Abstract
Bronchiectasis is defined by permanent and abnormal widening of the bronchi. This process occurs in the context of chronic airway infection and inflammation. It is usually diagnosed using computed tomography scanning to visualize the larger bronchi. Bronchiectasis is also characterized by mild to moderate airflow obstruction. This review will describe the pathophysiology of noncystic fibrosis bronchiectasis. Studies have demonstrated that the small airways in bronchiectasis are obstructed from an inflammatory infiltrate in the wall. As most of the bronchial tree is composed of small airways, the net effect is obstruction. The bronchial wall is typically thickened by an inflammatory infiltrate of lymphocytes and macrophages which may form lymphoid follicles. It has recently been demonstrated that patients with bronchiectasis have a progressive decline in lung function. There are a large number of etiologic risk factors associated with bronchiectasis. As there is generally a long-term retrospective history, it may be difficult to determine the exact role of such factors in the pathogenesis. Extremes of age and smoking/chronic obstructive pulmonary disease may be important considerations. There are a variety of different pathogens involved in bronchiectasis, but a common finding despite the presence of purulent sputum is failure to identify any pathogenic microorganisms. The bacterial flora appears to change with progression of disease.
Keywords: bronchiectasis, inflammation, obstructive lung disease, pathophysiology, pathology
Introduction
Bronchiectasis is defined by the presence of permanent and abnormal dilation of the bronchi.1,2 This usually occurs in the context of chronic airway infection causing inflammation. The main clinical manifestation is a productive cough. Bronchiectasis is currently nearly always diagnosed using high-resolution computed tomography (HRCT) scanning. The main diagnostic features are: 1) internal diameter of a bronchus is wider than its adjacent pulmonary artery; 2) failure of the bronchi to taper; and 3) visualization of bronchi in the outer 1–2 cm of the lung fields.3 This review will describe the pathophysiology of noncystic fibrosis (CF) bronchiectasis.
With the widespread availability of HRCT it has been realized that bronchiectasis remains a common and important cause of respiratory disease. It has been estimated that there are at least 110,000 adults in the United States with this condition.4 In addition, there is overlap with chronic obstructive pulmonary disease (COPD) with two studies reporting an incidence of bronchiectasis in COPD as being 29%5 and 50%,6 respectively.
Bronchiectasis is characterized by mild to moderate airflow obstruction7–11 that tends to worsen over time.12–14 The most widely known model of the development of bronchiectasis is Cole’s “vicious cycle hypothesis”.15 In this model, Cole proposed that an environmental insult often on a background of genetic susceptibility impaired muco-ciliary clearance resulting in persistence of microbes in the sinobronchial tree and microbial colonization. The microbial infection caused chronic inflammation resulting in tissue damage and impaired mucociliary motility. In turn this led to more infection with a cycle of progressive inflammation causing lung damage. The current view is that the two factors required for the development of this condition are persistent infection and a defect in host defense.
There are no well established animal models of bronchiectasis nor have there been studies performed in the early stages of the disease. Bronchiectasis is also a very heterogeneous condition and can be considered the end result of a variety of different factors. As a consequence the pathophysiologic processes are still not well defined. This review will discuss different aspects in which this condition may develop.
Pathology
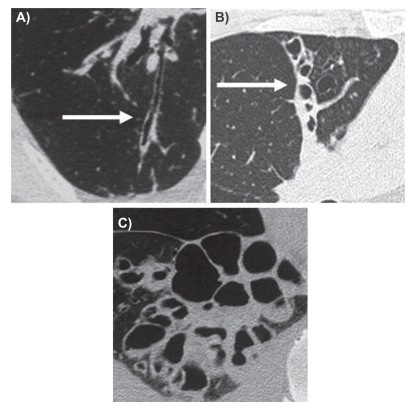
Most studies of the pathology of bronchiectasis were reported between 1930 and 1960 as there was access to significant quantities of operative and postmortem lung specimens at this time. Reid categorized bronchiectasis as having three main phenotypes: 1) tubular characterized by smooth dilation of the bronchi; 2) varicose in which the bronchi are dilated with multiple indentations; and 3) cystic in which dilated bronchi terminate in blind ending sacs.16 The current major form seen on HRCT is the tubular form of bronchiectasis. The CT appearance of these three different forms of bronchiectasis is demonstrated in Figure 1.
Figure 1.
HRCT examples of Reid’s three forms of bronchiectasis: A) tubular B), varicose, and C) cystic.
Abbreviation: HRCT, high-resolution computed tomography.
Arguably the most definitive study of the pathology of bronchiectasis was performed by Whitwell17 who studied 200 consecutive operative lung specimens. This study demonstrated marked inflammation of the bronchial wall, principally in the smaller airways. Bronchial dilation was characterized by deficiency/loss of elastin and more advance disease by destruction of muscle and cartilage. The specific mechanism of how this loss of tissue leads to bronchial dilatation is not known. There was variable bronchial wall fibrosis, atelectasis and peribronchial pneumonic change. Whitwell classified bronchiectasis into three different types: follicular, saccular, and atelectatic. Follicular bronchiectasis was the dominant form and this corresponded to tubular bronchiectasis (the main form commonly seen).
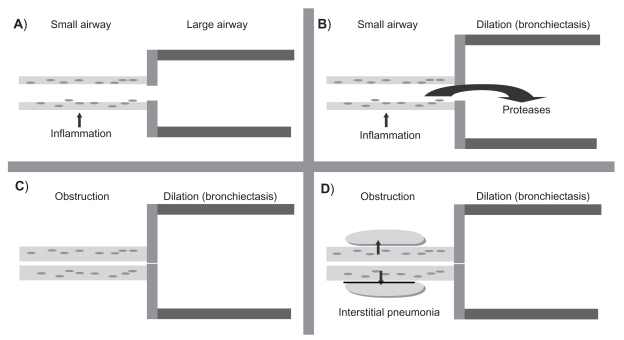
Follicular bronchiectasis was characterized by the presence of lymphoid follicles in the bronchial wall. The inflammatory process commenced in the small airway. This small airway inflammation caused the release of mediators such as proteases which damaged the large airways causing loss of elastin and other components such as muscle and cartilage which resulted in bronchial dilation. With progression of the disease lymphoid follicles enlarged in size and caused airflow obstruction to the small airways. The final event was spread of the inflammation beyond the airways to cause interstitial pneumonia. This process is demonstrated in Figure 2. Recently Hogg and colleagues18 described similar lymphoid follicles in chronic obstructive pulmonary disease (COPD) which had a strong association with bronchial wall thickening and airflow obstruction.
Figure 2.
Pathologic changes in follicular bronchiectasis as described by Whitwell. A) The first process involves infection of the small airways. B) This leads to the release of inflammatory mediators such as proteases which damage the large airways resulting in bronchial dilation and bronchiectasis. C) Infection drives progressive inflammation in the small airways which become thicker from a combination of cell-mediated inflammatory infiltrate and lymphoid follicles resulting in obstruction. D) The final process involves the spread of inflammation beyond the airways resulting in interstitial pneumonia.
The dominant cell types involved in the inflammatory process in bronchiectasis are neutrophils, lymphocytes, and macrophages.19 Neutrophils are the most prominent cell type in the bronchial lumen20,21 and release mediators, particularly proteases/elastase which cause bronchial dilation (ie, bronchiectasis).22,23 The infiltrate in the cell wall is predominantly composed of macrophages and lymphocytes.20,24 Studies have reported that the main lymphocyte is the T cell24,25 and these are cells that are likely to produce the lymphoid follicles described by Whitwell.
Emphysema may also occur in bronchiectasis. Loubeyre and colleagues26 reported that half of a cohort of subjects had localized emphysema in association with bronchiectasis. This presumably corresponds to the interstitial pneumonia described by Whitwell that spreads into the parenchyma of the lungs to cause localized damage.
Distribution of bronchiectasis
The distribution of bronchiectasis may be associated with different pathophysiologic processes eg, allergic bronchopulmonary aspergillosis is classically associated with central bronchiectasis. Bronchiectasis has been described as being localized (ie, confined to one lobe) or generalized. Most commonly it is generalized and seems to be most common in the lower lobes.2,27 The involvement of the lower lobes may reflect gravity dependent retention of infected secretions.
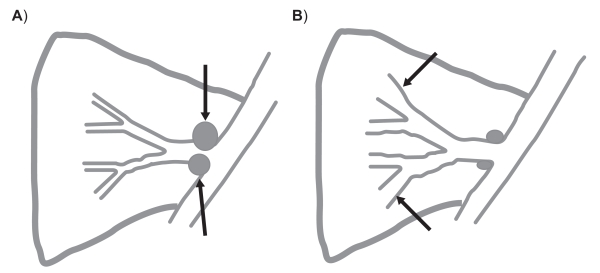
Right middle lobe bronchiectasis has been well described in the context of tuberculosis.17,28–32 The right middle lobe bronchus is long, often bends sharply at its bifurcation and is of relatively small caliber. A collar of lymph nodes also surrounds the proximal bronchus and any condition that causes a prolonged enlargement of these nodes may lead to obstruction and secondary bronchiectasis. This may also occur in malignancy33 and in nontuberculous mycobacterial infection (often with the lingula lobe).34 This event is demonstrated in Figure 3.
Figure 3.
Pathologic process in the right middle lobe with mycobacterial infection. A) Infection causes enlargement of peribronchial lymph nodes resulting in obstruction. B) The obstruction results in bronchiectasis that persists when the nodes return to normal size.
It is generally not known if bronchiectasis starts in one section of the respiratory tract and then spreads or if it begins as a generalized process. Childhood bronchiectasis has a high proportion of subjects who have chronic rhinosinusitis as well.10,27,35 One report described the apparent spread of sinus infection to the lungs resulting in bronchiectasis.36
Lung function
A number of studies have described the lung function in bronchiectasis. Patients usually have mild to moderate air-flow obstruction. It has also been demonstrated recently that there is a progressive decline in lung function over time with loss of forced expiratory volume in one second (FEV1).12–14 This is a paradoxical finding as the characteristic feature of bronchiectasis is airway dilation.
A CT/lung function study found that airflow obstruction in bronchiectasis was predominantly due to small and medium airway involvement with features of decreased attenuation and mucosal wall thickening. The decreased attenuation was a marker of small airway constrictive bronchiolitis. The small airway constrictive bronchiolitis is diagnosed by the presence of a mosaic pattern on expiratory films.37
The pathology studies by Whitwell explain the finding of airflow obstruction in bronchiectasis. These studies show the large airways become dilated but the small and medium airways are characterized by airflow obstruction predominantly arising from thickening of the bronchial wall. As most of the respiratory tree is composed of medium and small airways the net effect of the inflammatory process in bronchiectasis is airflow obstruction (Figure 2).
Etiologic factors
There have been a large number of factors that have been described as causative for bronchiectasis. A problem with assigning these factors as being causative is that subjects have usually had their lung disease for a long time (often more than 10 years) and the attribution may rely on long-term retrospective recall. It may perhaps be more appropriate to consider them as risk factors rather than the definitive cause. The etiologic factors that have been described generally all have some role in impairing host defence to infection. Some important etiologic factors are discussed below. A list of etiologic factors is given in Table 1.
Table 1.
Etiologic/risk factors associated with bronchiectasis
| Postinfective (postpneumonia, whooping cough, measles, mycobacterial infection) |
| Mucociliary disorder (immotile cilia, Kartagener’s syndrome, Young’s syndrome) |
| Obstructive (foreign body, mycobacterial infection, obstructing cancer) |
| Immune disorder (hypogammaglobulinemia, HIV infection, cancer, allergic bronchopulmonary aspergillosis, transplant rejection) |
| Rheumatic/inflammatory disease (rheumatoid arthritis, inflammatory bowel disease) |
| Extremes of age |
| Malnutrition/socioeconomic disadvantage |
| Chronic obstructive pulmonary disease |
| Aspiration |
| Alpha1-antitrypsin deficiency |
| Miscellaneous (yellow nail syndrome) |
Postinfectious
The most common cause of bronchiectasis is the literature is postinfectious. This is not a well defined entity but the usual context appears to be one acute infectious episode which is thought to result in bronchiectasis. To the author’s knowledge, the development of bronchiectasis after one infectious episode have not been definitively demonstrated in a cohort of patients. At some stage all bronchiectasis patients become colonized with bacteria but this appears to be a little different from acute process in the postinfectious entity that is described in the literature. A possible mechanism for postinfectious bronchiectasis is a significant infection in early childhood which causes structural damage to the developing lung and permits bacterial infection which is not cleared. Over time persistent infection may then result in bronchiectasis.
One well characterized form of bronchiectasis occurs in the context of mycobacterial infection and this form is particularly prevalent in the right middle lobe with nontuberculous mycobacterium.17,38,39 The mechanism of this form of bronchiectasis appears to arise from lymph node obstruction. The acute infection causes enlargement of peribronchial nodes which obstruct the bronchus and result in secondary bronchiectasis. This bronchial dilation persists when the mycobacterial infection resolves and the nodes return to normal size. This process is demonstrated in Figure 3.
Muco-ciliary clearance
Muco-ciliary clearance is a key defence mechanism against pulmonary infection. Its compromise is important in the development of the vicious cycle of bronchiectasis as proposed by Cole.15 Cystic fibrosis is associated with defective muco-ciliary clearance but this area is beyond the scope of the current review. There has been renewed interest recently in ciliary defects and this has been recently reviewed.40–42 The most prominent cilial disorder is primary ciliary dyskinesia (PCD) which combines upper and lower respiratory tract infection, male infertility and in approximately 50%, situs inversus. PCD arises primarily from a defect in the dynein arms which are necessary for normal cilial beating. This area has been the subject of recent discoveries.43,44 Bronchiectasis is a prominent manifestation of PCD. The definitive diagnosis of PCD requires electron microscopy to demonstrate loss of dynein and this requires specialized expertise available only in a limited number of centers. Screening can be undertaken more easily using nasal nitric oxide and in vivo tests of ciliary motility such as the saccharin test. Young’s syndrome is another condition in which the primary defect is thought to be defective mucous function.45
Malnutrition/socioeconomic
Bronchiectasis has been described as being a major problem in developing nations although the prevalence has not been well defined. Authors from South America,46 Hong Kong/China,47 India,48 and Turkey49 all report that bronchiectasis is an important local problem. It is certainly a major problem in certain indigenous populations such as Australian aborigines, New Zealand Maori, and Alaskan natives.50–52 A common feature of these groups is malnutrition and social disadvantage which are likely to be associated with impaired immune function (Torzillo and Chang, pers comm.).53 Malnutrition/socioeconomic disadvantage is not commonly listed as an etiologic factor for bronchiectasis, but in selected populations it may be important.
Extremes of age
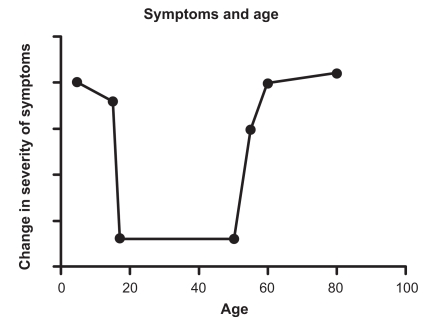
The immune system is less effective in young children and elderly adults which results in an increased incidence of infection in these two groups.54,55 Bronchiectasis has most commonly been described as commencing in childhood, particularly in the first five years of life, with chronic productive cough and unresolved infection.2,10,27 Field performed a long-term prospective follow-up on 225 patients with bronchoscopy. She found that 74% of the cohort had the onset of chronic respiratory infection in the first five years of life and subjects tended to significantly improve in late adolescence regardless of treatment.27 Our group has studied adult patients with bronchiectasis who had the onset of symptoms in childhood.2 Most of these subjects (about two-thirds) described the improvement of their symptoms in late adolescence and then a worsening of their symptoms from the age of 50 to 60 years when they represented for medical assessment. These two studies suggest that a common feature of childhood-onset bronchiectasis may be an improvement with adulthood and then clinical deterioration again beyond the age of 50 years. This is shown in Figure 4.
Figure 4.
Change in severity of symptoms over time in those with childhood-onset bronchiectasis. A common finding in subjects whose symptoms began in childhood is improvement in late adolescence and then deterioration again from about the age of 50 years.
We have also described the phenotype of patients who first develop bronchiectasis in adulthood.35 The onset of chronic chest suppuration cough was uncommon from the ages of 15–50 years. Eighty-one percent of adult-onset patients had the onset of chronic chest suppuration over the age of 50 years. Weycker and colleauges report a prevalence of bronchiectasis as being 4.2 per 100,000 persons aged 18 to 34 years and 272 per 100,000 in those aged over 75 years.4 Therefore extremes of age may be important in the development and clinical manifestations of bronchiectasis. Increasing age has been recently described as being an important factor in COPD.56
Chronic obstructive pulmonary disease
A cardinal feature of COPD is the presence of chronic bronchitis. Recently two studies have reported a high incidence of bronchiectasis in COPD patients. O’Brien and colleagues5 studied 110 patients with COPD who presented with an acute exacerbation in a primary care setting and found HRCT evidence of bronchiectasis in 29% of the cohort. Patel and colleagues6 studied 54 patients with severe COPD for the presence of bronchiectasis. They found that 50% of the patients had co-existent bronchiectasis and this was associated with bacterial airway colonization and inflammatory markers and longer duration of exacerbations.6 Patients with COPD and bronchiectasis tend to have more dyspnea, worse lung function, and a lack of upper airway involvement when compared to other bronchiectasis subjects.35
There is also some overlap in the pathology of COPD and bronchiectasis. Both conditions have neutrophils and T lymphocytes as the predominant inflammatory cell,19,57 protease release causes pulmonary damage and lymphoid follicles have a role in airflow obstruction.17,18
Immune dysfunction
The term immune dysfunction covers a wide variety of causes but some conditions associated with bronchiectasis are primarily categorized as immune dysfunction disorders. These can be considered in terms of primary immune deficiencies such as hypogammaglobulinemia, human immunodeficiency virus (HIV), interferon gamma receptor deficiency, and type I major histocompatibility complex deficiency.2 Detection of hypogammaglobulinemia is important as replacement therapy may have therapeutic implications. The clinical significance of immunoglobulin subclass deficiency is controversial.
Bronchiectasis may occur in the late stages of lung transplant rejection. Allergic bronchopulmonary aspergillosis (ABPA) is a classic cause of bronchiectasis and this is important to diagnose as there are specific ramifications for treatment. Bronchiectasis may also occur in subjects with very high levels of immunoglobulin E but without ABPA.36
Mycobacterial infections have been recognized to be associated with bronchiectasis, particularly in older women where the combination of mycobacterium avium complex (MAC) infections causing obstruction from lymphad-enopathy with right middle lobe bronchiectasis is a well described syndrome.58,59 It should also be remembered that MAC infections are opportunistic and may cause infection in other chronic lung diseases such as COPD and therefore infection with MAC may be secondary to bronchiectasis. It is not clear from the literature what the effect of secondary MAC colonization is on the progression of bronchiectasis. In four children who had disseminated mycobacterial infection, researchers recently found a mutation defect in the gene for interferon gamma receptor, the primary protective cytokine against intracellular mycobacterial infection.60
Rheumatological/inflammatory conditions
There is a well described association between bronchiectasis and rheumatoid arthritis. In rheumatoid arthritis the incidence of bronchiectasis has been described to be 1%–3%. Recent studies of patients with rheumatoid arthritis have described the prevalence of bronchiectasis on HRCT in such patients as being up to 30%.61,62 Bronchiectasis is described as preceding and occurring after the development of rheumatoid arthritis. Bronchiectasis may also occur in association with Sjogren’s syndrome63 and Churg–Strauss syndrome.64 It is possible that immune suppression may predispose to chronic airway infection.64 Bronchiectasis also occurs in subjects with inflammatory bowel disease.65
Alpha1-antitrypsin deficiency
Alpha1-antitrypsin deficiency (AAT) is associated with increased risk of COPD and bronchiectasis. Parr and colleagues studied the prevalence of airways disease in AAT-deficient subjects and found that 70 of 74 subjects had radiological evidence of bronchiectasis and 20 subjects were classified as having clinically significant bronchiectasis.66
Microbiology
A large number of different pathogens have been isolated in studies of microbiologic flora in bronchiectasis. There is often significant variation between different locations. The main findings from recent studies have been that Haemophilus influenzae is the most common pathogen (range 29%–70%) followed by Pseudomonas aeruginosa (range 12%–31%).7,10,67–71 The other major finding of these studies is that 30%–40% of sputum samples despite being good quality and purulent will fail to grow any pathogenic bacteria; and this applies even when bronchoscopy and protected brush/bronchoalveolar lavage is used. The bronchi also have a dynamic turnover of pathogens. A two-year prospective study found that a proportion of patients were continually colonized by Branhamella catarrhalis (the name Branhamella has recently been changed to Moxarella) but there was a continous turnover of strains every 2–3 months.72 In COPD, there is evidence for considerable turnover of bacteria with the acquisition of new strains demonstrated to be associated with exacerbations.73
An important finding of bronchiectasis is that there appears to be a change in microbial flora with severity of disease.71 Typically subjects with the best preserved lung function are most likely to have no pathogenic bacteria isolated. As lung function declines H. influenzae becomes predominant and finally in patients with the most severe disease the usual pathogen isolated is P. aeruginosa.
Specific pathogens
H. influenzae is the most common isolate and is nearly always the nontypeable form (NTHi). P. aeruginosa is associated with more sputum, more extensive bronchiectasis, more hospitalizations and worse quality of life. Nontuberculous mycobacterial infection may be important in bronchiectasis but studies have reported variable prevalence of 2%71,74 to 10%.75
The role of viral infection in bronchiectasis is not well defined. Becroft76 identified adenovirus as a risk factor for the development of bronchiectasis in young children. Influenzae A infection in vitro inhibits neutrophil function in bronchiectasis subjects.77 Viral infections have a role in exacerbations of COPD but this has not been defined for bronchiectasis.78
Effects of bacterial pathogens on the respiratory tract
Bacterial pathogens may exert a number of direct effects on the respiratory tract that impairs host defence. The most well described effects in bronchiectasis are inhibition of the mucociliary clearance. Mediators released by H. influenzae, P. aeruginosa, and Streptococcus pneumoniae may directly interfere with ciliary function, damage ciliated epithelium, and inhibit mucous transport.79 Bacteria release products such as glycoproteins which attract neutrophils. H. influenzae has the capacity to cause direct damage to airway epithelium and is also able to invade into the bronchial wall and interstitium of the lung.80,81 P. aeruginosa has the capacity to form biofilms.82 Biofilms occur particularly in advanced disease and form an impenetrable matrix around the Pseudomonas, shielding it from the effects of the immune system and antibiotics and allowing the bacterial infection to cause severe damage to the underlying lung.
Conclusions
Bronchiectasis is characterized by airway inflammation. The inflammation appears to arise as a combination of immune deficiency and persistent infection. As proposed by Cole this inflammatory process is progressive and results in a cycle of worsening pulmonary damage. Patients develop progressive obstructive lung disease. The airflow obstruction appears to arise predominantly from involvement of the small airways in which the bronchial wall is infiltrated by inflammatory cells particularly lymphocytes which may form lymphoid follicles.
Bronchiectasis is a heterogeneous condition and there are a large number of etiologic factors have been described. Because of the long-term nature of the disease it is often hard to be clear about the exact role of such factors in the pathogenesis. It may be more appropriate to consider them as being risk factors rather than the single cause of longstanding airway infection.
The microbiology of bronchiectasis is complex and varies significantly between different studies. An important finding is that despite purulent sputum and optimal collection techniques (eg, bronchoscopy) there is often failure to isolate a pathogenic microorganism. The role of viruses is not well understood. A better understanding of the microbiology of bronchiectasis would have direct implications for patient treatment.
Bronchiectasis is a complex condition and the pathophysiology is still not well understood. Defining the inflammatory process particularly before there is significant lung disease may be helpful in developing better strategies of treatment.
Acknowledgments
The author would like to thank Associate Professor Peter Holmes and Professor Stephen Holdsworth for their help with this work. The author reports no conflicts of interest in this work.
References
- 1.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 2.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med. 2006;100:2183–2189. doi: 10.1016/j.rmed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.McGuinness G, Naidich DP. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002;40:1–19. doi: 10.1016/s0033-8389(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 4.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;4:205–209. [Google Scholar]
- 5.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–642. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 7.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–961. doi: 10.1378/chest.108.4.955. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CB, Jones PW, O’Leary CJ, Cole PJ, Wilson R. Validation of the St. George’s Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156:536–541. doi: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest. 1998;113:1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 10.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–1284. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 11.Angrill J, Agusti C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–1632. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 12.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Gallagher M, Holmes PW. Outcome in Adult Bronchiectasis. COPD: Journal of Chronic Obstructive Pulmonary Diseases. 2005;2:27–34. doi: 10.1081/copd-200050685. [DOI] [PubMed] [Google Scholar]
- 13.Twiss J, Stewart AW, Byrnes CA. Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thoraxi. 2006;61:414–418. doi: 10.1136/thx.2005.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, Roman-Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132:1565–1572. doi: 10.1378/chest.07-0490. [DOI] [PubMed] [Google Scholar]
- 15.Cole PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. [PubMed] [Google Scholar]
- 16.Reid L. Reduction in bronchial subdivisions in bronchiectasis. Thorax. 1950;5:223–247. doi: 10.1136/thx.5.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitwell F. A study of the pathology and pathogenesis of bronchiectasis. Thorax. 1952;7:213–219. doi: 10.1136/thx.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 19.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J. 2008;31:396–406. doi: 10.1183/09031936.00069007. [DOI] [PubMed] [Google Scholar]
- 20.Eller J, Lapa e Silva JR, Poulter LW, Lode H, Cole PJ. Cells and cytokines in chronic bronchial infection. Ann N Y Acad Sci. 1994;725:331–345. doi: 10.1111/j.1749-6632.1994.tb39816.x. [DOI] [PubMed] [Google Scholar]
- 21.Loukides S, Bouros D, Papatheodorou G, Lachanis S, Panagou P, Siafakas NM. Exhaled H2O2 in steady-state bronchiectasis: relationship with cellular composition in induced sputum, spirometry, and extent and severity of disease. Chest. 2002;121:81–87. doi: 10.1378/chest.121.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Khair OA, Davies RJ, Devalia JL. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]
- 23.Zheng L, Tipoe G, Lam WK, et al. Up-regulation of circulating adhesion molecules in bronchiectasis. Eur Respir J. 2000;16:691–696. doi: 10.1034/j.1399-3003.2000.16d21.x. [DOI] [PubMed] [Google Scholar]
- 24.Lapa e Silva JR, Guerreiro D, Noble B, Poulter LW, Cole PJ. Immunopathology of experimental bronchiectasis. Am J Respir Cell Mol Biol. 1989;1:297–304. doi: 10.1165/ajrcmb/1.4.297. [DOI] [PubMed] [Google Scholar]
- 25.Gaga M, Bentley AM, Humbert M, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax. 1998;53:685–691. doi: 10.1136/thx.53.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loubeyre P, Paret M, Revel D, Wiesendanger T, Brune J. Thin-section CT detection of emphysema associated with bronchiectasis and correlation with pulmonary function tests. Chest. 1996;109:360–365. doi: 10.1378/chest.109.2.360. [DOI] [PubMed] [Google Scholar]
- 27.Field E. Bronchiectasis: a long-term follow-up of medical and surgical cases from childhood. Arch Dis Child. 1961;36:587–603. doi: 10.1136/adc.36.190.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham EA, Burford TH, Mayer TH. Middle lobe syndrome. Postgrad Med. 1948;4:29. doi: 10.1080/00325481.1948.11693655. [DOI] [PubMed] [Google Scholar]
- 29.Brock RC. Post-tuberculosis broncho-stenosis and bronchiectasis of the middle lobe. Thorax. 1950;5:5–11. [Google Scholar]
- 30.Fretheim B. The so-called middle lobe syndrome. Thorax. 1952;7:156–158. doi: 10.1136/thx.7.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albo RJ, Grimes OF. The middle lobe syndrome: a clinical study. Dis Chest. 1966;50:509–518. doi: 10.1378/chest.50.5.509. [DOI] [PubMed] [Google Scholar]
- 32.Bombarda S, Figueiredo CM, Seiscento M, Terra Filho M. Pulmonary tuberculosis: tomographic evaluation in the active and post-treatment phases. Sao Paulo Med J. 2003;121:198–202. doi: 10.1590/S1516-31802003000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertelsen S, Struve-Christensen E, Aasted A, Sparup J. Isolated middle lobe atelectasis: aetiology, pathogenesis, and treatment of the so-called middle lobe syndrome. Thorax. 1980;35:449–452. doi: 10.1136/thx.35.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin DL. Radiology of pulmonary Mycobacterium avium-intracellulare complex. Clin Chest Med. 2002;23:603–612. doi: 10.1016/s0272-5231(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 35.King PT, Holdsworth S, Farmer MW, Freezer N, Villanueva E, Holmes P. Phenotypes of bronchiectasis: onset of productive cough in childhood and adulthood. COPD. 2009;6:130–136. doi: 10.1080/15412550902766934. [DOI] [PubMed] [Google Scholar]
- 36.King P. Bronchiectasis and chronic rhinosinusitis. Respir Med CME. 2008;1:284–285. [Google Scholar]
- 37.Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198–204. doi: 10.1136/thorax.55.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995;19:353–360. doi: 10.1097/00004728-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Fujita J. Radiological findings of non-tuberculous mycobacteria respiratory infection. Kekkaku. 2003;78:557–561. [PubMed] [Google Scholar]
- 40.Bush A, Chodhari R, Collins N, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92:1136–1140. doi: 10.1136/adc.2006.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escudier E, Duquesnoy P, Papon JF, Amselem S. Ciliary defects and genetics of primary ciliary dyskinesia. Paediatr Respir Rev. 2009;10:51–54. doi: 10.1016/j.prrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Hogg C. Primary ciliary dyskinesia: when to suspect the diagnosis and how to confirm it. Paediatr Respir Rev. 2009;10:44–50. doi: 10.1016/j.prrv.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Omran H, Kobayashi D, Olbrich H, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–616. doi: 10.1038/nature07471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colantonio JR, Vermot J, Wu D, et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature. 2009;457:205–209. doi: 10.1038/nature07520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman KJ, Teichtahl H, De Kretser DM, et al. Screening Young syndrome patients for CFTR mutations. Am J Respir Crit Care Med. 1995;152:1353–1357. doi: 10.1164/ajrccm.152.4.7551394. [DOI] [PubMed] [Google Scholar]
- 46.Marostica PJ, Fischer GB. Non-cystic-fibrosis bronchiectasis: a perspective from South America. Paediatr Respir Rev. 2006;7:275–280. doi: 10.1016/j.prrv.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8:691–702. [PubMed] [Google Scholar]
- 48.Sethi GR, Batra V. Bronchiectasis: causes and management. Indian J Pediatr. 2000;67:133–139. doi: 10.1007/BF02726189. [DOI] [PubMed] [Google Scholar]
- 49.Karadag B, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non-cystic-fibrosis bronchiectasis in children: a persisting problem in developing countries. Respiration. 2005;72:233–238. doi: 10.1159/000085362. [DOI] [PubMed] [Google Scholar]
- 50.Singleton R, Morris A, Redding G, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol. 2000;29:182–187. doi: 10.1002/(sici)1099-0496(200003)29:3<182::aid-ppul5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Med J Aust. 2002;177:200–204. doi: 10.5694/j.1326-5377.2002.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 52.Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis “too high” for a developed country. Arch Dis Child. 2005;90:737–740. doi: 10.1136/adc.2004.066472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chinen J, Shearer WT. 6. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2008;121:S388–S392. doi: 10.1016/j.jaci.2007.06.003. quiz S417. [DOI] [PubMed] [Google Scholar]
- 54.English BK, Schroeder HW, Wilson CB. Immaturity of the fetal and neonatal immune system. In: Rich RR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HW, editors. Clinical Immunology, Principles and Practice. London, UK: Mosby; 2001. pp. 40.10–40.41. [Google Scholar]
- 55.Weksler ME, Szabo P. Aging and the immune system. In: Rich RR, Fleicher TA, Shearer WT, Kotzin BL, Schroeder HW, editors. Clinical Immunology; Principles and Practice. London, UK: Mosby; 2001. pp. 41.41–48. [Google Scholar]
- 56.Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61:1043–1047. doi: 10.1136/thx.2006.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 58.Wagner RB, Johnston MR. Middle lobe syndrome. Ann Thorac Surg. 1983;35:679–686. doi: 10.1016/s0003-4975(10)61085-5. [DOI] [PubMed] [Google Scholar]
- 59.De Boeck K, Willems T, Van Gysel D, Corbeel L, Eeckels R. Outcome after right middle lobe syndrome. Chest. 1995;108:150–152. doi: 10.1378/chest.108.1.150. [DOI] [PubMed] [Google Scholar]
- 60.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 61.Cortet B, Flipo RM, Remy-Jardin M, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:815–819. doi: 10.1136/ard.54.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassan WU, Keaney NP, Holland CD, Kelly CA. High resolution computed tomography of the lung in lifelong non-smoking patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:308–310. doi: 10.1136/ard.54.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larche MJ. A short review of the pathogenesis of Sjogren’s syndrome. Autoimmun Rev. 2006;5:132–135. doi: 10.1016/j.autrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.King P. Churgh-Strauss syndrome and bronchiectasis. Respir Med Extra. 2007;3:26–28. [Google Scholar]
- 65.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 66.Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176:1215–1221. doi: 10.1164/rccm.200703-489OC. [DOI] [PubMed] [Google Scholar]
- 67.Roberts DE, Cole P. Use of selective media in bacteriological investigation of patients with chronic suppurative respiratory infection. Lancet. 1980;1:796–797. doi: 10.1016/s0140-6736(80)91295-7. [DOI] [PubMed] [Google Scholar]
- 68.Pang J, Chan HS, Sung JY. Prevalence of asthma, atopy, and bronchial hyperreactivity in bronchiectasis: a controlled study. Thorax. 1989;44:948–951. doi: 10.1136/thx.44.11.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho PL, Chan KN, Ip MS, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114:1594–1598. doi: 10.1378/chest.114.6.1594. [DOI] [PubMed] [Google Scholar]
- 70.Angrill J, Agusti C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Klingman KL, Pye A, Murphy TF, Hill SL. Dynamics of respiratory tract colonization by Branhamella catarrhalis in bronchiectasis. Am J Respir Crit Care Med. 1995;152:1072–1078. doi: 10.1164/ajrccm.152.3.7663786. [DOI] [PubMed] [Google Scholar]
- 73.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 74.Wickremasinghe M, Ozerovitch LJ, Davies G, et al. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005;60:1045–1051. doi: 10.1136/thx.2005.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowler SJ, French J, Screaton NJ, et al. Nontuberculous mycobacteria in bronchiectasis: Prevalence and patient characteristics. Eur Respir J. 2006;28:1204–1210. doi: 10.1183/09031936.06.00149805. [DOI] [PubMed] [Google Scholar]
- 76.Becroft DM. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24:72–82. doi: 10.1136/jcp.24.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pang G, Clancy R, Cong M, Ortega M, Zhigang R, Reeves G. Influenza virus inhibits lysozyme secretion by sputum neutrophils in subjects with chronic bronchial sepsis. Am J Respir Crit Care Med. 2000;161:718–722. doi: 10.1164/ajrccm.161.3.9812047. [DOI] [PubMed] [Google Scholar]
- 78.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 79.Wilson R, Cole PJ. The effect of bacterial products on ciliary function. Am Rev Respir Dis. 1988;138:S49–S53. doi: 10.1164/ajrccm/138.6_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 80.Moller LV, Timens W, van der Bij W, et al. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157:950–956. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 81.Bandi V, Apicella MA, Mason E, et al. Nontypeable haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 82.Davies JC, Bilton D. Bugs, biofilms, and resistance in cystic fibrosis. Respir Care. 2009;54:628–640. doi: 10.4187/aarc0492. [DOI] [PubMed] [Google Scholar]