Abstract
Myofibroblasts have recently been identified as major mediators of tumor necrosis factor-α (TNF-α)-associated colitis, but the precise mechanism(s) involved remains incompletely understood. In particular, the possibility that TNF-α signaling cross talks with other proinflammatory mediators, including bradykinin (BK), has not been examined in these cells. Here we show that treatment of 18Co cells, a model of human colonic myofibroblasts, with BK and TNF-α induced striking synergistic COX-2 protein expression that was paralleled by increases in the levels of transcripts encoding COX-2 and microsomal prostaglandin E synthase 1 (mPGES-1) and by the production of PGE2. COX-2 expression in 18Co cells treated with BK and TNF-α was prevented by the B2 BK receptor antagonist HOE-140, the preferential protein kinase C (PKC) inhibitors Ro31-8220 and GF-109203X, and Gö-6976, an inhibitor of conventional PKCs and protein kinase D (PKD). In a parallel fashion, TNF-α, while having no detectable effect on the activation of PKD when added alone, augmented PKD activation induced by BK, as measured by PKD phosphorylation at its activation loop (Ser744) and autophosphorylation site (Ser916). BK-induced PKD activation was also inhibited by HOE-140, Ro31-8220, and Gö-6976. Transfection of 18Co cells with small interfering RNA targeting PKD completely inhibited the synergistic increase in COX-2 protein in response to BK and TNF-α, demonstrating, for the first time, a critical role of PKD in the pathways leading to synergistic expression of COX-2. Our results imply that cross talk between TNF-α and BK amplifies a PKD phosphorylation cascade that mediates synergistic COX-2 expression in colonic myofibroblasts. It is plausible that PKD increases COX-2 expression in colonic myofibroblasts to promote an inflammatory microenvironment that supports tumor growth.
Keywords: 18Co cells, prostaglandin E2, protein kinase C, phosphorylation
the dynamic interaction that occurs between the gastrointestinal (GI) epithelium and its underlying mesenchymal elements is critical for both normal GI functions and disease states. Myofibroblasts are a subpopulation of cells located in the lamina propria of the GI tract that interact with adjacent cells in a paracrine fashion through the secretion of cytokines, growth factors, and inflammatory mediators, including prostanoids (37). Through these interactions, myofibroblasts are involved in key cellular processes such as the regulation of epithelial proliferation/differentiation, mucosal repair, and fibrosis (37). They also participate in immune and inflammatory responses (42) and have been implicated in the underlying pathophysiology that characterizes both ulcerative colitis and colorectal cancer (18, 37, 60).
Myofibroblasts are a major source of the inducible isoform of cyclooxygenase (COX-2) (2, 25, 31, 45), the rate-limiting enzyme in arachidonate metabolism that catalyzes the biosynthesis of prostaglandin (PG)H2, the precursor of prostanoids including PGs and thromboxanes (26). Mounting evidence demonstrates that PGs play an important role in inflammation and cancer in the GI tract (7, 13, 18, 26, 60). Interestingly, increased numbers of myofibroblasts, in parallel with elevated levels of COX-2, are seen in the stroma underlying adenomas and carcinomas (1, 2, 8, 45, 62). Therefore, understanding the regulation of COX-2 expression in colonic myofibroblasts may provide insights into the pathogenesis of inflammation-associated cancer in the gut. Despite their importance, the cell signaling pathways that underlie myofibroblast function, particularly in the setting of inflammation, remain incompletely understood.
Many hormones, chemokines, eicosanoids, and inflammatory mediators, induce phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce two second messengers: inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] and diacylglycerol (DAG). Ins(1,4,5)P3 triggers the release of Ca2+ from internal stores (6), whereas DAG directly activates protein kinase C (PKC), a multigene family that encodes at least 10 distinct isoforms that are differentially expressed in cells and tissues (30, 40, 51). PKC isoforms mediate numerous cellular responses elicited by proinflammatory peptides, including the synthesis and release of chemokines and eicosanoids that amplify the inflammatory response (10, 23, 31, 63, 64). However, the mechanisms by which PKC-mediated signals are propagated to critical downstream targets remain incompletely understood in most cell types, and this has not been explored in intestinal myofibroblasts.
Protein kinase D (PKD) is the founding member of a new protein kinase family that occupies a unique position in the signal transduction pathways initiated by DAG and PKC (41). PKD is not only a direct DAG target but also lies downstream of PKCs in a phosphorylation cascade (21, 58, 66, 67) implicated in the regulation of a variety of biological responses, including inflammation (10), oxidative stress (49, 50, 56, 57), cell proliferation (47, 65), and carcinogenesis (9, 15, 22, 29, 33, 43). The regulation and biological function of PKD in response to proinflammatory mediators have not been previously investigated in human myofibroblasts. In particular, PKD until now has not been implicated in the induction of COX-2 in myofibroblasts or in any other cell type.
Bradykinin (BK) and tumor necrosis factor-α (TNF-α) are important proinflammatory mediators implicated in the pathophysiology underlying both ulcerative colitis (4, 5, 12, 48) and colorectal cancer (36, 50a). BK is an endogenous kinin with potent proinflammatory, nociceptive, and vasoactive properties produced by the kallikrein-kinin system (12, 48). BK-initiated signaling events occur via two distinct cell surface G protein-coupled receptors (GPCRs), the constitutively present B2 receptor and the inducible B1 receptor, both of which act through Gαq subunits to propagate signals via PLCβ-mediated hydrolysis of PIP2 (40). TNF-α is a 17-kDa proinflammatory cytokine that plays a pivotal role in regulating the inflammatory signaling cascades characteristic of ulcerative colitis (4) and has been strongly implicated in colitis-associated colon cancer (20, 36). Binding of TNF-α to its receptors, TNF-α receptor 1 (TNFR1) and TNF-α receptor 2 (TNFR2), triggers the formation of a multiprotein complex [TNF receptor 1-associated death domain (TRADD), receptor-interacting protein (RIP), TNF-α receptor-associated factor 2 (TRAF-2)] that initiates downstream signaling via phosphorylation cascades that culminate in the activation of MAP kinases and the transcription factor NF-κB (reviewed in Ref. 53). Myofibroblasts have recently been identified as major mediators of TNF-α-associated colitis, but the precise mechanism(s) involved remains incompletely understood (4). In particular, the possibility that TNF-α signaling cross talks with other proinflammatory mediators, including BK, has not been examined in these cells.
In the present study, we show striking synergistic effects between BK and TNF-α in cultures of human colonic subepithelial myofibroblasts (18Co cells) leading to dramatic COX-2 expression and PKC/PKD axis activation. A salient feature of the results presented here is that knockdown of PKD in these cells via specific small interfering RNAs (siRNAs) prevented the synergistic increase in COX-2 expression induced by BK and TNF-α. Thus we show, for the first time, that PKD plays a critical role in mediating COX-2 expression in response to potent proinflammatory mediators in 18Co cells, a model of human colonic myofibroblasts.
MATERIALS AND METHODS
Cell culture.
18Co cells (CRL-1459) were purchased from American Type Culture Collection (Rockville, MD). These cells share many of the structural and functional characteristics of in situ colonic subepithelial myofibroblasts, including a reversible stellate morphology, α-smooth actin expression, and the presence of multiple cell surface receptors (52). 18Co cells provide a model to elucidate the physiological and pathophysiological functions of intestinal subepithelial myofibroblasts and, accordingly, have been used extensively to study colonic myofibroblast function in a variety of settings (24, 32, 44). 18Co cells were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere containing 10% CO2 and 90% air. For experimental purposes, cells were plated in 35-mm dishes (1 × 105 cells/dish), grown in DMEM containing 10% FBS for 5–7 days until confluent, and used from passages 8–14.
[3H]bradykinin binding.
18Co cells were incubated either in the absence or presence of TNF-α (8.3 ng/ml) for either 4 h or 18 h in serum-free DMEM. Cells were then washed twice with phosphate-buffered saline (PBS) containing 1 mg/ml bovine serum albumin and 20 mM HEPES, pH 7.2 (binding buffer), and incubated for 1 h at 4°C in this binding buffer containing [3H]BK (4 pmol/ml, specific activity 80 Ci/mmol). Nonspecific binding was determined in the presence of 10 μM unlabeled BK. The incubation was terminated by washing the dishes five times with ice-cold binding buffer. After extraction with a solution containing 0.1 M NaOH and 1% SDS, radioactivity was measured by liquid scintillation counting.
Intracellular calcium measurements.
18Co cells grown on glass coverslips for 4–5 days were preincubated with TNF-α (8.3 ng/ml) for 4 h and then washed in Hanks' balanced salt solution supplemented with 0.03% NaHCO3, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, and 0.1% BSA (pH 7.4) (Hanks' buffer). After washing, cells were incubated with 5 μM fura-2 tetra-acetoxymethyl ester (fura-2 AM) from a stock of 1 mM in dimethyl sulfoxide for 10 min in a 37°C incubator. Cells were then washed again with Hanks' buffer and left at room temperature for an additional 5 min. The fura-loaded cells were introduced in a cuvette containing the incubation medium (Hanks' buffer), and the cuvette was placed into a Hitachi (Tokyo, Japan) F-2000 fluorospectrophotometer. The incubation medium in the cuvette was continuously stirred at 37°C. The excitation wavelengths were set at 340 and 380 nm, and the emission wavelength was set at 510 nm. Maximum fluorescence was determined after membrane permeabilization by the addition of 37.5 μM digitonin. Minimum fluorescence was measured after the Ca2+ in the solution was chelated by the addition of EGTA at a final concentration of 25 mM. A Kd of 224 nM was used for the Ca2+ dissociation constant from fura 2 in the cells at 37°C. Intracellular Ca2+ concentration ([Ca2+]i) was determined automatically by the cation measurement software of the F-2000 fluorospectrophotometer. BK was added as indicated in the individual experiments.
Immunoblotting and detection of PKD and COX-2.
Confluent 18Co cells, treated with different agonists, antagonists, inhibitors, and siRNA as indicated in the individual experiments, were lysed in 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (20 mM Tris·HCl, pH 6.8, 6% SDS, 2 mM EDTA, 4% 2-mercaptoethanol, 10% glycerol) and boiled for 10 min. After SDS-PAGE, proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A at 4°C for 5 h with a Bio-Rad transfer apparatus. The transfer buffer consisted of 200 mM glycine, 25 mM Tris, 0.01% SDS, and 20% CH3OH. For detection of proteins, membranes were blocked with 5% nonfat dried milk in PBS (pH 7.2) and then incubated for 2 h with the desired antibodies diluted in PBS (pH 7.2) containing 3% nonfat dried milk. Primary antibodies bound to immunoreactive bands were visualized by enhanced chemiluminescence (ECL).
Real-time PCR.
18Co cells, maintained as described above, were washed with 4 ml of PBS (GIBCO, Grand Island, NY) and harvested with 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was extracted with 0.2 ml of chloroform, centrifuged at 12,000 g for 15 min at 4°C, and precipitated with 0.5 ml of 2-propanol at 12,000 g for 10 min at 4°C. The RNA pellet was washed with 75% ethanol at 7,500 g for 5 min at 4°C, dissolved in 30 μl of RNA Storage Solution containing 1 mM sodium citrate, pH 6.4 (Ambion, Austin, TX), and stored at −20°C for subsequent analysis. RNA concentration was quantified on a spectrophotometer (GeneQuant Pro, Amersham Biotechnology, Piscataway, NJ) reading dual wavelengths of 260 nm and 280 nm.
After RNA extraction, total RNA samples (25 ng) were reverse transcribed and cDNAs were amplified with a TaqMan Gold RT-PCR kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Transcripts encoding human COX-2, microsomal prostaglandin E synthase 1 (mPGES-1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control were quantified by real-time PCR analysis with an ABI Prism 7700 Sequence Detection System (PE Biosystems, Foster City, CA). The human primers used are as follows: COX-2: sense 5′-GGC TCA AAC ATG ATG TTT GCA-3′, antisense 5′-CCT CGC TTA TGA TCT GTC TTG A-3′, and probe 5′-TCT TTG CCC AGC ACT TCA CGC ATC AGT TT-3′; mPGES-1: sense 5′-CGG CAA CTG CTT GTC TTT CT-3′ and antisense 5′-GGA GGG GAG AGC CTT CCT-3′. The human GAPDH primer and probe set were acquired from Applied Biosystems. Thermal cycling conditions for reverse transcription and amplification activation were set at 48°C for 30 min and 95°C for 10 min, respectively. PCR denaturing was set at 95°C at 15 s and annealing/extending at 60°C at 60 s for 40 cycles.
Enzyme-linked immunosorbent assay.
PGE2 was quantified from the supernatant of serum-starved, confluent 18Co cells after treatment conditions according to EIA kit instructions (Prostaglandin E2 EIA kit, Cayman Chemical, Ann Arbor, MI). The collected supernatant was centrifuged at 5,000 g for 5 min to remove cell debris. Absorbance readings were set between 405 and 420 nm on a spectrophotometer.
PKD siRNA transfection.
SMART pool PKD siRNA duplexes were purchased from Dharmacon (Lafayette, CO). The PKD siRNA pool was designed to target the mRNA of human PKD (NM_002742) and consists of four selected siRNA oligonucleotides. The sequences were as follows: oligo 1, CGGCAAAUGUAGUGUAUUAUU; oligo 2, GAACCAACUUGCACAGAGAUU; oligo 3, GGUCUGAAUUACCAUAAGAUU; oligo 4, GGAGAUAGCCAUCCAGCAUUU. siCONTROL nontargeting siRNA no. 3 (D-001210-03-20) was used as the control. 18Co cells were plated at ∼70–80% confluence in a 12-well plate with DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic at 37°C in a humidified atmosphere containing 10% CO2. After 24 h, each well was replaced with 400 μl of DMEM + 10% FBS (no antibiotic). Added to this was a mixture containing the Mirus TKO-IT transfection agent and PKD siRNA or control nontargeting siRNA (total volume: 500 μl/well; total transfection agent: 4 μl/well; siRNA: 50 nM). After incubation for 72 h, cells were used for experiments and subsequently analyzed by Western blot.
Materials.
BK, HOE-140, and the PKC inhibitor GF-109203X were purchased from Sigma (St. Louis, MO). TNF-α was purchased from R&D Systems (Minneapolis, MN). COX-2 antibody was purchased from Cell Signaling Technology (Beverly, MA). The PKC inhibitors Ro31-8220 and Gö-6976 were purchased from Calbiochem (La Jolla, CA). PKD C-20 and total ERK2 polyclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-PKD polyclonal antibodies pSer916 (Millipore, Billerica, MA) and pSer744 (Cell Signaling Technology) detect PKD when it is phosphorylated on Ser916 or Ser744, respectively. ECL detection was performed with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies obtained from GE Healthcare (Piscataway, NJ). [3H]BK (specific activity 80 Ci/mmol) was obtained from PerkinElmer (Waltham, MA).
RESULTS
Bradykinin and TNF-α lead to synergistic COX-2 expression in 18Co cells.
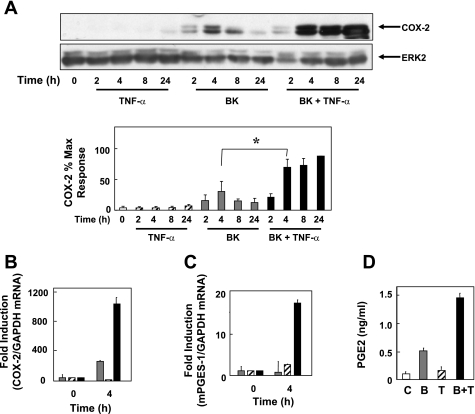
To determine whether COX-2 expression in human colonic myofibroblasts is regulated by proinflammatory mediators, 18Co cells were treated with BK and TNF-α, either alone or in combination, and COX-2 protein was assessed by Western blot analysis. There was no detectable COX-2 protein in unstimulated 18Co cells (Fig. 1A). Treatment of these cells with 100 nM BK induced COX-2 expression that was evident after 2 h, with maximal expression at 4 h followed by a gradual decline over a 24-h time period. Treatment with TNF-α (8.3 ng/ml) stimulated low levels of COX-2 expression that were far lower than those seen in the BK-treated cells. The salient feature of the results shown in Fig. 1 is that combined treatment of myofibroblasts with BK and TNF-α led to a striking synergistic increase in COX-2 protein expression that was evident after 4 h and was dramatically augmented and sustained over a 24-h time period (Fig. 1A).
Fig. 1.
Bradykinin (BK) and tumor necrosis factor-α (TNF-α) induce synergistic cyclooxygenase (COX)-2 expression in 18Co myofibroblasts. A: 18Co cells were washed and equilibrated in serum-free medium for 30 min, followed by treatment with 8.3 ng/ml TNF-α, 100 nM BK, or both for various times (2, 4, 8, and 24 h, as indicated). Lysates of these cells were then analyzed by SDS-PAGE and Western blot using a polyclonal antibody that detects COX-2 protein. Shown is a representative autoluminogram; similar results were obtained in 3 independent experiments. Autoluminograms were quantified by densitometric scanning. Results shown are means ± SE (n = 3) and are expressed as % of maximum level of COX-2 expression. Protein loading was quantified by using an antibody that detects ERK2, and COX-2 expression was corrected to the density of the ERK2 bands. *Statistical significance (P < 0.05). B and C: 18Co cells were treated with 100 nM BK (gray bars), 8.3 ng/ml TNF-α (hatched bars), or both (filled bars) for 4 h, and mRNA encoding COX-2 (B) or microsomal prostaglandin E synthase 1 (mPGES-1, C) were quantified by RT-PCR, as described under materials and methods, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. Results shown are means ± SE of 3 independent experiments and are expressed as fold induction of COX-2 or mPGES-1 mRNA compared with GAPDH mRNA. D: cultures of 18Co cells were incubated in serum-free medium with 100 nM BK (B, gray bar), 8.3 ng/ml TNF-α (T, hatched bar), or both (B+T, filled bar) for 4 h, with untreated cells (open bar) serving as a control (C). PGE2 released into the medium was quantified by ELISA per manufacturer's instructions.
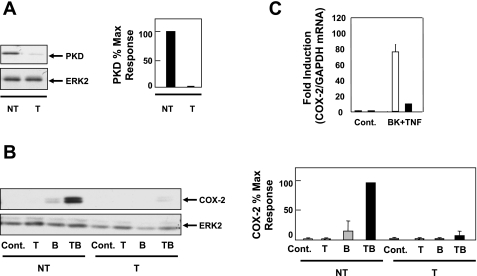
To characterize further the synergistic effects between BK and TNF-α on COX-2 expression in human colonic myofibroblasts, 18Co cells were treated with 100 nM BK, 8.3 ng/ml TNF-α, or both for 4 h and the level of mRNA encoding COX-2 was quantified by real-time PCR analysis. In line with the striking increase in COX-2 protein induced by BK and TNF-α (Fig. 1A), treatment with the combination of these proinflammatory mediators produced a prominent synergistic accumulation of COX-2 mRNA (Fig. 1B). These results show that the enhancement of COX-2 protein expression induced by BK and TNF-α is paralleled by upregulation of transcripts encoding COX-2.
COX-2 converts arachidonic acid to PGH2, leading to the production of PGs, including PGE2, which is implicated in colon cancer. mPGES-1 is the first committed step toward the synthesis of PGE2. To determine the effect of proinflammatory mediators on mPGES-1 mRNA levels, 18Co cells were treated with BK, TNF-α, or both for 4 h and transcripts encoding mPGES-1 were quantified by RT-PCR (Fig. 1C). Treatment with BK and TNF-α induced synergistic increase in the level of mPGES-1 mRNA, which was markedly higher than the level of mPGES-1 mRNA seen after treatment with either BK or TNF-α alone.
We also determined whether exposure to proinflammatory mediators stimulates PGE2 production from 18Co cells. As shown in Fig. 1D, treatment of these cells with BK stimulated PGE2 production, whereas TNF-α had only a small effect. Exposure to both TNF-α and BK induced synergistic production of PGE2, as quantified by ELISA. Thus the proinflammatory mediators BK and TNF-α induced synergistic COX-2 protein expression that was paralleled by increases in the levels of transcripts encoding COX-2 and mPGES-1 and by the production of PGE2 in human colonic myofibroblasts.
TNF-α enhances BK-induced COX-2 expression leading to synergistic COX-2 protein accumulation.
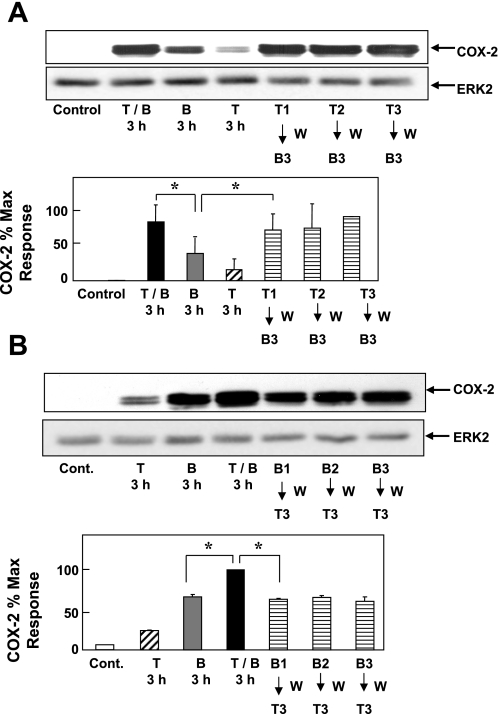
We next determined whether the synergistic effect induced by BK and TNF-α on COX-2 protein expression required simultaneous exposure to both agonists or, alternatively, whether it could be resolved in stages, shown by sequential treatment with the proinflammatory agonists. To distinguish between these possibilities, 18Co cells were treated with TNF-α (8.3 ng/ml) for 1–3 h, washed extensively, and then treated with BK (100 nM for 3 h). The reverse experiment was performed by treating 18Co cells with BK for 1–3 h, followed by washing and treatment with TNF-α for 3 h. For comparison, 18Co cells were exposed to TNF-α and BK simultaneously for 3 h. As shown in Fig. 2, simultaneous treatment with TNF-α and BK led to synergistic COX-2 protein expression in colonic myofibroblasts, as described above. Treatment of 18Co cells with TNF-α followed by BK (Fig. 2A) also induced synergistic expression of COX-2 protein. In contrast, exposure of 18Co cells to BK followed by TNF-α failed to induce any detectable synergistic increase in COX-2 expression (Fig. 2B). These results, suggesting that TNF-α signaling augments BK-induced COX-2 expression, prompted us to identify elements in BK-induced signaling that play a key role in promoting COX-2 expression synergistically with TNF-α.
Fig. 2.
Transient exposure of 18Co cells to TNF-α synergistically enhances BK-induced COX-2 protein accumulation. A: 18Co cells were treated with TNF-α (T1–3, 8.3 ng/ml) for 1–3 h, washed (W) with DMEM, and then treated with BK (B3, 100 nM) for 3 h. COX-2 expression was analyzed by Western blot, and results were compared with 18Co cells treated with TNF-α (T, 8.3 ng/ml) and BK (B, 100 nM), either alone or in combination (T/B), for 3 h. Result shown is a representative autoluminogram. Quantification of COX-2 expression was performed by densitometric scanning. Results shown are means ± SE (n = 3) and are expressed as % of maximum level of COX-2 expression. Protein loading was quantified by using an antibody that detects ERK2, and COX-2 expression was corrected to the density of the ERK2 bands. *Statistical significance (P < 0.05). B: 18Co cells were treated with BK (B1-3, 100 nM) for 1-3 h, washed (W) with DMEM, and then treated with TNF-α (T3, 8.3 ng/ml) for 3 h. Cell lysates were analyzed by SDS-PAGE and Western blot using anti-COX-2 antibody, and results were compared with 18Co cells treated with TNF-α (8.3 ng/ml) and BK (100 nM), either alone or in combination, for 3 h. Autoluminogram shown is representative of at least 3 independent experiments. Results shown are means ± SE (n = 3) and are expressed as % of maximum level of COX-2 expression, quantified by densitometric scanning. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05).
BK induces COX-2 expression via B2 receptor and PKC.
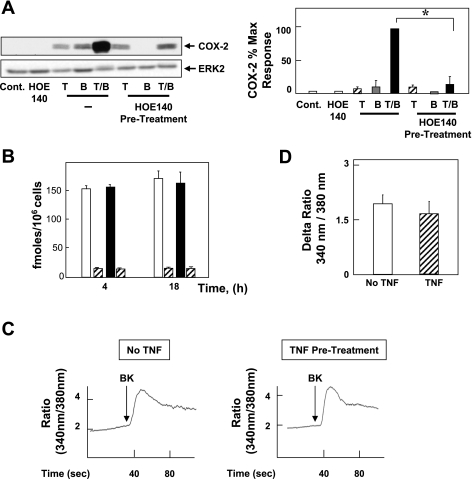
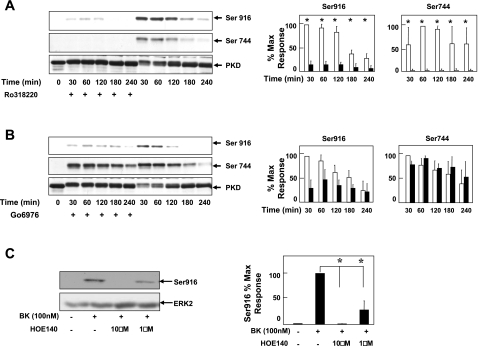
BK-mediated signaling is initiated by its binding to one of two distinct cell surface GPCRs (inducible B1 and constitutively present B2). To determine whether inhibition of the B2 receptor would affect synergistic COX-2 expression, confluent 18Co cells were pretreated with the B2 receptor-specific antagonist HOE-140 (10 μM), followed by treatment with BK, TNF-α, or both for 6 h. As shown in Fig. 3A, pretreatment with HOE-140 prevented both BK-induced COX-2 expression and the synergistic expression of COX-2 in response to BK and TNF-α. HOE-140 had no effect on COX-2 expression induced by TNF-α. These results indicate that the B2 BK receptor mediates expression of COX-2 and synergistic interaction with TNF-α.
Fig. 3.
BK induces COX-2 expression via constitutive B2 receptor; TNF-α does not affect [3H]BK binding or BK-mediated intracellular Ca2+ concentration ([Ca2+]i). A: 18Co cells were pretreated with or without the B2 receptor-specific antagonist HOE-140 (10 μM) for 1 h before stimulation with 8.3 ng/ml TNF-α, 100 nM BK, or both for 6 h, as indicated. Cell lysates were analyzed by SDS-PAGE and Western blot using anti-COX-2 antibody. Result shown is a representative autoluminogram; similar results were obtained in 3 independent experiments. COX-2 expression was quantified by densitometric scanning. Results shown are means ± SE (n = 3) and are expressed as % of maximum level of COX-2 expression. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05). B: 18Co cells were incubated either in the absence (open bars) or in the presence (filled bars) of 8.3 ng/ml TNF-α for either 4 h or 18 h Cells were washed twice with phosphate-buffered saline (PBS) containing 1 mg/ml bovine serum albumin and 20 mM HEPES, pH 7.2 (binding buffer) and incubated for 1 h at 4°C in this binding buffer containing [3H]BK (4 nM, 4 pmol/ml). Nonspecific binding (hatched bars) was determined in the presence of 10 μM unlabeled BK. The incubation was terminated by washing the dishes 5 times with ice-cold binding buffer. After extraction with a solution containing 0.1 M NaOH and 1% SDS, radioactivity was measured by liquid scintillation counting. C: BK (100 nM, indicated by arrow) was added to fura-loaded 18Co cells, and increase in [Ca2+]i was monitored as described in materials and methods for untreated cells (left) and cells preincubated with 8.3 ng/ml TNF-α for 4 h (right). Tracings are representative of 5 separate experiments. D: peak, maximal increment in [Ca2+]i in response to BK (100 nM) in cells incubated without (open bars) or with (hatched bars) 8.3 ng/ml TNF-α for 4 h before BK stimulation.
We next determined whether treatment of 18Co cells with TNF-α increases B2 BK receptors, a plausible mechanism leading to augmentation of BK-induced COX-2 expression by TNF-α. This possibility was assessed by means of binding studies using [3H]BK as the radioligand. Cultures of 18Co cells were treated with or without TNF-α (8.3 ng/ml) for either 4 or 18 h, washed extensively, and then incubated with [3H]BK at 4°C in the absence or presence of excess of unlabeled BK to determine the specific binding of [3H]BK to the cells. As can be seen in Fig. 3B, treatment of 18Co cells with TNF-α did not induce any significant change in specific binding of [3H]BK to the cells. We conclude that TNF-α signaling augments BK-induced COX-2 expression at a postreceptor locus. This conclusion was further substantiated by measuring the elevation of [Ca2+]i induced by BK, one of the earliest events induced by the Gq-coupled B2 receptor. In line with the results indicating that treatment with TNF-α did not increase BK receptor expression, treatment of 18Co cells with TNF-α did not affect the rapid increase in [Ca2+]i induced by BK in these cells (Fig. 3, C and D).
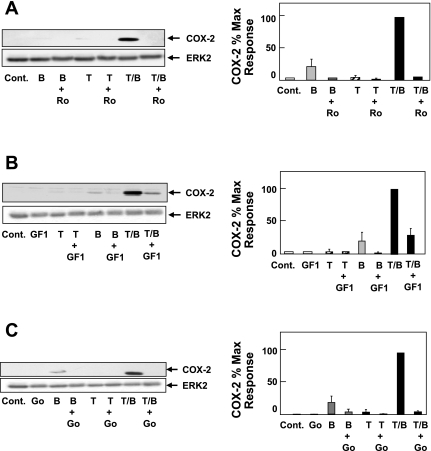
Isoforms of the PKC family are major downstream targets of BK-mediated signaling via Gq-coupled B2 receptors in other cell systems. We next determined the role of PKC in the synergistic expression of COX-2 in response to BK and TNF-α in colonic myofibroblasts. Cultures of 18Co cells were pretreated for 60 min with the preferential PKC inhibitors Ro31-8220 at 2 μM or GF-109203X (also known as bisindolylmaleimide I) at 5 μM, before stimulation with TNF-α and BK for 6 h. Cell treatment with either Ro31-8220 or GF-109203X inhibited the synergistic expression of COX-2 induced by BK and TNF-α (Fig. 4, A and B). These results suggest that PKC isoforms play a fundamental role in the synergistic enhancement of COX-2 expression mediated by TNF-α and BK.
Fig. 4.
BK induces COX-2 expression via protein kinase C (PKC). Cultures of 18Co cells were pretreated for 1 h with the PKC inhibitors Ro31-8220 (Ro, 2 μM; A) and GF-109203X (GF1, 5 μM; B) or the PKC/protein kinase D (PKD) inhibitor Gö-6976 (Go, 10 μM; C). Cultures were then challenged with TNF-α (8.3 ng/ml), BK (100 nM), or both for 6 h and lysed. Cell extracts were analyzed by SDS-PAGE and Western blot using anti-COX-2 antibody. Results shown are representative autoluminograms; similar results were obtained in at least 3 independent experiments for each condition. COX-2 expression was quantified by densitometric scanning. Results shown are means ± SE and are expressed as % of maximum level of COX-2 expression. Equal protein loading was verified with an antibody that detects ERK2.
However, the mechanism(s) by which PKC-mediated signals are propagated to downstream targets and contribute to synergistic COX-2 expression in colonic myofibroblasts is not known. The PKD family has emerged as major downstream targets of PKCs in a phosphorylation cascade that has been implicated in multiple biological responses (see Ref. 41 for review). In this context, we also found that Gö-6976 (10 μM), which inhibits the catalytic activity of classic PKC isoforms (but not novel or atypical PKCs) and PKD (see below), also prevented synergistic accumulation of COX-2 protein in response to BK and TNF-α (Fig. 4C). Given the results shown in Fig. 4, we next determined whether PKD plays a role in mediating COX-2 expression induced by BK and TNF-α.
BK induces PKD activation via B2 receptor and novel PKCs.
To evaluate a possible role of PKD in the synergistic expression of COX-2 in colonic myofibroblasts in response to BK and TNF-α, we first tested whether BK induces PKD activation in these cells. Confluent 18Co cells were treated with BK for various times and lysed. Activation of PKD was assessed by Western blot analysis of the cell lysates using site-specific primary antibodies that detect the phosphorylated state of Ser744 located in the activation loop of PKD (21, 55) and the autophosphorylated state of PKD on Ser916 (27). Treatment of 18Co cells with BK led to a rapid and sustained PKD activation, as judged by phosphorylation on Ser744 and Ser916 (Fig. 5, A and B). To determine the role of PKC in BK-induced PKD activation, 18Co cells were pretreated with the preferential PKC inhibitor Ro31-8220 (2 μM) for 60 min, followed by treatment with BK. Ro31-8220 completely inhibited BK-induced phosphorylation at the activation loop (Ser744) and on the autophosphorylation site (Ser916), in line with a critical role of PKC isoforms in mediating PKD activation (Fig. 5A). In contrast, treatment of 18Co cells with Gö-6976, an inhibitor of conventional but not novel PKCs, failed to inhibit PKD phosphorylation on Ser744 induced by BK (Fig. 5B), suggesting that novel PKC isoforms were responsible for phosphorylation at the activation loop of PKD. In line with a direct inhibitory effect of Gö-6976 on PKD catalytic activity (14), cell exposure to this compound markedly decreased PKD autophosphorylation on Ser916 induced by BK.
Fig. 5.
BK induces PKD activation via B2 receptor and novel PKCs. A and B: 18Co myofibroblasts were washed and equilibrated in serum-free medium for 30 min, pretreated for 1 h with either 2 μM Ro31-8220 (A) or 10 μM Gö-6976 (B) followed by treatment with 100 nM BK for various times (0–240 min). Cell cultures were lysed with 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting performed with antibodies that detect the phosphorylated state of Ser916 or Ser744. In addition, Western blot analysis with an antibody directed against the COOH-terminal region of PKD (PKD-C20) is also shown. Regarding the apparent difference in total PKD in some of the lanes, it should be pointed out that Ser916 (in COOH-terminal region of PKD) lies in the epitope recognized by the PKD-C20 antibody and that the phosphorylation of this residue (as induced by BK) was found to interfere with the binding of PKD-C20 antibody to its epitope. In line with this explanation, the results show that immunoreactivity with PKD-C20 was inversely related to Ser916 phosphorylation. Results shown are representative of at least 3 independent experiments. PKD phosphorylation at Ser744 and Ser916 either in the absence (open bars) or presence (filled bars) of R031-8220 or Gö-6976 was quantified by densitometric analysis and shown as means ± SE, expressed as % of maximum level of phosphorylated Ser916 or Ser744. C: 18Co cells were pretreated without or with the B2 BK receptor antagonist HOE-140 for 60 min at 1 μM or 10 μM followed by treatment with 100 nM BK. PKD phosphorylation at Ser916 was analyzed by Western blot. Membranes were stripped and reprobed for ERK2 to verify equal protein loading. Result shown is a representative autoluminogram; similar results were obtained in at least 3 independent experiments. Ser916 phosphorylation was quantified by densitometric scanning. Results shown are means ± SE and are expressed as % of maximum level of phosphorylated Ser916. Equal protein loading was verified with an antibody that detects actin. *Statistical significance (P < 0.05).
To identify the BK receptor involved in the activation of PKD in colonic myofibroblasts, 18Co cells were pretreated with the B2 receptor antagonist HOE-140 at either 1 μM or 10 μM before stimulation with the agonist and PKD activation was assessed by autophosphorylation on Ser916. As shown in Fig. 5C, pretreatment with HOE-140 inhibited BK-induced PKD activation, with complete inhibition occurring at 10 μM. These results demonstrate that BK triggers PKD activation via the Gq-coupled B2 receptor in colonic myofibroblasts and raised the possibility that PKD could mediate cross talk between BK and TNF-α.
TNF-α augments BK-mediated PKD activation.
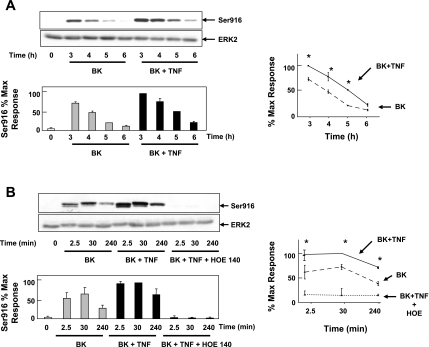
As a first step to examine the role of PKD in the synergistic effects of proinflammatory mediators, we determined whether TNF-α enhances the intensity and/or duration of BK-induced PKD activation in colonic myofibroblasts. Confluent 18Co cells were treated with TNF-α for 4–18 h, and PKD activation was assessed by Western blotting using site-specific antibodies that detect the phosphorylated state of Ser744 or Ser916. TNF-α, added alone at various concentrations (8–25 ng/ml), did not induce any detectable increase in PKD phosphorylation in 18Co cells (data not shown). However, pretreatment of 18Co cells with TNF-α enhanced the intensity and duration of BK-mediated PKD activation (Fig. 6A). Pretreatment with the B2 receptor antagonist HOE-140 (10 μM) completely inhibited the enhanced PKD activation after treatment with BK and TNF-α (Fig. 6B). These experiments demonstrate that TNF-α augments BK B2 receptor-mediated PKD activation in 18Co cells, while having no detectable effects on PKD activation in these cells when added alone.
Fig. 6.
TNF-α augments BK-mediated PKD activation. A: 18Co cells were incubated in the presence or absence of TNF-α (25 ng/ml) for 18 h, followed by stimulation with BK (100 nM) over various times (3, 4, 5, or 6 h, as indicated). Cells were lysed, and extracts were analyzed by SDS-PAGE and Western blotting using an antibody that detects the phosphorylated state of COOH-terminal autophosphorylation site (Ser916). Result shown is a representative autoluminogram; band intensity was analyzed by densitometric scanning of 3 independent experiments and is depicted in graphic form, bottom and right, presented as means ± SE and expressed as % of maximum level of phosphorylated Ser916. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05). B: 18Co cells were pretreated without or with the B2 BK receptor antagonist HOE-140 (10 μM) for 1 h and then without or with 100 nM BK for 2.5, 30, or 240 min. Some cultures were pretreated for 4 h with TNF-α (8.3 ng/ml), as indicated. Cell lysates were analyzed by SDS-PAGE and Western blot using pS916 site-specific antibody. Western blot shown is representative of at least 3 independent experiments. Band intensity was analyzed by densitometric scanning and is depicted in graphic form, bottom and right, presented as means ± SE, and expressed as % of maximum level of phosphorylated Ser916. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05).
Synergistic COX-2 expression induced by BK and TNF-α is mediated by PKD.
The stimulatory effects produced by TNF-α and BK on COX-2 expression and PKD activation and the inhibitory effects on both responses by preferential PKC/PKD inhibitors reinforced the hypothesis that PKD plays a critical role in mediating synergistic COX-2 expression in response to TNF-α and BK. To test this hypothesis directly, we determined whether siRNA-mediated knockdown of PKD protein in 18Co cells abrogates the synergistic expression of COX-2 in response to the proinflammatory mediators. After the optimal concentration of PKD siRNA (50 nM), conditions for transfection, and incubation time (72 h) in 18Co cells were determined, lysates of cells treated with targeting or nontargeting PKD siRNAs were analyzed by Western blotting to assess the level of PKD. As shown in Fig. 7A, siRNA targeting PKD produced a robust depletion of PKD protein in 18Co cells.
Fig. 7.
PKD mediates synergistic COX-2 expression induced by BK and TNF-α. A: 18Co cells were transfected with either nontargeting small interfering RNA (siRNA) (NT) or with PKD siRNA (T) at 50 nM in the presence of 4 μl of Mirus TKO-IT transfection agent for 72 h. Cells were lysed, and level of PKD protein was analyzed by Western blotting using the anti-PKD-C20 antibody. Antibody against ERK2 was used to verify equal protein loading. Similar results were obtained in 4 independent experiments. Band intensity was analyzed by densitometric scanning and is presented as means ± SE (n = 4) and expressed as % of maximum level of PKD. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05). B: 18Co cells were transfected with 50 nM siRNA targeting PKD or with a nontargeted sequence also at 50 nM, as described in A, followed by stimulation with 100 nM BK, 8.3 ng/ml TNF-α, or both for 4 h. Cell lysates were analyzed by SDS-PAGE and Western blotting using anti-COX-2 antibody. Antibody against ERK2 was used to verify equal protein loading. Western blot shown is representative of 4 independent experiments. Band intensity was analyzed by densitometric scanning and is presented as means ± SE (n = 4) and expressed as % of maximum level of COX-2. C: after transfection of 18Co cells with either PKD siRNA (filled bar) or a scrambled nontargeted sequence (open bar), cells were treated with both 100 nM BK and 8.3 ng/ml TNF-α for 4 h and COX-2 mRNA levels were quantified by RT-PCR and compared with untreated cells (Cont). GAPDH mRNA was used as an internal control. Results shown are means ± SE of 3 independent experiments and are expressed as fold induction of COX-2 mRNA compared with GAPDH mRNA.
Having demonstrated that PKD protein can be strikingly depleted in 18Co cells via transfection with PKD siRNA, we next determined the role of PKD in mediating synergistic COX-2 expression in response to BK and TNF-α. As shown in Fig. 7B, knockdown of PKD in 18Co cells completely prevented the synergistic increase in COX-2 protein induced by treatment with BK and TNF-α. In addition, PKD depletion drastically reduced COX-2 mRNA levels induced by BK and TNF-α (Fig. 7C). The results show that PKD plays a critical role in mediating synergistic expression of COX-2 protein and mRNA induced by BK and TNF-α in 18Co myofibroblasts.
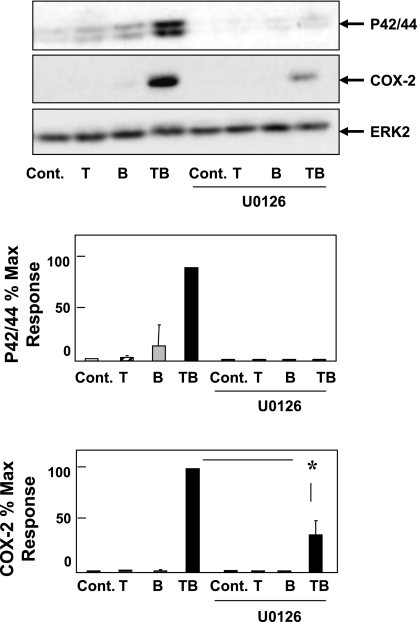
In other cell types, including fibroblasts, one of the mechanisms by which PKD mediates GPCR-induced signaling is by increasing the duration of MEK/ERK/p90 ribosomal S6 kinase (p90RSK) activation (47), one of the major pathways leading to COX-2 transcriptional activation (28, 46, 59). It is known that TNF-α induces only weak stimulation of ERK signaling in most cells (53). However, we found that treatment of 18Co cells with TNF-α, under conditions that enhanced PKD activation, resulted in marked prolongation of BK-induced ERK signaling (data not shown), and consequently we hypothesized that the ERKs could play a role in relaying the PKD signal. To examine this possibility, 18Co cells were incubated for 1 h in the absence or presence of 5 μM U-0126, a selective inhibitor of MEK, and subsequently challenged with TNF-α, BK, or both. After 4 h, the cells were lysed and extracts were analyzed by immunoblotting using a site-specific antibody that detects the activated form of ERK1/2 phosphorylated on Thr202 and Tyr204 and another antibody to detect COX-2 protein. As shown in Fig. 8, exposure of 18Co cells to the combination of TNF-α and BK produced synergistic stimulation of ERK activation and, as expected from the previous results shown in this study, a striking increase in COX-2 protein expression.
Fig. 8.
PKD-mediated synergistic COX-2 expression is inhibited by U-0126. 18Co cells were treated with TNF-α (8.3 ng/ml), BK (100 nM), or both for 6 h with or without preincubation with U-0126 (5 μM), a selective MEK inhibitor, for 60 min. Combined treatment with TNF-α and BK led to synergistic COX-2 expression, as previously described, and also markedly augmented ERK phosphorylation. Preincubation with U-0126 inhibited ERK phosphorylation and significantly reduced COX-2 expression in response to TNF-α and BK. Result shown is a representative autoluminogram; band intensity was analyzed by densitometric scanning of 3 independent experiments, presented as mean ± SE, and expressed as % of maximum level of p42/44 and COX-2. Equal protein loading was verified with an antibody that detects ERK2. *Statistical significance (P < 0.05).
The salient feature of the results shown in Fig. 8 is that exposure to U-0126, at a concentration that completely prevented ERK activation, markedly reduced COX-2 expression in response to stimulation with the combination of TNF-α and BK. We verified that cell treatment with U-0126 did not inhibit BK-induced PKD activation (scored by Ser916 phosphorylation), demonstrating that ERK signaling is downstream of PKD (results not shown). Collectively, these results suggest that the ERK pathway contributes to PKD signaling leading to COX-2 expression.
DISCUSSION
The association between chronic inflammation and the development of cancer is a well-established but incompletely understood phenomenon (20). Numerous epidemiologic, cell culture, and animal models have supported this association, with one of the best-known examples seen in patients with ulcerative colitis. Although the fundamental mechanism is not known, the pathophysiology likely involves complex interactions between neighboring cells of the inflammatory microenvironment (34). These interactions are regulated by an intricate network of signaling pathways that, in a particular context and combination of interacting cells, may predispose to tumor development. In this context, there has been a growing awareness of the importance of the underlying intestinal stroma on the development of colon cancer (1, 2, 45, 62). However, the signal transduction pathways involved remained incompletely understood.
In the present study, we show that treatment of 18Co cells, a model of human colonic myofibroblasts, with the potent proinflammatory mediators TNF-α and BK induces a synergistic increase in the expression of COX-2 protein. Stimulation of myofibroblasts with TNF-α and BK also induced a synergistic increase in the levels of mRNA encoding COX-2 and mPGES-1 and in the production of PGE2. Intestinal myofibroblasts are recognized as a major source of stromal-derived COX-2, and increased numbers of these cells have been seen underlying adenomas and carcinomas in parallel with elevated levels of COX-2 (1, 2, 45, 62). A large body of evidence supports a causal relationship between COX-2 expression, PGE2, and colon carcinogenesis (7, 13, 36, 60), and recent results indicate that TNF-α is a major contributor to colitis-associated cancer (36) through as yet unidentified mechanisms. The BK signaling system has also been associated with colitis in experimental models (3, 19) and in human tissues (48). Interestingly, our results showed that exposure of myofibroblasts to TNF-α alone induced a relatively small increase in COX-2 expression, but exposure to this cytokine dramatically amplified the ability of BK to induce COX-2 expression and PGE2. Consequently, it is conceivable that interactions between the signaling pathways initiated by TNF-α with other inflammatory mediators, including BK, may provide a mechanism for exaggerated cellular responsiveness (e.g., COX-2 expression) that drives the chronic inflammation seen in ulcerative colitis and also predisposes to the development of colitis-associated cancer.
In an effort to elucidate signaling pathways involved in colonic myofibroblasts, we found that the increase in COX-2 expression induced by BK and TNF-α in these cells was prevented by the B2 receptor antagonist HOE-140 and the PKC inhibitors Ro31-8220, GF-109203X, and Gö-6976, indicating that PKCs have a major role in mediating COX-2 induction in myofibroblasts. These results are consistent with the notion that PKC isoforms mediate numerous cellular responses elicited by proinflammatory peptides, including the synthesis and release of chemokines and eicosanoids that amplify the inflammatory response (10, 23, 31, 63, 64). However, the mechanism(s) by which PKC-mediated signals are propagated to critical downstream targets remains incompletely understood in most cell types, and this has not been explored in intestinal myofibroblasts.
Many studies have demonstrated that PKD is rapidly activated by GPCR agonists through a Gq/PLC/PKC-dependent pathway. At baseline, PKD is maintained in a catalytically inactive state through the suppressive actions of its cysteine-rich domain and pleckstrin homology domain (16, 54, 58). PKD is activated by phosphorylation at sites (Ser744 and Ser748) located in the activation loop of the catalytic domain (17, 21, 39, 41, 55). Our results showed that BK-induced PKD phosphorylation on Ser744 and Ser916, a major autophosphorylation site, was prevented by PKC inhibitors (Ro31-8220, GF-109203X). Furthermore, treatment of the cells with Gö-6976, an inhibitor of classic PKCs and PKD, greatly diminished autophosphorylation on Ser916 but did not prevent phosphorylation on Ser744. These results confirmed that PKD is directly inhibited by Gö-6976 and suggest that PKD phosphorylation on the activation loop is mediated by novel (Gö-6976 insensitive) PKCs. Interestingly, exposure of myofibroblasts to TNF-α alone did not produce any detectable increase in PKD phosphorylation either on Ser744 or on Ser916, but this cytokine greatly increased the intensity and duration of BK-induced PKD phosphorylation.
Accumulating evidence demonstrates that PKD plays an important role in several cellular processes and activities, including signal transduction, chromatin organization, Golgi function, gene expression, immune regulation and cell survival, adhesion, motility, differentiation, and DNA synthesis and proliferation (reviewed in Refs. 40, 41). However, none of the previous studies demonstrated a link between PKD and COX-2 expression. Our results, from multiple pharmacological interventions, suggested that PKD could be a point of integration of inputs from TNF-α and BK leading to dramatic COX-2 expression in colonic myofibroblasts. To test this possibility further, we used siRNA targeting PKD in myofibroblasts and then challenged these cells with BK and TNF-α. Under conditions that resulted in siRNA-mediated depletion in PKD protein, the synergistic effect of these proinflammatory mediators on COX-2 expression was completely blocked. These results, together with the potent inhibitory effects on COX-2 expression and PKD activation produced by preferential PKC inhibitors (Ro31-8220, GF-109203X) and a direct PKD inhibitor (Gö-6976), imply that PKD is a critical downstream target that links TNF-α and BK to COX-2 expression.
In a variety of cell types, COX-2 gene expression is upregulated by several transcription factors, including cAMP-response element-binding protein (CREB), CCAAT/enhancer-binding protein (C/EBP), activator protein 1 (AP-1), and NF-κB (see Ref. 11) and repressed by histone deacetylases (HDACs), including HDAC4 (61). A mechanism by which PKD mediates Gq-coupled receptor signaling is by increasing the duration of the MEK/ERK/p90RSK activation (47), one of the major pathways leading to COX-2 transcriptional activation via AP-1 (28, 46, 59). Here we show that suppression of ERK activation markedly reduced COX-2 expression in response to stimulation with the combination of TNF-α and BK, suggesting that the ERK pathway contributes to PKD signaling leading to COX-2 expression. Recently, PKD has been implicated in the regulation of NF-κB and CREB activity and in promoting nuclear extrusion of class II HDACs (41). Thus PKD could act as a switch of COX-2 expression in colonic myofibroblasts through different transcriptional pathways, a proposition that warrants further experimental work.
In conclusion, our results demonstrate potent synergistic effects between TNF-α and BK leading to COX-2 expression through PKD in colonic myfibroblasts. Given that TNF-α, BK, COX-2, and myofibroblasts are increasingly implicated in intestinal inflammation and cancer, the cross talk between TNF-α and BK, identified here, leading to synergistic enhancement of COX-2 expression through PKD provides an attractive mechanism potentially leading to colitis-associated cancer. A better understanding of the contribution of stromal cells to inflammation and tumorigenesis could lead to new therapeutic approaches and novel treatment strategies. In this context, PKD emerges as a potential novel target for the prevention and therapy of inflammatory bowel diseases and colorectal cancer.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R0–1-DK-55003, R0-1-DK-56930, and P30-DK-41301 to E. Rozengurt.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126: 829– 836, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res 10: 5870– 5879, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arai Y, Takanashi H, Kitagawa H, Wirth KJ, Okayasu I. Effect of icatibant, a bradykinin B2 receptor antagonist, on the development of experimental ulcerative colitis in mice. Dig Dis Sci 44: 845– 851, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205: 331– 337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrie A, Regueiro M. Biologic therapy in the management of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 13: 1424– 1429, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11– 21, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 310: 1504– 1510, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chapple KS, Cartwright EJ, Hawcroft G, Tisbury A, Bonifer C, Scott N, Windsor ACJ, Guillou PJ, Markham AF, Coletta PL, Hull MA. Localization of cyclooxygenase-2 in human sporadic colorectal adenomas. Am J Pathol 156: 545– 553, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Deng F, Singh SV, Wang QJ. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res 68: 3844– 3853, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in nontransformed human colonic epithelial cells through NF-kappaB. Am J Physiol Cell Physiol 292: C767– C777, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 29: 4325– 4339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devani M, Vecchi M, Ferrero S, Avesani EC, Arizzi C, Chao L, Colman RW, Cugno M. Kallikrein-kinin system in inflammatory bowel diseases: intestinal involvement and correlation with the degree of tissue inflammation. Dig Liver Dis 37: 665– 673, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dingzhi W, Raymond ND. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett 267: 197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase C isoenzymes. FEBS Lett 392: 77– 80, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res 62: 1632– 1640, 2002 [PubMed] [Google Scholar]
- 16.Iglesias T, Rozengurt E. Protein kinase D activation by mutations within its pleckstrin homology domain. J Biol Chem 273: 410– 416, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem 273: 27662– 27667, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Inatomi O, Andoh A, Yagi Y, Ogawa A, Hata K, Shiomi H, Tani T, Takayanagi A, Shimizu N, Fujiyama Y. Matrix metalloproteinase-3 secretion from human pancreatic periacinar myofibroblasts in response to inflammatory mediators. Pancreas 34: 126– 132, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Isordia-Salas I, Pixley RA, Li F, Sainz I, Sartor RB, Adam A, Colman RW. Kininogen deficiency modulates chronic intestinal inflammation in genetically susceptible rats. Am J Physiol Gastrointest Liver Physiol 283: G180– G186, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Itzkowitz SH, Yio X. Inflammation and cancer. IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287: G7– G17, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem 283: 12877– 12887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E. Insulin potentiates Ca2+ signaling and phosphatidylinositol 4,5-bisphosphate hydrolysis induced by Gq protein-coupled receptor agonists through an mTOR-dependent pathway. Endocrinology 148: 3246– 3257, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Koon HW, Zhao D, Zhan Y, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves protein kinase Cdelta activation. J Pharmacol Exp Ther 314: 1393– 1400, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Laurens K, Thomas TM, Jane EC, Sylvia LFP, Ian RS. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology 130: 127– 136, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol Gastrointest Liver Physiol 273: G1341– G1348, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol 42: 55– 80, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase C mu. J Biol Chem 274: 26543– 26549, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Mifflin RC, Saada JI, Di Mari JF, Adegboyega PA, Valentich JD, Powell DW. Regulation of COX-2 expression in human intestinal myofibroblasts: mechanisms of IL-1-mediated induction. Am J Physiol Cell Physiol 282: C824– C834, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res 101: 1237– 1246, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J 370: 361– 371, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohama T, Okada M, Murata T, Brautigan DL, Hori M, Ozaki H. Sphingosine-1-phosphate enhances IL-1beta-induced COX-2 expression in mouse intestinal subepithelial myofibroblasts. Am J Physiol Gastrointest Liver Physiol 295: G766– G775, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Pacheco II, MacLeod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 295: G748– G759, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Paolucci L, Rozengurt E. Protein kinase D in small cell lung cancer cells: rapid activation through protein kinase C. Cancer Res 59: 572– 577, 1999 [PubMed] [Google Scholar]
- 34.Peek RM, Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a National Cancer Institute-sponsored meeting. Cancer Res 65: 8583– 8586, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118: 560– 570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol 289: G2– G7, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rey O, Reeve JR, Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cepsilon. J Biol Chem 279: 34361– 34372, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589– 602, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 280: 13205– 13208, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol 177: 5968– 5979, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Scholz RP, Regner J, Theil A, Erlmann P, Holeiter G, Jahne R, Schmid S, Hausser A, Olayioye MA. DLC1 interacts with 14-3-3 proteins to inhibit RhoGAP activity and block nucleocytoplasmic shuttling. J Cell Sci 122: 92– 102, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res 66: 846– 855, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10−/− mice. Gastroenterology 118: 337– 345, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem 273: 22120– 22127, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in Swiss 3T3 cells. J Biol Chem 279: 16883– 16893, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, Wilczok T, Colman RW. Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 289: G361– G366, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol 24: 2614– 2626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J 22: 109– 120, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-α as a tumour promoter. Eur J Cancer 42: 745– 750, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J 376: 545– 552, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valentich JD, Popov V, Saada JI, Powell DW. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol Cell Physiol 272: C1513– C1524, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45– 65, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Waldron RT, Iglesias T, Rozengurt E. The pleckstrin homology domain of protein kinase D interacts preferentially with the eta isoform of protein kinase C. J Biol Chem 274: 9224– 9230, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem 276: 32606– 32615, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem 279: 27482– 27493, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Waldron RT, Rozengurt E. Oxidative stress induces protein kinase D activation in intact cells: involvement of Src and dependence on protein kinase C. J Biol Chem 275: 17114– 17121, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem 278: 154– 163, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res 65: 1822– 1829, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Wang D, Dubois RN. Prostaglandins and cancer. Gut 55: 115– 122, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang WL, Lee YC, Yang WM, Chang WC, Wang JM. Sumoylation of LAP1 is involved in the HDAC4-mediated repression of COX-2 transcription. Nucl Acids Res 36: 6066– 6079, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wendum D, Comperat E, Boelle PY, Parc R, Masliah J, Trugnan G, Flejou JF. Cytoplasmic phospholipase A2 alpha overexpression in stromal cells is correlated with angiogenesis in human colorectal cancer. Mod Pathol 18: 212– 220, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Xu K, Chang CM, Gao H, Shu HKG. Epidermal growth factor-dependent cyclooxygenase-2 induction in gliomas requires protein kinase C-delta. Oncogene 28: 1410– 1420, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates interleukin-8 expression through modulation of I kappa B alpha phosphorylation and p65 transcriptional activity: involvement of protein kinase C alpha. Mol Pharmacol 67: 2025– 2031, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Zhukova E, Sinnett-Smith J, Rozengurt E. Protein kinase D potentiates DNA synthesis and cell proliferation induced by bombesin, vasopressin, or phorbol esters in Swiss 3T3 cells. J Biol Chem 276: 40298– 40305, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J 15: 6220– 6230, 1996 [PMC free article] [PubMed] [Google Scholar]
- 67.Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J Biol Chem 272: 23952– 23960, 1997 [DOI] [PubMed] [Google Scholar]