Abstract
Parathyroid hormone (PTH) plays a critical role in the regulation of renal phosphorous homeostasis by altering the levels of the sodium-phosphate cotransporter NaPi2a in the brush border membrane (BBM) of renal proximal tubular cells. While details of the molecular events of PTH-induced internalization of NaPi2a are emerging, the precise events governing NaPi2a removal from brush border microvilli in response to PTH remain to be fully determined. Here we use a novel application of total internal reflection fluorescence microscopy to examine how PTH induces movement of NaPi2a out of brush border microvilli in living cells in real time. We show that a dynamic actin cytoskeleton is required for NaPi2a removal from the BBM in response to PTH. In addition, we demonstrate that a myosin motor that has previously been shown to be coregulated with NaPi2a, myosin VI, is necessary for PTH-induced removal of NaPi2a from BBM microvilli.
Keywords: apical total internal reflection fluorescence microscopy, parathyroid hormone
alterations in serum phosphorous and parathyroid hormone (PTH) levels are a cardinal feature of chronic and end-stage renal disease and are associated with considerable morbidity and mortality (8, 18). PTH is a critical regulator of phosphorous and calcium homeostasis. In the proximal tubule, PTH causes a decrease in the apical membrane abundance of the renal sodium-phosphate cotransporters NaPi2a and NaPi2c (6), while in the distal convoluted tubule and collecting duct, this hormone alters the expression and functional properties of the renal calcium channel TRPV5 (14, 37). While it is known that PTH reduces the abundance of NaPi2a in the brush border membrane (BBM) of renal proximal tubule cells (3, 16, 24, 29), details of precisely how PTH causes NaPi2a to move from the apical microvillar membrane to intermicrovillar clefts and from there into the cell interior remain to be fully described. The aim of this study is to further elucidate the molecular mechanisms of how PTH causes redistribution of NaPi2a from the top of the microvillar membrane to its base using total internal reflection fluorescence (TIRF) microscopy (TIR-FM), a high-resolution imaging technique particularly suited to examining processes at or near surfaces, which we have applied to the apical membrane of renal proximal tubular cells. The apical surface of renal proximal tubule cells is covered with densely packed microvilli which greatly increase the resorptive surface area. Each brush border microvillus is a specialized structure containing a central actin core, scaffolding or PDZ proteins thought to link membrane proteins to the actin cytoskeleton and myosin motors including myosin VI (5, 7, 21, 22, 31, 39, 40). The way in which these microvillar components interact to move NaPi2a out of the brush border microvillus in response to PTH remains unknown. To examine trafficking within the BBM, we have developed a novel application of TIR-FM to the apical surface of renal proximal tubular cells. TIR-FM or evanescent field microscopy allows selective examination of events within 100 nm of the coverslip (4, 36). In this technique, incident laser light is totally internally reflected, producing a field of electromagnetic radiation called the evanescent wave that illuminates objects within ∼100 nm of the coverslip. Traditionally, this approach has been applied to the basolateral surface. However, we have established a technique to use TIR-FM to visualize events at the apical surface of living cells. This high-resolution approach is superior to conventional confocal microscopy in that the immediate surface of the cell adjacent to the coverslip is visualized as opposed to contributions from fluorophores within the entire cell. This makes TIR-FM ideal for examining processes at the cell surface such as trafficking of NaPi2a in renal proximal tubule cells in response to PTH.
Given the fact that the central actin core is a key feature of the brush border microvillus, we decided to examine whether a dynamic actin cytoskeleton is required for NaPi2a removal from the brush border in response to PTH. Here using an actin inhibitor and TIR-FM we demonstrate that a dynamic actin cytoskeleton is needed for NaPi2a-induced trafficking in response to PTH.
Since actin is involved in the movement of NaPi2a from the top to the base of microvilli in response to PTH, we investigated whether a myosin motor is required for removal of NaPi2a from the microvillar brush border. Myosin VI is the only unconventional (toward the minus end) myosin discovered to date (11, 17, 20) and thus could act to pull cargo toward the cell interior. In addition, previous work has shown that myosin VI is present in renal brush border microvilli (7, 39, 40). Furthermore, a variety of stimuli associated with alterations in blood pressure or proximal tubular flow including hypertension, captopril, and a high-salt diet have been shown to cause redistribution of myosin VI from the top to the base of brush border microvilli (22, 39, 40), providing indirect evidence that myosin VI may be involved in trafficking events in the BBM. To directly examine whether myosin VI is involved in trafficking of NaPi2a in brush border microvilli in response to PTH, we have used a dominant-negative myosin VI mutant that lacks the motor domain to inactivate endogenous myosin VI. We show herein that PTH fails to induce removal of NaPi2a from brush border microvilli in cultured proximal tubule cells that coexpress the dominant-negative myosin VI motor, suggesting that myosin VI is required for NaPi2a internalization from the brush border in response to PTH.
MATERIALS AND METHODS
Cell culture.
Opossum kidney proximal tubule cells that are responsive to PTH (OKP cells) were a gift from Dr. J. Cole (Univ. of Memphis, Memphis, TN) and were grown in DMEM-F-12 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. For experimental work, cells were seeded on porous membrane inserts (Corning, Lowell, MA). At 90% confluency, cells were transfected overnight using Lipofectamine 2000 (Invitrogen) according to the instructions provided by the manufacturer. The next day the cells were placed in DMEM-F-12 supplemented with 0.2% FBS to synchronize the cells.
Plasmid constructs.
The green fluorescent protein (GFP)-NaPi-2a plasmid was a gift from Dr. H. Mürer (Univ. of Zürich, Zurich, Switzerland). Yellow fluorescent protein (YFP)-NaPi-2a was generated by cutting NaPi-2a from this plasmid using BglII and SacII and inserting it into a similarly digested enhanced (e)YFP-C1 plasmid (Clontech, Mountain View, CA). GFP-tagged myosin VI tail construct was a gift from Dr. T. Hasson (University of San Diego, La Jolla, CA). Cyan fluorescent protein (CFP)-tagged myosin VI tail was generated by cutting the myosin VI tail fragment from the GFP plasmid using KpnI and BamHI and ligating it into a similarly digested pAmCyan1-C1 vector from Clontech (Mountain View, CA).
Apical TIRF.
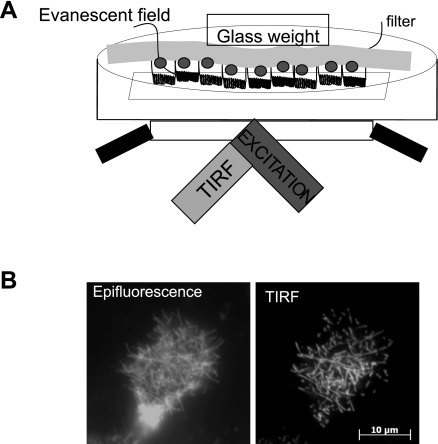
A schematic of the experimental setup is shown in Fig. 1. OKP cells were grown to confluence on 12-mm, 0.4-μm membrane inserts (Corning, Lowell, MA). Membranes with confluent transfected cell layers were cut out of the plastic support and placed apical face down in a dish with a coverslip bottom (no. 1.5, MatTek, Ashland, MA) that contained 2 ml of the appropriate cell culture medium prewarmed to 37°C. To bring the apical cell surface into TIRF, a glass weight consisting of a tissue culture ring 8 mm in diameter (BiOptechs, Butler, PA) was placed on top of the membrane. Samples were observed using a Zeiss TIRF microscope equipped with a ×100, 1.45 numerical aperture objective under the control of AxioVision V4.5 software (Carl Zeiss, Thornwood, NJ). Laser excitation was derived from a multiline argon ion laser run at the same current setting for all experiments. The power at the sample was controlled by a neutral density filter wheel. Excitation and emission wavelengths were selected using filter set for YFP (514 nm). The laser was aligned per manufacturer's instructions to achieve TIRF illumination. Images were taken using a Zeiss Axiocam MRm camera operating with 2 by 2 binning or a Photometrix QuantEM without binning. The microscope was also equipped with an epifluorescence lamp and filters and was housed in a microscope incubation chamber with temperature, humidity, and CO2 control. The sample dish itself was a push-fit into a custom holder that in turn mounts on an XY stage attached to the microscope. Components were a tight mechanical fit to minimize sample drift during the measurement. A vibration-isolated table and the temperature-controlled enclosure both serve to stabilize the system. In practice it was observed that after a period of settling, it was possible to capture time-lapse sequences over as long as 3 h without refocusing. Oxygen was provided by the ambient air which was supplemented by 5% CO2 and warmed to 37°C in an environmental chamber surrounding the specimen. Images were taken every 10 min.
Fig. 1.
Total internal reflection fluorescence (TIRF) microscopy (TIR-FM) allows selective examination of the apical membrane of renal proximal tubule cells. A: schematic representation of the apical TIRF setup. Opossum kidney proximal tubule (OKP) cells are transfected with yellow fluorescent protein (YFP)-NaPi2a and grown to confluence on filters. The filter is excised and placed apical surface down in a dish with a coverslip bottom. A glass weight is placed on top of the membrane to bring the cells into contact with the coverslip. TIRF excitation of the coverslip produces an evanescent field that illuminates ∼100 nm beyond the coverslip, thus illuminating the apical surface of the cells. B: comparison of images obtained of an OKP cell transfected with YFP-NaPi2a under epifluorescence (left) versus TIR-FM (right). Note that under TIR-FM illumination, only the apical brush border microvilli are visible, whereas under epifluorescence, fluorescent molecules below the apical surface also make a contribution, giving a lower resolution view of the apical membrane.
PTH-induced internalization of NaPi2a in OKP cells.
A stock solution of 10−4 M 1–34 PTH (Bachem, King of Prussia, PA) was prepared as described previously (30) and stored in small aliquots at −20°C until needed. This was diluted into the appropriate cell culture medium to a final concentration of 10−6 M. Two milliliters of this solution was placed into the cover glass bottomed dish and warmed until ready for introduction of the membrane. Once the cells were immersed, the sample was immediately set up for TIRF imaging. We used a saturating PTH concentration to minimize variations due to differing PTH receptor-ligand binding from cell to cell. Observational photobleaching was minimized by keeping the laser intensity low (5% or less of maximum laser power) and the exposure times short (50 ms). Images were collected at 10-min intervals over periods up to 3 h. We started data acquisition immediately a suitable cell has been located in TIRF, while the sample was still settling in Z. To ensure that a focused image was always captured, we acquired an image stack consisting of seven to eight planes at 250-nm intervals. This minimizes the total exposure of the sample when compared with manual refocusing. The plane of best focus was chosen for subsequent analysis.
Jasplakinolide treatment.
Jasplakinolide is a cell-permeable cyclic peptide that stabilizes actin filaments (9). A stock solution of 1 mM jasplakinolide (Alexis Biochemicals, San Diego, CA) in DMSO was prepared and stored at −20°C in 10-μl aliquots until needed. This stock was diluted in medium containing 0.2% FBS to a final concentration of 1 μM. The DMSO content was 0.1%. While the membranes were still mounted in their 12-well plate, cells were washed once with this medium and 1 ml was then added to the basal compartment and 0.5 ml added to the apical compartment. This was then placed back in the incubator for 30 min. At the end of this period, cells were washed four times in DMEM containing 0.2% FBS and prepared as above for imaging.
Confocal microscopy.
Confocal images were acquired using a Zeiss 510 laser-scanning confocal microscope. YFP NaPi2a was excited using a 488-nm laser. While it was not possible to exactly match the laser power and image exposure time, we did reproduce the z-stack and interimage delay protocols to that of the appropriate TIRF experiment.
Whole cell lysate from OKP cells.
OKP cells were grown to confluence in 100-mm dishes. The cells were washed in ice-cold PBS and then scraped into ice-cold lysis buffer (150 mM NaCl, 1.0% NP-40, 50 mM Tris, pH 8.0). The suspension was agitated at 4°C for 30 min and then centrifuged at 12,000 rpm for 20 min. The supernatant was kept for analysis.
Western blot analysis.
Cell lysates (20 μg total protein) were separated by 10% SDS-PAGE (Criterion, Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes (Bio-Rad). Membranes were blocked for 30 min at room temperature with 5% milk in PBST buffer (phosphate-buffered saline, 0.5% Tween, pH 7.4) before incubation with the primary antibody diluted in PBST-milk overnight at 4°C. The myosin VI antibody was purchased from Sigma (St. Louis, MO) and used at 1:1,000. After four washes with PBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat secondary antibodies (Pierce) for 1 h at room temperature. HRP was detected following 5 min of incubation in Supersignal West Pico Chemiluminescent Substrate or Supersignal West Dura Extended Duration Substrate (Pierce), using a charge-coupled device imaging system. Band intensities were quantified using Quantity One software.
Myosin VI RT-PCR in OKP cells.
Myosin VI primers for OKP cells were designed using homology to known myosin VI sequences from Monodelphis domestica and are shown in Table 1. Total RNA was prepared using the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions. RT-PCR was performed on an Eppendorf Mastercycler Gradient thermal cycler.
Table 1.
Primers used to isolate myosin VI using RT-PCR
| Name | Sequence |
|---|---|
| Forward 1 | GGA AGA TGG AAA GCC CGT T |
| Reverse 1b | ACT GTT CCT TTG GTG CCC |
| Forward 2 | TGG CTC TTA TAA ACC AAG |
| Reverse 2b | GTT CAC CAC ATG ATC AAA A |
Each pair of primers (forward 1 and reverse 1b, forward 2 and reverse 2b) gave products of the predicted size (1,215 bp and 1,187 bp, respectively). The PCR products were confirmed by sequencing.
Data analysis (statistics).
For analysis of microscopy images, the average intensity of a selected region of interest (ROI) was obtained at each time point in Axiovision version 4.5 software and corrected by the intensity measured in an ROI outside of the cell. Intensities were normalized to the initial value and plotted in GraphPad Prism 5 (GraphPad Software). A linear fit was applied to the data points obtained in the first 40 min after PTH addition to obtain the rates of internalization. Statistical analysis (one-way ANOVA with post hoc Bonferroni) was performed using GraphPad Prism 5. A P value <0.05 was considered statistically significant. The n values given in the figure legends refer to the number of different cells analyzed for each condition.
RESULTS
TIR-FM can be used to study trafficking at the apical surface.
To investigate the molecular mechanisms underlying the PTH-induced removal of NaPi2a from the proximal tubule brush border, we developed a novel application of TIR-FM to the apical surface of living cells. A schematic of the experimental setup is shown in Fig. 1A. Briefly, cells are grown on filter inserts to full differentiation before transfection with fluorescent protein-tagged constructs. For TIRF imaging the filter is excised, turned over onto a coverslip, and brought into close proximity of the coverslip using a glass weight. The method results in varying degrees of contact between the cells and the coverslip, but due to the inherent ruffling of the excised cell culture membrane substrate, it is always possible to find regions where cells are in the evanescent field yet are not squashed up against the coverslip. As can be seen in Fig. 1B, using this method it is possible to obtain high-resolution images of fluorescently tagged proteins within the brush border as compared with the epifluorescence image, where there is a significant contribution from subapical fluorescent molecules.
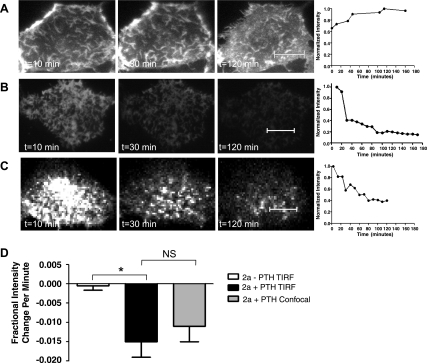
Apical TIR-FM can be used to monitor the effects of PTH on NaPi2a trafficking at the cell surface. Figure 2A shows a representative image of an OKP cell transfected with YFP-NaPi2a without any PTH treatment. Images are shown 10 min, 30 min, and 2 h after the start of the experiment. Figure 2B shows a cell transfected with YFP-NaPi2a that was treated with 10−6 M PTH. A decrease in the fluorescence brightness of the microvilli is seen both at 30 min and 2 h after PTH application. For the analysis of the PTH response, small ROIs outlining groups of microvilli were defined and their average intensity was plotted as a function of time, as illustrated in Fig. 2B. We assume that the average brightness of the ROI is proportional to the number of YFP-NaPi2a molecules within the microvillus. The intensity is normalized to the intensity at the first measurement. The rate of change (expressed as fractional change in intensity per minute), obtained by linear fitting over the first 40 min, was measured for multiple repeats of this experiment. The average of these measurements obtained for many cells is plotted in Fig. 2D, where a more negative slope indicates faster removal of YFP-tagged NaPi2a from the apical cell surface. The average initial slope for YFP-NaPi2a-transfected cells treated with PTH is significantly more negative than that of transfected cells that were not treated with PTH [−0.015 (SE 0.004) vs. −0.004 (SE 0.001), P = 0.0357], indicating that PTH induces removal of NaPi2a from the apical cell surface. The decrease in surface fluorescence intensity after PTH treatment is not due to photobleaching. OKP cells that were transfected with YFP-NaPi2a, fixed, and then treated with PTH showed no change in surface fluorescence intensity over the first 40 min (data not shown).
Fig. 2.
The time (t) course of parathyroid hormone (PTH)-induced removal of NaPi2a from the brush border membrane (BBM) is similar using confocal or TIR-FM imaging. A: representative images of an OKP cell transfected with YFP-NaPi2a and not treated with PTH. Images are shown at 10 min, 30 min, and 2 h after the start of imaging. A graph of normalized fluorescence intensity versus time for the entire time course of the experiment is shown to the right of the images. B: representative images of a YFP-NaPi2a-transfected OKP cell treated with 10−6 M PTH and imaged using TIR-FM. The entire time course is depicted graphically to the right of the images. C: representative images of an OKP cell transfected with YFP-NaPi2a, treated with 10−6 M PTH, and imaged using confocal microscopy. The entire time course is shown to the right. D: bar graph showing the average initial slope ± SE for cells transfected with YFP NaPi2a (2a) and not treated with PTH (open bar, n = 8), cells treated with 10−6 M PTH and imaged using TIRF (black bar, n = 14), and cells treated with 10−6 M PTH and imaged using confocal microscopy (gray bar, n = 9). A more negative slope indicates faster removal from the BBM (*P = 0.0357; NS, not significant). Scale bars represent 5 μm.
Confocal microscopy provides similar results as apical TIR-FM.
As a complementary method to TIR-FM, YFP-NaPi2a-transfected cells were treated with 10−6 M PTH and examined under confocal microscopy (Fig. 2C). PTH also caused a decrease in apical surface fluorescence intensity over time in cells transfected with fluorescently tagged NaPi2a. There was no statistical difference in the rate of change of apical fluorescence intensity versus time after PTH treatment of YFP-NaPi2a transfected cells observed using TIRF-FM compared with confocal microscopy [−0.015 (SE 0.004) vs. −0.011 (SE 0.004), P = not significant; Fig. 2D]. However, it was more difficult to determine the precise location of the BBM when confocal microscopy was used.
A dynamic actin cytoskeleton is necessary for PTH-induced removal of NaPi2a from the BBM.
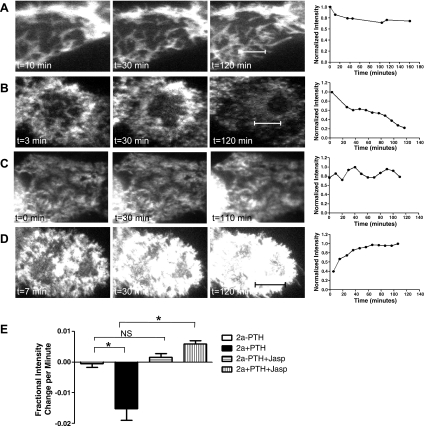
Each brush border microvillus contains a central actin core which undergoes slow but constant turnover, with actin monomers depolymerizing at the base of the actin bundle (the minus end) and repolymerizing at the top (the plus end). The rate of depolymerization is balanced by that of repolymerization so there is no net change in the length of the actin microvillar bundle. To determine whether a dynamic actin cytoskeleton is required for PTH-mediated removal of NaPi2a from brush border microvilli, YFP-NaPi2a-transfected OKP cells were treated with jasplakinolide, an agent that stabilizes actin filaments and halts treadmilling (10). Pretreatment of cells with jasplakinolide before application of PTH actually led to a significant increase in surface fluorescence over time, resulting in a positive slope for the fractional intensity change per minute in this group of cells [−0.015 (SE 0.004) vs. +0.006 (SE 0.001), P < 0.0001 compared with NaPi2a + PTH, Fig. 3E ]. In contrast, pretreatment of OKP cells with jasplakinolide alone did not lead to any significant change in YFP-NaPi2a fluorescence intensity over time (Fig. 3, C and E). Taken together, these data suggest that jasplakinolide inhibits the PTH-induced removal of NaPi2a from the BBM implying that a dynamic actin cytoskeleton is required for this process.
Fig. 3.
A dynamic actin cytoskeleton is required for PTH to induce NaPi2a removal from brush border microvilli. A: representative image of an OKP cell transfected with YFP-NaPi2a and not treated with PTH. The graph of the change in fluorescence intensity with time is shown to the right. The cells were imaged using TIR-FM. B: TIRF images of YFP-NaPi2a-transfected OKP cells treated with 10−6 M PTH. The graph of fluorescence intensity versus time is shown to the right. The negative slope indicates disappearance of fluorescent molecules from the cell surface and thus removal of YFP-NaPi2a from the brush border. C: TIRF images of OKP cell transfected with YFP-NaPi2a and treated with jasplakinolide alone. There is little change in surface fluorescence intensity over time. D: TIRF images of OKP cell transfected with YFP-NaPi2a and treated with jasplakinolide before PTH treatment. There is an increase in surface fluorescence intensity over time. E: average initial slopes of OKP cells transfected with YFP-NaPi2a and either not treated with PTH (white bar, n = 8), treated with PTH (black bar, n = 14), treated with jasplakinolide alone (horizontal hatched bar, n = 12), or treated with jasplakinolide before PTH (vertical hatched bar, n = 14). A more negative slope indicates faster removal of NaPi2a from the brush border (*P < 0.0001). Scale bars represent 5 μm.
Myosin VI is present in OKP cells.
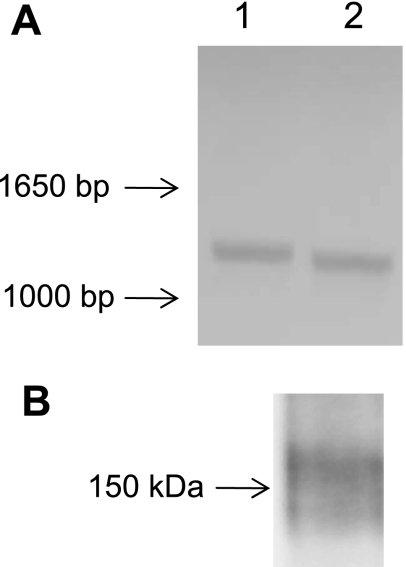
Since PTH-induced removal of NaPi2a from proximal tubule microvilli requires a dynamic actin cytoskeleton we questioned whether a myosin motor might be involved. Myosin VI is the only minus-end directed myosin motor discovered to date (11, 17, 20) and thus could act to pull cargo down the microvillus towards the cell interior. In addition, myosin VI has been found in the proximal tubule brush border in rat kidneys (7) and in OK cells (23). To confirm that myosin VI is present in OKP cells, we performed RT-PCR using the primers shown in Table 1. Products of the predicted size were found using primers specific for myosin VI (Fig. 4A). The presence of myosin VI protein in OKP cells was confirmed by Western blot analysis (Fig. 4B).
Fig. 4.
Myosin VI is present in OKP cells. A: PCR products of the predicted size are obtained using the forward 1 and reverse 1b primers (lane 1) and forward 2 and reverse 2 primers (lane 2). B: Western blot analysis of cell lysate from OKP cells probed with an anti-myosin VI antibody. A band at the predicted size (∼150 kDa) is obtained.
Myosin VI is required for PTH-induced removal of NaPi2a from BBM.
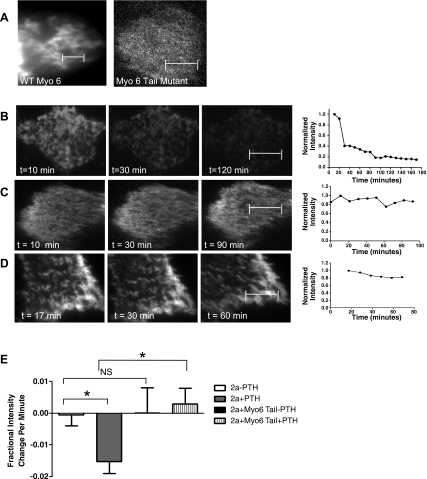
To test whether myosin VI is involved in the PTH-mediated trafficking of NaPi2a out of brush border microvilli, we cotransfected OKP cells with a CFP-tagged dominant-negative myosin VI tail and YFP-tagged NaPi2a. The dominant-negative myosin VI tail molecule has previously been shown to block myosin VI activity (2). The dominant-negative myosin VI tail consists of the cargo-binding portion of the myosin VI protein from which the motor has been removed. This mutant is thus capable of binding cargo but is immobile. The mutant competes with native myosin VI for cargo but cannot move the cargo, thus inhibiting endogenous myosin VI function. As can be seen in Fig. 5A, transfected wild-type myosin VI or dominant-negative tail mutant myosin VI is found in the BBM.
Fig. 5.
Inhibition of myosin VI inhibits PTH-induced removal of NaPi2a from the BBM. A: OKP cells transfected with green fluorescent protein (GFP)-tagged wild-type (WT) myosin VI (left) or dominant-negative myosin VI tail mutant (right) showing that the wild-type and mutant-transfected proteins are present in the BBM. B: representative images and graph of fluorescence intensity versus time for an OKP cell transfected with YFP-NaPi2a and treated with PTH. C: representative images and graph of fluorescence intensity versus time for an OKP cell cotransfected with YFP-NaPi2a and a dominant-negative myosin VI tail mutant and not treated with PTH. There is little change in the fluorescence intensity over time. D: representative images and graph of an OKP cell cotransfected with YFP-NaPi2a and a dominant-negative tail mutant and treated with PTH. The mutant inhibits PTH-induced removal of NaPi2a from the BBM. E: average initial slopes of fluorescence intensity versus time graphs for OKP cells transfected with YFP-NaPi2a and not treated with PTH (white bar, n = 8), PTH (gray bar, n = 14), cotransfected with YFP-NaPi2a and cyan fluorescent protein (CFP)-tagged dominant-negative myosin VI tail without PTH (black bar, n = 7), and YFP-NaPi2a and myosin VI tail plus PTH (hatched bar, n = 10). (*P = 0.001.) Scale bars represent 5 μm.
OKP cells were treated with PTH and observed using TIR-FM. PTH removal of NaPi2a from the cell surface was inhibited in cells containing the dominant-negative myosin VI tail [initial slope of +0.003 (SE 0.002) for NaPi2a + Myo6 tail + PTH vs. −0.015 SE (0.004) for NaPi2a + PTH; P = 0.001, Fig. 5, D and E], suggesting that myosin VI is required for this process. In contrast, there was no change in brush border fluorescence intensity of YFP-NaPi2a in cells cotransfected with the dominant-negative myosin VI tail mutant and not treated with PTH (Fig. 5, C and E), indicating that the myosin VI mutant alone has no effect on NaPi2a trafficking in the BBM.
DISCUSSION
We have applied a high-resolution imaging technique, TIR-FM, that was previously restricted to the basolateral membrane, to the apical surface of the cell. Using apical TIRF, we demonstrate that a dynamic actin cytoskeleton and the unconventional myosin motor myosin VI are required for removal of NaPi2a from the brush border microvillus in response to PTH.
It should be noted that in this study we are examining the most proximal events that occur in NaPi2a internalization in response to PTH—removal of the cotransporter from the most apical cellular compartment, the proximal tubule brush border. Using TIR-FM we are able to examine the initial step in PTH-induced trafficking of NaPi2a—the events occurring in brush border microvilli. We show here that the initial step in PTH-induced trafficking of NaPi2a in renal proximal tubular cells is movement of the cotransporters in the plane of the microvillar membrane to the base of the microvilli.
In OKP cells, PTH signals via the type 1 PTH receptor, which is found in both the apical and the basolateral membrane (19). Stimulation of apical PTH receptors activates protein kinase C-dependent pathways, whereas binding of PTH to basolateral receptors activates protein kinase A. Apical PTH receptors are assembled into a complex containing the PDZ (PSD-95, discs large, ZO-1) protein sodium-hydrogen exchange regulatory factor 1 (NHERF-1) and phospholipase C-β (26). NHERF-1 has been shown to be necessary for PTH-induced internalization of NaPi2a via apical PTH receptors (signaling through PKC) but not via basolateral PTH receptors (signaling via PKA) (12). In addition, recent work has shown that activation of either the PKC or the PKA pathway results in phosphorylation of a serine residue within NHERF-1 (38). This phosphorylation appears to cause dissociation of the NaPi2a/NHERF-1 complex, with NHERF-1 remaining at the apical membrane while NaPi2a is internalized after PTH treatment (15). Presumably, dissociation of the NaPi2a/NHERF-1 complex allows other scaffolding proteins to bind NaPi2a. Such proteins might include Disabled-2 (Dab-2) (34) or Gαi-interacting protein COOH terminus (GIPC) (1), both of which have been shown to bind myosin VI. Indeed, Dab-2, after binding to the cargo-binding domain of myosin VI, has been found to mediate dimerization of these motor proteins which would allow myosin VI to transport cargo in a directed manner (41). Alternatively, NaPi2a might bind directly to myosin VI. The mechanism whereby NaPi2a is linked to myosin VI within the microvillar membrane remains to be elucidated.
The OKP cell line provides an ideal system in which to study the molecular basis of these events by manipulation of individual components of the system. Thus we are able to study the role of actin in PTH-induced internalization of NaPi2a by examining the effects of jasplakinolide, a potent actin inhibitor. Such studies would not be feasible at the whole animal level due to the toxicity of actin inhibitors. Jasplakinolide alone appears to have no effect on YFP-NaPi2a fluorescence intensity over time, whereas jasplakinolide plus PTH treatment appears to increase the intensity of YFP-NaPi2a in the BBM over time, suggesting that the combination of both agents may cause an increase in exocytosis and/or an increase in the number of brush border microvilli. Further study is needed to investigate this phenomenon.
Actin has shown to be involved in the trafficking of several transporters and channels including aquaporin-2 (28), the sodium-hydrogen exchanger (13), and the ATP-sensitive potassium channel (33). In addition, a dynamic actin cytoskeleton has been shown to be necessary for myosin-based transport within cells (32). The precise mechanism whereby NaPi2a is linked to actin cytoskeleton remains to be determined but is thought to involve the PDZ protein NHERF-1 and the actin-binding protein ezrin (25, 26).
A novel finding of this study is the requirement for the unconventional myosin motor protein myosin VI in the initial step in PTH-induced trafficking of NaPi2a—removal of NaPi2a from brush border microvilli. An advantage to using a myosin VI mutant is that it specifically targets myosin VI as opposed to ATP depletion, which inhibits all myosin motors and might also interfere with signaling via the PTH receptor. Myosin VI has been shown to be involved in trafficking of the cystic fibrosis transmembrane conductance regulator (27, 35). The precise mechanism whereby myosin VI is linked to NaPi2a within the brush border microvillus remains to be determined. Spudich et al. (34) have shown that the targeting of myosin VI to clathrin-coated vesicles at the plasma membrane is mediated by binding to Dab-2 and phosphatidylinositol 4,5-bisphosphate. It is not known whether a similar mechanism is operational in the brush border microvilli of renal tubular epithelial cells.
One potential criticism of our apical TIR-FM technique is that the apposition of the microvilli may interfere with normal NaPi2a trafficking. However, we have validated our finding with time-lapse confocal microscopy where we obtain the identical results.
In summary, we have used a novel application of TIR-FM to image the apical brush border of renal proximal tubular cells. Using this method, we have determined that actin and myosin VI are involved in removal of the sodium-phosphate cotransporter NaPi2a from brush border microvilli in response to PTH. Further work is needed to determine the mechanisms linking NaPi2a to myosin VI motors after PTH treatment.
GRANTS
J. T. Blaine was supported by an AHA-Scientist Development Grant (award no. 0830394N). M. Levi and N. Barry were supported by a National Institutes of Health RO1 grant (DK066029). S. Y. Breusegem acknowledges the support of a post-doctoral fellowship from the American Heart Association, Pacific Mountain Affiliate (award no. 0520054Z) and Y. Caldas was supported by a National Institutes of Health (3R01 AG026529) minority fellowship.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Tama Hasson for the gift of the myosin VI dominant-negative tail mutant construct and Drs. Eleanor Lederer and Syed Khundmiri for gifts of the OK cell NaPi4 antibody.
REFERENCES
References
- 1.Aschenbrenner L, Lee T, Hasson T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol Biol Cell 14: 2728– 2743, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschenbrenner L, Naccache SN, Hasson T. Uncoated endocytic vesicles require the unconventional myosin, Myo6, for rapid transport through actin barriers. Mol Biol Cell 15: 2253– 2263, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacic D, LeHir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+//phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69: 495– 503, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Beaumont V. Visualizing membrane trafficking using total internal reflection fluorescence microscopy. Biochem Soc Trans 31: 819– 823, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Biber J, Gisler SM, Hernando N, Wagner CA, Murer H. PDZ interactions and proximal tubular phosphate reabsorption. Am J Physiol Renal Physiol 287: F871– F875, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch 458: 39– 52, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Biemesderfer D, Mentone SA, Mooseker M, Hasson T. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am J Physiol Renal Physiol 282: F785– F794, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607– 617, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869– 14871, 1994 [PubMed] [Google Scholar]
- 10.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275: 5163– 5170, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Buss F, Kendrick-Jones J. How are the cellular functions of myosin VI regulated within the cell? Biochem Biophys Res Commun 369: 165– 175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capuano P, Bacic D, Roos M, Gisler SM, Stange G, Biber J, Kaissling B, Weinman EJ, Shenolikar S, Wagner CA, Murer H. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol 292: C927– C934, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell 17: 2661– 2673, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJM, Hoenderop JGJ. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693– 1704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O'Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-Pi cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol 289: C159– C167, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dranitzki Elhalel M, Wald H, Rubinger D, Gal-Moscovici A, Inoue M, Levi M, Popovtzer MM. Regulation of NaPi-IIa mRNA and transporter protein in chronic renal failure: role of parathyroid hormone (PTH) and dietary phosphate (Pi). Pflügers Arch 449: 265– 270, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Frank DJ, Noguchi T, Miller KG. Myosin VI: a structural role in actin organization important for protein and organelle localization and trafficking. Curr Opin Cell Biol 16: 189– 194, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131– 2138, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun 328: 666– 678, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci 107: 3535– 3543, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Giral H, Breusegem SY, Halaihel N, Baile G, Catalan J, Carrodeguas JA, Barry NP, Levi M, Sorribas V. Interaction of MAP17 with NHERF3/4 induces translocation of the renal Na/Pi IIa transporter to the trans-Golgi. Am J Physiol Renal Physiol 292: F230– F242, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Leong PKK, Devillez A, Sandberg MB, Yang LE, Yip DKP, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol 290: F854– F863, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Cong R, Biemesderfer D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 295: C529– C537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, Biber J, Murer H, Kaissling B. Rapid downregulation of rat renal Na/Pi cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest 104: 483– 494, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahon MJ. Ezrin promotes functional expression and parathyroid hormone-mediated regulation of the sodium-phosphate cotransporter 2a in LLC-PK1 cells. Am J Physiol Renal Physiol 294: F667– F675, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mahon MJ, Segre GV. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem 279: 23550– 23558, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Nadia Ameen GA. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998– 1006, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Noda Y, Sasaki S. The role of actin remodeling in the trafficking of intracellular vesicles, transporters, and channels: focusing on aquaporin-2. Pflügers Arch 456: 737– 745, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Pfister MF, Lederer E, Forgo J, Ziegler U, Lotscher M, Quabius ES, Biber J, Murer H. Parathyroid hormone-dependent degradation of type II Na+/Pi cotransporters. J Biol Chem 272: 20125– 20130, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Reshkin SJ, Wuarin F, Biber J, Murer H. Parathyroid hormone-induced alterations of protein content and phosphorylation in enriched apical membranes of opossum kidney cells. J Biol Chem 265: 15261– 15266, 1990 [PubMed] [Google Scholar]
- 31.Rodman JSMM, Farquhar MG. Cytoskeletal proteins of the rat kidney proximal tubule brush border. Eur J Cell Biol 42: 319– 327, 1986 [PubMed] [Google Scholar]
- 32.Semenova I, Burakov A, Berardone N, Zaliapin I, Slepchenko B, Svitkina T, Kashina A, Rodionov V. Actin dynamics is essential for myosin-based transport of membrane organelles. Curr Biol 18: 1581– 1586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song DK, Ashcroft FM. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SUR subunit. J Biol Chem 276: 7143– 7149, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Spudich G, Chibalina MV, Au JSY, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol 9: 176– 183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swiatecka-Urban A, Boyd C, Coutermarsh B, Karlson KH, Barnaby R, Aschenbrenner L, Langford GM, Hasson T, Stanton BA. Myosin VI regulates endocytosis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 279: 38025– 38031, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Toomre D, Steyer JA, Keller P, Almers W, Simons K. Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy. J Cell Biol 149: 33– 40, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Abel M, Hoenderop JGJ, van der Kemp AWCM, Friedlaender MM, van Leeuwen JPTM, Bindels RJM. Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int 68: 1708– 1721, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Weinman EJ, Biswas RS, Peng Q, Shen L, Turner CL, EX, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412– 3420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yang LE, Leong PKK, McDonough AA. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. Am J Physiol Renal Physiol 293: F1197– F1208, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Yang LE, Maunsbach AB, Leong PKK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890– 2896, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell 138: 537– 548, 2009 [DOI] [PubMed] [Google Scholar]