Abstract
Cdc42GAP (GTPase activating protein) has been shown to regulate smooth muscle contraction as well as cell motility, adhesion, proliferation, and apoptosis. We have recently shown that Cdc42GAP activity is suppressed in smooth muscle cells during contractile activation, which is reversed by inhibitors of reactive oxygen species (ROS). Because p47phox, a regulatory subunit of NAD(P)H oxidase, has been implicated in smooth muscle signaling, we determined whether this subunit modulates Cdc42GAP activity in response to contractile stimulation. Transfection of smooth muscle cells with plasmids encoding short hairpin RNA (shRNA) against p47phox, but not plasmids for luciferase shRNA, inhibited the expression of p47phox. ROS production and the suppression of Cdc42GAP activity in response to stimulation with 5-hydroxytryptamine (5-HT) were attenuated in cells producing p47phox shRNA compared with cells producing luciferase shRNA. In contrast, the addition of hydrogen peroxide to p47phox-deficient cells suppressed the activity of Cdc42GAP. Furthermore, exposure to hydrogen peroxide led to a decrease in Cdc42GAP activity in an in vitro assay. Cdc42 activation, p21-activated kinase 1 (PAK1) phosphorylation at Thr-423 (an indication of PAK activation), and vimentin phosphorylation at Ser-56 in response to 5-HT activation were also attenuated in smooth muscle cells producing shRNA against p47phox. The knockdown of p47phox inhibited smooth muscle contraction during stimulation with 5-HT but not hydrogen peroxide. These results suggest that the p47phox subunit of NAD(P)H oxidase may mediate the agonist-induced GAP suppression by controlling ROS generation in smooth muscle cells during agonist stimulation. p47phox-regulated GAP affects smooth muscle contraction likely through the Cdc42/PAK1/vimentin pathway.
Keywords: contractile activation, 5-hydroxytryptamine, reactive oxygen species, cytoskeleton and small GTPases
smooth muscle contraction plays a fundamental role in regulating the functions of the hollow organs in the body such as the airways and blood vessels. Abnormal smooth muscle contraction is a key pathological feature of many diseases such as asthma and hypertension. Despite its important role, the mechanisms that regulate smooth muscle contraction are not completely understood. Actomyosin cross-bridge cycling has been thought as a sole mechanism that controls smooth muscle contraction (18). However, recent studies suggest that the vimentin intermediate filament network is also important for smooth muscle contraction. The depletion of vimentin by gene targeting or antisense oligodooxynucleotides attenuates contraction in smooth muscle cells/tissues (14, 34, 45). Vimentin deficiency also impairs the contractile capacity of nonmuscle cells such as fibroblasts (6). Furthermore, contractile stimulation triggers vimentin phosphorylation at Ser-56 in association with force development in smooth muscle (25, 37, 46). Vimentin phosphorylation in various cell types including smooth muscle cells is mediated by p21-activated kinase (PAK) (3, 25, 37, 46); depletion of PAK inhibits vimentin phosphorylation and smooth muscle contraction (25, 46).
It is known that PAK is a downstream effector of the small GTPase Cdc42, which is regulated by Cdc42 GTPase activating protein (GAP) (3, 24, 25, 37, 46). Our recent studies show that overexpression of Cdc42GAP inhibits Cdc42 activation, PAK phosphorylation, vimentin phosphorylation, and contraction in smooth muscle cells (24), suggesting that Cdc42GAP is an important upstream regulator of the vimentin network and force development. More importantly, the activity of Cdc42GAP is regulated in smooth muscle cells during contractile stimulation. For instance, stimulation with 5-hydroxytryptamine (5-HT), a well-known contractile agonist, results in the suppression of Cdc42GAP activity in smooth muscle cells (24). Furthermore, the agonist-induced GAP suppression is mediated by intracellular reactive oxygen species (ROS) that have been implicated in cell signaling in various cell types including smooth muscle cells (11, 22, 47). Treatment with pharmacological inhibitors of ROS restores GAP activity in smooth muscle cells during contractile activation (24). However, it is largely unknown how the contractile agonist modulates the production of ROS and thus Cdc42GAP in smooth muscle.
p47phox is a regulatory subunit of NAD(P)H oxidase that has been proposed to regulate ROS production in smooth muscle cells (11, 22). p47phox may play a role in controlling arterial smooth muscle contraction; knockout of this subunit in mice attenuates the ANG II-induced increase in arterial pressure (20, 23). However, the role of p47phox in regulating Cdc42GAP and its downstream events in smooth muscle cells is not well defined.
The objective of this investigation is to determine whether p47phox knockdown by RNA interference affects the activity of Cdc42GAP, Cdc42 activation, PAK phosphorylation, vimentin phosphorylation, and contraction during 5-HT activation. Our results suggest that the p47phox subunit of NAD(P)H oxidase plays a pivotal role in regulating the activity of Cdc42GAP and the GAP-mediated process during contractile activation of smooth muscle cells.
MATERIALS AND METHODS
Cell culture.
Canine tracheal smooth muscle cells were prepared and cultured using the method we previously described (25, 37). All animal protocols were approved by the Institutional Animal Care and Usage Committee.
Construction of plasmids encoding shRNA and cell transfection.
To construct plasmids encoding p47phox short hairpin RNA (shRNA), two sets of oligonucleotides were synthesized by Invitrogen (Carlsbad, CA). The sense sequences of p47phox shRNA 1 and shRNA 2 are 5′-CCATCGAAGTCATTCATAA-3′ and 5′-GCACTACGTCTACATGTTT-3′ (accession no. XM_844481), respectively. Oligonucleotides were ligated with pGSH1-mCherry (red fluorescence protein) plasmids (modified from pGSH1-GFP, Gene Therapy Systems, San Diego, CA) at BamH1 and NotI sites. The plasmids contain H1 RNA polymerase III promoter for shRNA production and CMV promoter for mCherry transcription. As a control, oligonucloetides (available from Gene Therapy Systems) for luciferase were ligated with the plasmids. These constructs were transformed into Eschericia coli (XL1-blue). E. coli carrying various cDNA constructs was grown in LB broth overnight. The bacterial cells were harvested by centrifugation at 3,430 g for 15 min at 4°C. The plasmids were purified using the Midiprep kit (Qiagen, Valencia, CA). Smooth muscle cells were transfected with plasmids using lipofectamine and plus reagents (Invitrogen) according to the manufacture's protocol. Cells were cultured in the medium for 2 days and placed in the serum-free medium 1 day before treatment.
Baculoviruses production, amplification, and recombinant protein expression and purification.
Recombinant Cdc42GAP and Cdc42 were expressed in a baculovirus expression system. Briefly, cDNAs encoding these proteins were subcloned into the baculovirus expression vector pDEST10 (Invitrogen). Recombinant baculoviruses were generated in the monolayer of Sf21 cells (Invitrogen). Viral stocks recovered after two cycles of amplification (3 days culture/cycle) were used to produce high yields of viruses. For expression of recombinant proteins, High Five cells (5 × 106 cells per 100-mm dish, Invitrogen) were infected with 0.25 ml (multiplicity of infection = 5) of viral stocks. Three days after infection, cells were harvested by centrifugation at 1,000 g for 5 min at 4°C. The cell pellet was washed with PBS (pH 7.4) and then suspended in 1 ml of ice-cold lysis buffer (10 mM Tris·HCl, pH 7.5, 500 mM NaCl, 0.1% Nonidet P-40, 10% glycerol, 2 mM PMSF, 15 mM imidazole, 2 mM β-mercaptoethanol, 20 μg/ml aprotinin, and 20 μg/ml leupeptin) and incubated on ice for 15 min. Cell debris was removed after centrifugation at 10,000 g for 10 min. The supernatant was loaded to preequilibrated Ni-NAT spin column (Qiagen). The loaded column was washed twice using wash buffer (10 mM Tris·HCl, pH 7.5, 200 mM NaCl, 0.2% Nonidet P-40, 10% glycerol, 2 mM PMSF, 15 mM imidazole, and 2 mM β-mercaptoethanol). Proteins were then eluted using buffer containing 10 mM Tris·HCl, pH 7.5, 100 mM NaCl, 0.1% Nonidet P-40, 10% glycerol, 2 mM PMSF, 250 mM imidazole, and 2 mM β-mercaptoethanol. The eluted protein was dialyzed against buffer (20 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1 mM DTT, 1 mM NaN3, and 40% glycerol) and stored at −20°C.
Immunoprecipitation and immunoblot analysis.
Cells were treated with lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, pH 8.3, 1 mM PMSF, 0.5 mM aprotinin, 2 mM benzamidine, 2 mM sodium pyrophosphate, 2 mM molybdate, and 2 mM sodium orthovanadate) at 4°C for 1 h. Supernatant was collected after centrifugation for 15 min at 13,600 g and then incubated with 50 μl of 10% protein A-Sepharose (Sigma, St. Louis, MO) for 30 min. Precleared samples were incubated overnight at 4°C with Cdc42GAP antibody (custom made by Biosynthesis, Dallas, TX; peptide sequence: SDDSKSSSPEPVTHLKWDD) followed by the addition of 10% protein A-Sepharose for 3 h. The entire immunoprecipitation procedure was performed at 4°C. Samples were washed three times in Tris-buffered saline. Immunoblot analysis was carried out using the procedures described previously (25, 37). Phospho-PAK1 (Thr-423)/PAK2 (Thr-403) antibody and PAK1 antibody were purchased from Cell Signaling (Beverly, MA). Phospho-vimentin (Ser-56) antibody was custom made by SynPep (Dublin, CA) (37). Vimentin antibody (clone, RV202) and Cdc42 antibody (clone, 21) were purchased from BD Biosciences (San Jose, CA) (25, 37). Polyclonal p47phox antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Analysis of small GTPase activation.
Activation of the small GTPase was determined using the pull-down assay as described previously (24, 38). Briefly, cells were mixed with lysis buffer containing 50 mM Tris, pH 7.5, 10 mM MgCl2, 0.3 mM NaCl, and 2% Nonidet P-40. The mixtures were centrifuged at 5,900 g for 5 min at 4°C. The resulting supernatant was reacted with PAK-PBD (p21-activated kinase binding domain) (Cytoskeleton, Denver, CO) beads in binding buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl, and 2% Nonidet P-40). GTP-bound Cdc42 (active) selectively binds to PAK-PBD beads. The beads were collected after centrifugation at 4,500 g for 3 min at 4°C and washed twice with wash buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, and 40 mM NaCl). The beads were boiled in SDS sample buffer [1.5% dithiothreitol, 2% SDS, 80 mM Tris, pH 6.8, 10% (vol/vol) glycerol, and 0.01% bromphenol blue] to release GTP-bound small GTPases, which was separated by 15% SDS-PAGE. Blots of the samples were probed using specific antibodies.
Assessment of Cdc42GAP activity.
Recombinant Cdc42 (2.5 μg) was preloaded with GTP in 10 μl of loading buffer containing 2 mM GTP, 20 mM Tris·HCl, pH 7.5, 25 mM NaCl, 0.1 mM DTT, and 5 mM EDTA for 10 min at 30°C. MgCl2 (20 mM) was added into the mixture to stop the loading, and the GTP-loaded Cdc42 was kept on ice for future use. For in vitro GAP assay, the reaction was initiated by adding the preloaded GTP-Cdc42 into solution containing 0.6 μM Cdc42GAP, 20 mM Tris, pH 7.5, 100 mM NaCl, and 0.2 mg/ml BSA. After incubation for 10 min, mixtures were transferred into ice-cold buffer (50 mM Tris, pH 7.5, 50 mM NaCl, and 5 mM MgCl2) and incubated with PAK-PBD beads at 4°C on a rotator for 2 h. GTP-bound Cdc42 was released from the beads and assessed by immunoblot analysis as described above.
For in vivo GAP assay, Cdc42GAP was immunoprecipitated from cell lysates with Cdc42GAP antibody. Samples were then washed three times in wash buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton X-100. The immune complex containing Cdc42GAP was resuspended in the reaction buffer (see above), and GTP-preloaded Cdc42 was added to the mixture at 20°C for 10 min. The amount of GTP-bound Cdc42 was then determined by the pull-down assay.
Determination of intracellular ROS production.
ROS generation in cells was estimated using the ROS fluorescent indicator 2′,7′-dichlorofluorescein diacetate (DCF-DA), which is cell permeable and not fluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within cells (24, 42). Smooth muscle cells were loaded with 10 μM DCF-DA (Invitrogen/Molecular Probes, Carlsbad, CA) for 30 min followed by washing, and intensity of the DCF signal was monitored and analyzed by fluorescent microscopy. The time of image capturing and intensity gaining in green (for DCF signal) and red (for mCheryy) channels were optimally adjusted and kept constant for all experiments to standardize the fluorescence intensity measurements among experiments. For each independent experiment, five cells were randomly selected for observation. The fluorescent intensity of the signals was calculated using Multi Gauge software (Fujifilm, Stamford, CT). Measurements from five cells were averaged for a single data point for each treatment.
Assessment of single cell contraction.
Confluent smooth muscle cells were released from dishes with trypsination (24, 29). Cells were resuspended in DMEM at a concentration of 3.6 × 105 cells/ml and were mixed with neutralized collagen solutions (2.5 mg/ml) at 1:1 ratio. The mixture of 30 μl containing ∼1,000 cells was plated onto glass-bottom dishes (MatTek, Ashland, MA). The dishes were then placed in a CO2 incubator at 37°C for 1 h. The polymerized mixtures were overlaid with 2 ml of DMEM containing 10% FBS and incubated in a CO2 incubator overnight. They were serum starved for 24 h before treatment with the contractile stimulus. Images of cells were taken before and after stimulation using a digital fluorescence microscope (Leica, Mannheim, Germany). The cell length was measured before and after various treatments using Multigauge software.
Statistical analysis.
All statistical analysis was performed using Prism 4 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way analysis of variance followed by post test (Tukey's multiple comparison test). Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
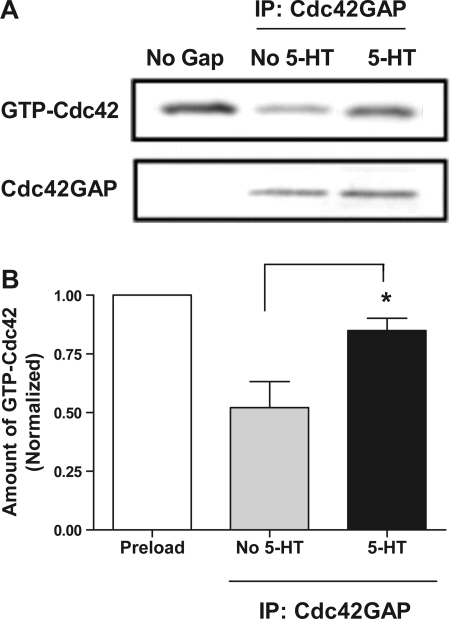
Stimulation with 5-HT attenuates the activity of Cdc42GAP in smooth muscle cells.
To evaluate whether contractile stimulation affects the activity of Cdc42GAP, cultured smooth muscle cells were exposed to 10 μM 5-HT for 15 min or they were unstimulated. Cdc42GAP was immunoprecipitated from cell extracts using Cdc42GAP antibody, and the activity of endogenous Cdc42GAP was determined using the method described in materials and methods.
Treatment with 5-HT led to a decrease in Cdc42GAP activity in smooth muscle cells. The amount of Cdc42 remaining bound to GTP after treatment with Cdc42GAP precipitates from unstimulated cells was relatively lower compared with preloaded Cdc42, suggesting a constitutive activity of Cdc42GAP in these cells (Fig. 1A). The amount of GTP-bound Cdc42 was higher in response to treatment of Cdc42GAP precipitates from stimulated cells, indicating a reduced activity of Cdc42GAP (Fig. 1A). The average amount of Cdc42 remaining bound to GTP after exposure to GAP precipitates from stimulated cells was significantly higher than that from unstimulated cells (Fig. 1B, P < 0.05, n = 4). These results were consist with our previous observation in smooth muscle cells (24).
Fig. 1.
Stimulation with 5-hydroxytryptamine (5-HT) suppresses the activity of Cdc42GAP in smooth muscle cells. A: Cdc42GAP immunoprecipitated from 5-HT (10 μM, 15 min) stimulated cells or unstimulated cells was added to preloaded Cdc42 for 10 min. GTPase activating protein (GAP) activity was then determined using the method described in materials and methods. Representative blots showing that higher amount of GTP-Cdc42 treated with Cdc42GAP precipitates from stimulated cells compared with unstimulated cells, suggesting that 5-HT stimulation inhibits the activity of Cdc42GAP in smooth muscle cells. B: amounts of GTP-Cdc42 treated with Cdc42GAP precipitates are normalized to the level of preloaded GTP-Cdc42 (without exposure to GAP immunoprecipitates). *Significantly higher GTP-Cdc42 levels after treatment with GAP precipitates from stimulated cells than the levels from unstimulated cells (P < 0.05). Values are means ± SE (n = 4).
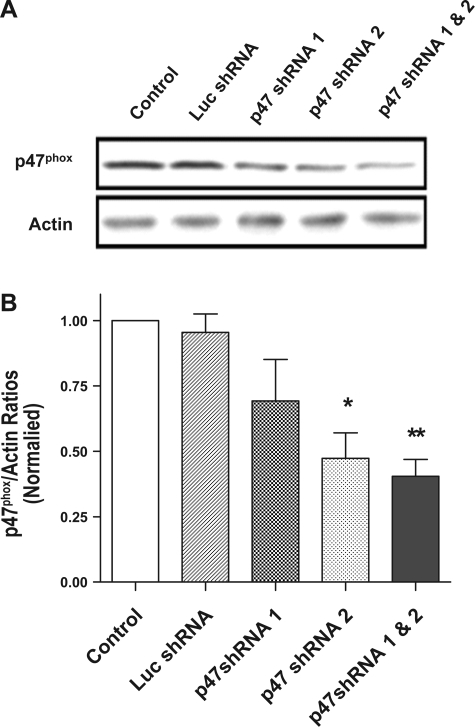
Transfection with plasmids encoding p47phox shRNA inhibits the expression of p47phox in smooth muscle cells.
Our recent studies show that the activity of Cdc42GAP is regulated by intracellular ROS (24). To determine the role of p47phox [a cytoplasmic subunit of NAD(P)H oxidase], we attempted to inhibit the expression of the subunit and then assess the effects of p47phox knockdown on Cdc42GAP activity. Cultured smooth muscle cells were transfected with plasmids encoding p47phox shRNA or luciferase shRNA (control) or they were untransfected. Immunoblot analysis was used to assess the expression of the protein in cells 72 h after transfection.
The expression of p47phox protein in smooth muscle cells is inhibited by RNA interference. In cells producing luciferase shRNA, the amount of p47phox was similar to that in cells treated with the empty vector. However, the level of the subunit was reduced in cells generating p47phox shRNA 1 or p47phox shRNA 2 compared with cells treated with the vector. Furthermore, the amount of p47phox was further reduced in cells cotransfected with plasmids encoding p47phox shRNA 1 and 2. The amount of smooth muscle α-actin was similar in cells under the different treatments (Fig. 2). Ratios of p47phox over α-actin in cells producing p47phox shRNA 2 or p47phox shRNA 1 and 2 were significantly lower than those in cells treated with the vector or in cells generating luciferase shRNA (P < 0.05, n = 4). Because cotreatment with p47phox shRNA 1 and p47phox shRNA 2 generated the best knockdown effect, the pooled shRNAs were used in subsequent studies.
Fig. 2.
Knockdown of p47phox gene in smooth muscle cells by short hairpin RNA (shRNA). A: blots of protein extracts from untransfected smooth muscle cells and cells transfected with plasmids encoding luciferase (Luc) shRNA, p47phox shRNA 1, p47phox shRNA 2, or p47phox shRNA 1 and 2 were probed with antibodies against p47phox and α-actin. The level of p47phox protein was lower in cells producing p47phox shRNAs than in untransfected cells and in cells expressing luciferase shRNA. However, the amount of α-actin was similar in all five treatments. B: ratios of p47phox over α-actin in cells producing various shRNAs were normalized to ratios obtained in untransfected cells. Values are means ± SE (n = 4). Significantly lower protein ratios in cells producing p47phox shRNA 2 or p47phox shRNA 1 and 2 compared with protein ratios in untransfected cells or in cells expressing luciferase shRNA (*P < 0.05, **P < 0.01).
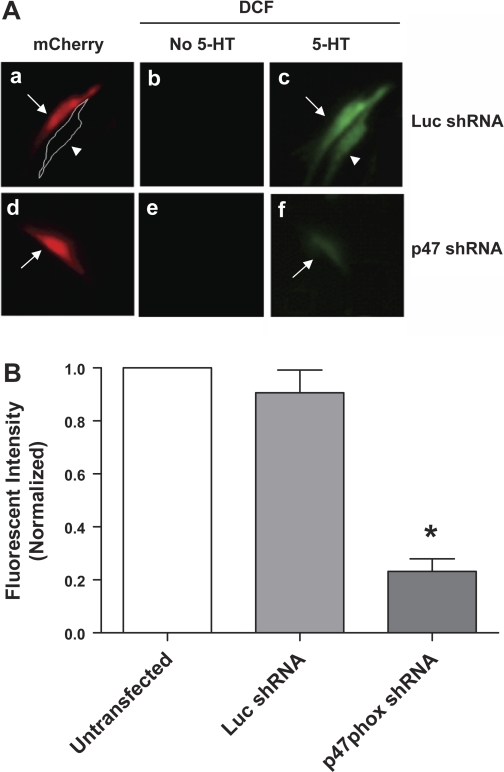
Knockdown of p47phox attenuates ROS production in smooth muscle cells during activation with 5-HT.
Contractile activation has been shown to induce ROS generation in smooth muscle cells (22, 24). To explore whether p47phox plays a role in ROS production in smooth muscle cells, we sought to determine whether p47phox knockdown affects ROS generation in cells using the ROS fluorescent indicator 2′,7′-dichlorofluorescein diacetate (DCF-DA), which is cell permeable and not fluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within cells (24, 42). Cells that had been transfected with plasmids encoding luciferase shRNA or p47phox shRNA were loaded with 10 μM DCF-DA for 30 min followed by washing, and intensity of the DCF signal was monitored and analyzed by fluorescent microscopy.
In untransfected cells and cells expressing luciferase shRNA, stimulation with 10 μM 5-HT for 15 min enhanced the intensity of the DCF signal. In contrast, the 5-HT-induced increase in the DCF signal was reduced in cells producing p47phox shRNA (Fig. 3). These results suggest that the silencing of p47phox diminishes the increase in the ROS generation in response to stimulation with 5-HT.
Fig. 3.
The generation of reactive oxygen species (ROS) induced by 5-HT is attenuated by the knockdown of p47phox. A: cells that had been transfected with plasmids encoding luciferase shRNA or p47phox shRNA were loaded with the ROS indicator 2′,7′-dichlorofluorescein diacetate (DCF-DA, 10 μM) for 30 min followed by washing. These cells were stimulated with 10 μM 5-HT for 10 min, or they were unstimulated. The red signal for mCherry and the green signal for DCF were monitored by fluorescent microscopy. a, two cells treated with plasmids encoding luciferase shRNA are shown. The red signal (mCherry) was detected in a cell suggesting successful transfection. Another cell was untransfected cell that did not display red fluorescence, and the shape of the cell was outlined by a white line. b, green signal (DCF) was barely detected in the two cells also shown in a, suggesting very little ROS production in these unstimulated cells. c, strong green fluorescence was observed in the two cells also shown in a, indicating that stimulation with 5-HT induces ROS production in untransfected cells and cells producing luciferase (Luc) shRNA. d, the cell treated with plasmids encoding p47phox shRNA displayed red fluorescence, suggesting effective transfection. e, the DCF signal was undetectable in the unstimulated cell also shown in d. f, the intensity of the DCF signal in the cell also shown in d was reduced in response to stimulation with 5-HT, suggesting that the 5-HT-stimulated ROS generation was inhibited in cells producing p47phox shRNA. Arrows, transfected cells; arrow heads, untransfected cells. B: intensities of the DCF signal from cells expressing Luc shRNA or p47phox shRNA are normalized to the intensity from untransfected cells. *Significantly lower values from cells expressing p47phox shRNA compared with intensities from untransteced cells and cells expressing Luc shRNA (n = 5, P < 0.01).
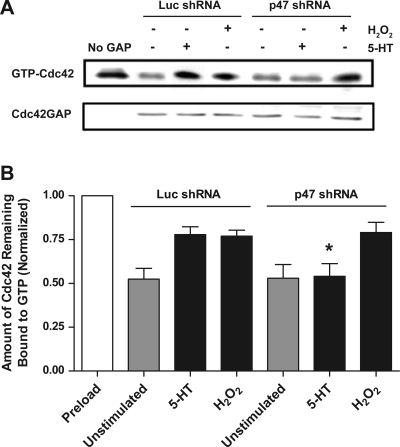
Silencing of p47phox reverses the suppression of Cdc42GAP activity in response to stimulation with 5-HT.
We evaluated the functional role of the p47phox subunit in the regulation of the agonist-mediated Cdc42GAP activity. Cells producing luciferase shRNA or p47phox shRNA were stimulated with 10 μM 5-HT for 15 min. The activity of Cdc42GAP was determined using the procedures described in materials and methods.
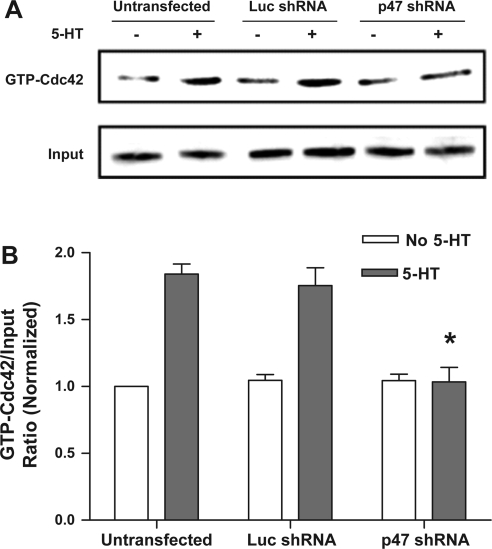
Knockdown of p47phox inhibits the 5-HT-induced suppression of Cdc42GAP in smooth muscle cells. In cells producing luciferase shRNA, the amounts of Cdc42 remaining bound to GTP during 5-HT stimulation were higher compared with unstimulated cells. The level of GTP-bound Cdc42 in response to 5-HT stimulation was reduced to near unstimulated level in cells expressing p47phox shRNA (Fig. 4A). Cdc42GAP activity during 5-HT stimulation in cells producing p47phox shRNA was significantly higher than in cells producing luciferase shRNA (Fig. 4B, P < 0.05, n = 4–5). Knockdown of p47phox did not affect the activity of Cdc42GAP before stimulation with 5-HT.
Fig. 4.
Knockdown of p47phox disinhibits the activity of Cdc42GAP during 5-HT stimulation but not exposure to hydrogen peroxide. Cells producing luciferase shRNA or p47phox shRNA were stimulated with 10 μM 5-HT or 100 μM hydrogen peroxide for 15 min, or they were not stimulated. Cdc42GAP was immunoprecipitated from these cells, and GAP activity was then assessed. A: representative immunoblots showing the effects of p47phox shRNA on GAP activity during stimulation with 5-HT. Hydrogen peroxide-induced GAP suppression was not affected by knockdown of p47phox. B: amounts of GTP-Cdc42 treated with Cdc42GAP precipitates from cells producing luciferase shRNA or p47phox shRNA are normalized to the level of preloaded GTP-Cdc42. *Significantly higher GAP activity in response to 5-HT stimulation in cells producing p47phox shRNA than in cells expressing luciferase shRNA (P < 0.05). Values are means ± SE (n = 3–4).
As a control, we also evaluated the effects of p47phox knockdown on GAP activity in response to stimulation with hydrogen peroxide. Cells transfected with plasmids encoding shRNAs were exposed to 100 μM hydrogen peroxide for 15 min or left not exposed. GAP activity of these cells was then determined. The level of Cdc42 remaining bound to GTP under exposure to hydrogen peroxide was similar in cells producing luciferase shRNA and cells expressing p47phox shRNA (Fig. 4, A and B).
The activity of Cdc42GAP is directly inhibited by hydrogen peroxide in vitro.
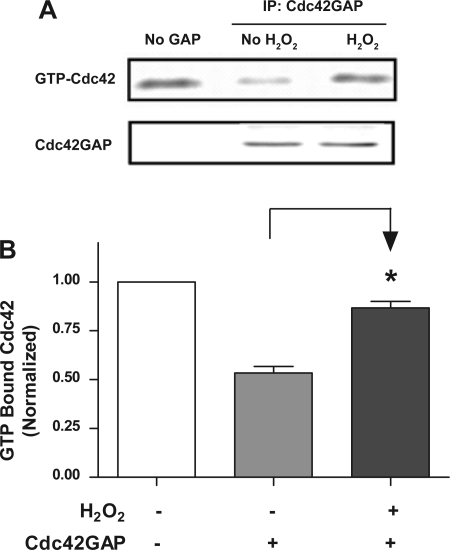
We evaluated the effects of hydrogen peroxide (a major ROS) on the GTP hydrolysis of Cdc42 by GAP in vitro. Cdc42GAP was pretreated with 100 μM hydrogen peroxide for 5–10 min, or it was not pretreated with hydrogen peroxide, after which GAP was reacted with preloaded Cdc42 for 10 min. The activity of Cdc42GAP was determined by using the GAP assay. The amount of Cdc42 remaining bound to GTP was lower in the presence of Cdc42GAP not pretreated with hydrogen peroxide, indicating that GAP enhances the transition of GTP-bound state to GDP-bound state (Fig. 5A), which is consistent with our previous results (24). However, the amount of GTP-bound Cdc42 exposed to GAP was increased in the presence of hydrogen peroxide (Fig. 5A). The average levels of GTP-Cdc42 after exposure to GAP pretreated with hydrogen peroxide were significantly higher than those exposed to GAP not pretreated with hydrogen peroxide (Fig. 5B, P < 0.05, n = 3). The results suggest that treatment with hydrogen peroxide directly inhibits Cdc42GAP activity.
Fig. 5.
Treatment with hydrogen peroxide inhibits the activity of Cdc42GAP in vitro. Cdc42GAP was pretreated with hydrogen peroxide for 5–10 min, or it was not pretreated with hydrogen peroxide. Afterwards, preloaded Cdc42 was exposed to Cdc42GAP for 10 min. The activity of Cdc42GAP was determined by using the experimental procedures described in materials and methods. A: representative blots illustrating the effects of hydrogen peroxide on GAP activity. The amount of GTP-Cdc42 was reduced after exposure to GAP not pretreated with hydrogen peroxide; however, the level of GTP-Cdc42 was increased when exposed to GAP pretreated with hydrogen peroxide. B: amounts of GTP-Cdc42 treated with Cdc42GAP are normalized to the level of preloaded GTP-Cdc42. *Significantly higher GTP-Cdc42 levels after treatment with GAP pretreated with hydrogen peroxide than those exposed to GAP not pretreated with hydrogen peroxide (P < 0.05). Values are means ± SE (n = 3).
The activation of Cdc42 induced by 5-HT is attenuated in p47phox-deficient cells.
To determine the role of p47phox in the regulation of Cdc42 activation, cells that had been transfected with plasmids encoding luciferase shRNA or p47phox shRNA were stimulated with 10 μM 5-HT for 15 min or left unstimulated. Cdc42 activation in these cells was determined using the pull-down assay. Stimulation with 5-HT triggered Cdc42 activation in untransfected cells and in cells expressing luciferase shRNA; however, Cdc42 activation during 5-HT stimulation was inhibited in cells producing p47phox shRNA (Fig. 6, P < 0.05, n = 3). Basal Cdc42 activity in unstimulated cells was not affected by p47phox silencing.
Fig. 6.
Silencing of p47phox inhibits Cdc42 activation in smooth muscle cells during 5-HT stimulation. Untransfected smooth muscle cells and cells producing luciferase shRNA or p47phox shRNA were stimulated with 10 μM 5-HT for 15 min, or they were unstimulated. Cdc42 activation in these cells was evaluated using the pull-down assay. A: representative immunoblots showing the reduced Cdc42 activation during 5-HT stimulation in cells expressing p47phox shRNA compared with untransfected cells and cells producing luciferase shRNA. B: ratios of GTP-Cdc42 to input in cells generating shRNAs are normalized to the ratios of untransfected and unstimulated cells. *Significantly lower ratios of GTP-Cdc42 to input in cells expressing p47phox shRNA than in untransfected cells and in cells producing luciferase shRNA (P < 0.05, n = 3).
Silencing of p47phox inhibits PAK1 phosphorylation at Thr-423 in response to stimulation with 5-HT.
The effects of p47phox knockdown on PAK1 phosphorylation at Thr-423 (an indication of PAK activation) in smooth muscle cells were evaluated. Blots of unstimulated or stimulated cells that had been treated with plasmids for luciferase shRNA or p47phox shRNA, or cells that had not been transfected were probed using phospho-PAK (Thr-423) antibody, stripped, and reprobed with use of PAK1 antibody.
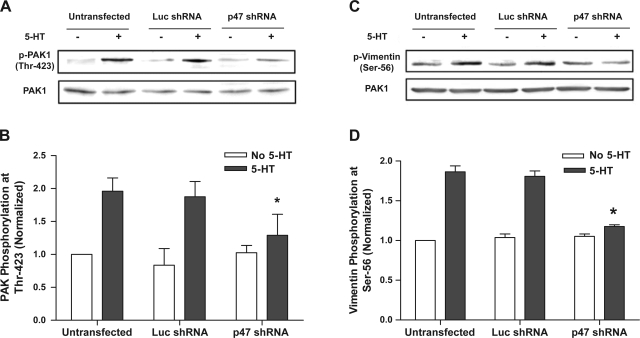
In untransfected cells and cells expressing luciferase shRNA, stimulation with 5-HT led to the enhancement of PAK1 phosphorylation by approximately twofolds, whereas the phosphorylation level of PAK1 stimulated by 5-HT (but not in unstimulated cells) was significantly lower in cells producing p47phox shRNA compared with untransfected cells and cells expressing luciferase shRNA (Fig. 7, A and B) (P < 0.05, n = 4).
Fig. 7.
p21-activated kinase 1 (PAK1) phosphorylation and vimentin phosphorylation upon stimulation with 5-HT are diminished in cells producing p47phox shRNA. Untransfected cells and cells expressing shRNAs were stimulated with 5-HT or left unstimulated. Phosphorylation of PAK1 and vimentin was determined by immunoblot analysis. Protein phosphorylation in response to 5-HT stimulation in cells producing shRNAs are normalized to the corresponding levels in unstimulated and untransfected cells. A: representative immunoblots illustrating reduced PAK1 phosphorylation at Thr-423 in cells producing p47phox shRNA upon 5-HT stimulation. B: *Significantly lower level of PAK1 phosphorylation stimulated by 5-HT in cells producing p47phox shRNA compared with untransfected cells and cells producing luciferase shRNA (P < 0.05, n = 4). C: immunoblots showing lower vimentin phosphorylation in response to 5-HT stimulation in cells producing p47phox shRNA. D: *significantly lower vimentin phosphorylation during 5-HT stimulation in cells expressing p47phox shRNA than in untransfected cells and in cells expressing luciferase shRNA (P < 0.05). Values are means ± SE (n = 4).
Knockdown of p47phox inhibits vimentin phosphorylation at Ser-56 during stimulation with 5-HT.
Vimentin phosphorylation has been shown to participate in the cellular processes that modulate smooth muscle contraction (24, 25, 34, 37, 41, 45, 46). Vimentin phosphorylation is catalyzed by PAK in in vitro studies and in smooth muscle cells in response to contractile activation (10, 25, 34, 46). To determine the functional role of p47phox in vimentin, immunoblot analysis was employed to determine vimentin phosphorylation of unstimulated cells and stimulated cells that had been treated with plasmids for luciferase shRNA or p47phox shRNA.
The levels of vimentin phosphorylation in response to 5-HT stimulation were augmented in untransfected cells and in cells producing luciferase shRNA. Knockdown of p47phox in smooth muscle cells attenuated 5-HT-induced vimentin phosphorylation but not basal vimentin phosphorylation without contractile stimulation (Fig. 7, C and D) (P < 0.05, n = 4).
Smooth muscle contraction induced by 5-HT is inhibited by silencing of p47phox.
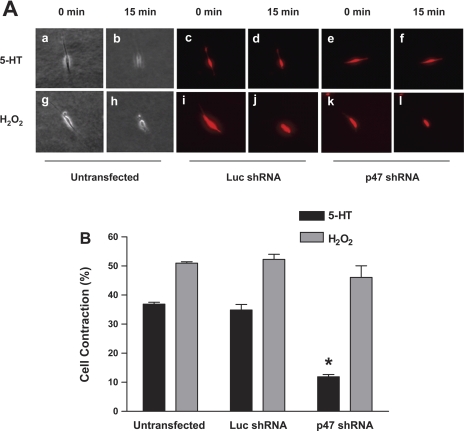
We assessed the role of p47phox in modulating smooth muscle contraction during chemical activation. Untransfected cells and cells producing shRNAs in the three-dimensional collagen matrix were stimulated with 5-HT. Cell contraction was evaluated using a digital fluorescent microscope. Stimulation with 5-HT induced the shortening of untransfected cells and cells producing luciferase shRNA. In contrast, 5-HT-induced contraction was inhibited in cells producing p47phox shRNA (Fig. 8).
Fig. 8.
Knockdown of p47phox attenuates cell contraction in response to stimulation with 5-HT, but not hydrogen peroxide. A: untransfected cells and cells producing shRNAs were cultured in the three-dimensional collagen matrix. The cells were stimulated with 5-HT or hydrogen peroxide for 15 min, and cell shortening was monitored using a digital fluorescent microscope. Contraction of untransfected cells are shown in phase contrast images (a, b, g, h). Contraction of transfected cells are shown in red (mCherry) images (c, d, e, f, i, j, k, l). B: contraction is calculated as follows: contraction (%) = (1 − cell length after stimulation/cell length before stimulation) × 100. *Significantly lower 5-HT induced contraction in cells expressing p47phox shRNA compared with that in untransfected cells and cells producing luciferase shRNA (P < 0.05, n = 12–18).
We also evaluated the effects of p47phox silencing on cell contraction during stimulation with hydrogen peroxide. Exposure to hydrogen peroxide led to contraction of untransfected cells and cells expressing luciferase shRNA as expected. More importantly, hydrogen peroxide induced contraction was not inhibited in cells producing p47phox shRNA (Fig. 8).
DISCUSSION
Cdc42GAP has recently emerged as a negative regulator of the vimentin framework and contraction through the Cdc42/PAK pathway in smooth muscle cells (24). In this report, we have demonstrated 1) knockdown of p47phox in smooth muscle cells attenuates ROS production and reverses the suppression of Cdc42GAP during contractile activation; 2) the activity of GAP is directly inhibited by the treatment with hydrogen peroxide; 3) silencing of p47phox inhibits Cdc42 activation, PAK phosphorylation, vimentin phosphorylation, and contraction in cells in response to agonist stimulation. We propose that p47phox is an important upstream regulator of Cdc42GAP and the GAP-mediated cellular response in smooth muscle during contractile stimulation.
The small GTPase Cdc42 serve as a molecular switch to regulate various biological responses including vimentin dynamics, smooth muscle contraction, actin polymerization, cell motility, cell cycle progression, and gene expression (2, 9, 36, 38, 43). Exposure of endothelial cells to vascular endothelial growth factor activates guanine nucleotide exchange factors (GEFs), thereby promoting the activity of the small GTPases (5, 8, 32). In the present study, quiescent cells possess constitutive activity of Cdc42GAP, whereas its activity decreases in association with cell contraction upon agonist activation. These findings suggest that Cdc42GAP possesses inherent activity and prevents Cdc42 activation in unstimulated cells. Agonist stimulation attenuates the activity of Cdc42GAP, facilitating the activation of Cdc42 and cell contraction.
In this report, stimulation with 5-HT promotes ROS generation in smooth muscle cells, which is consistent with our previous results (24). NAD(P)H oxidases have been implicated in the regulation of ROS production in smooth muscle cells by transferring electrons from NAD(P)H to molecular oxygen to produce superoxide. It has been proposed that smooth muscle NAD(P)H oxidase is composed of the membrane-associated catalytic complex including gp91phox homologue (NOX1 or NOX4) and p22phox, and cytoplasmic regulatory subunits containing p47phox, p67phox, p40phox, and Rac. However, the expression and role of these subunits may vary dependent on cell types and external stimulation (22, 47). In the present study, the expression of p47phox is present in airway smooth muscle cells, and knockdown of p47phox inhibits ROS production in response to activation with 5-HT. Since p47phox is a regulatory subunit of NAD(P)H oxidases, lack of this subunit may impair the activation of NAD(P)H oxidases reducing ROS generation in smooth muscle cells during contractile stimulation.
Our previous studies demonstrate that GAP suppression during contractile activation in smooth muscle cells is blocked by ROS inhibitors, suggesting a role of ROS in the regulation of GAP activity (24). In this report, GAP activity is directly inhibited by hydrogen peroxide in the in vitro biochemical experiment. The new finding indicates that ROS in cells may initiate the oxidation of Cdc42GAP, which may induce conformational changes and inhibit GAP activity. This assumption is supported by previous findings by other investigators, in which the activity of protein kinase A is directly modulated by redox (17). Additionally, the knockdown of p47phox reversed the 5-HT-induced GAP suppression; however, hydrogen peroxide-induced GAP suppression is not affected by p47phox silencing (Fig. 4). Moreover, 5-HT-induced ROS production is also attenuated by p47phox silencing. These results strongly suggest that the lack of p47phox may diminish the generation of ROS, which may reduce the oxidation of GAP and increase GAP activity in cells stimulated by 5-HT.
Although the small GTPases Cdc42, Rac1, and Rho are potential targets of GAP, our recent studies demonstrate that the overexpression of Cdc42GAP selectively inhibits the activation of Cdc42, but not Rac1 and Rho, in smooth muscle cells. It is known that Cdc42 activity is regulated by the balance between GEFs and GAP. GEFs enhance Cdc42 activity by promoting the binding of GTP to the small GTPase. GAP enhances the GTP hydrolysis of Cdc42 and inhibits its activation. Thus the overexpression of GAP may overcome the effects of GEFs and accelerate the GTP hydrolysis of Cdc42 in these cells. In this report, 5-HT-induced activation of Cdc42 is inhibited in p47phox-deficient cells, which may be attributed to increased GAP activity resulting from the reduction of ROS production. Furthermore, activation of PAK, a known downstream effector of Cdc42 (24, 37), during contractile activation is also attenuated by p47phox silencing. The results suggest that p47phox-regulated GAP is sufficient to modulate the functional status of Cdc42 and PAK1 in smooth muscle cells.
Our recent findings demonstrate that the overexpression of Cdc42GAP diminishes vimentin phosphorylation at Ser-56 and smooth muscle contraction (24). Cdc42GAP has also been implicated in the regulation of various cell functions in nonmuscle cells (33, 44, 49). Our present studies show that the knockdown of p47phox attenuates vimentin phosphorylation and contraction in smooth muscle cells in response to agonist activation. These results strongly suggest that the p47phox subunit of NAD(P)H oxidase may regulate the vimentin network and smooth muscle contraction. Because vimentin phosphorylation is mediated by PAK, it is very likely that contractile stimulation may activate NAD(P)H oxidase, which in turn increase the production of ROS in cells. ROS may inhibit GAP activity and increase vimentin phosphorylation and cell contraction mediated by Cdc42/PAK. In addition, Cdc42 is known for its ability to regulate cortical actin assembly facilitating the linkage of actin filaments to β-integrins, a key component of the membrane-associated dense plaque responsible for force transmission between the contractile apparatus and extracellular matrix in smooth muscle (13, 38). Thus the decreased Cdc42 activity by p47phox knockdown could impair cortical actin polymerization, which may inhibit the attachment of actin filaments to integrins and adhesion of integrins to extracellular matrix affecting the force transmission and smooth muscle contraction. However, Cdc42GAP deficiency has been shown to impair cortical actin assembly and adhesion of hematopoietic stem/progenitor cells (44), suggesting that Cdc42 hyperactivation by Cdc42GAP depletion inhibits the actin polymerization and adhesion of these cells. Thus the effects of Cdc42 activation on cortical actin filament assembly and adhesion may be cell type dependent.
A recent study by Dr. Murphy's group has shown that p47phox knockout inhibits the relaxation of mouse airway smooth muscle (4), which is complementary to the present observation. Because airway hyperresponsiveness has been attributed to increased airway smooth muscle contraction and decreased smooth muscle relaxation (7, 21), the results from the two different groups suggest that p47phox may play an important role in regulating both contraction and relaxation of airway smooth muscle. Thus p47phox may be a biological target for the development of new treatment of airway smooth muscle disease such as asthma.
There are several possibilities by which vimentin phosphorylation may affect smooth muscle contractility. Vimentin filaments are able to harbor several smooth muscle contraction associated molecules such as p130 Crk-associated substrate (CAS) and Ca2+/calmodulin-dependent protein kinase II (CamKII), preventing the activation of these molecules. Vimentin phosphorylation may increase partial disassembly of vimentin filaments in smooth muscle cells, resulting in the dissociation of CAS and CamKII from the vimentin network and facilitating the activation of these molecules during contractile activation (1, 24, 26, 28, 30, 35, 37, 39, 40, 46). In addition, vimentin phosphorylation may affect the spatial reorganization of the vimentin framework in smooth muscle cells during agonist stimulation (24, 25, 34, 46). Molecular structure of vimentin consists of an NH2-terminal head domain containing several phosphorylation sites, a central α-helical rod domain, and a COOH-terminal tail domain (15, 27). Phosphorylation on the head domain may change the assembly/disassembly process and induce spatial rearrangement of vimentin filaments (16, 25, 31, 37, 48). Because vimentin filaments connect to cytoplasmic dense bodies (to which actin filaments also attach) and to desmosomes (intercellular junctions), it is also possible that the spatial reorientation of vimentin filaments induced by contractile agonists may facilitate the reorganization of contractile elements and/or the intercellular and intracellular force transmission, which may be a part of the cellular processes that coordinate force development in smooth muscle (12, 19, 25, 34, 37, 45, 46).
Summary.
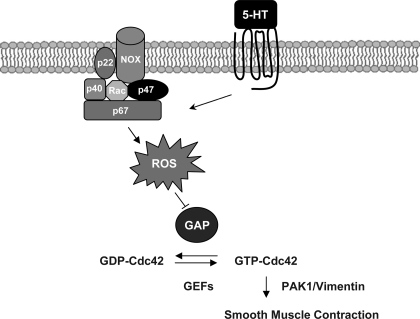
The role of p47phox in regulating Cdc42GAP in smooth muscle cells is not well characterized. In this report, knockdown of p47phox in smooth muscle cells attenuates ROS production and disinhibits the activity of Cdc42GAP during stimulation with 5-HT. Moreover, p47phox-regulated Cdc42GAP affects contraction likely via the Cdc42/PAK/vimentin axis in cells in response to agonist stimulation. Thus the activity of Cdc42GAP and its downstream cellular events are mediated by the p47phox subunit of NAD(P)H oxidase in smooth muscle cells during contractile stimulation (Fig. 9).
Fig. 9.
Proposed mechanism of Cdc42GAP regulation in smooth muscle cells. Contractile activation may activate NAD(P)H oxidases, which consist of the membrane associated catalytic complex including gp91phox homologue (NOX1 or NOX4) and p22phox, and cytoplasmic regulatory subunits containing p47phox, p67phox, p40phox and Rac. Activated NAD(P)H oxidases in turn stimulate the generation of ROS in cells. Increased ROS may suppress the activity of Cdc42GAP, which leads to Cdc42 activation and facilitate smooth muscle contraction via the PAK1/vimentin pathway. Cdc42 activity may also be regulated by GEFs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-75388 from the (to D. D. Tang).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Ruping Wang for technical assistance.
REFERENCES
- 1.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits C.AS-mediated process and constriction in resistance arteries. Circ Res 101: 420– 428, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspenstrom P. Effectors for the Rho GTPases. Curr Opin Cell Biol 11: 95– 102, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem 72: 743– 781, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chitano P, Wang L, Mason SN, Auten RL, Potts EN, Foster WM, Sturrock A, Kennedy TP, Hoidal JR, Murphy TM. Airway smooth muscle relaxation is impaired in mice lacking the p47phox subunit of NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 294: L139– L148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Cote JF, Vuori K: GEF. What? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 17: 383– 393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111: 1897– 1907, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J 12: 1252– 1256, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res 313: 3285– 3297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol 83: 851– 856, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7: 91– 97, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal 8: 1443– 1445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 15: 600– 616, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576– C587, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrion D, Terzi F, Matrougui K, Duriez M, Boulanger CM, Colucci-Guyon E, Babinet C, Briand P, Friedlander G, Poitevin P, Levy BI. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J Clin Invest 100: 2909– 2914, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem 73: 749– 789, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8: 562– 573, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling: kinase versus phosphatase inactivation. J Biol Chem 282: 22072– 22079, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kamm KE, Stull JT. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol 51: 299– 313, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun 285: 1077– 1083, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511– 515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA, Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 164: 141– 148, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lee MY, Griendling KK. Redox signaling, vascular function, hypertension. Antioxid Redox Signal 10: 1045– 1059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109: 1307– 1313, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Li QF, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol 297: C299– C309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 281: 34716– 34724, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase I.I. is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res 97: 541– 549, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Mucke N, Wedig T, Burer A, Marekov LN, Steinert PM, Langowski J, Aebi U, Herrmann H. Molecular and biophysical characterization of assembly-starter units of human vimentin. J Mol Biol 340: 97– 114, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Ogden K, Thompson JM, Hickner Z, Huang T, Tang DD, Watts SW. A new signaling paradigm for serotonin: use of Crk-associated substrate in arterial contraction. Am J Physiol Heart Circ Physiol 291: H2857– H2863, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol 217: 162– 171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278: C537– C545, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 313: 2098– 2109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sternweis PC, Carter AM, Chen Z, Danesh SM, Hsiung YF, Singer WD. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv Protein Chem 74: 189– 228, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Szczur K, Xu H, Atkinson S, Zheng Y, Filippi MD. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood 108: 4205– 4213, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Tang DD. Invited review: intermediate filaments in smooth muscle. Am J Physiol Cell Physiol 294: C869– C878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang DD. p130 Crk-associated substrate (CAS) in vascular smooth muscle. J Cardiovasc Pharmacol Ther 14: 89– 98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther 13: 130– 140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 388: 773– 783, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722– 51728, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Tang DD, Tan J. Role of Crk-associated substrate in the regulation of vascular smooth muscle contraction. Hypertension 42: 858– 863, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal wiskott-aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem 280: 23380– 23389, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Tsuda T, Griendling KK, Alexander RW. Angiotensin II stimulates vimentin phosphorylation via a Ca2+-dependent, protein kinase C-independent mechanism in cultured vascular smooth muscle cells. J Biol Chem 263: 19758– 19763, 1988 [PubMed] [Google Scholar]
- 42.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, Dudek SM, Pendyala S, Garcia JG, Natarajan V. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem 282: 23284– 23295, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Villalonga P, Ridley AJ. Rho GTPases and cell cycle control. Growth Factors 24: 159– 164, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Yang L, Filippi MD, Williams DA, Zheng Y. Genetic deletion of Cdc42GAP reveals a role of Cdc42 in erythropoiesis and hematopoietic stem/progenitor cell survival, adhesion, and engraftment. Blood 107: 98– 105, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Li Q, Tang DD. Role of vimentin in smooth muscle force development. Am J Physiol Cell Physiol 291: C483– C489, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L240– L248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolin MS. Reactive oxygen species, and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539– H549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi T, Goto H, Yokoyama T, Sillje H, Hanisch A, Uldschmid A, Takai Y, Oguri T, Nigg EA, Inagaki M. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol 171: 431– 436, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell 17: 4675– 4685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]