Abstract
The ability to dynamically image cellular and subcellular structures in a live animal and to target genes to a specific cell population in a living tissue provides a unique tool to address many biological questions in the proper physiological context. Here, we describe a powerful approach that is based on the use of rat submandibular salivary glands, which offer the possibility to easily perform intravital imaging and deliver molecules from the oral cavity, and plasmid DNA, which offers the advantage of rapid manipulations. We show that, under different experimental conditions, a reporter molecule can be rapidly expressed in specific compartments of the glands: 1) in the intercalated ducts, when plasmid DNA is administered alone, and 2) in granular ducts, striated ducts, and, to a lesser extent, acini, when plasmid DNA is mixed with replication-deficient adenovirus subtype 5 particles. Remarkably, we also found that gene expression can be directed to acinar cells when plasmid DNA is administered during isoproterenol-stimulated exocytosis, suggesting a novel mechanism of plasmid internalization regulated by compensatory endocytosis. Finally, as a practical application of these strategies, we show how the expression of fluorescently tagged molecules enables the study of the dynamics of various organelles in live animals at a resolution comparable to that achieved in cell cultures.
Keywords: intravital two-photon microscopy, compensatory endocytosis, membrane traffic
in the last five years, the development of intravital microscopy has enabled imaging of various cellular and subcellular processes in living animals, allowing, for the first time, dynamic studies in physiological conditions (7, 19, 23, 32, 34, 43). A breakthrough to this approach that would open the door to new and exciting discoveries would be the ability to transfer or ablate genes in a specific tissue. The major strategy to genetically manipulate animals has been the use of transgenic or knockdown models, which offer the possibility to control the levels of expression of the transgenes, to selectively target the tissue of interest when specific promoters are available, and to induce expression or deletion of a gene at a certain point in time (9). However, in terms of resources and time, the high costs to generate animal models make this approach prohibitive, especially for screening purposes. A viable alternative is the direct delivery of a gene, small interfering RNA (siRNA) or short hairpin RNA (shRNA), to the target organ of the adult animal. This strategy has some advantages: 1) the possibility to express genes in a relatively short period of time, thus avoiding compensatory mechanisms or effects during the development, 2) the possibility to target specific tissues, and 3) limited cost. For delivery of a gene into the organ of a live animal, there are two main approaches: the use of viral vectors and the use of plasmid DNA (20). Although viral vectors are the most efficient vehicles for gene transfer, the targeted tissue often develops immunopathological reactions to the virus (22, 39). On the other hand, the major limitation of non-viral-based gene transfer is the low efficiency of transduction. For this reason, the use of “naked DNA” has been combined with different physical and chemical methods, such as hydrodynamic delivery, electroporation, and gene gun transfer, or the use of DNA complexes with cationic polymers and lipids (20).
The salivary glands, which are major secretory organs, produce saliva, a mixture of water, proteins, and electrolytes; the functions of the salivary glands range from lubrication to protection of the oral epithelium, facilitation of digestion, and antimicrobial activity (12, 26). To image and study different cellular processes at a subcellular level, we recently used the submandibular salivary glands (SMGs) as a model system and established various experimental procedures based on intravital two-photon microscopy (TPM) (23). Because they are easily accessible through the intraoral cannulation of the major excretory (Wharton's) duct, the SMGs are a useful target for transgene expression. Indeed, successful gene transfer into rat and mouse SMGs has been shown using viral-based (1, 2, 4, 6, 24, 33, 41) and non-viral-based (11, 18, 29, 30) approaches. Viral vectors show diverse tropism for acinar and ductal cells in mouse SMGs. For example, adenoviral, vaccinia, and lentiviral vectors are able to infect acinar and ductal cells, herpes simplex type I vector infects only acinar cells with low efficiency, and a retroviral vector, the murine leukemia virus, failed to infect salivary glands unless cell proliferation was induced (38). Although non-viral-based approaches, such as the use of plasmid DNA alone or in combination with replication-deficient adenovirus subtype 5 (rAd5) particles, have been shown to be effective in expressing human recombinant growth hormone in rat salivary glands, the efficiency of gene expression and the nature of the cells targeted were not established (11).
To address these questions, we used as reporter molecules various fluorescent proteins, taking advantage of the high-resolution imaging provided by TPM and confocal microscopy. Here, we show that when plasmid DNA is injected intraorally into the SMGs of live rats, it is expressed in a specific population of cells in the parenchyma of the glands, namely, the intercalated ducts (ID). In the presence of rAd5, the efficiency of transduction is increased up to 7- to 10-fold, with the cells expressing pVenus being primarily located in granular and striated ducts (hereafter referred to as “large ducts”) and, to a lesser extent, in acini. Remarkably, we discovered that compensatory endocytosis, a process necessary to retrieve the massive amount of membranes delivered during stimulated secretion, can be exploited to internalize plasmid DNA into acinar cells, offering an additional tool to control gene expression. Finally, we show how various genes expressed using these methods can be used to gain dynamic information on various intracellular compartments in the live animal.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (150–250 g body wt; Harlan Laboratories, Frederick, MD) were acclimated for 1 wk before they were used for the procedures. Water and food were provided ad libitum. All the experiments were approved by the National Institute of Dental and Craniofacial Research (NIDCR) Animal Care and Use Committee.
Plasmids and viruses.
To characterize the expression of plasmid DNA, we used the following plasmids: pEGFP-C1 and pEGFP-N1 (Clontech), pYFP-N1, mCherry-N2, and Venus A206K (Dr. G. Patterson, National Institute of Child Health and Human Development) and pTag-RFP (Evrogen). Since we did not see any differences in terms of efficiency of expression, we used primarily Venus A206K for its brightness. Other plasmids used in this study were as follows: TGN38-Venus and TGN38-mCherry (Dr. G. Patterson), green fluorescent protein (GFP)-clathrin (Dr. L. Greene, National Heart, Lung, and Blood Institute), GFP-Rab-5 and vesicle-associated membrane protein-8-yellow fluorescent protein (VAMP-8-YFP, Dr. Julie Donaldson, National Heart, Lung, and Blood Institute), and aquaporin-5-YFP. All plasmids were purified using a QIAfilter Plasmid Maxi Kit (Qiagen, Valencia, CA). The Label IT Tracker CX-rhodamine nucleic acid labeling kit (Mirus, Madison, WI) was used according to the manufacturer's instructions. rAd5 encoding no protein (C. Goldsmith, NIDCR) was prepared and purified as previously reported (6). Viral titer was determined by measurement of the optical absorbance at 260 nm (14, 15). Adeno-associated virus subtype 5 (AAV5) particles were kindly provided by Dr. G. DiPasquale (NIDCR). VSV-G-pseudotyped lentiviral particles encoding no protein were prepared as previously reported (28). For the inactivation of the adenovirus, rAd5 was exposed to UV light [at 6 cm from a UV lamp (model XX-15S, UVP, Upland, CA)] for various amounts of time or incubated at 45°C for 1 h (37).
In vivo gene delivery into SMGs.
For the in vivo gene delivery, plasmid DNA was diluted with saline at the appropriate concentration with or without the viral particles. The animals were anesthetized by an intramuscular injection of a mixture of 100 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 20 mg/kg xylazine (Akorn, Decatur, IL). A fine polyethylene (PE-5) cannula (Strategic Applications, Libertyville, IL) was inserted intraorally in the orifice of Wharton's duct and sealed with glue (Elmer's Products, Columbus, OH). The plasmid DNA (100 μl/gland) was slowly injected to avoid damage, and the cannulas were removed after 10 min. When needed, agonists or antagonists of muscarinic or adrenergic receptors were administered subcutaneously. Isoproterenol, pilocarpine, carbachol, phenylephrine, propranolol, and atropine were purchased from Sigma (St. Louis, MO).
For gene delivery by gravity, the anesthetized animal was cannulated with 35-cm-long PE-5 tubing, and the syringes containing the solution were elevated to 25 cm. The solution was allowed to diffuse into the glands for 1 h (25).
Immunocytochemistry.
SMGs were snap frozen in liquid nitrogen using optimum cutting temperature compound (Sakura Finetek USA, Torrance, CA), cut (20 μm thick) on silanated glass slides, and fixed for 15 min in 2% formaldehyde at room temperature. Cryosections were incubated with blocking solution for 45 min and then with the appropriate primary antibody or with Alexa 488-phalloidin (Invitrogen, Carlsbad, CA) or tetramethylrhodamine isothiocyanate-phalloidin (Sigma). The primary antibodies were as follows: rabbit anti-lysosome-associated membrane protein (LAMP)-1 (Novus Biologicals, Littleton, CO), rabbit anti-Ki67 (Abcam, Cambridge, MA), and goat anti-CAR (R & D Systems, Minneapolis, MN). Alexa-conjugated secondary antibodies were obtained from Invitrogen.
TPM and confocal microscopy.
An inverted confocal microscope (model IX81, Olympus America, Center Valley, PA) was modified to perform TPM, as previously described (23). As a laser source, a tunable Ti:sapphire femtosecond laser (Chameleon Ultra II, Coherent Laser Group, Santa Clara, CA) was used, and the power was modulated using a combination of neutral density filters (Chroma Technology, Rockingham, VT). A beam expander (LSM Technologies, Stewartstown, PA) was used to modulate the size of the beam that was then directed into a scanning head (Fluoview 1000, Olympus America). The emitted signal was aimed into a custom-made set of three nondescanned detectors (LSM Technologies). A 680-nm barrier filter (Chroma Technology) prevented the scattered IR light from reaching the detectors. Two dichroic mirrors and the barrier filters were purchased from Chroma Technology, and three cooled photo multipliers (PMTs) were purchased from Hamamatsu (Bridgewater, NJ). The first PMT (510-nm dichroic mirror, 400- to 480-nm barrier filter) detected the endogenous fluorescence and the second harmonic signal. FITC and Alexa 488 were detected on the second PMT (570-nm dichroic mirror, 505- to 560-nm barrier filter). CX-rhodamine and Texas Red were detected on the third PMT (590- to 650-nm barrier filter).
For time-lapse images of the live animal, an objective inverter (LSM Technologies) was used with the animal in the upright configuration, whereas the excised glands were imaged in the inverted setting (23). For time-lapse imaging, acquisition speed was set to 0.3 frames/s. All images and movies were acquired using a UPLSAPO ×60, numerical aperture (NA) 1.2 objective; an XLUMPFL20XW ×20, NA 0.95 water immersion objective; or a UPLSAPO ×10, NA 0.4 objective (Olympus America).
For live imaging, a carbomer-940-based gel (Snowdrift Farm, Tucson, AZ) was used to couple the sample to the objective, as described previously (32). The temperature of the exposed glands was controlled using an objective heater (Bioptechs, Butler, PA), and the body temperature of the animal was maintained by heated pads (Kent Scientific, Torrington, CT), as described previously (23). Oregon Green 488 (70 kDa) and Texas Red-dextran were purchased from Invitrogen and used at the appropriate concentrations. For imaging of the isolated SMGs, the rats were euthanized by exposure to carbon dioxide, and the glands were excised and placed in ice-cold PBS before imaging.
Image processing.
When needed, background noise was reduced by application to each image of one or two rounds of a 2 × 2 pixel low-pass filter by using Metamorph (Molecular Devices). Brightness, contrast, and gamma correction were applied. For the movies, adjustments in the alignment of the frames were performed using ImageJ (W. Rasband, National Institutes of Health) plug-in Stackreg. Volume rendering was performed using Imaris 5.6 and 6.0 64-bit (Bitplane). The final preparation of the images was conducted with Adobe Photoshop CS. Movies were assembled with Metamorph and compressed with Quicktime Pro.
Quantitative analysis.
For quantification of the efficiency of gene expression, 10 random images per gland were collected at 930-nm excitation using a ×10 objective. The field of view (1.61 mm2) was chosen to entirely enclose the parenchyma. The number of fluorescent cells per field of view was estimated using a computer-assisted method (Metamorph). The efficiency of transduction was calculated by dividing the number of cells per field of view by the area (number of cells/mm2). For each experiment, two glands per animal and two animals per condition were quantified. Each experiment was repeated at least three times. The significance of differences between samples was evaluated by Student's t-test. The average size of the lumen of the acinar canaliculi, ID, and large ducts was measured in cryosections labeled with phalloidin to highlight the actin. Ten randomly selected structures were chosen per section.
RESULTS
In rat SMGs, plasmid DNA is expressed in the ID; in the presence of rAd5, it is expressed primarily in granular and striated ducts.
To follow the expression of plasmid DNA by light microscopy, we tested different soluble fluorescent proteins as reporter molecules (see materials and methods). Since the nature of the protein expressed did not affect our results, we primarily used the monomeric variant of the yellow fluorescent protein pVenus, which offers many advantages for intravital imaging, such as greater stability, brightness, and low toxicity (27). Plasmid DNA encoding for pVenus was retrogradely injected into Wharton's duct of rat SMGs in the presence or absence of rAd5 particles. At 16 h after the injection, the glands were excised and imaged by TPM at 930-nm excitation to ensure the optimal detection of pVenus. Under these conditions, the elastic fibers enclosing the glands were also revealed by a combination of two-photon and second harmonic emissions, thus providing a valuable reference point for the surface of the organ (23). In the absence of rAd5, single cells expressing pVenus were observed immediately below the elastic fibers (Fig. 1). When rAd5 was coinjected with plasmid DNA, clusters of cells expressing pVenus were observed, and the efficiency of transduction, reported here as the number of cells expressing pVenus per area unit, increased sevenfold (from 3.9 ± 0.2 to 27.8 ± 2.3 cells/mm2; Fig. 2A). In both cases, pVenus localized in the cytosol (Fig. 1B) or the nuclei (not shown), and no expression was detected when saline or rAd5 alone was injected (data not shown). Furthermore, no expression was detected when the plasmid DNA, regardless of the presence of rAd5, was directly injected into the glands (not shown). Among the plasmids tested, those sharing the backbone of the pEGFP series of cloning vectors gave the best results in terms of reproducibility and efficiency of transduction (not shown). The minimal amount of rAd5 that enhanced the expression of pVenus was 3 × 109 particles/gland, whereas other viruses, such as AAV5 (Fig. 2B) or a VSV-G-pseudotyped lentivirus (data not shown), did not elicit stimulation. Injection of the virus did not trigger an immune response, nor did it damage the glands (see supplemental Fig. 1 in the online version of this article). Furthermore, the rAd5-induced enhancement of pVenus expression required that the proteins of the capsid were intact, since heat-inactivated, but not UV-inactivated, rAd5 (37) failed to stimulate pVenus expression (Fig. 2B). Although in the absence of rAd5 the levels of expression of pVenus did not change over time or with increasing amounts of the injected plasmid DNA, in the presence of rAd5, the maximum level of expression was reached after 16 h and with use of 12 μg of DNA (Fig. 2, C and D). The expression of pVenus in the presence of rAd5 declined after 48 h and was no longer detectable after 1 wk (Fig. 2C; data not shown).
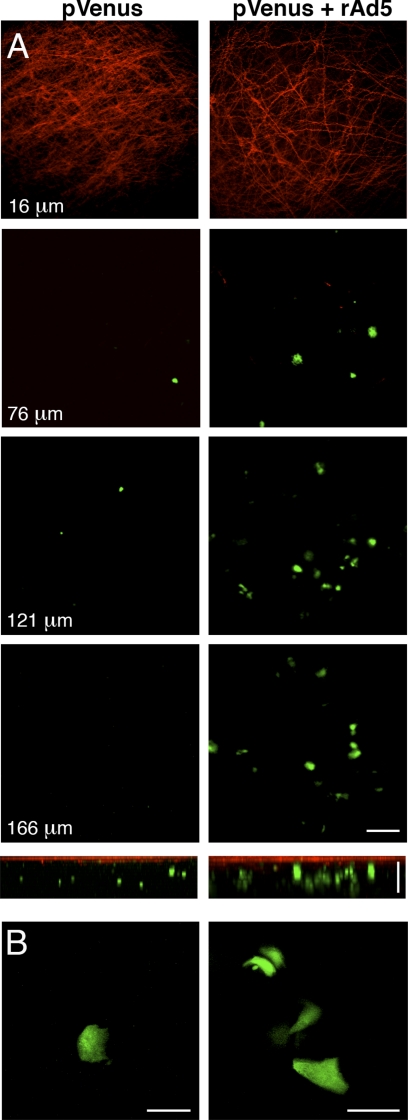
Fig. 1.
Plasmid DNA transduction in rat submandibular salivary glands (SMGs) is enhanced in the presence of replication-deficient adenovirus subtype 5 (rAd5) particles. Plasmid DNA (12 μg) encoding for pVenus was injected into Wharton's ducts of anesthetized rats alone (pVenus) or in the presence of 3 × 1010 particles of rAd5 (pVenus + rAd5). At 16 h after the injection, SMGs were exposed and imaged by 2-photon microscopy (TPM) using a ×20 water immersion objective [numerical aperture (NA) 0.95, 930-nm excitation]. A, top: z stacks of transduced SMGs. Consecutive images were obtained at ≤200 μm below the surface of the gland. Elastic fibers are shown in red and pVenus-expressing cells in green. Scale bar, 100 μm. A, bottom: volume rendering. z bar, 100 μm. B: SMGs imaged using a ×60 water immersion objective (NA 1.2) to show cytosolic distribution of pVenus. Scale bars, 20 μm.
Fig. 2.
Plasmid DNA-mediated expression of pVenus is transient, and its enhancement is specific for rAd5. A–C: 12 μg of plasmid DNA encoding pVenus were retroductally injected alone or in the presence of rAd5. SMGs were dissected and imaged by TPM (as described in Fig. 1 legend), and the number of cells expressing pVenus were scored and reported as cells/mm2. A: presence of rAd5 induced a 7-fold increase in pVenus expression compared with control. Values are means ± SE of 8 independent experiments. ***P < 0.001. B: plasmid DNA was injected alone (Ctrl) or in the presence of rAd5 (3 × 109 and 3 × 1010 particles), adeno-associated virus subtype 5 (AAV5, 3 × 109 particles/gland), or heat-inactivated or UV-light-treated rAd5 (3 × 1010 particles/gland). Statistical significance was calculated using t-test: **P < 0.01, ***P < 0.001, +++P < 0.001. C: time course of pVenus expression in SMGs. Plasmid DNA was injected as described above, SMGs were imaged 8, 16, 30, 48, and 72 h after injection, and the number of cells expressing pVenus was scored. D: dose response of plasmid DNA. pVenus (4, 12, or 24 μg) was injected intraorally into SMGs in the absence or presence of rAd5 and analyzed after 16 h to evaluate the number of cells expressing pVenus. In B–D, values are means ± SE of 3 independent experiments.
Next, we checked whether the distribution of the cells expressing pVenus was homogeneous throughout the tissue. To overcome the depth limitations of the two-photon microscope, we physically sectioned the gland longitudinally and transversely (see supplemental Fig. 2A). When plasmid DNA was administered alone, the distribution of the cells expressing pVenus was homogeneous throughout the gland (see supplemental Fig. 2B, open bars). However, in the presence of rAd5, cells expressing pVenus were more abundant in the central part of the gland (up to 6- to 7-fold; see supplemental Fig. 2B, solid bars). This area of the gland is particularly enriched in ductal structures (see Fig. 5B), suggesting that, in the presence of rAd5, a different population of cells could be targeted. Indeed, the SMG is a complex organ that is formed by heterogeneous populations of cells organized in various structures (Fig. 3A) (40). To determine which populations of cells in the gland expressed the transduced genes, we exploited a unique property of TPM. Indeed, we previously showed that when the exposed glands are imaged using 740-nm excitation, various endogenous molecules are excited, revealing the cellular architecture of the parenchyma of the glands (23). In the first 20–30 μm below the layer occupied by the elastic fibers, the acini can be easily identified for their characteristic morphology and shape (Fig. 3C, left). Further below, large ducts appeared as very bright structures, since, on the basolateral side, they are highly enriched in mitochondria (40), which contain NADH, a molecule that emits very efficiently upon two-photon excitation (Fig. 3C, right). Although the acini could still be distinguished at this depth, their resolution was largely diminished as a result of high light scattering (Fig. 3C, right). Remarkably, the morphology of acini and large ducts, as revealed by TPM, matched that achieved by conventional hematoxylin-eosin staining (Fig. 3B), indicating that this approach offers the advantage of rapidly identifying the cells expressing the transduced gene directly in the live animal without the need of any processing. For identification of the nature of the cells expressing pVenus, plasmid DNA was administered in the absence or presence of rAd5, and the glands were exposed and imaged at 740-nm excitation. The same field of view was then imaged at 930-nm excitation to detect the cells expressing pVenus. In the presence of rAd5, pVenus was expressed primarily in large ducts and also, although to a lesser extent, in acini (Fig. 3E). In the absence of rAd5, pVenus was not expressed in acini or in large ducts (Fig. 3D, arrows). In the absence of rAd5, the diameter of cells expressing pVenus was 10–15 μm; in the presence of rAd5, the diameter of cells expressing pVenus was 15–20 μm, when localized in acini, or 20–30 μm, when localized in large ducts. Interestingly, in rat SMGs, ID were formed by 10 ± 2-μm-diameter cells (40). To test the hypothesis that cells expressing pVenus in the absence of rAd5 were indeed ID, we tried to label the transfected cells with specific markers, such as lactoferrin, lysozyme, or cytokeratin 5 (21). Unfortunately, the available antibodies did not work for immunocytochemistry in rat, nor were they specific for the ID, at least in our experimental conditions. Thus, to identify these cells, we used a morphological approach based on the fact that acini, ID, and large ducts have very distinctive features (40). The isolated glands were processed for immunocytochemistry and probed with fluorescently labeled phalloidin to label the actin cytoskeleton. Actin in the salivary gland epithelium is localized primarily in the apical domain, lining the acinar canaliculi and the ductal system (Fig. 4A). In large ducts, actin shows distinctive apical meshlike structures (Fig. 4A, inset 3, and Fig. 4B). In acini (Fig. 4A, insets 1 and 2) and in the ID (Fig. 4A, inset 1; see supplemental movie 1 in the online version of this article), actin uniformly labels the apical domain, highlighting the lumen of the acinar canaliculi and the ducts, respectively, which differ primarily in size (0.6 ± 0.1 μm for the canaliculi and 2.4 ± 0.7 μm for the ID, see materials and methods). Furthermore, in the acini, actin uniformly lined the subapical domain of the plasma membrane (Fig. 4A, inset 2), whereas in the ID, actin showed a wavy and more irregular pattern (Fig. 4A, inset 1). In the absence of rAd5, cells expressing pVenus faced the lumen of a 2.0 ± 0.4-μm-diameter duct, suggesting that the targeted cells were indeed in the ID (Fig. 4, C and F; see supplemental movie 2). In the presence of rAd5, pVenus was expressed in large ducts, on the basis of the characteristic actin structure and the size of the lumen (4.6 ± 2.3 μm; Fig. 4E), or in 0.9 ± 0.3-μm-diameter cells facing an actin-enriched lumen, consistent with acinar cells (Fig. 4D).
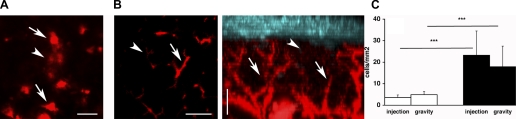
Fig. 5.
Plasmid DNA is internalized from the apical pole of salivary gland epithelium. A and B: SMGs of anesthetized rats were exposed, and 5 μg of CX-rhodamine-labeled pVenus (red) were administered into Wharton's duct by injection (A) or gravity infusion (B). Glands were imaged by TPM using a ×20 water immersion objective and excitation at 930 nm. Single sections (A and B, left) and volume rendering (B, right) are shown. Scale bars, 20 μm. A: labeled DNA is in ducts (arrows) and diffused in stroma (arrowhead). B: labeled DNA is retained in ductal system (arrow) and in acinar canaliculi (arrowhead). C: 12 μg of plasmid DNA encoding for pVenus were administered by injection or gravity infusion in the absence (open bars) and presence (solid bars) of rAd5. After 16 h, the number of cells expressing pVenus was scored as described in Fig. 2 legend. Values are means ± SE of 3 independent experiments. Statistical significance was calculated using t-test: ***P < 0.001.
Fig. 3.
Two-photon analysis of exposed SMGs reveals that pVenus is expressed in large ducts when administered with rAd5. A: schematic diagram of SMG structure. Main secretory units are acini (Ac), which constitute 63% (volume) of SMG parenchyma. Ductal system is formed by intercalated ducts (ID, 2% vol), which join granular convoluted ducts (GCD, 18% volume) and then striated ducts (SD, 4% volume). GCD and SD cells are characterized by intensive invaginations of basolateral plasma membrane and high content of mitochondria. Myoepithelial cells (Myo) wrap acini and ID. B: hematoxylin-eosin-stained rat SMGs. Acini (shown by dotted line and arrow) and large ducts (arrowhead) are clearly visible. C: architecture of exposed glands revealed by TPM (740-nm excitation). Images were acquired at 20 μm (left) and 50 μm (right) below the surface of the glands using a ×60 water immersion objective (Olympus). Acini (shown by dotted line and arrows) and large ducts (arrowhead) are shown. D and E: TPM images of glands 16 h after administration of plasmid DNA in the absence (D) and presence (E) of rAd5. The same field of view was imaged using 740-nm excitation to reveal parenchyma (red at left and gray at right) and then using 930-nm excitation to reveal pVenus-expressing cells (green at left). In the absence of rAd5 (D), pVenus was expressed in cells (arrows) located outside acini (dotted line) and large ducts (arrowheads). In the presence of rAd5 (E), pVenus was expressed in cells (arrows) located in large ducts (top, arrowheads) or in acini (bottom, dotted line). Scale bars, 50 μm.
Fig. 4.
Immunocytochemistry of exposed rat SMGs reveals that pVenus is expressed in ID when administered in the absence of rAd5. Rat SMGs were left untreated (A and B) or injected with plasmid DNA in the absence (C and F) and presence (D and E) of rAd5. At 16 h after injection, glands were processed for immunocytochemistry to label actin and imaged by confocal microscopy. A and B: maximal projections of a series of consecutive stacks of rat SMGs labeled with Alexa 488-phalloidin (in red) and imaged by confocal microscopy. A: low-magnification image. Scale bar, 50 μm. Inset 1: actin lines subapical domain of ID, showing a complex pattern (ID, arrowheads). Inset 2: actin lines subapical domain of narrow acinar canaliculi (Ac, arrows). Inset 3: actin staining in a cross section of a large duct (GCD/SD, *). B: side view of a large duct showing typical apical meshlike organization of actin. Scale bar, 10 μm. C–E: tetramethylrhodamine isothiocyanate-phalloidin staining (red) of SMGs transduced with pVenus (green) in the absence (C) and presence (D and E) of rAd5. C: pVenus-expressing cell (arrow) faces lumen of ID (arrowhead). In the same field of view, an acinus (Ac) and a large duct (GCD/SD) are shown. Right: high-magnification view of cell facing ID (arrowhead). D: pVenus-expressing cells (arrows) facing lumen of acinar canaliculi (arrowheads). Right: high-magnification view. E: pVenus-expressing cells facing lumen of a large duct (*). Scale bars, 10 μm. F: isosurface derived from volume rendering of cell shown in C using Imaris 6.2 (Bitplane). ID are in white, acinar canaliculi in red, and pVenus-expressing cell in green (see supplemental movie 2).
Next, we checked whether, under our experimental conditions, the expression of pVenus was affected by the integrity of the transepithelial barrier in the ductal system. Indeed, it has been shown that fluid injected via syringe into Wharton's duct induces an increase in the intraductal pressure, which leads to a transient impairment of the transepithelial barrier, which in turn allows molecules to access the basolateral side of the epithelium and the stroma (44). Plasmid DNA was labeled with CX-rhodamine and injected through Wharton's duct in the presence or absence of rAd5. Under these conditions, we noted that the labeled DNA filled ductal structures and acinar canaliculi but also leaked into the stroma (Fig. 5A; see supplemental movie 3). Thus we used an alternative and more gentle way to administer molecules through Wharton's duct, namely, slow diffusion mediated by gravity (25). Under these conditions, the plasmid DNA was retained in the ductal system (Fig. 5B) and in the acinar canaliculi (Fig. 5B) without leakage into the stroma. When the plasmid encoding for pVenus was administered by slow diffusion in the presence or absence of rAd5, the efficiency of pVenus expression was comparable to that obtained by injection of the plasmid with the syringe (Fig. 5C), strongly indicating that the transient disruption of the transepithelial barrier does not affect the expression of plasmid DNA.
Exploiting compensatory endocytosis to enhance the expression of the plasmid DNA in acinar cells.
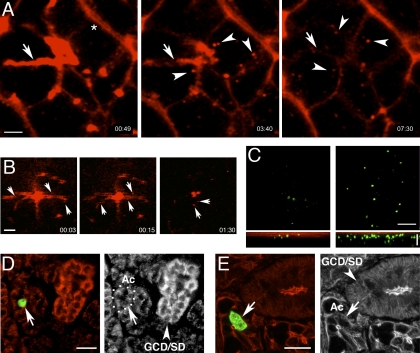
We have shown that plasmid DNA can be selectively targeted to the ID or to the large ducts and, to a smaller extent, to the acini. We sought to determine whether the uptake of plasmid DNA into the acini could be enhanced. It has been suggested that, during exocytosis, membranes delivered during the release of the secretory granules are retrieved by compensatory endocytosis (3, 13, 36, 42). To test whether this process occurs in rat SMGs, we administered, by gravity, a fluorescently labeled 10-kDa dextran in the presence or absence of isoproterenol, an agonist of the β2-adrenergic receptor, which regulates stimulated exocytosis (5, 8, 17, 31). Strikingly, in the isoproterenol-treated glands, dextran was internalized in vesicular structures from the apical side (Fig. 6A; see supplemental movie 4); in control glands, the uptake was minimal (not shown). Although the 10-kDa dextran diffused from the tight junction to the basolateral side of the acini because of its small size (4 nm diameter), we did not observe internalization from there (Fig. 6A; see supplemental movie 4). Remarkably, labeled DNA was also internalized from the apical pole of the acini when injected in the presence of isoproterenol (Fig. 6B). To check whether, under these conditions, genes were expressed in acinar cells, plasmid DNA was injected into Wharton's duct by syringe or by slow diffusion with subcutaneous administration of isoproterenol. Strikingly, regardless of the modality of administration, pVenus was expressed primarily in acinar cells in the animals injected with isoproterenol (Fig. 6, D and E). The level of expression increased three- to fourfold with respect to control animals (Figs. 6C and 7A) and was homogeneous throughout the tissue (see supplemental Fig. 2C). _Although the number of cells expressing pVenus was maximal at 0.5 mg/kg isoproterenol (6- to 7-fold), this concentration was not tolerated by the animals; thus we subsequently used 0.25 mg/kg isoproterenol (Fig. 7B). The expression of pVenus was transient (Fig. 7C), and it was not stimulated by muscarininc or α1-adrenergic receptors, as shown by the lack of effect of carbachol, pilocarpine, or phenylephrine (Fig. 7D). Furthermore, the isoproterenol-dependent stimulation was completely inhibited by pretreatments with an antagonist of the β-adrenergic receptors (propranolol) and only mildly affected by an antagonist of the muscarinic receptors (atropine; Fig. 7E). Isoproterenol has been previously described to transiently modify the permeability of the tight junctions up to 6 h when injected into the rat SMGs (16, 25). To determine whether the increase in pVenus expression could be linked to this phenomenon, rather than to compensatory endocytosis, we injected isoproterenol and then administered the plasmid DNA after 1, 4, and 8 h. At 16 h after the DNA injection, the glands were imaged, and pVenus was expressed in cells only when isoproterenol was administered together with the plasmid DNA (not shown).
Fig. 6.
Plasmid DNA is internalized by isoproterenol-stimulated compensatory endocytosis. A and B: SMGs of anesthetized animals were exposed, and 5 μg of 10-kDa Texas Red-dextran (A) or 5 μg of CX-rhodamine-labeled pVenus (B) were administered by gravity. Glands were injected with 0.25 mg/kg isoproterenol, and time-lapse images were obtained by intravital TPM using a ×60 water immersion objective at 930-nm excitation. A: part of 10-kDa dextran is cleared from the duct as a result of isoproterenol-stimulated secretion; remaining dextran is internalized in vesicular structures, which appear from the apical domain (arrowheads, see also supplemental movie 4). Note dextran labeling basolateral domain (*) and the absence of basolateral-derived vesicles. B: labeled DNA is cleared from the duct and internalized in small vesicles from the apical pole upon isoproterenol treatment (arrows). Scale bars, 5 μm. C: plasmid DNA transduction is increased upon isoproterenol stimulation of compensatory endocytosis. After a subcutaneous injection of saline (left) or isoproterenol (right), 12 μg of a plasmid DNA encoding for pVenus were injected into Wharton's ducts of anesthetized rats. After 16 h, SMGs were imaged by TPM to detect cells expressing pVenus, as described in Fig. 1 legend. Bottom: volume rendering of transduced SMGs. Scale bars, 100 μm. D and E: injection of 12 μg of plasmid DNA into SMGs was followed by subcutaneous injection of 0.25 mg/kg isoproterenol. After 16 h, glands were exposed and imaged by TPM (D) or processed for immunocytochemistry to label actin and imaged by confocal microscopy (E), as described in Fig. 3 and 4 legends. Arrowheads, pVenus-expressing cells; dotted lines, acini. Scale bars, 50 μm.
Fig. 7.
Characterization of isoproterenol-stimulated plasmid DNA transduction in rat SMGs. A–D: 12 μg of plasmid DNA (open bars and symbols) were administered by injection (A–D) or by gravity infusion (A), as described in Fig. 6 legend. Isoproterenol (ISOP) or saline (as control) was injected subcutaneously. After 16 h, SMGs were dissected and imaged by TPM, as described in Fig. 1 legend. Number of cells expressing pVenus were scored and reported as number of cells per mm2. A: after administration of plasmid DNA, isoproterenol was injected (0.25 mg/kg sc). Regardless of modality of DNA administration, isoproterenol (solid bars) induced a 3-fold increase in the level of pVenus expression compared with control (open bars). Values are means ± SE of 11 independent experiments. ***P < 0.001. B: isoproterenol dose response. Different doses of isoproterenol were injected subcutaneously. C: time course of pVenus expression in SMGs. Plasmid DNA was injected with 0.25 mg/kg isoproterenol (pVenus + ISOP) or saline (pVenus), as described above, and SMGs were imaged at 8, 16, 30, 48, and 72 h after injection to count the number of cells expressing pVenus. D: stimulation of muscarinic and adrenergic receptors. Injections of plasmid DNA were followed by subcutaneous injections of agonists of different receptors: 0.25 mg/kg isoproterenol, 0.5 mg/kg pilocarpine (PILO), 5 μg/kg carbachol (CARB), and 100 μg/kg phenylephrine (PHEN). Increase in the level of transduction was observed only upon stimulation by isoproterenol. E: inhibition of isoproterenol-dependent stimulation. Before injection of plasmid DNA and isoproterenol, rats were treated with 0.5 mg/ml atropine (ATROP) or 25 mg/ml propranolol (PROP). In B–E, values are means ± SE of 3 independent experiments. In D and E, statistical significance was calculated using t-test: ***P < 0.001, +++P < 0.001.
Markers for various subcellular compartments can be expressed and properly targeted in the rat SMGs, enabling dynamic imaging of subcellular compartments.
Finally, we wanted to check whether, using the approaches described in this study, genes other than pVenus could be expressed, properly targeted, and imaged dynamically in rat SMGs. We injected various plasmids encoding proteins implicated in different aspects of membrane trafficking, such as the coat protein clathrin (Fig. 8, A and B), the trans-Golgi protein TGN38 (Fig. 8, C and D), the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein vesicle-associated membrane protein VAMP8, the small GTPase Rab-5, and the water channel aquaporin-5 (not shown). GFP-clathrin, a coat protein involved in endocytosis and in trafficking from the trans-Golgi network (TGN), was, indeed, localized at the plasma membrane and in the perinuclear area, as previously described (Fig. 8, A and B; see supplemental movie 5) (35), whereas TGN38-Venus was localized in the typical perinuclear tubulovesicular structure described in cell cultures (Fig. 8, C and D; see supplemental movie 6) (10) and colocalized with TGN46, another marker of the TGN (Fig. 8D). The overexpression of TGN38-Venus or GFP-clathrin did not alter the morphology of the TGN (Fig. 8, B and D) or the distribution of endogenous clathrin (not shown). Furthermore, we coexpressed GFP-clathrin and TGN38-mCherry (Fig. 8E). The two molecules were found closely associated, and their pattern was very similar to that of endogenous clathrin and TGN46 (Fig. 8F). Strikingly, the dynamics of the subcellular compartments labeled by these two molecules and their three-dimensional organization could be imaged in the live animal (see supplemental movies 7 and 8, respectively). These data show that the procedures that we described in the present study can, indeed, be used for the expression of multiple molecules in live animals and that subcellular compartments highlighted by fluorescently tagged molecules can be imaged dynamically at a resolution similar to that of cell cultures.
Fig. 8.
Fluorescently tagged markers of various subcellular organelles can be expressed in SMGs and localize properly. A–E: 12 μg of a plasmid DNA encoding for GFP-clathrin (A and B), TGN38-Venus (C and D), or TGN38-mCherry and GFP-clathrin (E) were injected into Wharton's ducts of anesthetized rats in the presence of 3 × 1010 particles of rAd5. A, C, and E: at 16 h after the injection, SMGs from anesthetized rats were exposed and imaged by intravital TPM using a ×60 water immersion objective (NA 1.2, 930-nm excitation). Maximal projections of a series of 5 consecutive stacks of acinar cells expressing green fluorescent protein (GFP)-clathrin (A, green), TGN38-Venus (C, green), or both [E, GFP-clathrin (green) and TGN8-mCherry (red); see time-lapse sequences in supplemental movies 4 and 5]. B and D: SMGs were excised, processed for immunocytochemistry, labeled for TGN46 (red), a marker for the trans-Golgi network (TGN), and imaged by confocal microscopy. B: GFP-clathrin (green) is associated with endogenous TGN46 (red). D: TGN38-Venus colocalizes with endogenous TGN46 (red). F: control nontransfected gland stained for endogenous clathrin (green) and TGN46 (red). Scale bars, 10 μm.
DISCUSSION
The ability to express genes in a specific cell population of an adult animal, combined with the ability to perform intravital imaging, provides a powerful tool to study a variety of biological processes in the proper physiological context. The use of fluorescently tagged molecules labeling specific subcellular compartments, combined with the use of dominant-negative mutants, siRNA, or shRNA, would enable us to perform, in vivo, a detailed molecular analysis that is achievable only in cell cultures. Prompted by our interest in developing a fast method to express genes in the salivary glands of live rodents, we have characterized a variety of approaches based on the use of plasmid DNA. These approaches offer the possibility to perform large screenings of molecules, since they do not involve genetically modified animals or the generation of viral vectors and the preparation of viral particles, which can be time consuming.
Although in rat SMGs the use of plasmid DNA alone or in combination with rAd5 has been described, neither the efficiency of transduction nor the cells expressing the transgenes were determined (11, 18, 29, 30). Here, we show three different strategies to transduce plasmid DNA to specific compartments of the rat SMGs: 1) the use of plasmid DNA alone, which enables the expression of the reporter gene in the ID; 2) the use of plasmid DNA mixed with rAd5 particles, which facilitates the expression primarily in large ducts and, to a lesser extent, in acini; and 3) the use of plasmid DNA in combination with isoproterenol-stimulated exocytosis, which enables targeting to primarily acini. Although we have not rigorously investigated the mechanisms regulating the transduction of plasmid DNA under the conditions tested in this study, our data suggest that, at least in the presence of isoproterenol, compensatory endocytosis might be the likely process utilized for the transduction in acinar cells. Other endocytic pathways might be implicated in the uptake of plasmid DNA in the presence and absence of rAd5.
Furthermore, our study shows that any fluorescently tagged molecules can be rapidly expressed and imaged in live animals at a high resolution. Indeed, we have shown that the dynamics and the three-dimensional organization of large subcellular compartments, such as the TGN, and smaller compartments, such as clathrin endocytic vesicle and endosomes, can be studied in live animals. This implies that the same approaches routinely utilized in cell cultures can be applied in a more physiological context.
In conclusion, we have shown a very powerful approach to selectively target genes to the various components of rat SMGs. Although the efficiency of transduction is too low to be used for gene therapy (1–2% of the cells of the parenchyma), the absolute number of cells expressing a transgene that can be accessed and imaged is very high (102–103). This, combined with the ability to image a large number of samples in a reasonable amount of time, will enable the study of many aspects of the physiology of the salivary glands in live animals and, more generally, will allow elucidation of the molecular machinery of key cellular processes, such as membrane trafficking, dynamics of the cytoskeleton, and signal transduction. The use of plasmid DNA under the three conditions explored here can be extended to organs that allow direct access through a major duct, such as the other exocrine glands (parotid, lacrimal glands, and pancreas). Furthermore, future studies that characterize the delivery of siRNA or shRNA will enable the ablation of genes in specific cell populations.
GRANTS
This research was supported by the National Institute of Dental and Craniofacial Research Intramural Research Program.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Julie Donaldson, Dr. Silvio Gutkind, and Sarah Lamb for critical reading of the manuscript and all the members of the Oral and Pharyngeal Cancer Branch for invaluable assistance.
REFERENCES
- 1.Adriaansen J, Perez P, Goldsmith C, Zheng C, Baum BJ. Differential sorting of human parathyroid hormone after transduction of mouse and rat salivary glands. Hum Gene Ther 19: 1021– 1028, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccaglini L, Shamsul Hoque AT, Wellner RB, Goldsmith CM, Redman RS, Sankar V, Kingman A, Barnhart KM, Wheeler CJ, Baum BJ. Cationic liposome-mediated gene transfer to rat salivary epithelial cells in vitro and in vivo. J Gene Med 3: 82– 90, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Barg S, Machado JD. Compensatory endocytosis in chromaffin cells. Acta Physiol (Oxf) 192: 195– 201, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, Chiorini JA, Voutetakis A, Leakan RA, Van Waes C, Mitchell JB, Delporte C, Wang S, Kaminsky SM, Illei GG. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta 1758: 1071– 1077, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Castle JD. Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann NY Acad Sci 842: 115– 124, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Delporte C, Miller G, Kagami H, Lillibridge CD, O'Connell BC, Atkinson JC, Baum BJ. Safety of salivary gland-administered replication-deficient recombinant adenovirus in rats. J Oral Pathol Med 27: 34– 38, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905– C916, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Fujita-Yoshigaki J, Tagashira A, Yoshigaki T, Furuyama S, Sugiya H. A primary culture of parotid acinar cells retaining capacity for agonists-induced amylase secretion and generation of new secretory granules. Cell Tissue Res 320: 455– 464, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Otin AL, Guillou F. Mammalian genome targeting using site-specific recombinases. Front Biosci 11: 1108– 1136, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Girotti M, Banting G. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for rat TGN38. J Cell Sci 109: 2915– 2926, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Goldfine ID, German MS, Tseng HC, Wang J, Bolaffi JL, Chen JW, Olson DC, Rothman SS. The endocrine secretion of human insulin and growth hormone by exocrine glands of the gastrointestinal tract. Nat Biotechnol 15: 1378– 1382, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res 84: 500– 509, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51: 773– 786, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Green M, Pina M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology 20: 199– 207, 1963 [DOI] [PubMed] [Google Scholar]
- 15.Green M, Pina M, Kimes R, Wensink PC, MacHattie LA, Thomas CA., Jr Adenovirus DNA. I. molecular weight and conformation. Proc Natl Acad Sci USA 57: 1302– 1309, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto S, Murakami M, Kanaseki T, Kobayashi S, Matsuki M, Shimono M, Segawa A. Morphological and functional changes in cell junctions during secretory stimulation in the perfused rat submandibular gland. Eur J Morphol 41: 35– 39, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Herzog V, Farquhar MG. Luminal membrane retrieved after exocytosis reaches most Golgi cisternae in secretory cells. Proc Natl Acad Sci USA 74: 5073– 5077, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honigman A, Zeira E, Ohana P, Abramovitz R, Tavor E, Bar I, Zilberman Y, Rabinovsky R, Gazit D, Joseph A, Panet A, Shai E, Palmon A, Laster M, Galun E. Imaging transgene expression in live animals. Mol Ther 4: 239– 249, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458: 524– 528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami S, Higuchi Y, Hashida M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci 97: 726– 745, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Korsrud FR, Brandtzaeg P. Characterization of epithelial elements in human major salivary glands by functional markers. J Histochem Cytochem 30: 657– 666, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 10: 935– 940, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic 9: 1801– 1810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastrangeli A, O'Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol Gastrointest Liver Physiol 266: G1146– G1155, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Mazariegos MR, Tice LW, Hand AR. Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol 98: 1865– 1877, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445– 469, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87– 90, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 93: 11382– 11388, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niedzinski EJ, Chen YJ, Olson DC, Parker EA, Park H, Udove JA, Scollay R, McMahon BM, Bennett MJ. Enhanced systemic transgene expression after nonviral salivary gland transfection using a novel endonuclease inhibitor/DNA formulation. Gene Ther 10: 2133– 2138, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Niedzinski EJ, Olson DC, Chen YJ, Udove JA, Nantz MH, Tseng HC, Bolaffi JL, Bennett MJ. Zinc enhancement of nonviral salivary gland transfection. Mol Ther 7: 396– 400, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Peter B, Van Waarde MA, Vissink A, s-Gravenmade EJ, Konings AW. Degranulation of rat salivary glands following treatment with receptor-selective agonists. Clin Exp Pharmacol Physiol 22: 330– 336, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Rothstein EC, Nauman M, Chesnick S, Balaban RS. Multi-photon excitation microscopy in intact animals. J Microsc 222: 58– 64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuni Y, Zheng C, Cawley NX, Cotrim AP, Loh YP, Baum BJ. Sorting of growth hormone-erythropoietin fusion proteins in rat salivary glands. Biochem Biophys Res Commun 373: 136– 139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandoval RM, Molitoris BA. Quantifying endocytosis in vivo using intravital two-photon microscopy. Methods Mol Biol 440: 389– 402, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem 66: 511– 548, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Segawa A, Loffredo F, Puxeddu R, Yamashina S, Testa Riva F, Riva A. Exocytosis in human salivary glands visualized by high-resolution scanning electron microscopy. Cell Tissue Res 291: 325– 336, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Seth P, Rosenfeld M, Higginbotham J, Crystal RG. Mechanism of enhancement of DNA expression consequent to cointernalization of a replication-deficient adenovirus and unmodified plasmid DNA. J Virol 68: 933– 940, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shai E, Falk H, Honigman A, Panet A, Palmon A. Gene transfer mediated by different viral vectors following direct cannulation of mouse submandibular salivary glands. Eur J Oral Sci 110: 254– 260, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther 10: 964– 976, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Tamarin A, Sreebny LM. The rat submaxillary salivary gland. A correlative study by light and electron microscopy. J Morphol 117: 295– 352, 1965 [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Baum BJ, Yamano S, Mankani MH, Sun D, Jonsson M, Davis C, Graham FL, Gauldie J, Atkinson JC. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res 79: 701– 708, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci 9: 1019– 1027, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med 204: 489– 495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida Y, Takai N, Uchihashi K, Kakudo Y. Sialographic damage in rat submandibular gland. Oral Surg Oral Med Oral Pathol 59: 426– 430, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.