Abstract
Albino Sprague–Dawley rats are known to undergo photoreceptor degeneration after exposure to constant light, but the molecular mechanism(s) by which the photoreceptors degenerate is not fully understood. We hypothesized that cytotoxic oxysterols are generated in situ in the retina under such conditions and may be involved in the degenerative mechanism. Thus, photodamaged and control rat retinas were analyzed for oxysterols by liquid chromatography mass spectroscopy. Elevated levels of two known cytotoxic oxysterols, 7-ketocholesterol (7KCh) and 7αβ-hydroxycholesterol (7HCh), were found in the photodamaged retinas, at levels six-fold and 50-fold greater, respectively, than those found in non photodamaged controls. Notably, two key intermediates, 5,6α,β-epoxycholesterol (5,6-epoxyCh) and 7αβ-hydroperoxy-cholesterol, were also identified, indicating that the formation of 7KCh and 7HCh is mediated by a free radical mechanism. By immunohistochemistry, 7KCh was localized to the ganglion cell layer, photoreceptor inner segments and retinal pigment epithelium (RPE), which coincides with the localization of ferritin in the retina. Exposure of a mixture of ferritin and low-density lipoprotein to intense white light in vitro produced similar oxysterol species as seen in vivo. We propose that the increased levels of 7KCh and 7HCh, especially in photoreceptor inner segments and RPE, may arise due to ferritin-catalyzed reactions and may be important contributors to the photoreceptor degeneration observed in photodamaged rats.
INTRODUCTION
The retina is a complex tissue that contains at least 10 different cell types and faces unique challenges not encountered by other neurological tissues or organs. Prolonged exposure to light, a high metabolic rate, high oxygen flux and the presence of high levels of polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA, 22:6n-3), contribute to a unique environment that has necessitated the evolution of specific mechanisms to protect the retina from photo-oxidative insult while also providing for the uptake and active metabolism of highly photosensitive lipids (1, 2). Recently, we have demonstrated that the retina is capable of avid low-density lipoprotein (LDL) uptake from the blood (3), providing blood-borne cholesterol to all the cellular layers of the retina. The retina also has a robust lipid exchange system that employs both endogenous (4) and exogenous sources (3) to maintain its dynamic steady-state composition. Within the retina the lipids appear to be transported by the same kinds of molecules utilized in systemic lipid transport (5). The retina expresses the “reverse cholesterol transport” proteins apolipoprotein A1 (apoA1), ATP-binding cassette transporter subfamily A member 1 (ABCA1), scavenger receptors BI and BII (SR-BI and SR-BII), cholesterylester transfer protein (CETP) and lethicincholesterol acyltransferase (LCAT) in specific locations, suggesting the existence of a high-density lipoprotein-mediated intraretinal lipid transport process (5). It also expresses significant amounts of apolipoprotein E (apoE) (6) and small, but measurable, amounts of apolipoprotein B (apoB) (3, 7). The reason(s) for this rapid and vigorous lipid movement is unclear. However, considering the well-documented susceptibility of most classes of unsaturated lipids to photo-oxidation and the unique photo-oxidative environment in which the retina resides, there may be a connection between these processes. Despite more than four decades of research, the exact compendium of lipid molecules in the retina that are likely to become photo-oxidized and the enzymatic pathway(s) involved in metabolizing these molecules are not fully understood.
One of the most extensively studied and most highly cytotoxic of the oxidized lipids is 7-ketocholesterol (7KCh) (8). 7KCh is commonly found in oxidized LDL (oxLDL), which is a major component of atherosclerotic plaques, and is suspected of causing foam cell formation in macrophages due to its cytotoxicity (8, 9). 7KCh also has been shown to be responsible for the majority of the cytotoxicity associated with oxLDL when supplied to cultured retinal pigment epithelium (RPE) cells (10, 11). In the oxLDL particles 7KCh is mostly found in the form of steryl esters, covalently coupled to unsaturated fatty acids (12). Oxidation of these steryl esters occurs via the Fenton reaction, which is catalyzed by transition metals, particularly copper and iron (9, 12). Both of these metals are found in atherosclerotic plaques in appreciable quantities (13). Dietary sources, especially dairy products, initially had been suspected as major contributors to the 7KCh found in body tissues (14), but subsequently it has been demonstrated that dietary oxysterols are quickly metabolized by the liver and excreted as bile acids (15). Thus, it seems that most of the 7KCh found in atherosclerotic plaques is made in situ by copper and/ or iron catalyzed oxidation.
Girotti and coworkers have elegantly characterized two known mechanisms for oxidizing cholesterol to 7KCh: one is free radical-mediated and the other is singlet oxygen-mediated (16, 17). The free radical-mediated mechanism is characterized by the formation of 7αβ-hydroperoxides (7OOHCh), 7αβ-hydroxycholesterol (7HCh) and 5,6αβ-epoxycholesterol (5,6-epoxyCh). The 7OOHCh are reactive intermediates and readily form 7HCh and 7KCh. Hence, these latter two oxysterols are signature molecules for the free radical-dependent oxidation of cholesterol. Although 7HCh is generally considered a stable end product, it can also further oxidize to 7KCh (12). The epoxide of 5,6-epoxCh can also open and further oxidize to form cholest-5-en-3β,5,6-triol (i.e. 5,6-dihydroxycholesterol). The singlet oxygen mechanism is characterized by the formation of 5α-hydroperoxide and the 6αβ-hydroperoxides, which are reactive intermediates that can rearrange to form 7OOHCh and, subsequently, 7KCh. The free radical mechanism requires a transition metal catalyst (typically Fe+2 or Cu+2). One biologically significant form of iron is ferritin, which has been shown to enhance lipid peroxidation in photoreceptor outer segment (POS) membranes in the presence of light (18, 19), and also is relatively abundant in the retina (20, 21). Hence, we suspect that ferritin may be implicated in the formation of 7KCh in the retina.
In contrast to the free radical-dependent mechanism, the singlet oxygen mechanism requires a photosensitizer (16, 22). This mechanism of direct photo-oxidation of free cholesterol may also be active in the retina, mediated by endogenous photosensitizers such as pyridinium bis-retinoid (A2E) or all-trans retinoic acid (ATRA). However, previous studies (23, 24) have shown that A2E and ATRA are poor photosensitizers even when ionized with blue light. Results from studies in our laboratory (I. R. Rodriguez and S. L. Coon, unpublished) also have demonstrated that A2E and ATRA are only about 1% as efficient as hematoporphyrin (22) in generating 7KCh using white light. Cholesteryl esters can be oxidized by both the free radical-mediated and the singlet oxygen-mediated mechanisms, with oxidation of the cholesteryl and/or the fatty acyl moieties (16, 22). The free radical-mediated mechanism is generally accepted to be involved in the formation of oxLDL in atherosclerotic plaques (9, 13). Recently we have reported 7KCh in the primate retina associated with lipoprotein deposits in Bruch’s membrane, the choriocapillaris and capillary endothelial cells of the neural retina (25). The location of the 7KCh further suggests that its formation occurs via a free radical-mediated mechanism.
In the present study we have used the photodamaged albino rat as a model to identify the mechanism of 7KCh formation in the retina. Using liquid chromatography-mass spectroscopy (LCMS) we have identified cholesterol oxidation intermediates that previously have been shown to be signature molecules specific to a free radical-mediated mechanism (16, 17). We also present evidence that implicates ferritin and/or other iron-carrying proteins in the mechanism of 7KCh formation in the retina.
MATERIALS AND METHODS
Materials
Cholesterol, β-sitosterol, 7KCh, 7α-hydroxycholesterol, 7β-hydroxycholesterol, 5,6α-epoxycholesterol, 5,6β-epoxycholesterol and 5α,6β-dihydroxycholesterol (cholest-5-en-3β,5α,6β-triol) were purchased from Steraloids, Inc. (Newport, RI). Stock solutions (10 mm) for standard sterols were prepared in 45% (wt/vol) aqueous hydroxypropyl-β-cyclodextrin (Sigma/Aldrich, St. Louis, MO). The solvents for LCMS (water, acetonitrile and methanol) were “Optima” grade and purchased from Fisher Scientific (Fair Lawn, NJ). Adult female albino rats (Sprague–Dawley) were purchased from Harlan (Indianapolis, IN), and were maintained for a minimum of two weeks under dim cyclic light (12 h light/12 h dark, 20–24 l×, fluorescent light), fed water and standard commercial rodent chow ad lib prior to use. Conditions were in compliance with those specified by the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee (IACUC). A mouse monoclonal antibody monospecific for 7KCh (anti-7KCh) was obtained from the Japanese Institute for the Control of Aging (Shizuoka, Japan). Appropriate secondary antibodies and immunodetection reagents were used as purchased from Vector Laboratories, Ltd. (Burlingame, CA). Horse spleen ferritin Type I was purchased from Sigma-Aldrich. LDL was prepared from fresh human serum as previously described (26).
Retinal light damage paradigm
Exposure of rats to intense constant light (retinal light damage paradigm) was performed as previously described (27). In brief, adult female Sprague–Dawley (albino) rats were dark-adapted overnight, and then placed in a light damage apparatus and subjected continuously for 24 h to green light (1700 l×, 490–580 nm), applied uniformly (360°) in a temperature-controlled chamber (kindly provided by D.T. Organisciak, Wright State University School of Medicine, Dayton, OH). Rats were allowed to recover for 0, 24 or 48 h in dim cyclic light (40 l×, 12 h light/12 h dark), and were then killed by sodium pentobarbital overdose. The retinas from each eye were rapidly removed and snap-frozen in liquid nitrogen in a polypropylene microfuge tube, and stored at −85°C in darkness until ready for analysis. The contralateral eye from one set of animals was used for immunohistochemical analysis (see below).
Extraction of lipids from retinas
Rather than saponifying the tissue, which tends to variably degrade 7KCh, the retinas were directly extracted with organic solvents and then taken to dryness under a stream of argon gas as previously described (28). Prior to extraction an internal standard of β-sitosterol (5 nmol) was added to each sample. Extracted lipids were then redissolved in methanol and analyzed directly (see below).
High pressure liquid chromatography and mass spectroscopic (LCMS) analysis of lipids and detection of oxysterols
The LCMS analyses were performed as previously described (25). In brief, the reverse-phase chromatography was performed using an Xterra RP18 column (2.0 × 250 mm) running at 0.25 mL min−1 at 60°C in a Waters model 2695 HPLC instrument equipped with a 2996 photodiode array detector (Waters Corp., Milford, MA). The HPLC instrument was attached to a QTOF Micro mass spectrometer (Waters Corp.). The samples were ionized using an IonSABRE atmospheric pressure chemical ionization (APCi) probe. The oxysterols were eluted using a linear gradient with initial conditions of water/acetonitrile (1:1, by vol) and progressing to 100% acetonitrile in 20 min. Mobile phase was held at 100% acetonitrile for 5 min and then the solvent was switched to 100% methanol for 10 min to elute cholesteryl esters. All solvents contained 0.1% formic acid and the entire solvent flow was diverted to the APCi probe. The IonSABRE APCi probe was set to 500°C and the source temperature was 120°C. At the source the corona was set at 10 µA, the sample cone at 20 V and the extraction cone at 3 V. The cone gas flow was set at 50 L h−1 and the dissolvation gas flow was set at 600 L h−1.
Immunohistochemical localization of 7KCh in retina
Immunohistochemical localization of 7KCh was performed as previously described (25). In brief, frozen sections were prepared and incubated with mouse anti-7KCh (1:100) overnight at 4°C. The sections were developed using the Vectastain ABC peroxidase kit (Vector Laboratories, Ltd.) according to the manufacturer’s instructions.
Ferritin-mediated oxidation of LDL in vitro
LDL (1 mg, lipid and protein weight) was mixed with 1 mg of ferritin in a glass tube (6 × 50 mm) in a total volume of 200 µL of water. The mixture was exposed to 1500 l× of white light for 48 h. Two controls, each containing the same amount of LDL, were employed: one with ferritin and not exposed to light and another without ferritin and exposed to light. The lipids were then extracted as previously described (28), dried under argon and redissolved in 100 µL of methanol. The extracts were analyzed by LCMS using the same conditions used for the rat retinas (see above).
Hematoporphyrin-mediated oxidation of sterols
The unstable 7αβ-hydroperoxides were prepared by photo-oxidizing cholesterol using hematoporphyrin IX (Frontier Scientific, Logan, UT) (22). A 10 mm solution of cholesterol containing 200 µm hematoporphyrin was irradiated with 1500 l× of visible light for 96 h. The oxidized mixture was separated by HPLC using the conditions above. The 7OOHCh was identified by retention time and characteristic mass spectra features.
RESULTS
Measurement of 7KCh and other intermediates in the photodamaged rat retina
In order to determine whether light damage conditions could cause an increase in 7KCh levels in the retina in vivo, albino Sprague–Dawley rats were exposed to constant intense green light for 24 h. This paradigm is well-known to promote extensive histological damage (“retinal light damage” or “photodamage”) to the neural retina, particularly in the superior central zone (29). The eyes were then harvested at 0, 24 and 48 h after the light exposure, and the levels of 7KCh and other oxysterol intermediates were measured by LCMS. Two rats were used for each time point and each eye was independently analyzed. In addition, to determine the background levels of 7KCh in Sprague–Dawley rats 24 other eyes were analyzed from control rats that were not subjected to photodamage treatment maintained under dim cyclic light (12 h light/12 h dark, 20–40 l×).
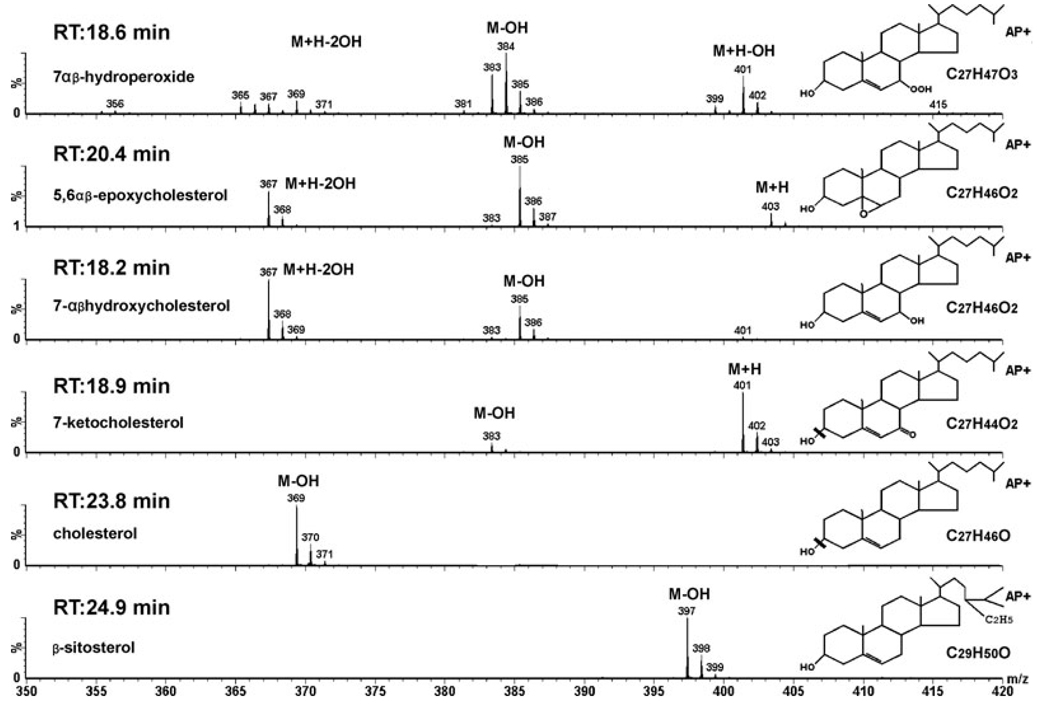
As determining the identity of the intermediates is critical to understanding the in vivo mechanism of cholesterol photo-oxidation, we demonstrate the MS spectra of authentic standards and their structures in Fig. 1. The identification of the oxysterols is based upon their retention times and distinguishing ions of their mass spectra. Under the chromatographic conditions used, the α- and β-epimers of these oxysterols are not well separated. The ions detected are all positively charged ions. The 7αβ-hydroperoxides (7OOHCh) form several ions, but the most interesting are the m/z 401 (M+H-OH) and 384 (M+H-2OH), which are diagnostic as they only are observed together for the hydroperoxides. The 5,6αβ-epoxides (5,6-epox) form a signature three-ion combination consisting of m/z 403 (M+H), 385 (M-OH) and 367 (M+H-2OH) species, which also distinguishes them from the other oxysterols. The 7HCh forms mainly an m/z 367 ion (M+H-2OH) with a small amount of an m/z 385 ion (M-OH). The 367 and 385 ions are seen with all of the cholesteryl diols, including the common side chain hydroxylated diols such as 24 and 27 hydroxycholesterol. However, these latter diols elute earlier than 7HCh and are not present in significant amounts in the retina. 7KCh predominantly forms an m/z 401 ion (M+H) and a small amount of an m/z 383 ion (M-OH). Cholesterol and the β-sitosterol internal standard form M-OH ions of m/z 369 and 397, respectively. The corresponding retention times are also important in the identification of these oxysterol species. In addition, MS/MS was also performed on the main ions for further validation (data not shown).
Figure 1.
Mass spectra and retention times of oxysterol standards. The retention time, spectra and structure are shown for each of the authentic oxysterols of interest. With the exception of the 7αβ-hydroperoxides (7-OOHCh) all of the other sterols were commercially available (see Materials and Methods). The 7-OOHCh was prepared by photo-oxidizing cholesterol using hematoporphyrin. AP+ refers to the type of ionization probe (APCi) running in positive mode (collecting positively charged ions).
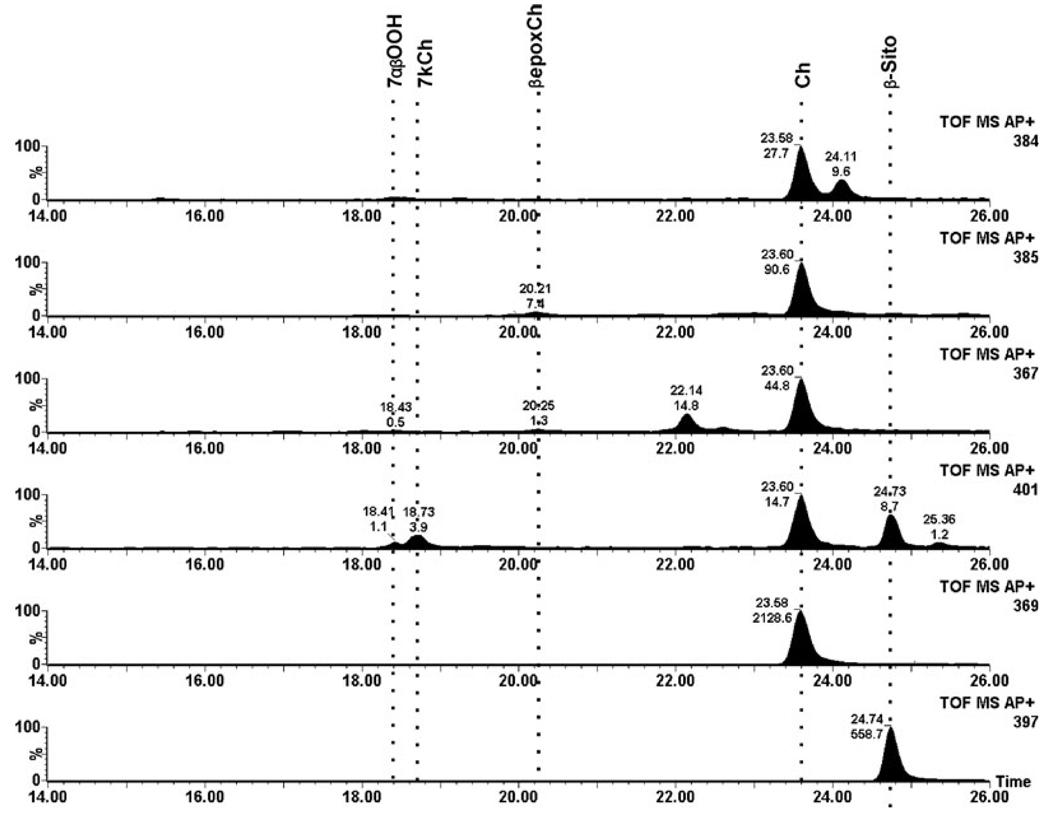
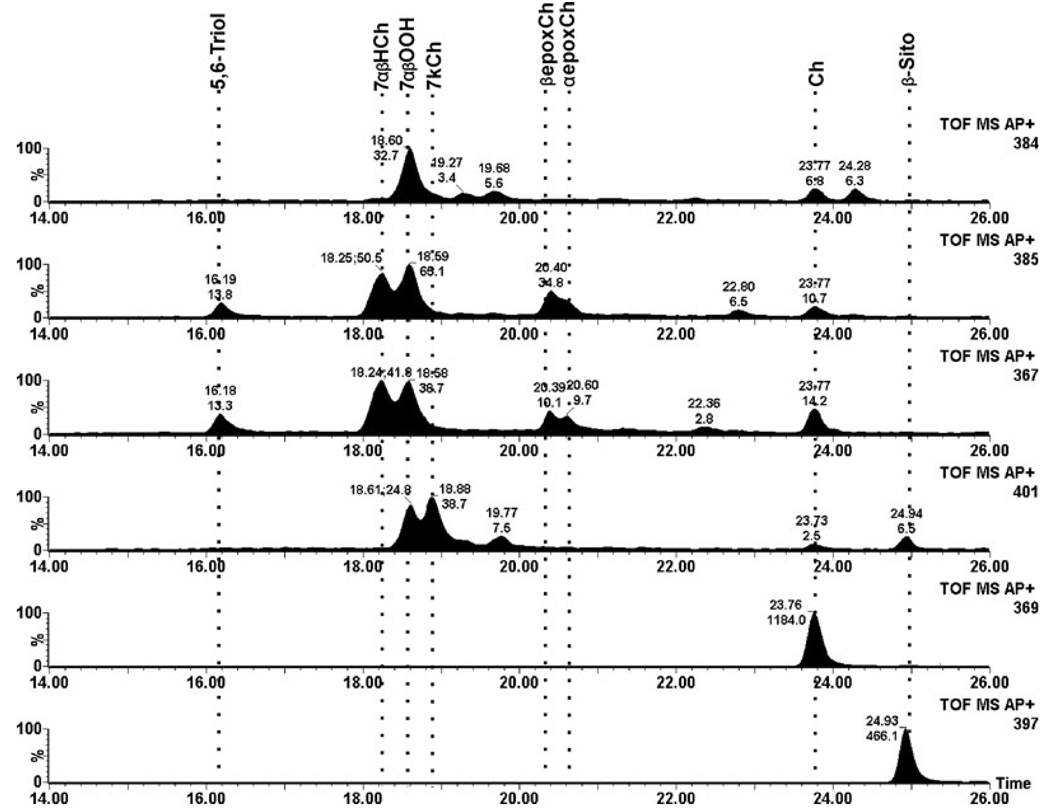
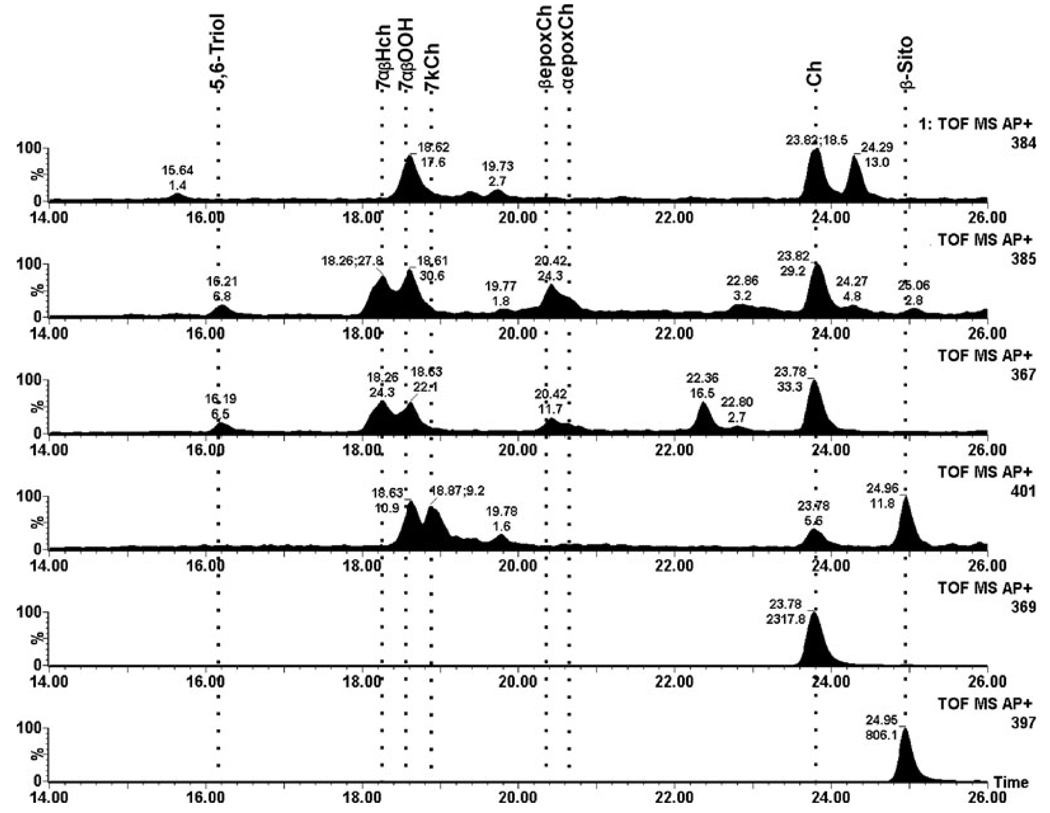
In control rat retinas very low levels of 7KCh and only traces of 7HCh and 5,6-epoxide intermediates were detected (Fig. 2). The total ion current for the dominant ion for each of the oxysterols is shown; cholesterol and the internal β-sitosterol standard are also shown for comparison. In striking contrast, retinal lipids from the photodamaged rats extracted immediately after termination of the light treatment (0 h time point) exhibited dramatically greater levels of 7HCh and 7KCh as well as a variety of the intermediates (Fig. 3). The levels of 7KCh were, on average, six-fold greater than in nonphotodamaged controls. The most dramatic change occurred in the levels of 7HCh, which were approximately 50-fold increased in the photodamaged rat retinas compared to unexposed controls (cf. Fig. 2 and Fig. 3). The actual numerical data are given in Table 1. After 24 h postlight damage, the oxysterol levels decreased to approximately two-fold over control values and then changed little by 48 h (Table 1).
Figure 2.
Total ion current (TIC) of a representative nonphotodamaged control rat retina. Each panel shows the TIC for each of the ions used to identify and quantify the specific oxysterol. From the bottom up, β-sitosterol internal standard (m/z 397), cholesterol (m/z 369), 7KCh (m/z 401), 7HCh (m/z 367), 5,6-epoxCh (m/z 385) and 7-OOHCh (m/z 384). The locations of the different sterols of interest are shown by dotted lines. TOF MS AP+ defines that this is a time of flight instrument (TOF) collecting MS spectra using an APCi probe in positive mode (collecting positive ions).
Figure 3.
Total ion current (TIC) of a representative rat retina analyzed immediately after photodamage treatment. Each panel shows the TIC for each of the ions used to identify and quantify the specific oxysterol. From the bottom up, β-sitosterol internal standard (m/z 397), cholesterol (m/z 369), 7KCh (m/z 401), 7HCh (m/z 367), 5,6-epoxyCh (m/z 385) and 7-OOHCh (m/z 384). The locations of the different sterols of interest are shown by the dotted lines.
Table 1.
Levels of 7-ketocholesterol (7KCh) and 7αβ-hydroxycholesterol (7HCh) in rat retinas.
| 7KCh (pmol nmol−1 Ch) | 7HCh (pmol nmol−1 Ch) | |
|---|---|---|
| Control | 1.6 | ND |
| 0 h | 12.7 | 25.0 |
| 24 h | 2.1 | 8.5 |
| 48 h | 2.3 | 8.6 |
Values are expressed in picomoles per nanomole of cholesterol (Ch). Measurements for the photodamaged retinas were from two different rats and each eye was analyzed independently; values represent an average of four individual measurements. Values for controls (no light treatment) represent an average of six individual retinas from six rats. The levels of 7HCh in control retinas were too low to accurately quantify under the conditions used in this study.
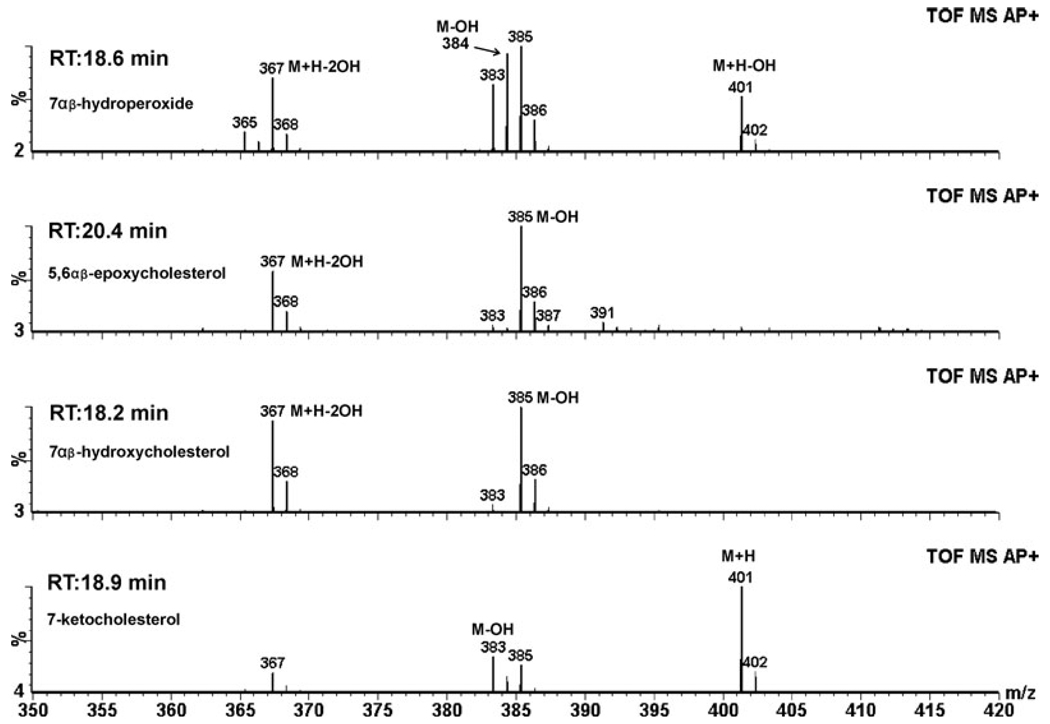
The MS spectra for selected peaks of interest in Fig. 3 are shown in Fig. 4 and can be compared to the spectra of the standards shown in Fig. 1; these spectra and the retention times verify the identification of the oxysterol intermediates as shown. By 48 h, the oxysterol levels were decreased but their relative composition was similar to the 0 h measurements (Table 1, Fig. 5).
Figure 4.
Mass spectra of the main peaks from the photodamaged rat retina. The retention time and mass spectra of the different oxysterols of interest from Fig. 3 confirm the identification of oxysterols generated by photodamage.
Figure 5.
Total ion current of a representative rat retina analyzed 48 h after photodamage treatment. Figure layout equivalent to that in Fig. 2 and Fig. 3.
Increased immunoreactivity for 7KCh in photodamaged rat retina in vivo
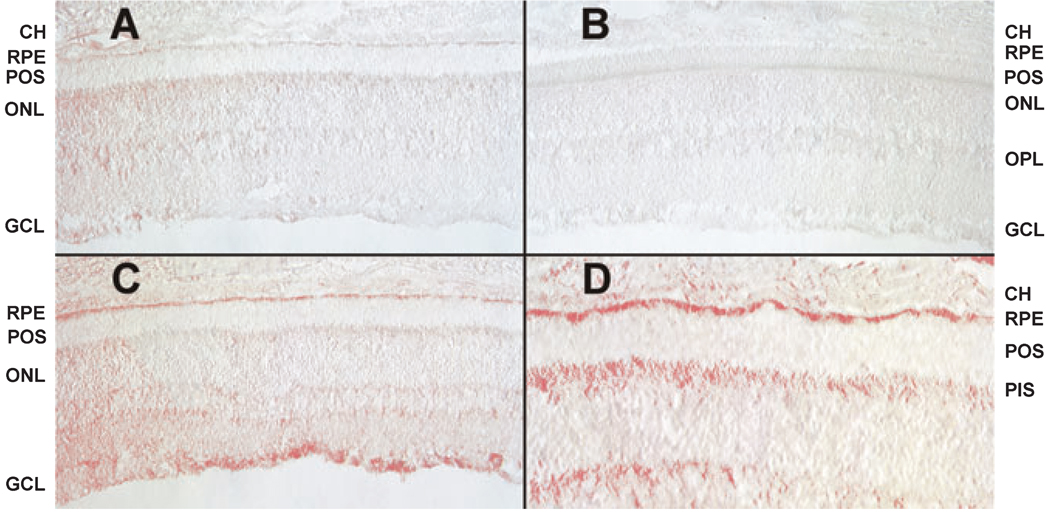
It has been well established that albino rats receiving the type of light-damage treatment employed in this study undergo a massive retinal degeneration within 3 weeks posttreatment (27, 29). Therefore to determine whether the increased levels of the toxic 7KCh and 7HCh could be contributing to the degeneration, immunohistochemical localization of 7KCh was performed (Fig. 6) on eyes that had been fixed immediately after 24 h of photodamage (Fig. 6C,D). Two independent controls were used, an unexposed (nonphotodamaged) rat retina (Fig. 6A) and a photodamaged rat retina where no primary antibody was employed in the immunohistochemical analysis (Fig. 6B). The results indicate that photodamage increases the levels of 7KCh in the ganglion cells, the photoreceptor inner segments and the RPE. Importantly, ferritin exhibits a similar distribution in the rat retina (20, 21).
Figure 6.
Immunohistochemistry of rat retinas analyzed immediately after photodamage treatment. Immunohistochemistry was performed using a commercially available anti-7KCh antibody as previously described (17). (A) Nonphotodamaged control rat retina. (B) Photodamaged rat retina (no primary antibody) control. (C) Photodamaged rat retina (low magnification). (D) Photodamaged rat retina (higher magnification). CH, choriocapillaris; RPE, retinal pigment epithelium; POS, photoreceptor outer segments; PIS, photoreceptor inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; GCL, ganglion cell layer.
Photo-oxidation of LDL by ferritin
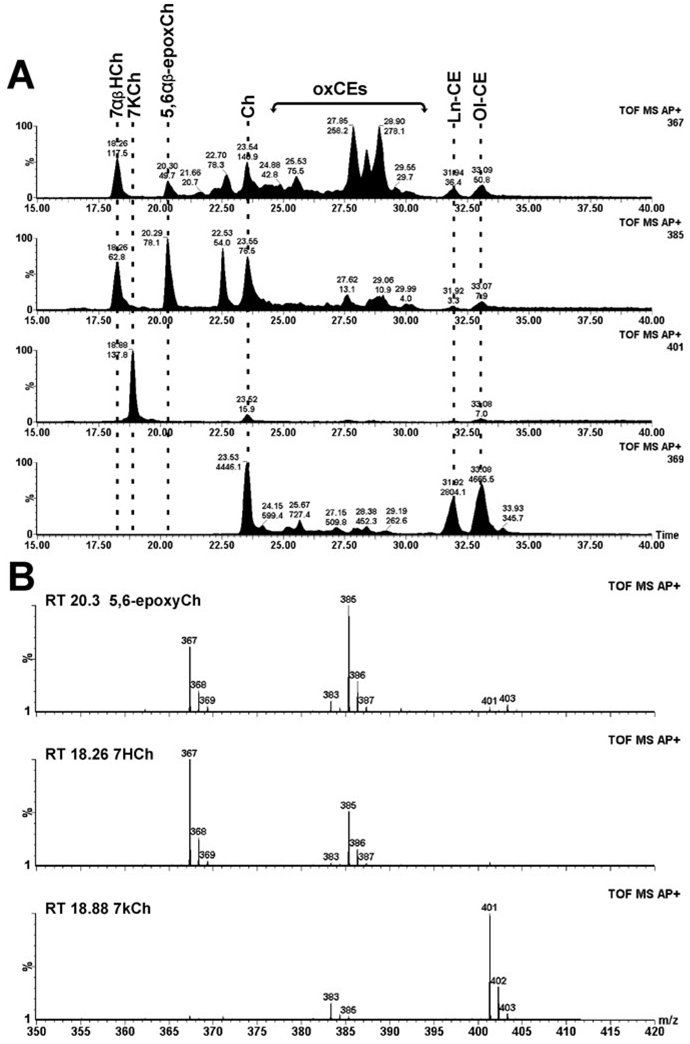
Other investigators have previously observed that ferritin can catalyze lipid peroxidation in bovine photoreceptors in the presence of visible light (19). Moreover, exposure of ferritin to visible light promotes release of free iron (18). As ferritin seems to colocalize to the regions of the retina where 7KCh formation occurs during photodamage (Fig. 6C,D), we examined the ability of ferritin and visible light to oxidize LDL in vitro. As shown in Fig. 7, 7HCh, 7KCh and the 5,6-epoxide were only formed in the light-treated, ferritin-containing sample. The control LDL sample, containing ferritin but not exposed to light, had only traces of these oxysterols (data not shown). Interestingly, we did not observe 7-OOHCh in these samples, suggesting that these species may be converted to 7KCh and 7HCh more quickly than in vivo.
Figure 7.
Analysis of LDL after photo-oxidation using ferritin. (A) Total ion current of the different ions of interest extending the chromatograph to show the cholesteryl esters. The locations of the different sterols of interests are marked by dotted lines. Ol-CE, cholesteryl oleate; Ln-CE, cholesteryl linoleate; ox-CE, various unidentified oxidized cholesteryl esters; and others previously defined. (B) Mass spectra and retention times used to identify 7KCh, 7HCh and 5,6-epoxyCh.
DISCUSSION
Lipid peroxidation has been implicated in the process by which the retinas of albino rats undergo photodamage, based upon results obtained from several laboratories (30–33). However, the focus in those studies typically was on oxidation of PUFAs, especially DHA and arachidonic acid (20:4n-6), the two most prevalent PUFAs in retina and retinal POS (34). With the exception of one study (33), the involvement of sterol oxidation in the photodamage mechanism heretofore has not been considered. In the present study we demonstrate that photodamaging the albino rat retina generates significant levels of two known cytotoxic oxysterols, 7KCh and 7HCh (Fig. 2 and Fig. 3). The presence of these cholesterol-derived oxysterols and identification of two other key intermediates (7-OOHCh and 5,6-epoxyCh) indicate that these cytotoxic oxysterols were generated via a free radical-mediated mechanism (16, 17, 22). This mechanism, as distinguished from a singlet oxygen-mediated mechanism (22), requires a transition metal catalyst (typically Fe+2 or Cu+2) (9, 12, 16, 17). Thus, the identification of these particular oxysterol species in the photodamaged rat retina also implicates transition metals in the photo-oxidation and, hence, in the mechanism of light-induced retinal damage in this rat model.
However, transition metal-catalyzed oxidation of cholesterol and cholesterol esters does not require light (9, 12). This suggests that light may not be directly involved in oxidizing cholesterol in the retina; rather, it may facilitate the release of bound copper and/or iron in situ, which then promotes oxidation. Ferritin has been previously shown to release bound iron when exposed to light (18) and can induce lipid peroxidation in POS (19). Ferritin also has been localized to the same areas of the retina (20, 21) where we observed the largest amounts of 7KCh formation after exposure to damaging intense light (Fig. 6). Moreover, iron chelation by desferriox-amine has been shown to protect albino rats from light-induced damage (35), and iron chelation has also been shown to protect cultured RPE cells from peroxide and blue light-induced oxidation (36). Our in vitro experiments using LDL as a model lipid complex demonstrated that ferritin promoted cholesterol oxidation (7KCh formation) when exposed to visible light (Fig. 7).
The mode of toxicity of 7KCh has been extensively studied and seems to involve mitochondrial membrane depolarization and subsequent apoptosis (37–39). However, the process by which 7KCh induces the mitochondrial changes that lead to apoptosis is not well understood. One potential apoptosis mechanism involves ceramide, which has been shown to increase in U937 cells in response to 7βHCh and 7KCh exposure (39). Recently, 7KCh was identified and localized in the retina associated with lipoprotein deposits in the choriocapillaris and Bruch’s membrane as well as in the lumen of the capillary endothelial cells in the neural retina (25). 7KCh was also found to be a potent inducer of vascular endothelial growth factor (VEGF) and IL-8 in cultured RPE and microvascular endothelial cells (25). The 7KCh-mediated VEGF induction was determined to be independent of HIF-1α, but was partially mediated by liver X receptors (LXRs). The 7KCh-mediated IL-8 induction was mediated by LXRs and NF-κB, but the influence of NF-κB on VEGF induction is more complex and needs further investigation (25).
In summary, our results have demonstrated that conditions that cause retinal light damage in the albino rat generate 7KCh and 7HCh via a free radical-mediated mechanism. This indicates that a transition metal is involved, most likely iron, because iron is abundant in the retina and is known to readily catalyze cholesterol oxidation. Iron chelation has been shown to protect retinal cells from light damage both in vivo and in vitro. The source of the iron is likely ferritin both because it is abundant in the retina and is present in the identical locations where we observed 7KCh formation after light-induced damage in the albino rat.
Acknowledgements
The authors thank Dr. Ernesto F. Moreira for performing the 7KCh immunohistochemistry. This work was supported by the National Eye Institute intramural research program (I.R.R.), by U.S.P.H.S. grant EY007361 (S.J.F.) and by a departmental Challenge Grant from Research to Prevent Blindness (S.J.F.). S.J.F. is the recipient of a Research to Prevent Blindness Senior Scientific Investigator Award.
REFERENCES
- 1.Gordon WC, Bazan NG. Cellular organization and biochemistry of the retina. In: Harding JJ, editor. Biochemistry of the Eye. London: Chapman and Hall; 1997. pp. 144–275. [Google Scholar]
- 2.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularized and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [Review] [DOI] [PubMed] [Google Scholar]
- 3.Tserentsoodol N, Sztein J, Campos M, Fliesler SJ, Gordiyenko NV, Fariss RN, Lee JW, Rodriguez IR. Uptake of cholesterol by the retina occurs primarily via an LDL-receptor mediated process. Mol. Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- 4.Fliesler SJ, Florman R, Rapp LM, Pittler SJ, Keller RK. In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett. 1993;335:234–238. doi: 10.1016/0014-5793(93)80736-e. [DOI] [PubMed] [Google Scholar]
- 5.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Internal lipid transport in the retina is dependent on HDL-like particles and class B scavenger receptors. Mol. Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 6.Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV. Local cellular sources of Apolipoprotein E in the human retina and retinal pigment epithelium: Implications for the process of drusen formation. Am. J. Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 7.Li C-M, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: Initial characterization. Invest. Ophthalmol. Vis. Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 8.Lyons MA, Brown AJ. Molecules in focus: 7-Ketocholesterol. Int. J. Biochem. Cell Biol. 1999;31:369–375. doi: 10.1016/s1357-2725(98)00123-x. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Brown AJ, Dean RT, Jessup W. Free and esterified oxysterol: Formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J. Lipid Res. 1996;37:320–355. [PubMed] [Google Scholar]
- 10.Ong JM, Aoki AM, Seigel GM, Sacerio I, Castellon R, Nesburn AB, Kenney MC. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochem. Res. 2003;6:883–891. doi: 10.1023/a:1023223409798. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez IR, Alam S, Lee JW. Cytotoxicity of oxidized low density lipoprotein in cultured RPE cells is dependent on the formation of 7-ketocholesterol. Invest. Ophthamol. Vis. Sci. 2004;45:2830–2837. doi: 10.1167/iovs.04-0075. [DOI] [PubMed] [Google Scholar]
- 12.Dzeletovic S, Babiker A, Lund E, Diczfalusy U. Time course of oxysterol formation during in vitro oxidation of low density lipoprotein. Chem. Phys. Lipids. 1995;78:119–128. doi: 10.1016/0009-3084(95)02489-6. [DOI] [PubMed] [Google Scholar]
- 13.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arterioscler. Thromb. Vasc. Biol. 2004;24:949–954. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen JH, Olsen CE, Duedahl C, Skibsted LH. Isolation and quantification of cholesterol oxides in dairy products by selected ion monitoring mass spectroscopy. J. Dairy Res. 1995;62:101–113. doi: 10.1017/s0022029900033719. [DOI] [PubMed] [Google Scholar]
- 15.Lyons MA, Samman S, Gatto L, Brown AJ. Rapid hepatic metabolism of 7-ketocholesterol in vivo: Implications for dietary oxysterols. J. Lipid Res. 1999;40:1846–1857. [PubMed] [Google Scholar]
- 16.Girotti AW, Korytowiski W. Cholesterol as a singlet oxygen detector in biological systems. Methods Enzymol. 2000;319:85–100. doi: 10.1016/s0076-6879(00)19011-1. [Review] [DOI] [PubMed] [Google Scholar]
- 17.Girotti AW. Cholesterol-derived hydroperoxides: Generation and reactivity in biological systems. In: Fliesler SJ, editor. Sterols and Oxysterols: Chemistry, Biology, and Pathobiology. Trivandrum, India: Research Signpost; 2002. pp. 121–139. [Review] [Google Scholar]
- 18.Ohishi K, Zhang XM, Moriwaki S, Hiramitsu T, Matsugo S. Iron release analyses from ferritin by visible light irradiation. Free Radic. Res. 2005;39:875–882. doi: 10.1080/10715760500177807. [DOI] [PubMed] [Google Scholar]
- 19.Ohishi K, Zhang XM, Moriwaki S, Hiramitsu T, Matsugo S. In the presence of ferritin, visible light induces lipid peroxidation of the porcine photoreceptor outer segment. Free Radic. Res. 2006;40:799–807. doi: 10.1080/10715760600555027. [DOI] [PubMed] [Google Scholar]
- 20.Yefimova MG, Jeanny JC, Guillonneau X, Keller N, Nguyen-Legros J, Sergeant C, Guillou F, Courtois Y. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Invest. Ophthalmol. Vis. Sci. 2000;41:2343–2351. [PubMed] [Google Scholar]
- 21.Hahn P, Dentchev T, Qian Y, Rouault T, Harris ZL, Dunaief JL. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol. Vis. 2004;10:598–607. [PubMed] [Google Scholar]
- 22.Korytowiski W, Bachowski GJ, Girotti AW. Photoperoxidation of cholesterol in homogeneous solution, isolated membranes, and cells: Comparison of the 5α-and 6β-hydroperoxides as indicators of singlet oxygen intermediacy. Photochem. Phtotobiol. 1992;56:1–8. doi: 10.1111/j.1751-1097.1992.tb09594.x. [DOI] [PubMed] [Google Scholar]
- 23.Pawlak A, Wrona M, Rózanowska M, Zareba M, Lamb LE, Roberts JE, Simon JD, Sarna T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem. Photobiol. 2003;77:253–258. doi: 10.1562/0031-8655(2003)077<0253:cotapo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Rózanowska M, Sarna T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [Review] [DOI] [PubMed] [Google Scholar]
- 25.Moreira EF, Larrayoz IM, Lee JW, Rodriguez IR. 7-Ketocholesterol is present in lipid deposits in the primate retina: Potential implication in the induction of VEGF and CNV formation. Invest. Ophthalmol. Vis. Sci. 2009;50:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Organisciak DT, Darrow RA, Barsalou L, Darrow RM, Lininger LA. Light-induced damage in the retina: Differential effects of dimethylthiourea on photoreceptor survival, apoptosis and DNA oxidation. Photochem. Photobiol. 1999;70:261–268. [PubMed] [Google Scholar]
- 28.Bligh EE, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Rapp LM, Williams TP. Damage to the albino rat retina produced by low intensity light. Photochem. Photobiol. 1979;29:731–733. doi: 10.1111/j.1751-1097.1979.tb07758.x. [DOI] [PubMed] [Google Scholar]
- 30.Weigand RD, Giusto NM, Rapp LM, Anderson RE. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest. Ophthalmol. Vis. Sci. 1983;24:1433–1435. [PubMed] [Google Scholar]
- 31.Organisciak DT, Darrow RA, Jiang Y-L, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest. Ophthalmol. Vis. Sci. 1996;37:2243–2257. [PubMed] [Google Scholar]
- 32.Tanito M, Yoshida Y, Kaidzu S, Ohira A, Niki E. Detection of lipid peroxidation in light-exposed mouse retina assessed by oxidative stress markers, total hydroxyoctadecanoic acid and 8-iso-prostaglandin F2α. Neurosci. Lett. 2006;398:63–68. doi: 10.1016/j.neulet.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 33.Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: Correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp. Eye Res. 2006;82:538–541. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [Review] [DOI] [PubMed] [Google Scholar]
- 35.Li ZL, Lam S, Tso MO. Desferrioxamine ameliorates retinal photic injury in albino rats. Curr. Eye Res. 1991;10:133–144. doi: 10.3109/02713689109001741. [DOI] [PubMed] [Google Scholar]
- 36.Lukinova N, Iacovelli J, Dentchev T, Wolkow N, Hunter A, Amado D, Ying G-S, Sparrow JR, Dunaief JL. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest. Ophthalmol. Vis. Sci. 2009;50:1440–1447. doi: 10.1167/iovs.08-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panini SR, Sinenksy MS. Mechanisms of oxysterol-induced apoptosis. Curr. Opin. Lipidol. 2001;12:529–533. doi: 10.1097/00041433-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Prunet C, Lamaire-Ewing S, Ménétrier F, Néel D, Lizard G. Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol and 7β-hydroxycholesterol induced cell death: A morphological and biochemical study. J. Biochem. Mol. Toxicol. 2005;19:311–326. doi: 10.1002/jbt.20096. [DOI] [PubMed] [Google Scholar]
- 39.Miguet C, Monier S, Bettaleb A, Athias A, Bessede G, Laubriet A, Lemaire S, Neel D, Gambert P, Lizard G. Ceramide generation occurring during 7β-hydroxycholesterol- and 7-ketocholesterol-induced apoptosis is caspase independent and is not required to trigger cell death. Cell Death Differ. 2001;8:83–99. doi: 10.1038/sj.cdd.4400792. [DOI] [PubMed] [Google Scholar]