Although neuroimaging studies confirm the frontal lobe’s involvement in language processes and auditory working memory1,2, the cellular and network basis of these functions is unclear. Physiological studies of the frontal lobe in non-human primates have focused on visual working memory and auditory spatial processing in dorsolateral prefrontal cortex (PFC)3,4, although the candidate PFC areas for non-spatial acoustic processing lie in the ventrolateral PFC (areas 12 and 45), which receives afferents from physiologically5,6 and anatomically7,8 defined auditory cortex. We recorded neuronal responses from ventrolateral PFC to auditory cues in awake monkeys under controlled conditions and report that the macaque ventrolateral PFC contains an auditory responsive domain in which neurons show responses to complex sounds, including animal and human vocalizations.
We made extracellular recordings to auditory and visual stimuli in the PFC of two monkeys (Macaca mulatta) while they performed a fixation task. Recording cylinders were implanted over the ventrolateral PFC overlying the inferior prefrontal convexity (areas 12 and 45) based on previous anatomical and physiological studies6,8-10. In monkey 1, we recorded from the left hemisphere; in monkey 2, the right hemisphere. During the neuronal recordings, the monkeys fixated a central point on a television monitor and either viewed visual stimuli or were presented with an auditory stimulus (65–80 dB SPL at the subject’s ear) from a speaker located just below the monitor (33 inches from the subject). At the end of the stimulus, the requirement for fixation was released and the monkey was given a juice reward. Once isolated, a single unit was tested with one or more of 10 lists of auditory and/or visual stimuli (10 stimuli per list). Each auditory list consisted of monkey and human vocalizations, environmental sounds (bells, whistles, sirens, etc.), band-passed or white noise, FM sweeps and tone bursts. Visual stimuli included both simple (monochrome shapes and bars) and complex (faces, objects and patterns) items. Units that were responsive to any stimulus were tested further with additional exemplars to determine specificity.
We recorded from 400 neurons in 90 recording tracks in the PFC during auditory stimulus presentation in the fixation task. Because we tested responsivity to visual stimuli, monitored eye movements with a scleral search coil, and required fixation during stimulus presentation, we were able to establish the selectivity of these neurons for acoustic stimuli and rule out visual or saccadic activity as an alternative explanation for the responses. Of the 400 neurons tested, 70 were significantly responsive to auditory stimuli (ANOVA, p < 0.05, determined by comparing responses during the stimulus period with intertrial interval responses). Only 5 of these (7%, 5/70) were also responsive to visual stimuli.
As with visually responsive neurons in the inferior convexity (IC), most auditory responsive cells were excitatory (n = 63), although a few inhibitory responses (n = 7) were noted. Using both statistical criteria and visual inspection, we classified auditory neurons into three categories on the basis of their responses: phasic neurons, which had brief responses that coincided with stimulus onset (n = 13 neurons; Fig. 1a) or offset (n = 4); tonic neurons (n = 15), which continued discharging beyond the initial onset period, occasionally lasting for the length of or beyond the acoustic stimulus presentation (Fig. 1b); and phasic-tonic neurons (n = 33), which contained a mixture of these categories, a phasic onset and a longer-lasting tonic component. In addition, some auditory responsive cells exhibited stimulus-synchronized discharges (n = 5) that appeared to be linked to temporal changes within the auditory stimulus (Fig. 1c and e, mv15).
Fig. 1.
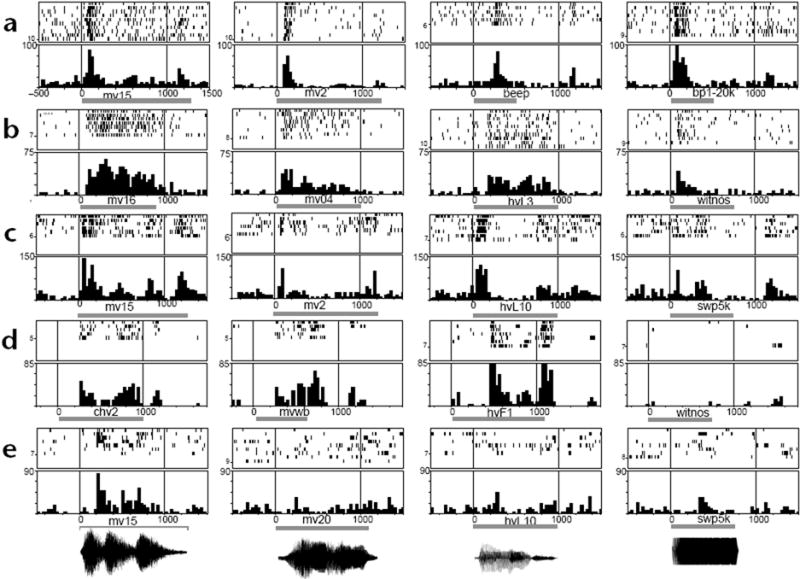
Prefrontal auditory neuron response profiles. Responses of 5 cells (a–e) to auditory stimuli are shown as raster (top panels) and post-stimulus time histograms (bottom panels). Gray bar below the histogram indicates onset and duration of auditory stimulus. Some sounds used are shown as waveforms below (e). Cell (a) gave a non-specific phasic onset response to all auditory stimuli tested, whereas auditory stimuli elicited a tonic response in cell (b) that lasted the length of the auditory stimuli. For some stimuli, cells (c, e) showed evidence of stimulus-synchronized activity (c, mv15; e, mv15). Whereas cells (a–c) responded to a variety of vocalization and non-vocalization stimuli, the responses of cells (d) and (e) were stronger for vocalizations compared to other complex stimuli, including white noise (d, witnos), and FM sweeps (e, swp5k). Whereas most PFC neurons responded to more than one auditory stimulus (66/70), cell (e) responded to only one stimulus, a vocalization (mv15) after testing with 32 complex stimuli. PSTH are calculated as spikes/s along the y-axis; bin width, 30 ms. All responses in (a–c) and the first 3 panels in (d) show a statistically significant increase (p < 0.05) over baseline responding in the inter-trial interval. For cell (e), only the response to mv15 was significant (p < 0.05). mv, monkey vocalization; hv, human vocalization (human vocalizations were spoken words); bp1-20K, band-passed noise range, 1–20 kHz; swp5k, FM sweep range 100–5,000 Hz.
Vocalizations proved to be the most effective search stimuli and evoked responses in 52/70 auditory neurons. Most of these cells responded to vocalization and some non-vocalization stimuli (Fig. 1a–c), although a small subset of cells responded only to vocalizations (n = 3; Fig. 1d and e). To explore the selectivity of PFC neurons for vocalizations, we tested 14 vocalization-responsive cells with a large battery of vocalization and non-vocalization stimuli. Monkey or human vocalizations elicited a stronger response in 71% (10/14) of these cells (assessed by comparing mean firing rates during the stimulus, using a Tukey test, p < 0.05). In contrast, few PFC neurons responded to pure tones. A total of 13 of the 70 auditory responsive units exhibited responses to tones and were tested with a range of pure tones from 0.2 kHz to 10 kHz. Only 2 cells exhibited sharp tuning (as assessed with Tukey HSD post hoc comparison), one at 10 kHz and the other at 0.2 kHz. A few units were responsive to tones over a broad range of lower frequencies (n = 3) and several cells (n = 3) were most responsive at frequencies above 2 kHz.
Most of the auditory neurons (57/70) were localized to a small portion (4 mm × 4 mm) of the recording area (Fig. 2). In both monkeys, visual responses were noted over a wider region of the ventrolateral PFC and were most commonly observed anterior to the inferior limb of the arcuate sulcus (AS), consistent with previous studies6-8. In contrast, the auditory responsive cells were tightly clustered in the ventrolateral part of the recording chamber and were antero-lateral to visual neurons in the inferior AS in the same animals (Fig. 2). No auditory cells were found outside this ventrolateral quadrant of the recording cylinder. Histological verification of our recording tracks revealed that auditory neurons were localized to the lateral surface of the IC (areas 12 lateral and 45) and lateral orbital cortex (area 12 orbital) (Fig. 2).
Fig. 2.
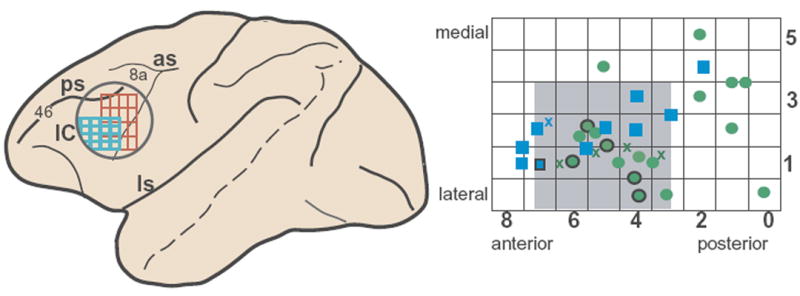
Location of auditory responsive neurons in the ventral PFC, below the principal sulcus (area 46), and anterior to the arcuate sulcus and area 8a, in areas 12 and 45. Left, macaque brain schematic indicating the recording cylinder (circle), the auditory responsive region in ventral PFC (blue grid) and the visual responsive region in the same animals (red grid). An enlargement of the auditory response grid at right shows the locations of auditory cells in the recording grid in x –y coordinates on the surface of ventral PFC (green circles, monkey 1; blue squares, monkey 2). Numbers on grid rows and columns correspond to medial–lateral and anterior–posterior coordinates where cells were located. 81% of the auditory cells were found within a 4 × 4 mm region (gray). Crosses (blue, monkey 1; green, monkey 2) correspond to locations of cells with auditory and visual responses (n = 5 cells). A total of 33 locations are shown (squares, circles and crosses) where 70 auditory cells were located. The locations of 10 cells (6 locations) that responded more strongly to vocalization than non-vocalization stimuli are portrayed by the circles and squares outlined in black. PCF, prefromtal cortex; IC, inferior convexity region; ls, lateral sulcus; los, lateral orbital sulcus; ps, principal sulcus; as acurate sulcus.
Previous physiological studies of the PFC in non-human primates have localized neurons with selective visual responses to face and object stimuli to the IC of the frontal lobe8-11. Although previous lesion and anatomical studies predicted the existence of an auditory domain in the ventral PFC of non-human primates, very few electrophysiological studies have observed responses to complex natural stimuli in this region12. Auditory-responsive neurons previously reported had weak responses, were seen sporadically, were not tested with complex acoustic stimuli, or eye movements and visual responses were not controlled for, as was done in this study12-14. Our findings of 70 discretely localized auditory responsive cells establishes an auditory domain in the non-human primate ventrolateral PFC (areas 12 lateral, 12 orbital and 45), anterolateral to the visual domain, in an area that receives projections from the auditory belt and parabelt cortex6-8. Although the number of auditory responsive cells across the entire recording cylinder seems small (70/400, 17.5%), their frequency rises to 35% (70/200) when electrode penetrations are restricted to the circumscribed area where auditory cells were most commonly encountered. This is not unreasonable, given the small number of stimuli in our sample relative to the inordinately large number of auditory stimuli to which the sensory systems can respond. Importantly, the modality specificity of these neurons is established, as 93% of the auditory responsive cells were not responsive to visual stimuli (65/70), or the result of visual saccades.
The localization of auditory responses to the ventral PFC in the macaque is suggestive of some functional similarities between this region and the inferior frontal gyrus of the human brain (including Broca’s area)15, where mnemonic, semantic and syntactic auditory processes have been localized1,2. Physiological identification of an auditory processing region in the ventral PFC of macaque monkeys may allow us to decipher the cellular mechanisms that underlie vocal communication in the frontal lobe.
Acknowledgments
This work was supported by NIMH (MH-38546), James S. McDonnell Foundation (JSMF 93-28), and Cure Autism Now.
References
- 1.Gabrieli JDE, et al. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price CJ. Trends Cogn Sci. 1998;2:281–288. doi: 10.1016/s1364-6613(98)01201-7. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M, Suzuki H. Brain Res. 1984;298:343–346. doi: 10.1016/0006-8993(84)91434-3. [DOI] [PubMed] [Google Scholar]
- 4.Vaadia E, et al. J Neurophysiol. 1986;56:934–952. doi: 10.1152/jn.1986.56.4.934. [DOI] [PubMed] [Google Scholar]
- 5.Rauschecker JP, Tian B, Hauser M. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- 6.Romanski LM, et al. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackett TA, Stepniewska I, Kaas JH. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- 8.Romanski LM, et al. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Wilson FAW, et al. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 10.O’Scalaidhe SP, et al. Science. 1997;278:1135–1138. doi: 10.1126/science.278.5340.1135. [DOI] [PubMed] [Google Scholar]
- 11.Goldman-Rakic PS. Phil Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 12.Benevento LA, et al. Exp Neurol. 1977;57:849–872. doi: 10.1016/0014-4886(77)90112-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanila H, et al. Behav Brain Res. 1993;53:63–71. doi: 10.1016/s0166-4328(05)80266-9. [DOI] [PubMed] [Google Scholar]
- 14.Newman JD, Lindsley DF. Exp Brain Res. 1976;25:169–181. doi: 10.1007/BF00234901. [DOI] [PubMed] [Google Scholar]
- 15.Deacon TW. Brain Res. 1992;573:8–26. doi: 10.1016/0006-8993(92)90109-m. [DOI] [PubMed] [Google Scholar]


