MPBS into the extracellular space (or SRS) between the photoreceptors and the RPE apical membrane. After stabilization of the detachment, IFNγ (40 μl of 100 ng/μl) was added to the anterior surface of the eye in the form of eye drops (Celluvisc). A series of three-dimensional OCT images were recorded at different time points.
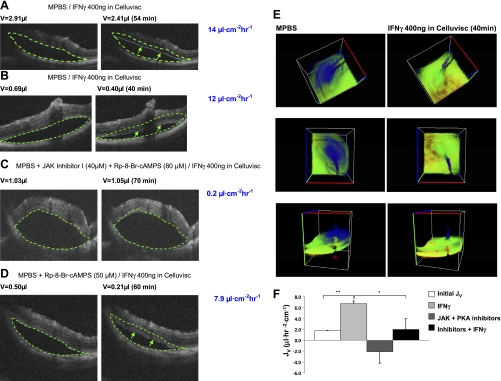
As shown in Fig. 9, A and B, addition of IFNγ to the anterior surface of the eye caused a decrease in detachment size (volume). The green dashed line in the left-hand image delineates the detachment size just before addition of IFNγ. This same traced area is superimposed on the right-hand side to help visualize the changes in detachment size over time. In Fig. 9, A and B, the green arrows point to the new position of the retinal border at 54 and 40 min, respectively. The fluid transport rates were calculated by reconstruction of the volume and area of the detachment. Figure 9E shows a three-dimensional rendering of the OCT sections from the two time points, at 0 and 40 min, in Fig. 9B (JV = 12 μl·cm−2·h−1). The en face view of the detachment (Fig. 9E, middle) shows a significant diminution of the detachment area and reduction of detachment height at 40 min. Tilted views of this detachment are shown in Fig. 9E, top and bottom.
Fig. 9.
Time course of volume change of retinal detachment measured by optical coherence tomography (OCT). MPBS, modified PBS. A–D: OCT images from 4 different experiments showing difference in detachment size before addition of IFNγ to the anterior surface (left) and after 40–70 min of IFNγ addition (right), in the absence or presence of JAK-STAT pathway and PKA inhibitors (C and D). A and B: 2 examples of a decrease in detachment size/volume after IFNγ treatment. C: IFNγ was added in the presence of JAK inhibitor I (40 μM) and Rp-8-Br-cAMPS (80 μM). D: IFNγ was added in the presence of Rp-8-Br-cAMPS alone. Arrows indicate boundary of the bleb for comparison with area enclosed in dashed line, which indicates starting volume. E: 3-dimensional sections of experiment summarized in B. Pseudocolor in blue indicates spatial extent of detachment at 0 and 40 min. Middle panel in E is an en face view. F: summary of all in vivo experiments (n = 3). *P < 0.05; **P < 0.01.
As shown in Fig. 9, C and D, the anterior surface IFNγ-induced changes in JV can be blocked completely or partially by injection of JAK-STAT pathway inhibitors into the SRS. In these experiments, we injected MPBS under the retina to create the initial detachment and included a cocktail of JAK inhibitor I and Rp-8-Br-cAMP or Rp-8-Br-cAMP blocker alone. As shown in Fig. 9D, the PKA inhibitor alone was not sufficient to completely block the effect of IFNγ (∼40% reduction in absorption rate). However, the cocktail of both inhibitors can completely block the effect of IFNγ. This result was corroborated in a total of three experiments, and the data are summarized in Fig. 9F.
DISCUSSION
The schematic model shown in Fig. 10 summarizes a set of intracellular signaling pathways and second messengers that can mediate IFNγ-induced fluid transport across epithelia. In hfRPE cells, acute (30-min) exposure to IFNγ increased net transepithelial fluid absorption from the retinal to the choroidal side of the tissue. In addition, chronic exposure to IFNγ significantly decreased RT and RPE mitochondrial membrane potential. These physiological responses were generated after activation of the JAK-STAT1 and p38 MAPK pathways and the elevation of PKA-cAMP and NO, which activated basolateral membrane Cl− channels (CFTR).
IFNγ cell signaling in human RPE.
Native human adult RPE and hfRPE cells constitutively express IRF-1 and IRF-2, but not ICSBP. The constitutive level of IRF-2 protein is much higher than that of IRF-1, but stimulation by IFNγ significantly increased IRF-1 protein levels, with no effect on IRF-2, despite a threefold increase in IRF-2 mRNA. These two transcription factors are mutually antagonistic (66), which may explain the strong inhibitory effect of IFNγ on RPE proliferation and migration, perhaps caused by an increase in the ratio of IRF-1 to IRF-2.
Cytokine responses are typically transient; several inhibitory mechanisms have been identified that downregulate STAT signaling (60, 62). For example, SOCS is a family of cytokine-inducible proteins that function to regulate the initiation, intensity, and duration of JAK-STAT signaling (45, 49, 75). PIAS is another family of proteins that can inhibit the transcriptional activity of STATs (60, 61). In hfRPE cells, IFNγ significantly upregulated STAT1 protein levels, as observed in other systems (27), but in hfRPE, the IFNγ-induced phosphorylation of STAT1 was observed over a much longer period of time (2–48 h). Stimulation with IFNγ does not affect PIAS1, but it does increase SOCS3 mRNA by sevenfold. These results suggest the absence of a negative-feedback mechanism, which may indicate a difference between fetal cultures and native cells. More interestingly, it may illustrate a particular function of IFNγ, which provides a sustained increase of fluid transport out of the SRS in the inflammatory state.
Pathways to CFTR.
The present experiments show that IFNγ-induced changes in JV were significantly inhibited by basal bath addition of CFTRinh-172 and that DIDS, a Ca2+-dependent Cl− channel blocker, had no effect on the increase in JV produced by IFNγ (43, 54). In previous experiments using human native fetal or bovine RPE, we showed that the basolateral membrane contains at least two anion channels, CFTR and a Ca2+-activated, DIDS-sensitive Cl− channel (7, 8, 54, 55). Together, all these data suggest a fundamentally important role for CFTR in mediating fluid transport across human RPE.
IFNγ did not change total cell cAMP in hfRPE cells. This result is puzzling and suggests two possibilities: 1) the entire response is mediated by IRF-1-induced NO production (12, 36), or 2) IFNγ induces a localized change in cAMP (14, 28) near the basolateral membrane, where the electrical changes are generated. This hypothesis is supported by the effects of PKA inhibitors, H-89 and Rp-8-Br-cAMPS, which blocked the IFNγ-induced JV increase. Electrophysiology experiments carried out in a modified fast-flow Ussing chamber further support this hypothesis (see materials and methods). Figure 8 shows that elevation of cell cAMP produced cell membrane voltage and resistance changes, all of which are consistent with cAMP activation of CFTR, and these electrical changes were blocked by CFTRinh-172. Similar TEP and RT changes were produced after the activation of the IFNγ receptor, indicating that cAMP is a critical part of this signaling pathway.
In addition, experiments with NO donors and iNOS inhibitors indicate that NO is also a significant contributor to the IFNγ response. This finding suggests the involvement of cGMP signaling, possibly mediated by NO activation of soluble guanylyl cyclase and cGMP-dependent protein kinase type II (PKGII), leading to the activation of CFTR (70). Consistent with this hypothesis, elevation of cell cGMP with 8-Br-cGMP significantly increased JV, and these effects were partially blocked by basal bath CFTRinh-172, a specific CFTR inhibitor, and by basal DIDS, which was previously shown to block basolateral membrane Cl− channels in mammalian RPE (7, 54, 55). Intracellular recordings in the present study show that elevation of cell cGMP (or cAMP) depolarized VB and probably increased basolateral membrane conductance, and these electrical responses were blocked by basal DIDS (or CFTRinh-172). The present experiments demonstrate that cGMP is a major regulatory determinate of fluid transport in RPE. The interactions between cGMP, cAMP, and CFTR in IFNγ signaling are complex and remain to be elucidated.
The present study shows that the IFNγ-induced increases in JV are significantly blocked by JAK-STAT or p38 MAPK pathway inhibitors, suggesting at least two separate intracellular signaling pathways for the regulation of fluid transport across human RPE. It is worth noting that TNFα or IL-1β per se can stimulate p38 MAPK phosphorylation in hfRPE cells but has no effect on JV (data not shown). After inhibition by the p38 inhibitor, CFTRinh-172 produced a further decrease of JV. Conversely, the p38 inhibitor had no further effect on JV after inhibition by basal bath CFTRinh-172, suggesting that p38 MAPK is part of the CFTR signaling pathway.
The effects of CHX, a protein synthesis inhibitor, suggest that the IFNγ-induced JV increase has two components: one involves protein synthesis, and one does not. The latter response is acute and is not blocked by CHX and, most likely, is mediated by downstream signals from the JAK-STAT or p38 MAPK pathway or by other second messengers that directly activate CFTR. Experiments using chronic treatment with CHX indicate that the IFNγ-induced increase in JV is driven, at least in part, by activation of nuclear transcription factors and protein synthesis.
Cytokine regulation of CFTR is complex and cell specific (5, 6, 9, 38). Kulka et al. (38) showed that IFNγ modulation of CFTR gene expression is mediated by STAT1-dependent and -independent pathways. In T84 cells, the IFNγ-mediated downregulation of CFTR was inhibited by a JAK2 inhibitor, but not by p38 or ERK inhibitors. In contrast, in rat and human mast cells, IFNγ upregulation of CFTR is p38 and ERK dependent and JAK2 independent. In the present study, IFNγ caused a small decrease in CFTR mRNA and protein level, but this change is not statistically significant. This small reduction may be attributed to the balance between CFTR degradation in the cell and recycling to the plasma membrane (38).
Physiological and clinical implications.
The in vivo experiments showed that addition of IFNγ to the anterior surface of the rat eye significantly increased the rate of fluid absorption from the SRS in <30 min. In rat, as in human, these IFNγ-induced increases in fluid absorption are blocked by JAK-STAT pathway inhibitors (Fig. 9). There are two pathways that a molecule can take from the ocular surface to the posterior part of the eye: 1) through the cornea, vitreous, and retina to the RPE apical surface or 2) through the conjunctiva, sclera, and choriocapillaris to the basolateral surface of the RPE. The latter route is more likely, given the size (15–25 kDa) and hydrodynamic radius (2.6 nm) of IFNγ (68) and the fact that the RPE is essentially impermeable to ≥20-kDa molecules (37). Since the sclera is highly permeable to molecules with molecular radius as large as 8 nm (2) and with the assumption that, as in human RPE, IFNγ receptors are mainly located at the basolateral membrane of rat RPE, these in vivo results strongly favor the scleral route to the RPE basolateral membrane.
IFNγ may be used therapeutically to prevent the pathological accumulation of fluid in the SRS, for example, in retinal degenerative diseases such as diabetic retinopathy and uveitis. In addition, the IFNγ-CFTR pathway in RPE is also activated by NO, which is continually produced in large amounts by the distal retina (52) and, perhaps, by the choriocapillaris (69). Therefore, normal retinal metabolism helps dehydrate the SRS and maintain a close anatomic relationship between the photoreceptors and RPE (1, 47). In the early stages of age-related macular degeneration (AMD), inflammation may help keep the RPE barrier intact by preventing RPE proliferation and migration, and, at the same time, choroidal cell growth may increase blood flow to the back of the eye and the clearance of pathogens or drusen.
In chronic inflammatory diseases such as posterior uveitis and AMD, the IFNγ-induced dehydration of the SRS would increase the activity of already accumulating chemokines and, thereby, help draw monocytes and neutrophils across the RPE to the SRS (17). Inhibition of RPE proliferation and migration by IFNγ also protects and maintains the RPE barrier. However, over long periods of unresolved inflammation, chronic exposure to IFNγ and other cytokines (e.g., IL-1β and TNFα) could induce a significant decrease in transepithelial paracellular resistance (20, 24) and the loss of transport potential (fluid absorption). These persistent changes, coupled with an IFNγ-driven increase in choroidal cell proliferation, suggest a pathway for the entry of choroidal neovascular blood vessels into the SRS via the RPE tight junctions. These mechanisms provide a basis for understanding the role of inflammation in choroidal neovascularization and AMD (4, 50, 65).
GRANTS
This work was supported by the National Institutes of Health Intramural Research Program.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Charles E. Egwuagu, Terry Machen, Reiko Horai, Bret Hughes, John O'Shea, Sharon Milgram, David D. Roberts, and Thomas Miller for helpful comments and criticism and Jeffrey Adijanto for technical help with the Ca2+ fluorescence imaging experiments.
REFERENCES
- 1.Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol 133: 603– 622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati J, Canakis CS, Miller JW, Gragoudas ES, Edwards A, Weissgold DJ, Kim I, Delori FC, Adamis AP. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci 41: 1181– 1185, 2000 [PubMed] [Google Scholar]
- 3.Bach EA, Aguet M, Schreiber RD. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol 15: 563– 591, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Barouch FC, Miller JW. The role of inflammation and infection in age-related macular degeneration. Int Ophthalmol Clin 47: 185– 197, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Baudouin-Legros M, Hinzpeter A, Jaulmes A, Brouillard F, Costes B, Fanen P, Edelman A. Cell-specific posttranscriptional regulation of CFTR gene expression via influence of MAPK cascades on 3′-UTR part of transcripts. Am J Physiol Cell Physiol 289: C1240– C1250, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Besancon F, Przewlocki G, Baro I, Hongre AS, Escande D, Edelman A. Interferon-γ downregulates CFTR gene expression in epithelial cells. Am J Physiol Cell Physiol 267: C1398– C1404, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Bialek S, Joseph DP, Miller SS. The delayed basolateral membrane hyperpolarization of the bovine retinal pigment epithelium: mechanism of generation. J Physiol 484: 53– 67, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol 106: 43– 50, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Brouillard F, Bouthier M, Leclerc T, Clement A, Baudouin-Legros M, Edelman A. NF-κB mediates up-regulation of CFTR gene expression in Calu-3 cells by interleukin-1β. J Biol Chem 276: 9486– 9491, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA. Ocular neovascularisation and excessive vascular permeability. Expert Opin Biol Ther 4: 1395– 1402, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37– 40, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR, Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 39: 150– 162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba H, Kojima T, Osanai M, Sawada N. The significance of interferon-γ-triggered internalization of tight-junction proteins in inflammatory bowel disease. Sci STKE 2006: pe1, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans 33: 1319– 1322, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dahan D, Evagelidis A, Hanrahan JW, Hinkson DA, Jia Y, Luo J, Zhu T. Regulation of the CFTR channel by phosphorylation. Pflügers Arch 443Suppl 1: S92– S96, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Darnell JE., Jr STATs and gene regulation. Science 277: 1630– 1635, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Economopoulou M, Hammer J, Wang FE, Fariss R, Maminishkis A, Miller SS. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci 50: 1454– 1463, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman JL, Miller SS. Epinephrine stimulates fluid absorption across bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci 32: 3033– 3040, 1991 [PubMed] [Google Scholar]
- 19.Fang Y, Yu S, Braley-Mullen H. Contrasting roles of IFN-γ in murine models of autoimmune thyroid diseases. Thyroid 17: 989– 994, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130: 55– 70, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chignell AH, Dumonde DC. Cytokines in human intraocular inflammation. Curr Eye Res 11Suppl: 187– 191, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev 79: S77– S107, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan JW, Mathews CJ, Grygorczyk R, Tabcharani JA, Grzelczak Z, Chang XB, Riordan JR. Regulation of the CFTR chloride channel from humans and sharks. J Exp Zool 275: 283– 291, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129: 550– 564, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hemmi S, Bohni R, Stark G, Di Marco F, Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon-γ receptor in human cells. Cell 76: 803– 810, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Hooks JJ, Chan CC, Detrick B. Identification of the lymphokines, interferon-γ and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci 29: 1444– 1451, 1988 [PubMed] [Google Scholar]
- 27.Hu X, Herrero C, Li WP, Antoniv TT, Falck-Pedersen E, Koch AE, Woods JM, Haines GK, Ivashkiv LB. Sensitization of IFN-γ Jak-STAT signaling during macrophage activation. Nat Immunol 3: 859– 866, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Gilmore E, Kultgen P, Barnes P, Milgram S, Stutts MJ. Local regulation of cystic fibrosis transmembrane regulator and epithelial sodium channel in airway epithelium. Proc Am Thorac Soc 1: 33– 37, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Hughes BA, Gallemore R, Miller SS. Transport Mechanisms in the retinal pigment epithelium. In: The Retinal Pigment Epithelium, edited by Marmor MF. New York: Oxford University Press, 1998, p. 103– 134 [Google Scholar]
- 30.Illek B, Fu Z, Schwarzer C, Banzon T, Jalickee S, Miller SS, Machen TE. Flagellin-stimulated Cl− secretion and innate immune responses in airway epithelia: role for p38. Am J Physiol Lung Cell Mol Physiol 295: L531– L542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, Liu J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-α-lipoic acid. Invest Ophthalmol Vis Sci 48: 339– 348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang C, Finkbeiner WE, Widdicombe JH, McCray PB, Jr, Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science 262: 424– 427, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Jilling T, Haddad IY, Cheng SH, Matalon S. Nitric oxide inhibits heterologous CFTR expression in polarized epithelial cells. Am J Physiol Lung Cell Mol Physiol 277: L89– L96, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Joseph DP, Miller SS. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol 435: 439– 463, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsoulidis E, Li Y, Mears H, Platanias LC. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J Interferon Cytokine Res 25: 749– 756, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Kelley TJ, Elmer HL. In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium. J Clin Invest 106: 403– 410, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koevary SB. Pharmacokinetics of topical ocular drug delivery: potential uses for the treatment of diseases of the posterior segment and beyond. Curr Drug Metab 4: 213– 222, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kulka M, Dery R, Nahirney D, Duszyk M, Befus AD. Differential regulation of cystic fibrosis transmembrane conductance regulator by interferon-γ in mast cells and epithelial cells. J Pharmacol Exp Ther 315: 563– 570, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci 48: 5722– 5732, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Limb GA, Little BC, Meager A, Ogilvie JA, Wolstencroft RA, Franks WA, Chignell AH, Dumonde DC. Cytokines in proliferative vitreoretinopathy. Eye 5: 686– 693, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261– 274, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci 47: 3612– 3624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maminishkis A, Jalickee S, Blaug SA, Rymer J, Yerxa BR, Peterson WM, Miller SS. The P2Y2 receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci 43: 3555– 3566, 2002 [PubMed] [Google Scholar]
- 44.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125– 137, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muhl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-γ. Int Immunopharmacol 3: 1247– 1255, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1: re4, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y, Benowitz L, Hafezi-Moghadam A, Miller JW. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci USA 104: 2425– 2430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev 8: 293– 312, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Nicola NA, Greenhalgh CJ. The suppressors of cytokine signaling (SOCS) proteins: important feedback inhibitors of cytokine action. Exp Hematol 28: 1105– 1112, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol 144: 618– 626, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin Med Res 4: 294– 309, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piehl L, Capani F, Facorro G, Lopez EM, de Celis ER, Pustovrh C, Hager A, Coirini H, Lopez-Costa JJ. Nitric oxide increases in the rat retina after continuous illumination. Brain Res 1156: 112– 119, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Platanias LC. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther 98: 129– 142, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci 33: 3513– 3527, 1992 [PubMed] [Google Scholar]
- 55.Quinn RH, Quong JN, Miller SS. Adrenergic receptor activated ion transport in human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci 42: 255– 264, 2001 [PubMed] [Google Scholar]
- 56.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol 23: 96– 101, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Seibert FS, Chang XB, Aleksandrov AA, Clarke DM, Hanrahan JW, Riordan JR. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochim Biophys Acta 1461: 275– 283, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23– S45, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Shi G, Maminishkis A, Banzon T, Jalickee S, Li R, Hammer J, Miller SS. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci 49: 4620– 4630, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res 16: 196– 202, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol 5: 593– 605, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3: 900– 911, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Soh J, Donnelly RJ, Kotenko S, Mariano TM, Cook JR, Wang N, Emanuel S, Schwartz B, Miki T, Pestka S. Identification and sequence of an accessory factor required for activation of the human interferon-γ receptor. Cell 76: 793– 802, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 67: 227– 264, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Swaroop A, Chew EY, Bowes Rickman C, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet 10: 19– 43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taniguchi T. Transcription factors IRF-1 and IRF-2: linking the immune responses and tumor suppression. J Cell Physiol 173: 128– 130, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Taniguchi T, Harada H, Lamphier M. Regulation of the interferon system and cell growth by the IRF transcription factors. J Cancer Res Clin Oncol 121: 516– 520, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Tobler SA, Holmes BW, Cromwell ME, Fernandez EJ. Benzyl alcohol-induced destabilization of interferon-γ: a study by hydrogen-deuterium isotope exchange. J Pharm Sci 93: 1605– 1617, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res 26: 205– 238, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EM, Bot AG, Edixhoven M, Boomaars WE, Lohmann SM, de Jonge HR. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proc Natl Acad Sci USA 95: 1466– 1471, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-γ. Curr Top Microbiol Immunol 316: 119– 154, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Voloboueva LA, Killilea DW, Atamna H, Ames BN. N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. FASEB J 21: 4077– 4086, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci 46: 4302– 4310, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willenborg DO, Staykova M, Fordham S, O'Brien N, Linares D. The contribution of nitric oxide and interferon-γ to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol 191: 16– 25, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Yoshimura A. Negative regulation of cytokine signaling. Clin Rev Allergy Immunol 28: 205– 220, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z, Reenstra W, Weiner DJ, Louboutin JP, Wilson JM. The p38 mitogen-activated protein kinase signaling pathway is coupled to Toll-like receptor 5 to mediate gene regulation in response to Pseudomonas aeruginosa infection in human airway epithelial cells. Infect Immun 75: 5985– 5992, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]