Abstract
Nerve injury increases the spinal cord expression and/or activity of voltage- and ligand-gated ion channels, peptide receptors, and neuro-immune factors that then drive dorsal horn neuron hyperexcitability. The intensity and duration of this central sensitization is determined by the net activity of local excitatory and inhibitory neurotransmitter systems, together with ongoing/evoked primary afferent activity and descending supraspinal control. Spinal endogenous inhibitory systems serve as opposing compensatory influences, and are gaining recognition for their powerful capacity to restrain allodynia and hyperalgesia. These include numerous G-protein coupled receptors (mu and delta opioid, α2-adrenergic, purinergic A1, neuropeptide Y Y1 and Y2, cannabinoid CB1 and CB2, muscarinic M2, GABAB, metabotropic glutamate type II-III, somatostatin) and perhaps nuclear receptors (PPARγ). Excessive down-regulation or defective compensatory up-regulation of these systems may contribute to the maintenance of neuropathic pain. An increasing number of pharmacotherapeutic strategies for neuropathic pain are emerging that mimic and enhance inhibitory neurotransmission in the dorsal horn.
INTRODUCTION
Peripheral nerve injury produces numerous neurobiological events in the peripheral and central nervous system that contribute to chronic pain. Over the past two decades, with research stemming from the development of new animal models of peripheral nerve injury, we have experienced an explosion of research directed towards an understanding of these pathophysiological neural changes. These include changes in the excitability or activation threshold of primary sensory neurons, increases in descending facilitation or decreases in descending inhibition from the brain, and increases in excitatory signaling or decreases in inhibitory signaling in the dorsal horn of the spinal cord [1,2].
This review begins with an update of the neural mechanisms thought to drive neuropathic pain. It then introduces the growing body of literature devoted to endogenous spinal inhibitory mechanisms, and discusses the current and emerging pharmacotherapeutic approaches that capitalize on the pharmacological plasticity of spinal inhibitory systems after peripheral nerve injury.
NEURAL MECHANISMS THAT DRIVE NEUROPATHIC PAIN
Ongoing Peripheral Nerve Activity
Normally, the spontaneous activity of primary afferent neurons is quite low. In patients with chronic peripheral neuropathy, however, direct microneurographic recordings demonstrated enhanced spontaneous firing of nociceptors innervating the painful region, indicating that abnormally active nociceptors contribute to neuropathic pain. Wall and colleagues found that spontaneous (stimulus-independent) activity and robust mechanical (stimulus-dependent) sensitivity develops in the afferent axonal sprouts innervating the neuroma. The abnormal impulses arising from these sites are called ectopic because they do not originate from the normal transduction elements of peripheral terminals of the primary afferent nociceptor[1]. Ectopic activity has been observed in animal models of neuropathic pain, and is thought to arise following either increased expression or redistribution from the soma to the site of injury. The resulting accumulation of sodium channels lowers action potential threshold and causes spontaneous ectopic discharge [1]. Ectopic activity is a key determinant in the generation and maintenance of positive neuropathic symptoms such as spontaneous pain [3].
Phenotypic and structural changes in DRG neurons and their central terminals
Nerve injury elicits a complex pattern of phenotypic changes in DRG neurons, including alterations in the expression of neurotransmitters, neuromodulators, receptors, ion channels, and structural proteins. While some of these changes are related to repair or regeneration, others may contribute to neuropathic pain. Early evidence suggested that nerve injury caused the phenotypic transformation of uninjured Aβ-fibers to a SP-synthesizing mode [4], which were thought to sprout from deeper non-nociceptive regions of the dorsal horn to more superficial nociceptive regions of lamina II [5], where pain transmission neurons are found to express a greater number of NK1 (SP) receptors. Abnormal activation of pain transmission neurons by stimulation of low threshold mechanoreceptors could then produce the touch-evoked pain that is so characteristic of many neuropathies [1]. However, recent rigorous data indicates that such sprouting is very limited [6], and found no evidence that Aβ afferent terminals contain or release SP in the dorsal horn [7]**.
Central sensitization of dorsal horn neurons
Prolonged or massive input from C-nociceptors triggers neuroplastic changes in dorsal horn nociresponsive neurons, leading to their increased spontaneous activity, expansion of receptive fields, and/or decreased threshold to subsequent afferent inputs [1]. This “central sensitization” involves a depolarization-induced influx of calcium through voltage-gated calcium channels, leading to the spinal release of neuropeptides and glutamate from nociceptors, and the subsequent triggering of secondary events such as nitric oxide synthesis, prostaglandin production, protein phosphorylation, and central glial activation. Normally, central sensitization induces an adaptive and protective response that helps to prevent further tissue damage. However, prolonged afferent input, such as sustained ectopic activity after nerve injury, can maintain central sensitization longer than necessary and this could underlie neuropathic pain [3]. In addition to neural mechanisms, the past few years have experienced an explosion of studies investigating the critical contribution of spinal microglia and astrocytes, Schwann cells, satellite cells in the DRG, and immune cells to the central sensitization associated with neuropathic pain. Numerous outstanding reviews have recently been devoted to this topic [8,9].
Supraspinal facilitation of central sensitization in neuropathic pain
Descending projections from the brainstem contribute to the maintenance of neuropathic pain states [2]. For example, the response to noxious mechanical stimulation of pain-facilitatory neurons in the rostral ventral medulla (RVM) increases after nerve injury [10]. Furthermore, selective destruction of facilitatory (mu-opioid receptor-expressing) RVM neurons increased both tactile allodynia and touch-evoked neural activity (Fos expression) within the dorsal horn [11]. Finally, silencing the RVM with local lidocaine injection exerted greater effects on facilitation than inhibition of stimulus-evoked activity of dorsal horn neurons in neuropathic rats, indicating that facilitatory (as opposed to inhibitory) influences from the brainstem predominate during neuropathic pain [12]. Ascending input spinal cord and/or descending input from cortical and sub-cortical structures can determine the magnitude and duration of descending facilitation, and this remains an important question in need of further research.
Nerve injury-induced loss of intrinsic inhibitory neurotransmission
Central sensitization is not necessarily pathological, and is normally kept in check by a balance of inhibitory controls. However, as discussed next, nerve injury may disrupt one or more endogenous pain inhibitory neurotransmitter systems, thus releasing the brake on central sensitization of dorsal horn neurons and causing stimulus-independent pain.
SPINAL NEUROTRANSMITTER INHIBITION OF NEUROPATHIC PAIN
Traditionally, the majority of pain research laboratories have sought to understand the molecular mechanisms that drive enhanced pain signaling in the dorsal horn, with the long-term objective of developing receptor antagonists as new analgesic drugs. These mechanisms include novel gene transcription, post-translational modifications, microglia or astrocyte activation, and alterations in voltage gated ion channels (Nav1.7-1.9, Cav2.2, alpha2-delta subunits), ligand-gated ion channels (neuronal nicotinic, GABAA), ionotropic glutamate receptors (AMPA, NMDA), peptide receptors (NK1), neurotrophins, lipid mediators, and neuro-immune mediators [1,8,13]. By contrast, an increasing number of laboratories have chosen to seek greater understanding of intrinsic pain inhibitory signaling in the spinal cord, with the long-term objective of developing agonists at targeted receptors. In addition to traditionally-studied candidates such as opioid, α2-adrenergic, and GABAB receptors, more recent attention has been given to alternative G-protein coupled receptors (GPCR), including those for NPY (Y1, Y2), adenosine (A1), cannabinoid (CB1, CB1), acetylcholine (M2), glutamate (groups II and III metabotropic), and somatostatin [14]. The remainder of this reviewer focuses on many of these systems, as well as an emerging non-GPCR target for neuropathic pain, namely, nuclear receptors [15].
Spinal Opioid Inhibition of Neuropathic Pain
Intrathecal morphine produces analgesia in both animals and humans, primarily through naloxone-reversible activation of the μ opioid receptor (MOR). In the dorsal horn, opioid agonists inhibit synaptic transmission at presynaptic terminals by blocking N-type calcium channels, thus reducing transmitter release, and at postsynaptic transmission neurons by opening G protein-coupled inwardly rectifying potassium (GIRK) channels and hyperpolarizing the membrane [14].
Once thought to be resistant to opioids, peripheral neuropathic pain responds moderately well to spinal or systemic opioid therapy [16,17]. However, doses must be pushed into ranges that produce significant side effects, and intrathecal morphine is relatively ineffective in neuropathic pain as compared to transient or inflammatory pain [18]. Recent studies have addressed the spinal mechanisms that underlie this difference. Namely, peripheral nerve section reduces μ opioid receptor (MOR) immunostaining and mRNA expression in injured small DRG neurons and their central terminals [18,19] (but see [20]). As a result, MOR agonists lose their ability to inhibit EPSCs and GIRKs on injured presynaptic terminals and their corresponding superficial dorsal horn neurons, respectively [18,21]. These findings indicate that peripheral nerve injury reduces opioid inhibition of spinal pain transmission from injured Aδ and C fiber nociceptors. This disinhibition could explain loss of opioid sensitivity in patients whose pain is generated by ectopic discharge from injured nociceptors. Indeed, nerve injury-induced loss of spinal MOR is associated with mechanical allodynia in rats [22].
Although the contribution of tonic endogenous opioid tone to transient or acute inflammatory pain is weak at best [23], the opioid receptor antagonist naloxone increases mechanical allodynia in animal models of neuropathic pain [24]. Furthermore, recent studies indicate that behavioral signs of neuropathic pain are enhanced in μ- or δ-deletion mutant mice [24,25]. Thus, as was originally proposed for clinical dental postoperative pain, opioids appear to orchestrate compensatory neuronal responses that tonically suppress mechanical allodynia in the setting of nerve injury [24,26].
Spinal noradrenergic neurotransmission and neuropathic pain
Spinal delivery of α2-adrenergic receptor agonists reduces behavioral signs of neuropathic pain in both animals and humans. Antinociceptive mechanisms include α2-presynaptic inhibition of dorsal root stimulation-induced release of pronociceptive neurotransmitters from primary afferent neurons, and α1 enhancement of inhibitory synaptic transmission from interneurons in the substantia gelatinosa leading to postsynaptic inhibition of spinal pain transmission neurons [27,28]. Epidural clonidine is approved in the US for the treatment of intractable neuropathic cancer pain, and manipulations that enhance norepinephrine concentrations in the spinal cord (amitriptyline and duloxetine) are first-line treatments for neuropathic pain [17]. Unlike the opioids, however, these compounds decrease neuropathic pain without altering transient nociceptive pain. To explain this, Eisenach and colleagues proposed that nerve injury induces the spinal release of BDNF, which then drives sprouting of terminals from descending noradrenergic fibers [29]**. An intriguing idea is that increased inhibitory NA innervation and release would lead to greater sensitivity to the analgesic effects of duloxetine. In addition, nerve injury increases the efficacy of G-protein coupling to spinal α2-adrenoceptors, which also could increase the antinociceptive potency of α2-adrenoceptor agonists [30].
Spinal norepinephrine is derived from the terminals of descending axons originating from noradrenergic brain nuclei, particularly the locus coeruleus (A6), but also A5 and A7 [31]. Further studies are required to determine whether endogenous tone from these supraspinal noradrenergic neurons is part of a tonic inhibitory system that restrains neuropathic pain, or whether they, like opioid receptor-containing neurons in the RVM (above), contribute to descending pain facilitation [32].
Spinal NPY and Neuropathic Pain
Neuropeptide Y and its Y1 and Y2 receptors are highly expressed at key sites of pain transmission, including lamina II of the spinal cord dorsal horn [33,34]. Because NPY inhibits the central release of CGRP from dorsal horn slices [35], these NPY receptors are poised to regulate pronociceptive neurotransmitter release from primary afferent neurons. Furthermore, peripheral nerve injury dramatically increases spinal NPY at the central terminals of low threshold DRG neurons and their central terminals, which appears to be associated with enhanced release in the dorsal horn [36]. As observed with noradrenergic systems, recent studies found that intrathecal administration of NPY decreased behavioral signs of neuropathic pain at doses that do not change transient pain. NPY reduced not only mechanical and cold hypersensitivity, but also spinal Fos expression, a molecular marker of neuronal activation. These anti-allodynic actions of NPY could be reversed either by deletion of the Y1 gene, or by pharmacological blockade of the Y1 or Y2 receptor [37,38]**. The mechanisms underlying the enhanced sensitivity to the analgesic actions of NPY in the setting of peripheral nerve injury remains an important question to address in future studies.
Endogenous NPY also exerts anti-allodynic actions, since germ-line NPY deletion mutant mice display exaggerated autotomy after sciatic nerve transection [39]. (Autotomy is a self-mutilation behavior possibly related to neuropathic pain sensation). Furthermore, unpublished data from our laboratory indicates that conditional NPY knockdown, or pharmacological intrathecal administration of Y2 receptor antagonists, dramatically increases cold and mechanical allodynia after SNI. As noted above for the endogenous opioid system, these findings suggest that endogenous spinal NPY exerts a compensatory, adaptive inhibition of cold and tactile hypersensitivity in the neuropathic state.
Spinal Adenosine Inhibition of Neuropathic Pain
Intrathecal administration of adenosine reduces allodynia in animals peripheral nerve injury, through both pre- and postsynaptic mechanisms involving Gi-coupled A1-adenosine receptors. At the central terminal of primary afferent neurons, adenosine decreases Ca currents, CGRP release and mEPSP frequency in cultured DRG neurons and/or spinal cord slices [40]. At postsynaptic sites, adenosine hyperpolarizes dorsal horn neurons [41]. Intrathecal adenosine also reduces clinical neuropathic pain, but therapeutic use is limited by major side effects including hyperalgesia [42].
Recent findings indicate that thiamine monophosphatase (TMP, also known as fluoride-resistant acid phosphatase, the transmembrane form of prostatic acid phosphatase), a classic marker of small-diameter DRG neurons and their central terminal in the dorsal horn whose expression plummets after nerve injury, is one of the ecto-5’-nucleotidase enzymes that can rapidly dephosphorylate AMP to adenosine [43]**. Intrathecal injection of TMP decreased behavioral signs of neuropathic pain in normal mice, but neither in A1R deletion mutant mice nor in the presence of an A1R antagonist [43]. These innovate results identify a new approach for pharmacological treatment of neuropathic pain: a treatment that selectively increases spinal TMP activity should generate inhibitory A1-adenosinergic tone that dampens the severity of neuropathic pain.
Cannabinoid receptor agonists and neuropathic pain
Several clinical studies indicate that cannabinoids such as delta(9)-tetrahydrocannabinol (THC) or Sativex (THC plus cannabidiol), reduce neuropathic pain, but these compounds also produce adverse effects such dizziness and sedation at therapeutic concentrations [44]. Two intriguing approaches based on recent innovative basic science studies are currently under investigation to overcome these limitations.
First, selective targeting of peripheral CB1 receptors may provide significant pain relief with fewer CNS side effects. Conditional deletion of CB1 on sensory neurons increased behavioral signs of neuropathic pain and decreased the antinociceptive effects of the CB1 agonist, WIN 55212-2 [45]**. These data indicate that CB1 expressed by nociceptor neurons, rather than CNS neurons, primarily mediates cannabinoid-induced inhibition of neuropathic pain. Peripherally-acting CB1 agonists are in development [13].
Second, spinal administration of the CB2 receptor agonist JWH-133 attenuated mechanical allodynia in neuropathic rats, an anti-allodynic that could be reversed with the CB2 antagonist SR144528 [46]*. Because CB2 is not normally expressed in the CNS, these results were initially puzzling, until it was discovered that nerve injury induces the de novo expression of CB2 on activated microglia [47]. Subsequently it was revealed that intrathecal JWH-133 reduced microglial activation as well as nerve injury-induced allodynia [46]. In summary, sensory neuron CB1 and spinal cord glial CB2 receptors represent important emerging targets for the treatment of neuropathic pain.
Spinal GABA and modulation of neuropathic pain
Interneurons containing γ-aminobutyric acid (GABA) or glycine inhibit the spinal transmission of noxious sensory signals, and numerous studies indicate that spinal GABAergic inhibition is reduced after experimental nerve injury [48]. How this occurs has become quite controversial in recent years, in some cases with the presentation of strong data that counter prevailing theory. First, caspase-3 and TUNEL were initially reported in the dorsal horn after nerve injury, indicating death of inhibitory neurons [48,49]. However, Polgar and Todd conducted rigorous anatomical studies indicating that loss of neurons in lamina I-III is minimal and is not required for the development of tactile allodynia, and noted that TUNEL positive nuclei belonged to microglia not neurons [50]. Second, spinal GABA and GABA immunoreactivity was initially found to decrease in the dorsal horn after nerve injury reduced [51], but this could not be confirmed with rigorous light and electron microscopic analyses [52]**. Third, nerve injury does not decrease GABAA receptor immunoreactivity [48,52]. Fourth, De Koninck and colleagues have suggested that nerve injury reverses the chloride concentration gradient in lamina I, effectively converting GABA from an inhibitory to an excitatory neurotransmitter [53]. However, this mechanism implies that the overall effect of GABA is pain facilitatory, which is difficult to reconcile with numerous reports that GABAA and GABAB receptor agonists reduce allodynia and hyperalgesia [54]. Fifth, diminished primary afferent nociceptor activity following nerve injury might reduce the excitability or burst firing patterns of GABAergic interneurons; however, recordings from spinal cord slices with roots attached indicate that input from Aδ- and C-fibers do not differ between neuropathic and sham-operated rats (input from Aβ-fibers could not be tested in this study) [55]. In order to capitalize on robust inhibitory GABAergic neurotransmitter in the dorsal horn, further studies are needed to determine the mechanisms underlying decreased GABAergic tone after nerve injury and its relationship to neuropathic pain.
Spinal muscarinic inhibition of neuropathic pain
Intrathecal or systemic administration of cholinesterase inhibitors produces analgesia in animals and humans, likely via M2 muscarinic receptors in the dorsal horn [14,56]. Nerve injury increases the expression of M2 cholinergic receptors on sensory afferent [57], suggesting that cholinergic inhibitory tone is augmented after peripheral nerve injury.
Spinal Nuclear Receptors and Inhibition of Neuropathic Pain
The gamma subtype of peroxisome proliferator-activated receptor (PPARγ), a nuclear receptor, was recently found to be expressed in the spinal cord [15]**. Activation of these receptors inhibits allodynia, since intrathecal administration of PPARγ ligands dose-dependently attenuated nerve injury-induced mechanical and thermal hypersensitivity in a PPARγ antagonist-reversible manner [15]. These data suggest that new or currently available drugs targeted at spinal PPAR-gamma (such as pioglitazone, which is approved for the treatment of diabetes by the United States Food and Drug Administration) may yield important therapeutic effects for the management of neuropathic pain.
Furthermore, a PPARγ antagonist itself rapidly increased the mechanical allodynia associated with nerve injury [58]**. Although additional studies are required to identify the molecules that intrinsically activate PPARγ in the dorsal horn, these studies suggest that endogenous PPARγ tone dampens the severity of neuropathic pain.
CONCLUSIONS
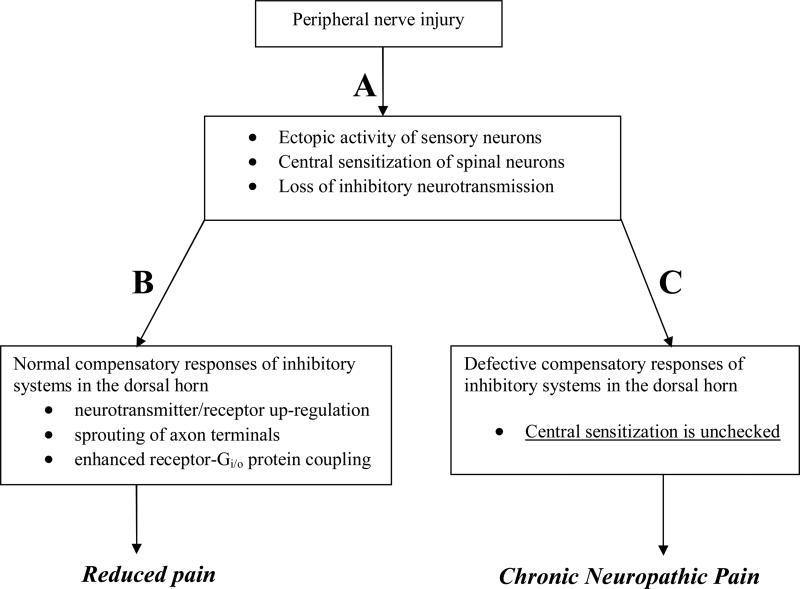
The past few years have experienced a generational shift in our efforts to understand the spinal mechanisms of neuropathic pain. As summarized in Table 1, trends in research focus include: 1) a shift from phenotypic and structural changes that drive pain to mechanisms of pharmacological plasticity that strengthen analgesia; 2) a shift from a few studies on neuron-immune interactions to an explosion of work on the reciprocal connections between neurons, glia, and immune cells in the dorsal horn that cause hypersensitivity; 3) a shift from loss of spinal GABAergic inhibition to reversal of GABAA-associated chloride channels; 4) a shift from an emphasis on GABA-, α2 receptor-, and opioid-mediated inhibition to a broader study of numerous other spinal inhibitory systems including cannabinoid, adenosine, NPY, and PPARγ; and 5) increasing recognition that the central sensitization that underlies neuropathic pain is opposed by compensatory influences involving the up-regulation of spinal inhibitory systems. Normally, such systems maintain a powerful capacity to restrain allodynia and hyperalgesia. Figure 1 proposes that if nerve injury does not induce these normal feedback inhibitory mechanisms, then central sensitization in the dorsal horn will remain unchecked, leading to neuropathic pain.
Table 1. Modification of endogenous inhibitory mechanisms in the spinal cord after nerve injury.
NPP = Neuropathic Pain. TMP = thiamine monophosphatase, which generates the inhibitory neurotransmitter, adenosine. Receptor down-regulation could induce loss of opioid sensitivity. Conversely, noradrenergic sprouting, enhanced α2 receptor-G protein coupling, and de novo CB2 expression could explain the enhanced efficacy of duloxetine, clonidine, and cannabinoids as analgesics for neuropathic pain, respectively.
| Mechanism | Recent Example | Proposed Impact on NPP |
|---|---|---|
| Receptor down-regulation | Mu-opioid | unimpeded |
| Enzyme down-regulation | TMP [43] | unimpeded |
| Reversal of chloride channel |
GABAA |
unimpeded |
| Sprouting of nerve endings | noradrenergic [29] | constrained |
| Receptor-G protein coupling | α2 receptor [30] | constrained |
| de novo expression in microglia | CB2 receptor | constrained |
| Neurotransmitter up-regulation | Neuropeptide Y | constrained |
| Receptor up-regulation | M2 receptors | constrained |
Figure 1.
Proposed sequence of events leading to: A) Early spinal nociceptive transmission and pain and; B) normal resolution of pain following compensatory up-regulation of inhibitory system;, or C) pathological development of neuropathic pain when compensatory mechanisms fail.
References
- 1.Taylor BK. Pathophysiologic mechanisms of neuropathic pain. Curr Pain Headache Rep. 2001;5:151–161. doi: 10.1007/s11916-001-0083-1. [DOI] [PubMed] [Google Scholar]
- 2.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 3.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;15:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 6.Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for sprouting of Abeta afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. J Neurosci. 2003;23:9491–9499. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Hughes DI, Scott DT, Riddell JS, Todd AJ. Upregulation of substance P in low-threshold myelinated afferents is not required for tactile allodynia in the chronic constriction injury and spinal nerve ligation models. J Neurosci. 2007;27:2035–2044. doi: 10.1523/JNEUROSCI.5401-06.2007. [Hughes, 2007. Using transganglionic labeling, stimulation of primary afferent, triple-label immunohistochemistry of spinal terminals, and an in situ assay of spinal SP release, this article strongly indicates that SP is not transported to central terminals and is not released into the dorsal horn following low threshold stimulation. This finding necessitates a reappraisal of the importance of the finding that nerve injury up-regulates SP in the cell bodies of axotomized Aβ afferents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neuroscience. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Dray A. Neuropathic pain: emerging treatments. Br J Anaesth. 2008;101:48–58. doi: 10.1093/bja/aen107. [DOI] [PubMed] [Google Scholar]
- 14.Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain. 2008;9:639–649. doi: 10.1016/j.jpain.2008.02.002. [Churi, 2008. Until recently, inhibitory neurotransmission in the dorsal horn appeared to be exclusively mediated by G protein-coupled receptors. This article clearly demonstrates that nuclear receptors (namely, PPARγ) can also contribute to the spinal inhibition of neuropathic pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr., Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki Y, Kohno T, Ji RR. Different effects of opioid and cannabinoid receptor agonists on C-fiber-induced extracellular signal-regulated kinase activation in dorsal horn neurons in normal and spinal nerve-ligated rats. J Pharmacol Exp Ther. 2006;316:601–607. doi: 10.1124/jpet.105.093583. [DOI] [PubMed] [Google Scholar]
- 22.Back SK, Lee J, Hong SK, Na HS. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain. 2006;123:117–126. doi: 10.1016/j.pain.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Taylor BK, Peterson MA, Basbaum AI. Continuous intravenous infusion of naloxone does not change behavioral, cardiovascular, or inflammatory responses to subcutaneous formalin in the rat. Pain. 1997;69:171–177. doi: 10.1016/s0304-3959(96)03265-4. [DOI] [PubMed] [Google Scholar]
- 24.Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100:912–921. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Nadal X, Banos JE, Kieffer BL, Maldonado R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci. 2006;23:830–834. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- 26.Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278:740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–484. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- **29.Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [Hayashida, 2008. First-line treatments for clinical neuropathic pain (antidepressants and antiepileptics) and spinal clonidine target or mimic noradrenergic analgesic systems. This article provides a mechanism of pharmacological plasticity to explain how these drugs can reduce neuropathic pain without exerting much effect on acute transient pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases alpha2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- 32.Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. doi: 10.1016/j.neuroscience.2009.02.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138:1361–1376. doi: 10.1016/j.neuroscience.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489:328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- 35.Gibbs J, Flores CM, Hargreaves KM. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience. 2004;125:703–709. doi: 10.1016/j.neuroscience.2004.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuphal KE, Solway B, Pedrazzini T, Taylor BK. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition. 2008;24:885–891. doi: 10.1016/j.nut.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi TJ, Zhang X, Berge OG, Erickson JC, Palmiter RD, Hokfelt T. Effect of peripheral axotomy on dorsal root ganglion neuron phenotype and autonomy behaviour in neuropeptide Y-deficient mice. Regulatory Peptides. 1998;75-76:161–173. doi: 10.1016/s0167-0115(98)00064-0. [DOI] [PubMed] [Google Scholar]
- 40.Mauborgne A, Polienor H, Hamon M, Cesselin F, Bourgoin S. Adenosine receptor-mediated control of in vitro release of pain-related neuropeptides from the rat spinal cord. Eur J Pharmacol. 2002;441:47–55. doi: 10.1016/s0014-2999(01)01619-3. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72:1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- 42.Martin TJ, Eisenach JC, Misler J, Childers SR. Chronic activation of spinal adenosine A1 receptors results in hypersensitivity. Neuroreport. 2006;17:1619–1622. doi: 10.1097/01.wnr.0000239949.37825.e9. [DOI] [PubMed] [Google Scholar]
- **43.Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [Zylka, 2008. TMP (FRAP) was known to be expressed in small diameter DRG neurons for almost 50 years, but absence of adequate antibodies and genetic sequence precluded the identification of its molecular and physiological functions. With the recognition that TMP was a membrane-bound isoform of the well-described prostatic acid phosphatase, these authors could rigorously study the participation of TMP to spinal nociceptive transmission.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- **45.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [Agarwal, 2007. This is the quintessential illustration of the extraordinary power of current transgenic mouse technology to address an important hypothesis in neuropathic pain research. The Cre/loxP system for conditional gene deletion was used to generate mice that lacked CB1 only in small sensory neurons, without affecting expression in the brain, spinal cord, or any other organ.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **46.Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108:722–734. doi: 10.1097/ALN.0b013e318167af74. [Romero-Sandoval, 2008 #4221. The past few years have yielded an incredible array of new data implicating activated microglia in the genesis of neuropathic pain. This article supports the interesting hypothesis that mimicry of an endogenous inhibitory mechanism on spinal microglia (CB2) represents a new global strategy for pharmacotherapy. Polgar, 2008 #4205. This article represents the nail in the coffin to the theory that neuropathic pain results from nerve injury-induced death of GABAergic neurons in the dorsal horn.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 48.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 52.Polgar E, Todd AJ. Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABA(A) receptors from synapses in laminae I-II of the ipsilateral spinal dorsal horn. Neuroscience. 2008;156:193–202. doi: 10.1016/j.neuroscience.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 54.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Schoffnegger D, Heinke B, Sommer C, Sandkuhler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol. 2006;577:869–878. doi: 10.1113/jphysiol.2006.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clayton BA, Hayashida K, Childers SR, Xiao R, Eisenach JC. Oral donepezil reduces hypersensitivity after nerve injury by a spinal muscarinic receptor mechanism. Anesthesiology. 2007;106:1019–1025. doi: 10.1097/01.anes.0000265163.22007.6d. [DOI] [PubMed] [Google Scholar]
- 57.Hayashida KI, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–268. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- **58.Fehrenbacher JC, LoVerme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK. Rapid Pain Modulation with Nuclear Receptor Ligands. Brain Research Reviews. doi: 10.1016/j.brainresrev.2008.12.019. in press. [Fehrenbacher, 2008. Until recently, direct transcriptional (genomic) mechanisms were thought to mediate the actions of nuclear receptor ligands such as 15d-PGJ2, palmitoylethanolamide and estradiol. This timely review describes both previously published and new data suggesting that these ligands may operate through rapid, non-genomic mechanisms to modulate inflammatory and neuropathic pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]