Abstract
Rapamycin-sensitive signaling is required for skeletal muscle differentiation and remodeling. In cultured myoblasts, the mammalian target of rapamycin (mTOR) has been reported to regulate differentiation at different stages through distinct mechanisms, including one that is independent of mTOR kinase activity. However, the kinase-independent function of mTOR remains controversial, and no in vivo studies have examined those mTOR myogenic mechanisms previously identified in vitro. In this study, we find that rapamycin impairs injury-induced muscle regeneration. To validate the role of mTOR with genetic evidence and to probe the mechanism of mTOR function, we have generated and characterized transgenic mice expressing two mutants of mTOR under the control of human skeletal actin (HSA) promoter: rapamycin-resistant (RR) and RR/kinase-inactive (RR/KI). Our results show that muscle regeneration in rapamycin-administered mice is restored by RR-mTOR expression. In the RR/KI-mTOR mice, nascent myofiber formation during the early phase of regeneration proceeds in the presence of rapamycin, but growth of the regenerating myofibers is blocked by rapamycin. Igf2 mRNA levels increase drastically during early regeneration, which is sensitive to rapamycin in wild-type muscles but partially resistant to rapamycin in both RR- and RR/KI-mTOR muscles, consistent with mTOR regulation of Igf2 expression in a kinase-independent manner. Furthermore, systemic ablation of S6K1, a target of mTOR kinase, results in impaired muscle growth but normal nascent myofiber formation during regeneration. Therefore, mTOR regulates muscle regeneration through kinase-independent and kinase-dependent mechanisms at the stages of nascent myofiber formation and myofiber growth, respectively.
Keywords: rapamycin, rapamycin-resistant, s6k1, mice, myogenesis
during development, cells in the somites undergo myogenic lineage commitment, myoblast proliferation, and terminal differentiation to form multinucleated myofibers. Skeletal myogenesis is guided by various environmental cues and regulated by distinct signal transduction pathways, which result in the activation of specific transcription factors and subsequent reprogramming of gene expression (26, 39). One of the adult skeletal muscle remodeling processes, regeneration after muscle damage, recapitulates embryonic myogenesis to a certain degree. Upon muscle injury, quiescent muscle precursor cells, or satellite cells, positioned between the basal lamina and the plasma membrane of muscle fibers become activated, proliferate, and differentiate to form new myofibers or repair existing myofibers (17).
The Ser/Thr kinase mammalian target of rapamycin (mTOR) is a master regulator of a wide range of biological processes, including cell growth, various types of cellular differentiation, and metabolism (12, 35, 40). As the cellular target of the bacteria macrolide rapamycin in complex with the ubiquitous protein FKBP12 (1), mTOR transduces signaling in response to nutrient availability, cellular energy sufficiency, mitogenic signals, and various types of stress signals. The best-known function of mTOR is regulation of protein synthesis through phosphorylation of the downstream targets S6K1 and 4E-BP1, which target ribosomal protein S6 and the cap binding complex, respectively (15, 16, 24). mTOR assembles two biochemically and functionally distinct protein complexes: the rapamycin-sensitive mTORC1 and the rapamycin-insensitive mTORC2, containing raptor and rictor, respectively (35).
Rapamycin-sensitive mTOR signaling has emerged as a key regulator of skeletal muscle differentiation and remodeling. Rapamycin had been known for some time to inhibit differentiation of several types of myoblasts in culture (7–9, 11). mTORC1 regulates myoblast differentiation at different stages via distinct molecular mechanisms. The initial stage of differentiation resulting in nascent myotube formation is controlled by mTOR through its regulation of IGF-II expression in a nutrient-dependent manner (11, 13), whereas a late-stage fusion leading to myotube maturation is regulated by mTOR through a yet-to-be-identified secreted factor (31). Whereas the kinase activity of mTOR is required for myotube maturation, mTOR initiates myoblast differentiation via an unexpected pathway that is independent of its kinase activity and independent of the previously established downstream effectors S6K1 and 4E-BP1 (11, 13). However, the kinase-independent myogenic function of mTOR has been disputed by data obtained in the same myoblast cell line C2C12 (37). This discrepancy may be attributed to clonal and subculture variation, a common pitfall of cell culture systems. Ultimately, in vivo evidence will be required to validate any regulatory mechanisms discovered in vitro.
Several lines of evidence suggest a critical involvement of mTOR in adult muscle remodeling. For instance, rapamycin inhibits IGF-induced myotube hypertrophy in vitro (32, 34). Compensatory myofiber hypertrophy in vivo and regrowth of myofibers after denervation-induced atrophy are also sensitive to rapamycin (3). Furthermore, mTORC1 regulates protein synthesis in response to mechanical stimulation of skeletal muscle ex vivo (18, 19). Mice with systemic s6k1 knockout have smaller myofibers, suggesting that S6K1 is required for muscle growth (29). Although S6K1 serves as a reliable readout of mTORC1 signaling, it is not the sole target of the rapamycin-sensitive functions of mTOR. Given that our in vitro studies have established an S6K1-independent role of mTOR in myoblast differentiation, direct examination of the function of mTOR and its kinase activity in an in vivo model would be highly desirable.
In this study, we investigate the regulation of skeletal muscle regeneration by mTOR in a mouse muscle injury model. Our studies reveal that rapamycin impairs both new fiber formation and myofiber growth during regeneration. Although rapamycin is considered a highly specific inhibitor for mTOR in cell culture when used at nanomolar concentrations, its direct targeting of FKBP12 (1) can potentially lead to disruption of a myriad of mTOR-independent functions at higher drug concentrations. As it is not feasible to control, nor measure, the concentration of rapamycin in tissues or cells in the intact animal, the effect of rapamycin in vivo cannot be presumed to be exclusively through inhibition of mTOR. To confirm the role of mTOR in the rapamycin-sensitive processes of regeneration, we have generated transgenic mice expressing a rapamycin-resistant (RR) mutant of mTOR (11) in skeletal muscle. To probe the requirement of mTOR kinase activity for its myogenic functions, we have also generated transgenic mice with muscle-specific expression of a rapamycin-resistant/kinase inactive (RR/KI) mTOR (11). Characterization of the regeneration potential of these transgenic mice has revealed kinase-independent regulation of new myofiber formation by mTOR and a requirement for mTOR kinase activity during the myofiber growth phase. These results provide genetic and in vivo evidence for the distinct regulatory mechanisms of mTOR in myogenesis as originally unraveled by in vitro studies.
MATERIALS AND METHODS
Antibodies and other reagents.
Antibodies against mTOR and phospho-Ser235/236-S6 were purchased from Cell Signaling Technology. Anti-tubulin was from Abcam. All secondary antibodies were from Jackson ImmunoResearch Laboratories. Anti-embryonic myosin heavy chain (eMHC) (F1.652) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development, National Institutes of Health and maintained by The University of Iowa, Department of Biological Sciences. Rapamycin was from LC Labs. All other chemicals were from Sigma Aldrich.
Plasmids.
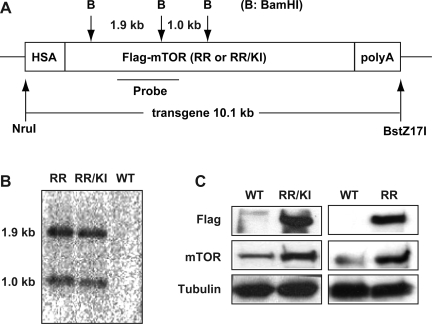
The pSA3 vector containing the 2.2-kb promoter of the human α-skeletal actin (HSA) gene (a generous gift from Dr. Karyn Esser, University of Kentucky) was used to construct the mTOR transgene expression plasmids. Human mTOR cDNA containing a sequence encoding the FLAG epitope at the 5′ end and Ser2035Thr (RR) or Ser2035Thr/Asp2357Glu (RR/KI) mutations was inserted into pSA3 downstream of the HSA promoter via NotI sites. The polyA signal from the BGH gene was inserted at the 3′ end of mTOR cDNA via the NotI site. NruI and PstZ17I were used to generate a 10.1-kb transgene fragment for pronuclear injection (see Fig. 3A).
Fig. 3.
Generation of transgenic mice expressing mammalian target of Rap (mTOR) mutants. A: diagram of the mTOR transgene expression vector is shown. FLAG-tagged mTOR [Rap resistant (RR) or RR/kinase inactive (KI)] was under the control of the human skeletal muscle actin (HSA) promoter. The NruI-BstZ17I fragment was used for pronuclear injection. BamHI sites and the probe used for Southern analysis of the transgene integration are shown. B: representative Southern blot for genomic DNA isolated from RR and RR/KI mice tails is shown. C: transgene expression was examined by Western analysis of limb muscle homogenates, using anti-FLAG and anti-mTOR antibodies. Anti-tubulin blot served as a loading control.
Generation of transgenic and knockout mice.
All animal experiments in this study followed protocols approved by the Animal Care and Use Committee at the University of Illinois at Urbana-Champaign. HSA-driven RR-mTOR and RR/KI-mTOR transgenic mice were produced by pronuclear injection of FVB zygotes at the Gene Targeting and Transgenic Facility at the University of Connecticut Health Center. Transgene integration in the founder lines was confirmed by PCR and Southern analysis (strategy depicted in Fig. 3A), and breeding was performed using wild-type FVB mice. All transgenic mice were maintained as hemizygotes.
S6k1 knockout mice in a C57BL/6 background were generated by blastocyst injection of previously reported embryonic stem cells with targeted disruption of the s6k1 gene (22) followed by backcrossing with C57BL/6 mice for eight generations. The mice were maintained as heterozygotes, which were bred to yield S6k1−/− and S6k1+/− mice for experiments. Genotyping was performed by PCR.
BaCl2 induced muscle injury and regeneration.
An established method of muscle injury by intramuscular injection of BaCl2 (4, 25) was employed in this study. The choice of BaCl2 among myotoxins was based on its low cost and easy accessibility. BaCl2 induces muscle fiber necrosis while completely preserving the basement membrane (4), and the subsequent muscle regeneration follows a process and timeline comparable to those induced by snake venoms or freezing (10, 14, 20, 21, 25, 33). Ten-week-old male mice were used in all the regeneration experiments. The following strains and/or genotypes of mice were characterized as described in results and the figure legends: wild-type (FVB and C57BL/6 as controls for mTOR transgenics and s6k1 knockouts, respectively), RR-mTOR, RR/KI-mTOR, s6k1+/−, and s6k1−/−. For each experimental condition (including controls), 3 to 10 mice were used to obtain the means ± SD data presented here. The animals were anesthetized by intraperitoneal injection of 300–400 μl of 2.5% avertin (100% avertin was made by mixing 10 g of tribromoethyl alcohol with 10 ml of tertiary amyl alcohol). BaCl2 (50 μl of 1.2% wt/vol in saline) was injected into the tibialis anterior (TA) or gastrocnemius muscle of a hindlimb. As controls, saline (50 μl) was injected similarly. Injected animals were caged singly throughout the experiments and monitored for their general levels of physical activity and any signs of health problems. Rapamycin was administered by intraperitoneal injection once daily at 1 mg/kg wt (100 μl in 5% Tween 80, 5% PEG-400, and 4% ethanol). The control mice received intraperitoneal injection of the vehicle. Food intake was measured using Rodent Cafe Type M feeder (OYC international). On days 1, 3, 7, 14, 21, and 28 after injury, the mice were euthanized and the TA muscles were collected, followed by cryosection and staining as described in Muscle tissue cryosection and analysis. In some instances as detailed in results and figure legends, mice were characterized 8 wk after injury. All mice were housed in a 12 h light/12 h dark cycle mouse facility, with the room temperature tightly controlled at 72°F. All the BaCl2 injections and muscle collection were performed around 10 am.
Muscle tissue cryosection and analysis.
TA muscles were isolated by dissection, frozen in liquid nitrogen-cooled 2-methylbutane, and embedded in TBS tissue freezing medium (Fisher Scientific). Sections of 10-μm thickness were made with a Microm HM550 cryostat at −20°C, placed on uncoated slides, and stained with hematoxylin and eosin (H&E). The stained slides were examined with a Leica DMI 4000B microscope, and the images were captured with a ×20 dry objective (Leica Fluotar, numerical aperture 0.4) using a RETIGA EXi camera. The images were then processed as 24-bit colored images using Adobe Photoshop CS2. An area of 614,400 μm2 at the center of degenerated region of each TA muscle was selected for scoring centrally nucleated regenerating myofiber numbers and cross-section area (CSA). CSA was measured using the Qcapture Pro51 software (QImaging).
Muscle tissue homogenization and Western blotting.
Mouse TA muscles were isolated, ground into powder in liquid nitrogen, and then lysed in a buffer containing 2% Triton X-100, 200 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM sodium orthovanadate, and a protease inhibitor cocktail (Sigma). After microcentrifugation at 14,000 rpm, the supernatant was mixed with 2× SDS sample buffer. The samples were boiled, run on SDS-PAGE, and transferred onto PVDF membrane (Millipore) followed by incubation with various antibodies according to the manufacturers' recommendations. Detection of horseradish peroxidase-conjugated secondary antibodies was performed with Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer Life Sciences).
Statistical analysis.
All data are presented as means ± SD. Statistical significance of the data was analyzed, when necessary, by performing paired two-tailed t-tests or two-way repeated measures analysis of variance (ANOVA) with Bonferroni's posttest as indicated in the figure legends, using GraphPad Prism 4.0. P values, whenever applicable, are indicated in the figure legends.
RESULTS
Rapamycin inhibits skeletal muscle regeneration.
Mice were injected with BaCl2 in the TA muscle, which was dissected and cryosectioned on various days up to 28 days after injury (AI). The injected muscle underwent pronounced necrosis on day 1 AI (Fig. 1A), followed by extensive immune cell infiltration and satellite cell proliferation by day 3 AI (data not shown), consistent with previously reported effects of both BaCl2 and snake venoms on mouse muscles (4, 23, 25). Formation of regenerating myofibers, identified by the presence of central myonuclei, was clearly evident by day 7 AI (Fig. 1A), and by day 21 the regeneration was typically complete and regenerated myofibers reached the size of undamaged mature fibers (Fig. 1A, top; also see Fig. 1B). eMHC expression was detected in all the centrally nucleated myofibers on day 7 (data not shown), confirming their nascent myofiber nature. This time course of regeneration is consistent with those previously reported by others examining muscle regeneration postinjury by BaCl2 (5, 21, 25), snake venoms (10, 23), or freezing (20). Systemic rapamycin administration (daily at 1 mg/kg body wt) drastically impaired the regeneration process, evidenced by the abnormal muscle architecture, the presence of necrotic fibers, and infiltration of mononucleated cells even at day 28 AI (Fig. 1A, bottom). When BaCl2 was injected into the gastrocnemius muscle, the regeneration process and rapamycin inhibition were indistinguishable from those of TA muscles (data not shown).
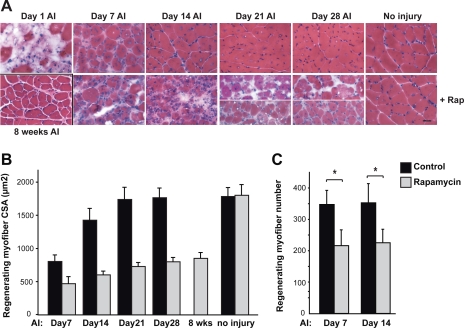
Fig. 1.
Muscle regeneration is impaired by rapamycin (Rap). Injury of the hindlimb tibialis anterior (TA) muscle was induced by BaCl2 injection in 10-wk-old male mice. The “no injury” control muscles received saline injection. Where indicated, Rap (1 mg/kg) was administered daily via intraperitoneal injection starting on day 1 after injury (AI). Vehicle was injected in all mice not receiving Rap. On various days AI, the animals were euthanized and TA muscles were isolated and cryosectioned. A: TA muscle cross-sections were stained with hematoxylin and eosin (H&E). Scale bar represents 50 μm. Two representative images are shown for day 21 and day 28 to demonstrate the abnormal muscle structure as well as smaller regenerating myofibers. B: cross-sectional area (CSAs) of regenerating myofibers, identified by their central nuclei on H&E-stained sections, were quantified. At least 100 regenerating myofibers were measured for each muscle section. C: number of regenerating myofibers was counted in an area of 614,400 μm2 for each sample. For all the data, the average results (n = 7 mice for each data point) are shown, with error bars representing SD. Paired two-tailed t-tests were performed to compare data from control and rapamycin-treated samples at each time point in C. *P < 0.01.
The average CSA of the regenerating myofibers were measured. By day 21 AI, regenerating myofibers had average CSA comparable with undamaged fibers (Fig. 1B, solid bars). Impairment of regeneration by rapamycin was clearly reflected by the smaller average CSA of the regenerating myofibers throughout the time course examined (Fig. 1B, shaded bars). The rapamycin dosage administered was sufficient to inhibit mTORC1 signaling in muscles, as indicated by a dramatic decrease of S6 phosphorylation (see Fig. 4B). Even after 8 wk, the regenerating myofibers in animals continually treated with rapamycin did not grow to the normal size (Fig. 1B), although the overall muscle structure did improve (Fig. 1A). Animals injected with rapamycin for up to 8 wk did not display any visible symptoms, and rapamycin by itself did not affect myofiber size or structure (Fig. 1, A and B), consistent with previously reported observations (3). In addition, food intake by the animals after injury was not affected by rapamycin injection (data not shown). There was also no visibly detectable change in physical activities of the mice after injury or with rapamycin injection. Taken together, our observations suggest that rapamycin-sensitive mTOR signaling may be required for skeletal muscle regeneration.
Fig. 4.
Characterization of mTOR transgenic mice. A: TA muscles from 10-wk-old wild-type (WT), RR, and RR/KI mice were isolated, cryosectioned, and stained with H&E. The average CSAs with SD are shown below the images (n = 4–8 mice). Scale bar represents 50 μm. B: limb muscle homogenates were isolated from mice injected with Rap (1 mg/kg) or vehicle and subjected to Western analysis for phospho-S6, a read-out for mTORC1 signaling activity. Anti-tubulin blot served as a loading control.
Rapamycin inhibits both nascent myofiber formation and myofiber growth during regeneration.
Since rapamycin did not completely block muscle regeneration (Fig. 1, A and B), we questioned whether rapamycin had any effect on the initial formation of regenerating myofibers. To this end, we quantified the number of regenerating myofibers on H&E-stained muscle sections. On both day 7 and day 14 AI, rapamycin-injected mice had significantly fewer regenerating myofibers in their injured TA muscles (Fig. 1C), suggesting that mTOR is at least partially responsible for the initial formation of new myofibers during regeneration.
The observations described in Fig. 1 implied that rapamycin might also block the growth of myofibers during a later stage of regeneration, a process that may be molecularly separable from the initial myofiber formation. To directly examine this possibility, we started administering rapamycin to BaCl2-injured mice on day 7 AI, when new myofiber formation had already completed (see regenerating myofiber number comparison between day 7 and day 14 AI in Fig. 1C), but the regenerating myofibers were still significantly smaller than mature myofibers (see Fig. 1B). Indeed, in the presence of rapamycin, regenerating myofibers did not grow to normal size (Fig. 2A compared with Fig. 1A, top). In fact, muscle maturation appeared to be blocked, rather than delayed, by rapamycin administration, as the average fiber size was still ∼25% smaller than normal when examined 8 wk after injury with continued daily rapamycin injection (Fig. 2B). Hence, mTOR appears to be required for myofiber growth during regeneration.
Fig. 2.
Rapamycin suppresses regenerating myofiber growth. Mouse TA muscles were injured by BaCl2 injection, followed by daily intraperitoneal injection of Rap (1 mg/kg) starting on day 7 AI. On the days indicated, the animals were euthanized and TA muscles were isolated and cryosectioned. A: TA muscle cross-sections were stained with H&E. Scale bar represents 50 μm. B: CSAs of regenerating myofibers were quantified. At least 100 regenerating myofibers were measured for each muscle section. The average results (n = 7 mice for each data point) are shown, with error bars representing SD. The control data (solid bars) are from Fig. 1B.
Generation and characterization of transgenic mice expressing mTOR mutants.
To seek genetic validation for the involvement of mTOR in the rapamycin-sensitive processes and to probe the role of mTOR kinase activity, we established transgenic mice expressing rapamycin-resistant mTOR with (RR) or without (RR/KI) its kinase activity in the skeletal muscle. Both RR- and RR/KI-mTOR were tagged with the FLAG epitope at the NH2-terminus. Transgene expression was driven by the HSA promoter (Fig. 3A). Transgene integration for both mTOR mutants was confirmed by Southern analysis (Fig. 3B) and PCR (data not shown). Both the HSA-RR and HSA-RR/KI lines were confirmed to express FLAG-tagged recombinant mTOR protein in skeletal muscle (Fig. 3C); no transgene expression was detected in the liver (data not shown). The level of recombinant protein expression was approximately two- to threefold of that of the endogenous mTOR (Fig. 3C, mTOR blot).
No obvious phenotype was observed from birth up to 8 mo of age in either RR or RR/KI transgenic mice. The weight and size of the transgenic animals and their limb muscles were indistinguishable from those of WT animals when age-matched comparisons were made (data not shown). Examination of the TA muscle revealed no difference in the average myofiber CSA among RR-mTOR, RR/KI-mTOR, and WT mice (Fig. 4A). Despite the higher levels of transgene expression compared with the endogenous protein, the kinase-inactive mTOR did not behave as a dominant-negative mutant, consistent with what we had observed with this mutant in cell culture with a similar degree of overexpession (our unpublished observations). To confirm the biochemical nature of the mTOR transgene products, we analyzed the homogenates of limb muscles from RR and RR/KI mice injected with rapamycin. As shown in Fig. 4B, rapamycin injection drastically inhibited S6 phosphorylation, a common readout of mTORC1 activity, in both WT and RR/KI muscles, whereas RR muscles displayed complete rapamycin resistance in S6 phosphorylation. The inability of RR/KI-mTOR to support S6 phosphorylation in the presence of rapamycin is expected as S6K1 activation requires mTOR kinase activity.
Muscle regeneration in RR-mTOR transgenic mice in the presence of rapamycin.
With the transgenic lines in hand, we first asked whether the expression of the recombinant RR-mTOR in skeletal muscle was able to reverse the rapamycin inhibitory effect on muscle regeneration. The RR-mTOR mice were subjected to BaCl2-induced injury in the TA muscle. In the absence of rapamycin, muscle regeneration in these mice was indistinguishable to that in wild-type (WT) mice (Fig. 5A, top, compared with Fig. 1A, top). When they were compared in the presence of rapamycin (administered from day 1 AI), the RR-mTOR muscles regenerated significantly better than WT muscles, as the overall muscle structure was completely restored by day 21 AI (Fig. 5A, bottom, compared with Fig. 1A, bottom). The RR regeneration of the RR-mTOR muscles was also evidenced by a higher number of nascent regenerating myofibers (Fig. 5B) and larger CSAs of the regenerating myofibers (Fig. 5C) compared with WT muscles. It was apparent, however, that both the formation of regenerating myofibers and their growth were delayed by rapamycin injection in the RR-mTOR mice, although eventually these muscles regenerated to the full extent (Fig. 5B, day 14; Fig. 5C, day 28) (to be discussed in discussion).
Fig. 5.
RR-mTOR supports RR muscle regeneration. RR-mTOR mice were subjected to TA muscle injury, Rap injection, and muscle isolation as described in Fig. 1. A: TA muscle cross-sections generated on various days AI were stained with H&E. Scale bar represents 50 μm. B: number of regenerating myofibers was counted within an area of 614,400 μm2 for each sample. C: CSAs of regenerating myofibers were quantified. At least 100 regenerating myofibers were measured for each muscle section. For all the quantification data, the average results (n = 7 mice for each data point) are shown with error bars representing SD. The WT data are from Fig. 1, B and C. In B, paired two-tailed t-tests were performed to compare RR to WT samples under the same conditions (same day and same treatment), *P < 0.05. In C, two-way repeated measures ANOVA was performed to analyze regeneration in the presence of rapamycin over the time course, and between WT and RR genotypes. Overall analysis: time, P < 0.0001; genotype, P < 0.0001; interaction, P < 0.0001. Significant difference between WT and RR at each time point: **P < 0.001.
The kinase activity of mTOR is dispensable for nascent myofiber formation but required for myofiber growth in muscle regeneration.
To probe the requirement of mTOR kinase activity in muscle regeneration in vivo, we took advantage of the RR mutation in mTOR, which allowed us to study the function of any mTOR mutants (in this case, the kinase-inactive mutant) while suppressing endogenous mTOR function with rapamycin. We subjected the RR/KI-mTOR mice to BaCl2-induced muscle injury and characterized muscle regeneration in the presence or absence of systemic rapamycin administration. In the absence of rapamycin, regeneration of the RR/KI muscles was identical to that of WT muscles in the recovery of muscle structure (Fig. 6A, top), regenerating myofiber number (Fig. 6B, solid bars), and myofiber size (Fig. 7A, solid bars). This confirmed that RR/KI-mTOR transgene expression did not exert any dominant-negative effect. When rapamycin had been administered from day 1 AI, the regeneration of RR/KI muscles was much improved compared with WT muscles as indicated by the overall recovery of muscle structure by day 21 AI (Fig. 6A, bottom, compared with Fig. 1A, bottom). Quantification of myofiber numbers revealed that RR/KI muscles formed more regenerating myofibers than WT muscles in the presence of rapamycin (Fig. 6B). Although the RR/KI muscles were partially sensitive to rapamycin (to be discussed later), it is important to note that the rate and degree of myofiber formation in the presence of rapamycin were indistinguishable between RR/KI and RR mice (compare Fig. 6B to Fig. 5B). These observations strongly suggest that the kinase activity of mTOR is dispensable for the initial formation of regenerating myofibers.
Fig. 6.
Defective muscle regeneration in RR/KI-mTOR transgenic mice in the presence of Rap. RR/KI-mTOR mice were subjected to TA muscle injury, rapamycin injection, and muscle isolation as described in Fig. 1. A: TA muscle cross-sections generated on various days AI were stained with H&E. Scale bar represents 50 μm. B: number of regenerating myofibers was counted within an area of 614,400 μm2 for each sample. The average results (n = 7 mice for each data point) are shown with error bars representing SD. Paired two-tailed t-tests were performed to compare RR/KI with WT samples under the same conditions (same day and same treatment). **P < 0.001. C: WT, RR-, and RR/KI-mTOR mice were subjected to muscle injury, Rap injection, and muscle isolation as indicated. Total RNA was extracted, and Igf2 mRNA was measured by qRT-PCR. Relative levels compared with those of day 1 AI are shown with error bars representing SD (n = 3).
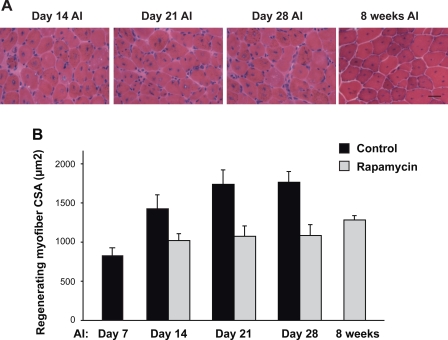
Fig. 7.
Kinase activity of mTOR is dispensable for nascent myofiber formation but required for myofiber growth in muscle regeneration. A: WT and RR/KI mice were subjected to TA muscle injury and Rap injection starting on day 1 AI, followed by muscle isolation and cryosection on days 7, 14, 21, 28 AI. B: WT, RR, and RR/KI mice were subjected to TA muscle injury and Rap injection starting on day 7 AI, followed by muscle isolation and cryosection on days 14, 21, 28 AI. CSAs of regenerating myofibers were quantified. At least 100 regenerating myofibers were measured for each muscle section. The average results (n = 7 mice for each data point) are shown, with error bars representing SD. The WT data are from Figs. 1B and 2B. In B, two-way repeated measures ANOVA was performed to analyze regeneration in the presence of Rap over the time course and between different genotypes. Overall analysis: time, P < 0.0001; genotype, P < 0.0001; interaction, P = 0.001. Significant difference between WT and transgenics at each time point: **P < 0.001.
Previously we had reported that mTOR regulates the initiation of C2C12 differentiation by controlling Igf2 mRNA expression in an mTOR kinase-independent manner (13). To investigate whether the same mechanism was involved in muscle regeneration in vivo, we examined the level of Igf2 mRNA in regenerating muscles by quantitative PCR (41). Igf2 levels in injured WT TA muscle peaked around day 7 AI (data not shown) as reported by others (30). This 13-fold increase of Igf2 was drastically inhibited by rapamycin administration (Fig. 6C, solid bars). In RR- and RR/KI-mTOR muscles, a similar degree of increase in Igf2 mRNA was observed on day 7 AI, but it was clear that this increase was partially resistant to rapamycin treatment (Fig. 6C, compare shaded and open bars to solid bars). The RR and RR/KI muscles behaved indistinguishably in this case. These observations are in full agreement with mTOR regulation of Igf2 expression in the absence of mTOR kinase activity during the early phase of muscle regeneration.
Although RR and RR/KI muscles displayed equal capacity in forming regenerating myofibers in the presence of rapamycin, the average myofiber size was affected by the genotype. As shown in Fig. 7A, rapamycin treatment severely diminished the average CSA of the regenerating fibers in RR/KI-mTOR muscles, to a similar degree as that of the fibers in WT muscles, suggesting that mTOR kinase activity might be necessary for the growth of regenerating myofibers. To directly investigate the requirement of mTOR kinase activity for myofiber growth, we administered rapamycin beginning on day 7 AI, when nascent myofibers had mostly formed but the average fiber size was significantly smaller than mature fibers (see Fig. 1, B and C). As shown in Fig. 7B, while regenerating myofibers in RR/KI mice continued to grow from day 7 to day 28 AI at a rate comparable to the growth of WT myofibers under normal conditions, rapamycin administration completely blocked RR/KI muscle growth. This block appeared to be permanent as no growth was evident even at 8 wk AI with continued daily rapamycin injection (data not shown). On the other hand, RR-mTOR myofibers underwent full RR growth (Fig. 7B). Therefore, the kinase activity of mTOR is required for myofiber growth and maturation.
S6K1 is dispensable for nascent myofiber formation but required for myofiber growth in muscle regeneration.
S6K1 is a major downstream effector of mTORC1 signaling. To assess the role of S6K1in muscle regeneration, we created and examined s6k1 knockout mice. Previously reported embryonic stem cells with targeted deletion of s6k1 (22) were used to generate systemic knockout animals, which were viable and fertile but smaller in size (data not shown), consistent with the report by Thomas and colleagues (38). The limb muscle weight (data not shown) and TA myofiber size (Fig. 8B, “uninjured”) in the s6k1−/− mice were also reduced compared with those in WT or heterozygous animals, in full agreement with the observations by Ohanna et al. (29).
Fig. 8.
S6K1 is dispensable for nascent myofiber formation but required for myofiber growth in muscle regeneration. WT, s6k1+/−, and s6k1−/− mice were subjected to TA muscle injury, muscle isolation, and H&E staining as described in Fig. 1. A: number of regenerating myofibers was counted within an area of 614,400 μm2 for each sample. No statistically significant difference was found when comparing muscles of three genotypes at each time point. B: CSAs of regenerating myofibers were quantified. At least 100 regenerating myofibers were measured for each muscle section. The average results (n = 4 mice for each data point) are shown, with error bars representing SD. Two-way repeated measures ANOVA was performed to analyze CSA data over regeneration time and between different genotypes. Overall analysis: time, P < 0.0001; genotype, P < 0.0001; interaction, P = 0.26. Significant difference between genotypes at each time point: *P < 0.05, **P < 0.001. Significant difference between Day 28 AI and uninjured: †P < 0.05.
s6k1−/−, s6k1+/−, and WT mice were subjected to BaCl2-induced TA muscle injury, and muscle regeneration was examined on various days after injury. The formation of regenerating myofibers, indicated by the number of centrally nucleated myofibers on day 7 and day 14 AI, was indistinguishable among the three types of muscles (Fig. 8A), suggesting that S6K1 is dispensable for the initial myofiber formation during regeneration.
Measurements of the CSA of the regenerating myofibers, however, revealed a significant difference between s6k1−/− and WT or the heterozygous animals. Whereas WT and s6k1+/− myofibers grew at a similar rate and reached the size of their uninjured counterparts by day 28 AI, the s6k1−/− myofibers appeared to be arrested at a size significantly smaller than that of the uninjured myofibers (Fig. 8B). Thus, in addition to the smaller average size of their myofibers before injury, the s6k1−/− muscles were impaired in the growth of regenerating myofibers. Taken together, we conclude that S6K1 plays a role in muscle growth but is not involved in the formation of nascent myofibers during regeneration.
DISCUSSION
Based on observations made in cell culture, we previously proposed that rapamycin-sensitive mTOR signaling regulates myoblast differentiation at two different stages by distinct mechanisms (11, 13, 31): the initiation of differentiation and nascent myotube formation are controlled by mTOR independent of its kinase activity and its target S6K1, whereas myotube growth and maturation require mTOR kinase activity. The current study was designed to examine mTOR function in muscle regeneration in vivo and to validate the molecular mechanisms of mTOR signaling previously discovered in vitro in a physiological setting. In a mouse model of BaCl2-induced muscle degeneration/regeneration, we have found that rapamycin inhibits both the formation of regenerating myofibers and the maturation of myofibers. This inhibitory effect of rapamycin is consistent with previously reported rapamycin inhibition of muscle regrowth after denervation-induced atrophy (3) and suggests the involvement of mTOR. To provide genetic evidence for mTOR function in the rapamycin-sensitive processes, we have generated and characterized transgenic mice with muscle-specific expression of RR-mTOR and its counterpart RR/KI-mTOR. RR regeneration in the RR-mTOR muscles has provided definitive evidence for the function of mTOR in regeneration.
We have observed that regeneration of the RR-mTOR muscles is partially sensitive to rapamycin, especially at earlier time points of regeneration. This delay may be explained by the nature of the HSA promoter that drives the mTOR transgene expression, which is highly active in differentiated myofibers but has low basal activity in satellite cells or myoblasts (27). Although we cannot definitively rule out the possibility that the RR-mTOR recombinant protein is only partially active, or that rapamycin has some mTOR-independent effect, our observations indicate that RR-mTOR can significantly overcome rapamycin inhibition in both regenerating myofiber formation and myofiber growth. Because of its delayed activation, the HSA promoter would not be suitable for driving transgene expression for the purpose of studying satellite cell activation and proliferation. Future efforts to generate transgenic mice expressing mTOR mutants under the control of satellite cell-specific promoters, such as that of Pax7, would allow investigation into the role of mTOR in earlier events of muscle regeneration.
Although the kinase activity of mTOR is essential for almost all reported mTOR functions, we previously observed that mTOR regulation of the initiation of C2C12 myoblast differentiation was independent of its kinase activity, and that this myogenic function is mediated by IGF-II (11, 13), which is critically involved in both satellite cell proliferation and differentiation (17). In the current study, we have found that the kinase-inactive RR/KI-mTOR supports myofiber formation in the presence of rapamycin as effectively as RR-mTOR. Furthermore, Igf2 mRNA expression during regeneration is inhibited by rapamycin and partially rescued by both RR- and RR/KI-mTOR transgene expression. Taken together, these in vivo observations faithfully recapitulate our in vitro findings and are consistent with a kinase-independent function of mTOR in the initial formation of myofibers during regeneration.
Rapamycin inhibits the continued growth of regenerating myofibers. Because of technical difficulties in quantifying nuclei numbers in centrally nucleated myofibers, we are not able to assess whether the myofiber growth examined in our study requires further addition of nuclei (i.e., fusion) or is only a result of mass increase. In any event, this growth requires mTOR in its kinase-active form, as it is supported by RR-mTOR, but not RR/KI-mTOR, in the presence of rapamycin. These observations fully corroborate our previous findings in vitro, that rapamycin-sensitive mTOR signaling is required for myotube maturation and hypertrophy (31, 32). We have also characterized s6k1−/− mice and found that growth of regenerating myofibers is impaired in these mice but the number of regenerating fibers is comparable to that in WT mice. These findings confirm the requirement of S6K1 for muscle growth (29) and provide in vivo validation for the S6K1-independent initiation of myoblast differentiation in vitro (11, 13). They are also in full agreement with the kinase-independent role of mTOR in regeneration since S6K1 is a major substrate of the mTOR kinase.
Most recently, it has been reported that muscle-specific ablation of raptor, the factor that defines the rapamycin-sensitive mTORC1, but not rictor, leads to a downshift of myofiber size distribution in skeletal muscles and signs of progressive muscle dystrophy (2). The effect of raptor knockout on myofiber size mirrors the effect of s6k1 knockout (29), confirming the role of mTORC1 in muscle size control. It would be reasonable to speculate that raptor−/− muscles would be able to form nascent myofibers upon injury like rapamycin-treated RR/KI-mTOR muscles and s6k1−/− muscles, since raptor function is tightly associated with mTOR kinase activity by recruiting substrates (such as S6K1) for mTOR (6, 28, 36). Of significance, daily rapamycin administration up to 2 mo in mice does not induce any detectable changes in muscle size or signs of muscle dystrophy (our unpublished observation) (3), even though the effect of rapamycin on regeneration upon muscle injury is quite dramatic. The discrepancy between pharmacological and genetic inhibition of mTORC1 can potentially be explained by the fact that gene deletions in animals, even when induced by Cre under the control of a muscle-specific promoter (HSA in the case of raptor deletion), often have developmental effects. As such, the mTOR transgenic mice combined with rapamycin treatment offer an experimental system that allows more precise probing of mTOR function in adult muscle remodeling and function without the complication of developmental influences. The comparison between RR- and RR/KI-mTOR transgenics also enables direct assessment of the functionality of the mTOR kinase activity. These mouse models created here provide useful tools for future investigation of mTOR function and signaling mechanisms in various aspects of muscle biology.
In conclusion, we have demonstrated that mTOR controls myofiber formation and myofiber growth during muscle regeneration via kinase-independent and kinase-dependent mechanisms, respectively. The expression of Igf2 during the early phase of regeneration is sensitive to rapamycin in an mTOR kinase-independent manner, whereas S6K1 is required for the mTOR kinase-dependent myofiber growth. Our results provide in vivo validation for the myogenic mechanisms of mTOR previously discovered in vitro. Of particular significance is the kinase-independent mechanism of mTOR at the initial stage of myogenesis, which has not been reported for any other functions of mTOR.
GRANTS
This work was supported by National Institutes of Health Grant AR48914 and American Diabetes Association to J. Chen.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Derek Miller for technical guidance, Ike Joewono for assistance in animal breeding, and Dr. Karyn Esser (University of Kentucky) for providing the pSA3 vector.
REFERENCES
References
- 1.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol 14: 483– 510, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411– 424, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014– 1019, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol 16: 225– 238, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol 268: 358– 371, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Choi KM, McMahon LP, Lawrence JC., Jr Two motifs in the translational repressor PHAS-I required for efficient phosphorylation by mammalian target of rapamycin and for recognition by raptor. J Biol Chem 278: 19667– 19673, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Conejo R, Valverde AM, Benito M, Lorenzo M. Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J Cell Physiol 186: 82– 94, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272: 6653– 6662, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem 274: 4341– 4346, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Deponti D, Francois S, Baesso S, Sciorati C, Innocenzi A, Broccoli V, Muscatelli F, Meneveri R, Clementi E, Cossu G, Brunelli S. Necdin mediates skeletal muscle regeneration by promoting myoblast survival and differentiation. J Cell Biol 179: 305– 319, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erbay E, Chen J. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem 276: 36079– 36082, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Erbay E, Kim JE, Chen J. Amino acid-sensing mTOR signaling. In: Nutrient and Cell Signaling, edited by Zempleni J, Dakshinamurti K: CRC, 2005, p. 353– 380 [Google Scholar]
- 13.Erbay E, Park IH, Nuzzi PD, Schoenherr CJ, Chen J. IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J Cell Biol 163: 931– 936, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink E, Fortin D, Serrurier B, Ventura-Clapier R, Bigard AX. Recovery of contractile and metabolic phenotypes in regenerating slow muscle after notexin-induced or crush injury. J Muscle Res Cell Motil 24: 421– 429, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Fumagalli S, Thomas G. S6 phosphorylation and signal transduction. In: Translational Control of Gene Expression, edited by Sonenberg N, Hershey JWB, Mathews MB. Cold Spring Harbor, NY: Cold Spring Harbor Lab, 2000, p. 695– 718 [Google Scholar]
- 16.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913– 963, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534– 551, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380: 795– 804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 5: 1391– 1396, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113: 483– 494, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol 174: 403– 413, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasome H, Papst P, Webb S, Keller GM, Johnson GL, Gelfand EW, Terada N. Targeted disruption of p70(s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc Natl Acad Sci USA 95: 5033– 5038, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefaucheur JP, Sebille A. The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul Disord 5: 501– 509, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2: 2, 2009 [DOI] [PubMed] [Google Scholar]
- 25.McArdle A, Edwards RH, Jackson MJ. Release of creatine kinase and prostaglandin E2 from regenerating skeletal muscle fibers. J Appl Physiol 76: 1274– 1278, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Naya FS, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11: 683– 688, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, Vertes D, Cao ML, De Backer F, Languille L, Roblot N, Joshi V, Gillis JM, Melki J. Intact satellite cells lead to remarkable protection against Smn gene defect in differentiated skeletal muscle. J Cell Biol 161: 571– 582, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461– 15464, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7: 286– 294, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics 11: 263– 272, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Park IH, Chen J. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem 280: 32009– 32017, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Park IH, Erbay E, Nuzzi P, Chen J. Skeletal myocyte hypertrophy requires mTOR kinase activity and S6K1. Exp Cell Res 309: 211– 219, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Richard-Bulteau H, Serrurier B, Crassous B, Banzet S, Peinnequin A, Bigard X, Koulmann N. Recovery of skeletal muscle mass after extensive injury: positive effects of increased contractile activity. Am J Physiol Cell Physiol 294: C467– C476, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009– 1013, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596– 603, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 13: 797– 806, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Shu L, Zhang X, Houghton PJ. Myogenic differentiation is dependent on both the kinase function and the N-terminal sequence of mammalian target of rapamycin. J Biol Chem 277: 16726– 16732, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200– 205, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75: 1241– 1244, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471– 484, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Yoon M-S, Chen J. PLD regulates myoblast differentiation through the mTOR—IGF-II pathway. J Cell Sci 121: 282– 289, 2008 [DOI] [PubMed] [Google Scholar]