Abstract
Allison, Milton J. (Dairy Cattle Research Branch, U. S. Department of Agriculture, Beltsville, Md.), M. P. Bryant, and R. N. Doetsch. Studies on the metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. I. Incorporation of isovalerate into leucine. J. Bacteriol. 83:523–532. 1962.—Ruminococcus flavefaciens strain C94, a cellulolytic rumen bacterium, requires either isobutyrate or isovalerate for growth. The organism was grown in the presence of C14-labeled isovalerate, and the metabolic fate of the labeled carbon was studied to obtain information on the functions of this growth factor. Radioactivity from isovalerate-1-C14 and isovalerate-3-C14 was found mainly in the protein and lipid fractions of the cells. The C14 in protein was all in leucine, indicating that a function of isovalerate was to serve as a carbon skeleton for leucine synthesis.
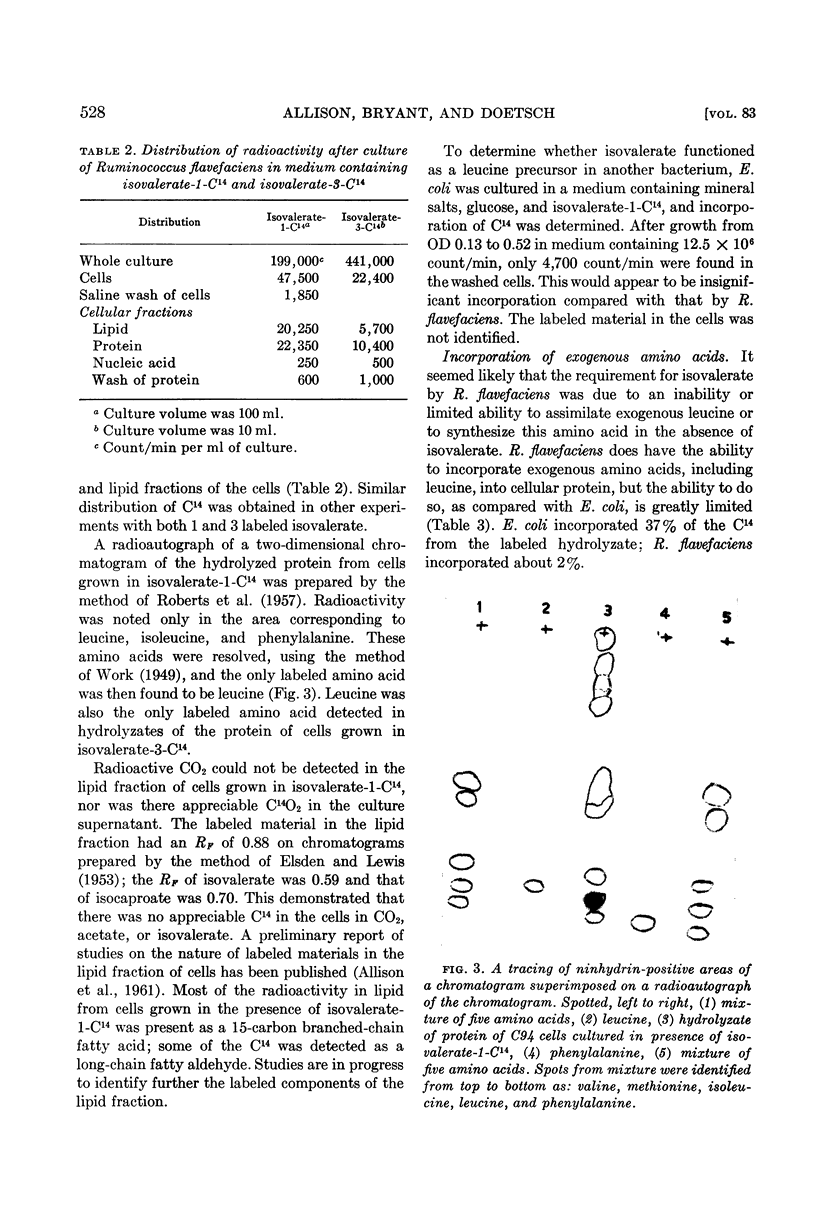
As C14 in leucine synthesized from isovalerate-1-C14 was entirely in carbon 2, the intact isovalerate molecule was apparently incorporated into leucine. This is evidence that leucine was synthesized by a mechanism different from that previously demonstrated in other microorganisms.
R. flavefaciens has a definite but limited ability to incorporate exogenous amino acids, including leucine. It incorporated 2% of the C14 during growth in uniformly labeled (UL) C14-Chlorella protein hydrolyzate; Escherichia coli incorporated 37% of the label under similar conditions. In another experiment, a limited amount of exogenous leucine-2-C14 was incorporated into protein of R. flavefaciens. The requirement for isovalerate was not replaced by dl-leucine or 2-ketoisocaproate. It is suggested that isovalerate or isobutyrate is required because R. flavefaciens has a limited ability to incorporate exogenous branched-chain amino acids and a limited ability to synthesize the isopropyl group found in these amino acids and in other components of the cell.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., BOLTON E. T., ALDOUS E. Utilization of carbon dioxide in the synthesis of proteins by Escherichia coli. I. J Biol Chem. 1952 Sep;198(1):165–172. [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Conversion of isovalerate to leucine by Ruminococcus flavefaciens. Arch Biochem Biophys. 1959 Sep;84:245–247. doi: 10.1016/0003-9861(59)90575-2. [DOI] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Volatile fatty acid growth factor for cellulolytic cocci of bovine rumen. Science. 1958 Aug 29;128(3322):474–475. doi: 10.1126/science.128.3322.474. [DOI] [PubMed] [Google Scholar]
- ANNISON E. F. Nitrogen metabolism in the sheep; protein digestion in the rumen. Biochem J. 1956 Dec;64(4):705–714. doi: 10.1042/bj0640705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F. Some observations on volatile fatty acids in the sheep's rumen. Biochem J. 1954 Jul;57(3):400–405. doi: 10.1042/bj0570400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYERS W. A. Nutrition and physiology of Ruminococcus flavefaciens. J Bacteriol. 1958 Nov;76(5):504–509. doi: 10.1128/jb.76.5.504-509.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., ROBINSON I. M. Characteristics of ruminal anaerobic celluloytic cocci and Cillobacterium cellulosolvens n. sp. J Bacteriol. 1958 Nov;76(5):529–537. doi: 10.1128/jb.76.5.529-537.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Studies on the Nitrogen Requirements of Some Ruminal Cellulolytic Bacteria. Appl Microbiol. 1961 Mar;9(2):96–103. doi: 10.1128/am.9.2.96-103.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHORITY B. A., JOHNSON R. R., BENTLEY O. G., MOXON A. L. Studies on the metabolism of valine, proline, leucine and isoleucine by rumen microorganisms in vitro. Arch Biochem Biophys. 1958 Nov;78(1):15–27. doi: 10.1016/0003-9861(58)90310-2. [DOI] [PubMed] [Google Scholar]
- EL-SHAZLY K. Degradation of protein in the rumen of the sheep. II. The action of rumen micro-organisms on amino acids. Biochem J. 1952 Aug;51(5):647–653. doi: 10.1042/bj0510647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSDEN S. R., LEWIS D. The production of fatty acids by a gram-negative coccus. Biochem J. 1953 Aug;55(1):183–189. doi: 10.1042/bj0550183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL E. R. Investigations on the microbiology of cellulose utilization in domestic rabbits. J Gen Microbiol. 1952 Nov;7(3-4):350–357. doi: 10.1099/00221287-7-3-4-350. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli J. K., Williams H. H., Thomas W. E., Ferris F. H., Maynard L. A. Synthesis of Amino Acids in the Rumen. Science. 1949 Aug 5;110(2849):144–145. doi: 10.1126/science.110.2849.144. [DOI] [PubMed] [Google Scholar]
- RAFELSON M. E., Jr The biosynthesis of leucine in Aerobacter aerogenes. Arch Biochem Biophys. 1957 Dec;72(2):376–379. doi: 10.1016/0003-9861(57)90214-x. [DOI] [PubMed] [Google Scholar]
- REISS O., BLOCH K. Studies on leucine biosynthesis in yeast. J Biol Chem. 1955 Oct;216(2):703–712. [PubMed] [Google Scholar]
- STRASSMAN M., LOCKE L. A., THOMAS A. J., WEINHOUSE S. A study of leucine biosynthesis in Torulopsis utilis. Science. 1955 Feb 25;121(3139):303–304. doi: 10.1126/science.121.3139.303. [DOI] [PubMed] [Google Scholar]
- WRIGHT D. E. The metabolism of carbon dioxide by Streptococcus bovis. J Gen Microbiol. 1960 Jun;22:713–725. doi: 10.1099/00221287-22-3-713. [DOI] [PubMed] [Google Scholar]




