Abstract
Inner ear development requires coordinated transformation of a uniform sheet of cells into a labyrinth with multiple cell types. While numerous regulatory proteins have been shown to play critical roles in this process, the regulatory functions of microRNAs (miRNAs) have not been explored. To demonstrate the importance of miRNAs in inner ear development, we generated conditional Dicer knockout mice by the expression of Cre recombinase in the otic placode at E8.5. Otocyst-derived ganglia exhibit rapid neuron-specific miR-124 depletion by E11.5, degeneration by E12.5, and profound defects in subsequent sensory epithelial innervations by E17.5. However, the small and malformed inner ear at E17.5 exhibits residual and graded hair cell-specific miR-183 expression in the three remaining sensory epithelia (posterior crista, utricle, and cochlea) that closely corresponds to the degree of hair cell and sensory epithelium differentiation, and Fgf10 expression required for morphohistogenesis. The highest miR-183 expression is observed in near-normal hair cells of the posterior crista, whereas the reduced utricular macula demonstrates weak miR-183 expression and develops presumptive hair cells with numerous disorganized microvilli instead of ordered stereocilia. The correlation of differential and delayed depletion of mature miRNAs with the derailment of inner ear development demonstrates that miRNAs are crucial for inner ear neurosensory development and neurosensory-dependent morphogenesis.
Keywords: RNA interference, microRNA, sensory epithelium, hair cell, inner ear development, conditional knockout, floxed Dicer
Introduction
The vertebrate ear’s ability to extract sound and angular and linear acceleration from mechanical stimuli requires an intricate three dimensional geometry that includes the strategic positioning of sensory epithelia and the appropriate histological organization of epithelial hair cells and supporting cells. In recent years, molecular analyses have revealed many factors that influence patterning, morphogenesis and histogenesis. For example, fibroblast growth factors (FGFs) and bone morphogenic proteins (BMPs) are essential factors that transduce early patterning signals into specific regional gene expression in developing prosensory epithelia, which in turn governs the morphogenesis of semicircular canals (Chang et al., 2004; Pauley et al., 2006; Pauley et al., 2003; Nichols et al., 2008). Likewise, molecular steps in histogenesis of the sensory epithelia have emerged (Fritzsch et al., 2006; Kelley, 2006) and indicate that coordinated transitions of Eya1, Sox2, and possibly Isl1 and Neurog1 gene expression are required to up-regulate Atoh1 in postmitotic precursors for hair cell differentiation (Kiernan et al., 2005; Matei et al., 2005; Radde-Gallwitz et al., 2004; Raft et al., 2007; Zou et al., 2008). Such developmental transitions are not only orchestrated by the regulatory functions of morphogens and transcription factors. Indeed, growing evidence demonstrates the widespread importance of non-coding RNAs in transcriptional and post-transcriptional regulation of eukaryotic gene expression (Amaral et al., 2008). In particular, the ~500 mammalian microRNAs (miRNAs) appear to play a substantial role in development, cell maintenance, and disease (Hobart, 2008; Makeyev and Maniatis, 2008).
Our prior work has shown that ~100 miRNAs are expressed in development and maturation of the inner ear (Weston et al., 2006). In addition, we have recently shown that hair cell-specific miRNAs are highly conserved across phyla and exhibit expression in known mechanosensory cells and neurosensory organs from C. elegans to mammals (Pierce et al., 2008), consistent with previous notions on the ancestry of mechanosensors suggested by the conservation of transcription factors (Fritzsch et al., 2007). If indeed these and other miRNAs play crucial roles in development as indicated by their abundant expression in the developing central nervous system (CNS) and mammalian inner ear (Kapsimali et al., 2007; Weston et al., 2006), it is reasonable to hypothesize that major histogenetic and morphogenetic defects will result when small RNA production is abrogated using a conditional approach to eliminated the RNA processing enzyme Dicer (Harfe et al., 2005).
We have therefore examined the effect of conditional Dicer knockout (KO) in Pax2-Cre transgenic mice (Ohyama and Groves, 2004), which enable deletion of floxed Dicer alleles in ear, kidney, and mid-hindbrain. Moreover, the early expression of Pax2-Cre in the otic placode provides for a broad examination of the importance of Dicer and its small RNA products in inner ear development. The data demonstrate that small RNAs, including miRNAs, are required for inner ear development, maintenance of sensory neurons, and differentiation of sensory epithelia. Of particular interest is the finding that residual mature miRNAs appear to enable partial hair cell differentiation, suggesting that no neurosensory component of the ear can form in the complete absence of miRNAs. These data are consistent with our hypothesis that miRNAs are integral components of inner ear developmental programs, although the model cannot differentiate the biological impact of miRNAs and other small RNA products of Dicer (i.e. siRNAs). Nevertheless, the data validate to an extent previously unknown the essential function of small RNAs in neurosensory organ development.
Materials and Methods
Generation of conditional Dicer knockout mice
Mice carrying floxed Dicer alleles (Dicerflox/flox; Harfe et al., 2005) were mated to mice carrying a Pax2-Cre transgene (Ohyama and Groves, 2004) to generate Pax2-Cre;Dicerflox/wt mice. Pax2-Cre;Dicerflox/wt mice were subsequently mated to Dicerflox/flox mice to generate Pax2-Cre;Dicerflox/flox mutant embryos. Offspring were genotyped by PCR analysis of tail DNA using Cre-specific primers (5′-GCCTGCATTACCGGTCGATGCAACGA and 5′-GTGGCAGATGGCGCGGCAACACCATT) that produce a 726 bp product, and Dicer-specific primers (5′-CCTGACAGTGACGGTCCAAAG and 5′-CATGACTCTTCAACTCAAACT) that produce a 420 bp product from the Dicerflox allele and a 351 bp product from the Dicerwt allele. Mice carrying a Dicerwt allele or lacking the Pax2-Cre transgene were used as control littermates. Embryos were harvested at E11.5, E12.5, E14.5, E17.5, and E18.5 from timed pregnant females counting from noon on the day the vaginal plug was found as E0.5. Embryos were perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) and tissues were isolated for analysis.
Primers used to discriminate the Dicerflox allele and the deletion allele (DicerΔ) by PCR amplification of DNA isolated from inner ear tissues were 5′-CCTGACAGTGACGGTCCAAAG and 5′-CCTGAGCAAGGCAAGTCATTC as previously described (Harfe et al., 2005).
In situ hybridization (ISH)
Whole-mount ISH detecting miRNA expression was performed as previously described (Weston et al., 2006) using locked nucleic acid (LNA) probes (Integrated DNA Technologies) labeled with digoxigenin (DIG; Roche). Briefly, tissues fixed 4% PFA in 0.1 M phosphate buffer (pH 7.4) were defatted with ethanol and digested with proteinase K prior to hybridization of 12 pmol LNA probe, washing and RNase A digestion, and probe detection using alkaline phosphatase (AP) conjugated sheep anti-DIG Fab fragment and BM Purple AP Substrate (Roche). Tissues were whole mounted in glycerol and viewed by differential interference contrast microscopy on a Nikon Eclipse 800 microscope. Two ears or brains were analyzed at each time point described.
LNA probes antisense to miR-124 (5′-GGCATTCACCGCGTGCCTTA), miR-183 (5′-CAGTGAATTCTACCAGTGCCATA), or miR-183* (5′-TTATGGCCCTTCGGTAATTCAC) contain LNA modifications at every third nucleotide position from the 5′ end. Such LNA probes have previously been shown to provide exceptional miRNA hybridization specificity that is sensitive to 1 or 2 nucleotide mismatches (Kloosterman et al., 2006; Pierce et al., 2008).
Whole-mount ISH detecting Fgf10 mRNA was similarly performed using a 552 nt riboprobe generated by in vitro transcription of PCR products derived from an Fgf10 cDNA clone using primers 5′-CACATTGTGCCTCAGCCTTTC and 5′-TAATACGACTCACTATAGGGTCCTCTCCTGGGAGCTC (Pauley et al., 2003). Two ears or brains were analyzed at each time point.
Neuronal tracing
Lipophilic dye-soaked filter strips were inserted into the brainstem to label afferents and efferents separately, or in the ear to fill afferents from the ear or discrete sensory epithelia to the brain as previously described (Jensen-Smith et al., 2007). Ears or brains were mounted in glycerol and viewed using a Zeiss LSM 510 confocal microscope. From two to six specimens were analyzed at each time point described.
Immunocytochemistry (ICC)
Antibodies for acetylated tubulin (Sigma), BDNF (R&D Systems), Sox2 (Millipore), MyoVIIa (Proteus Biosciences), and Caspase 3 (Chemicon) were used for ICC as previously described (Matei et al., 2005; Pauley et al., 2006). Briefly, tissues fixed with 4% PFA in 0.1 M phosphate buffer (pH 7.4) were defatted with ethanol, blocked with goat serum, and incubated for 1 day with primary antibody. Secondary antibody was conjugated to either Alexa 543 or Alexa 648. Tissues were mounted in glycerol and viewed using a Zeiss LMS 510 confocal microscope. From two to six specimens were analyzed at each time point described.
Scanning and transmission electron microscopy (SEM and TEM)
Three E17.5 ears and one E18.5 ear were post-fixed in 0.5% glutaraldehyde, followed by 0.5% osmium tetroxide. Ears were either embedded in epoxy resin to obtain ultrathin sections for TEM (E18.5) or critical point dried for SEM (E17.5). Critical point dried ears were mounted on stubs, sputter coated and viewed in a JEOL JSM-840A SEM. Ultrathin sections were counterstained with uranyl acetate and lead citrate and viewed in a JEOL JEM-1011 TEM as previously described (Ma et al., 2000).
Results
Conditional Dicer knockout mouse model
To investigate the function of small RNAs in mouse inner ear development, we have generated Pax2-Cre conditional Dicer knockout (KO) mice (i.e. Pax2-Cre;Dicerflox/flox). KO mice have a number of developmental defects owing to Dicer deletion in Pax2-Cre expression domains that likely contribute to late embryonic lethality at approximately E18.5. The Pax2-Cre transgene is strongly expressed in the mid-hindbrain, kidney, and otic placode at ~E8.5 (Ohyama and Groves, 2004). KO mice thus exhibit notable defects in mid-hindbrain development that include ablation of the cerebellum and tectum (Figs. 1A and 1B) and reduced kidney size (data not shown). Interestingly, KO mice also demonstrate obvious defects in craniofacial and eyelid development (Fig. 1A) outside the expected domain of Pax2-Cre expression. These defects are currently being investigated to determine whether they are direct or indirect effects of Pax2-Cre conditional Dicer KO.
Figure 1.
Developmental defects and miRNA depletion in Pax2-Cre Dicer KO mice. A, Overview of developmental defects. Depicted are external features of the head (left) and approximate midsagittal section (right). Notable defects exemplified by comparison of E18.5 Pax2-Cre Dicer KO and control littermates include abbreviated craniofacial development and abrogated eyelid and cerebellum (CB) development (denoted by the asterisk). B, Floxed allele recombination in Pax2-Cre control (Dicerflox/wt) and Dicer KO (Dicerflox/flox) mouse brain. Depicted is X-Gal staining indicating Pax2-Cre positive tissue in which the Rosa26-LacZ reporter (R26R) allele has been recombined to express functional β-galactosidase. Note the near complete loss of midbrain (MB) and cerebellum (CB) in the KO brain with normal formation of the forebrain, which shows no indication of Pax2-Cre expression. C, Depletion of neuronal specific miR-124 in Pax2-Cre Dicer KO brain and ganglia. ISH shows depletion of miR-124 in Pax2-Cre expression domains (Ohyama and Groves, 2004) including the mid-hindbrain boundary (MHB) and vestibular ganglia (VG). Also denoted are the hypothalamus (H), caudal midbrain (T), rhombomeres 1 and 2 (r1 and r2, respectively), cerebellum (CB), trigeminal motoneurons and ganglia (MV and TG, respectively), and otocyst (O).
To confirm the loss of functional Dicer and depletion of miRNAs, neuron-specific expression of miR-124 was examined in E11.5 KO mice and control littermates (e.g. Pax2-Cre;Dicerflox/wt). In situ hybridization (ISH) using a locked nucleic acid (LNA) probe for miR-124 demonstrates that expression is abundant in the control mouse throughout the mid-hindbrain and in sensory neurons of the trigeminal ganglia and vestibuloacoustic ganglia (Fig. 1C). However, miR-124 expression is absent in the KO mouse throughout the caudal midbrain and anterior hindbrain (i.e. rhombomere 1), and in the vestibular and acoustic ganglion neurons that derive from the otocyst. Importantly, hindbrain miR-124 expression remains in the hindbrain posterior to rhombomere 1, suggesting that the model does not directly perturb vestibular and cochlear efferents from rhombomere 4 (Bruce et al., 1997), or known effects of rhombomeres 5 and 6 on early patterning of the otocyst (Bok et al., 2007). These data suggest that functional Dicer and neuron-specific miRNAs are largely depleted within three days from the time of Pax2-Cre expression in the mid-hindbrain and otic placode. Moreover, the otocyst of the KO mouse is notably smaller at this early point in inner ear development (Fig. 1C).
To ensure that Pax2-Cre transgene expression and floxed allele recombination precisely recapitulates that previous observed (Ohyama and Groves, 2004) in the context of conditional Dicer KO, tissues from E17.5 control and KO mice carrying a Rosa26-LacZ reporter (R26R) allele (Soriano, 1999) were examined by X-gal staining. As expected, X-gal staining shows that Pax2-Cre expression supports recombination throughout the mid-hindbrain (Fig. 1B) and the inner ear (Fig. S1) of control mice and any remaining brain and ear tissues of KO mice. In particular, X-gal staining is observed in all sensory epithelia of control and KO mouse inner ears including the posterior crista as previously reported (Ohyama and Groves, 2004). However, it should be noted that some cells in the dorsoposterior part of the otic vesicle and non-sensory cells of the posterior crista may be reporter-negative (Ohyama and Groves, 2004). Nevertheless, the data suggest that Pax2-Cre expression domains retain their definition in the inner ear and midbrain of the Dicer KO model.
Overview of effects on inner ear development
The inner ear of mice consists of six sensory epithelia located within the vestibular and auditory organs that include the anterior, horizontal, and posterior cristae, utricular and saccular maculae, and the organ of Corti that runs the length of the cochlea (Fig. 2A). Conditional Dicer KO mice demonstrate a significant truncation of most inner ear structures at E17.5 (Fig. 2B). Although the KO inner ear exhibits a well developed posterior crista and canal, the horizontal crista and canal and the anterior crista are always absent, and the anterior canal is either absent or substantially reduced. A reduced utricle is always present (Figs. 2B and 2C) and a small saccule is sometimes present (Figs. 2B and 2D), either of which rarely contain misshapen otoconial masses. The cochlea presents only as a comma-shaped structure with no coils.
Figure 2.
Defects in inner ear morphogenesis and sensory epithelial development of Pax2-Cre Dicer KO mice. A, Stylized representation of normal inner ear morphology and sensory epithelia (colored). Indicated are the anterior, horizontal, and posterior cristae (AC, HC, and PC, respectively), utricular (U) and saccular (S) maculae, and organ of Corti in the cochlea (CO). Arrows indicate dorsal (D) and posterior (P) orientation for all images. B,C,D, Morphology of an E17.5 Pax2-Cre Dicer KO mouse inner ear. Invariably, each KO mouse inner ear lacks recognizable anterior and horizontal cristae and a horizontal canal, has a well-formed posterior crista and canal, and forms a utricle and an abbreviated cochlea consisting of approximately one half-turn. Rarely observed is a small saccule and misformed anterior canal. The utricle (C) may contain a single otoconial mass, and the saccule (D) if present may contain a misshapen otoconial mass. E, Normal sensory epithelial development indicated by X-gal staining of hair cells in Atoh1-LacZ mouse inner ear (i.e. Atoh1LacZ/wt; Fritzsch et al., 2005). The inset depicts to scale a conditional Dicer KO ear. F, Sensory epithelial development and innervation of the KO mouse inner ear. Sensory epithelia indicated by ICC detecting Sox2 invariably show a well-developed posterior crista, a utricular macula, and one or two sensory patches within the cochlea. Innervation shown by ICC detecting tubulin reveals fibers to the utricle, but not posterior crista. Few fibers innervate the cochlea but fail to target the sensory patch. Similar labeling of the normal cochlea is provide in Fig. S4D.
A comparison of normal inner ear and conditional Dicer KO inner ear provides perspective to the overall reduction in KO inner ear development. A developmentally normal inner ear at E18.5 from an Atoh1-LacZ mouse (i.e. Atoh1LacZ/wt; Fritzsch et al., 2005) shows X-gal staining of hair cells throughout the sensory epithelia (Fig. 2E) compared to scale with an E17.5 Dicer KO inner ear (Fig. 2E; inset). The truncation of KO inner ear sensory epithelial development is demonstrated by immunocytochemistry (ICC) detecting Sox2, which is specifically expressed in prosensory epithelia and in supporting cells following hair cell differentiation (Kiernan et al., 2005; Dabdoub et al., 2008). The E17.5 KO inner ear thus exhibits a well-developed posterior crista and relatively small utricular and cochlear patches of sensory epithelium development (Fig. 2F). Moreover, labeling of nerve fibers by ICC detecting tubulin shows that innervation of the E17.5 KO inner ear is severely reduced (Fig. 2F). Whereas a few fibers targeting the utricular macula remain, there is no innervation of the posterior crista and any fibers remaining to the cochlea fail to target the developing sensory epithelium (Fig. S2). These data demonstrate that conditional Dicer KO in the developing mouse inner ear results in profound morphogenic, histogenic and innervation defects.
Defects in sensory epithelial histogenesis
The histological development of sensory epithelia in the E17.5 KO mouse inner ear was further evaluated by ICC detecting brain-derived neurotrophic factor (BDNF) and myosin VIIa (MyoVIIa) markers for hair cells. The sensory epithelium of the cochlea expressing Sox2 most often appears as two distinct basal and apical patches (Fig. 3A). Relatively few cells within each patch demonstrate detection of BDNF (Figs. 3B–C), a documented hair cell factor that attracts nerve fibers (Tessarollo et al., 2004). Moreover, these presumptive hair cells fail to assume the precise organization of inner and outer hair cell rows seen in the normal organ of Corti (Fig. 3D). However, many cells within each patch appear MyoVIIa positive (Fig. 3E), suggesting they might represent developing hair cells.
Figure 3.
Sensory epithelial development in E17.5 Pax2-Cre Dicer KO mouse inner ear. Each panel depicts analysis by ICC detecting sensory epithelial Sox2 protein, and/or presumptive hair cell BDNF and MyoVIIa proteins as indicated. Shown are the two basal and apical sensory patches of the KO mouse cochlea (CO; A) with limited BDNF expression (B,C) compared to a segment of the organ of Corti of wild type cochlea (D) with characteristic inner and outer hair cell rows (ihc and ohc, respectively). The sensory patches of the KO mouse cochlea are positive for MyoVIIa indicative of hair cells (E). The utricular macula (U) and posterior crista (PC) exhibit abundant BDNF expression (F,G) and MyoVIIa expression (H,I,J). The posterior crista in optical section (J) demonstrates relatively normal organization and morphology of presumptive hair cells separated by a non-sensory cruciate eminence (CE). Arrows indicate dorsal (D) and posterior (P) directions.
The Sox2 positive sensory epithelia of the utricle and posterior crista demonstrate detection of relatively more BDNF positive and MyoVIIa positive cells (Figs. 3F–J). The utricular macula shows a circular morphology rather than the typical kidney-shaped sensory epithelium, whereas the posterior crista demonstrates a normal separation of the two hemicristae by a cruciate eminence. The sensory epithelia of the KO inner ear therefore demonstrate a range of morphological and histological definition from the well-developed posterior crista to the poorly-developed cochlea. Interestingly, the absence or presence of BDNF in developing hair cells does not strictly correlate with the lack of innervation indicated by tubulin ICC (Fig. 2F), suggesting that the inability of nerve fibers to properly target sensory epithelia is intrinsic to the neurons rather than a failure of developing hair cells to provide neurotrophic support. Overall, the conditional Dicer KO mouse phenotype is highly penetrant, with variation amongst specimens observed only in regard to the absence or presence of a discernible saccule, and the presence of 1 or 2 sensory epithelial patches in the cochlea.
Defects in hair cell development
To further examine developing hair cells in KO inner ear sensory epithelia, the detailed cellular anatomy was examined by tubulin ICC and electron microscopy. Tubulin ICC shows that most developing hair cells possess kinocilia (Fig. S2). The positioning of kinocilia shows that near normal hair cell polarity develops in the posterior crista and in the cochlea for one case where the organ of Corti demonstrates some organization into inner and outer hair cells. However, there was no evidence for formation of striolar polarity reversal in the utricular macula, where kinocilia positioning appeared mostly random.
Examination of E17.5 and E18.5 inner ear sensory epithelia by scanning electron microscopy (SEM) and transmission electron microscopy (TEM; Figs. 4A and 4B, respectively) shows that apical specializations were found to be fairly normal in the posterior crista. However, the utricular macula shows unusual organization of apical specializations presenting as multiple elongated microvilli in stark contrast to normal stereocilia development (Fig. S3). If developed, cochlear hair cells show unusual stereocilia organization having multiple clustered rows (Fig. 4B). Additionally, TEM demonstrates that both afferent and efferent synapses form in the utricular macula (Fig. 4B), but no innervation is detected in developing hair cells of the posterior crista or cochlea consistent with the absence of nerve fibers observed by tubulin ICC.
Figure 4.
Fine structure of Pax2-Cre Dicer KO mouse inner ear sensory epithelia. A, SEM of E17.5 KO mouse inner ear sensory epithelial apical specializations. Presumptive hair cells of the cochlea (CO), posterior crista (PC), and utricular macula (U) depicted at relative low (left) and high (right) magnification show disorganized microvillus extensions with only posterior crista hair cells exhibiting kinocilia and normal organization of stereocilia. B, Thin section TEM of E18.5 KO mouse inner ear sensory epithelia. Depicted are sections of each sensory epithelium at various magnification as indicated. Supporting cells and hair cells of the cochlea lack the distinctive normal cellular organization of the organ of Corti into inner and outer hair cell rows separated by pillar cells. Hair cells in the posterior crista show normal organization. The utricular macula exhibits presumptive hair cells showing mostly disorganized microvilli and is the only epithelium to show evidence of synapse formation with pre-synaptic vesicles (asterisk).
Defects in innervation
Our initial examination of the E17.5 conditional Dicer KO inner ear suggests a near absence of innervation (Fig. 2F), where only the utricular macula receives specific projections that contact developing hair cells (Fig. 4B). The data also show that neuron-specific miR-124 expression is absent by E11.5 (Figure 1C). Therefore, sensory neuron development was further examined (Fig. 5) using recently improved lipophilic dyes (Jensen-Smith et al., 2007) as in previously published studies on ear development (Farinas et al., 2001; Tessarollo et al., 2004). At E11.5, near normal innervation of the KO inner ear was observed with fibers extending toward the posterior crista (Fig. 5A), albeit in a less directed growth pattern than the control littermate (Fig. 5B). At E12.5, fibers do not progress in their projection to the posterior crista of the KO inner ear as they do in the control (Figs. 5C and 5D), and few fibers extended toward the utricle and the missing anterior and horizontal cristae in the KO inner ear (Fig 5C; inset). Similarly, central projections showed near normal vestibular fiber elongation along the hindbrain and toward the cerebellum at E11.5 that do not progress beyond rhombomere 1 into the cerebellum at E12.5 (Figs. 5E and 5F). These data suggest that the growth and pathfinding properties of neurons are affected within one day from loss of neuronal miRNA expression, which is evident approximately three days following conditional Dicer KO.
Figure 5.
Defects in inner ear afferent innervation and afferent projection into the brain of Pax2-Cre Dicer KO mice. A–D,G–J, Innervation of the ear in KO mice (A,C,G–J) and control littermate mice (B,D,G′-J′). E,F,K, Afferent projections into the brain in KO mice and control littermate (K′). All images show whole mounted ears or brains in which lipophilic dye was either inserted into the vestibular nucleus (A–D,H), the facial motoneurons/efferents and cochlear/vestibular nucleus (G,I,J), or the entire (E,F,K) or cochlear and vestibular parts of the ear (K′). Note that both afferent outgrowth (A,B) and initial growth of fibers into the brain (E) is essentially normal at E11.5. However, extension of fibers to the posterior crista (PC) and the rostral extension of the afferents in the brain beyond rhombomere 2 are compromised. At E12.5, afferent projections to the ear show severe truncation of fiber growth to the posterior crista (PC; compare C,D), and a reduction of fiber growth to the missing anterior and horizontal cristae is apparent (AC, HC; compare inset C,D). Afferent projections extend along the anterior-posterior axis of the vestibular nuclei (VN) into the brainstem but terminate at or near the first rhombomere (arrowhead) without extending into the cerebellum (F). By E17.5, most afferent neurons of the vestibular ganglion (VG) have disappeared (compare G,G′ and H,H′). All fibers enter the KO ear through the anterior vestibular nerve branch and typically extend to the utricle (U) with few fibers extending to the saccule (S) or cochlea (CO; H–J). In contrast, control littermate ears show substantial afferent and efferent projections to the vestibular and cochlear organs (H′-J′). The central projection of the KO ear displays fibers that ramify from the entry point in almost all directions with the exception of the descending tract along the vestibular nuclei (VN) that is normally organized (compare K,K′). Rostral vestibular fibers do not stay confined to vestibular nuclei but branch into the cochlear nucleus (CN) that should receive a distinct projection from the cochlea (labeled in red in K′). Also indicated are the geniculate ganglion (GG), facial branchial motoneurons (FBM), and spiral ganglia (SG).
The sparse innervation of the KO inner ear is further demonstrated by tubulin ICC (Figs. S2 and S4). At E17.5, few fibers are observed to reach the sensory epithelium of the utricle or, if present, the saccule. Fibers to the cochlea are much reduced and extend only in the base toward developing hair cells. The apical fibers typically extend along the cochlea without targeting the developing sensory epithelium. Most obvious is the complete absence of any innervation of the posterior crista, which is otherwise the most normal developed sensory epithelium of the KO inner ear.
A closer examination of E17.5 KO inner ear innervation indicated the presence of very few vestibular neurons to the utricle (~15–35 in two different mutants) that could be traced after lipophilic dye injection into the brainstem (Figs. 5G–I). We also examined the number of neurons labeled after lipophilic dye injection into the ear and found ~30 small neurons scattered along the nerve fibers reaching toward the brain. Only an occasional efferent fiber was found at this late stage (E17.5) to reach to the inner ear (Fig. 5J), implying near complete loss of efferent innervation in the KO inner ear (Bruce et al., 1997; Fritzsch, 1999; Simmons, 2002). The central projection of KO inner ears showed vestibular afferent fibers with a normal projection along the descending vestibular nucleus (Fig. 5K). However, no fibers extended anterior into the cerebellum (Fritzsch et al., 2002) consistent with the complete loss of this part of rhombomere 1 in the conditional Dicer KO (Fig. 1). Instead, rostral vestibular afferents radiated in all directions around the nerve root and extended into the cochlear nucleus (Fig. 5K).
Consistent with the fact that Pax2-Cre is not expressed in the hindbrain rhombomeres from which facial branchial motoneurons and efferent neurons originate (Karis et al., 2001), an examination of E12.5 control and conditional Dicer KO mouse brainstem by ISH shows similar sensory neuron expression of fibroblast growth factor 10 (Fgf10) mRNA (Fig. 6). Accordingly, lipophilic dye staining of hindbrain efferents shows normal development at this early stage (Figs. 7A and S5), although there is a severe reduction of afferent and efferent fibers in the auditory nerve (Figs. 7B–D). Further examination of the ear shows that conditional Dicer KO in auditory afferents initially has a more profound effect on the afferents than hindbrain efferents projecting to the ear (Figs. 7E–G). These data suggest that loss of afferent neuronal miRNA expression affects cues along sensory neurons that are required for efferent fiber growth (Fritzsch et al., 1998; Matei et al., 2005). Moreover, analysis of cell death by ICC detecting caspase 3 shows that regions of Pax2-Cre expression and conditional Dicer KO in the cerebellum (Fig. 6C; inset), midbrain (Figs. 8A–C), and inner ear (Figs. 8D,E) exhibit a substantial number of apoptotic cells consistent with analysis in other conditional Dicer KO models (Davis et al., 2008).
Figure 6.
Brainstem development in E12.5 control and conditional Dicer KO mice. Whole-mounted brains show the distribution of Fgf10 expression by ISH in branchial motoneurons and efferents (A,B). Indicated is expression in trigeminal (V), facial (FBM), olivo-cochlear efferents (OC), and nucleus ambiguous branchial motoneurons (NA). The cerebellum (CB) shows Fgf10 expression in the control, but not in the KO brain. The KO cerebellum instead exhibits numerous birefringent cells in dark field illumination (C). ICC detecting caspase 3 with Hoechst staining of nuclei shows that these cells are macrophages that contain several nuclei of dying cells (inset). Note the absence of apoptosis in the floor plate of rhombomere 1, indicating that only proliferating and/or differentiating neurons are affected by Dicer KO.
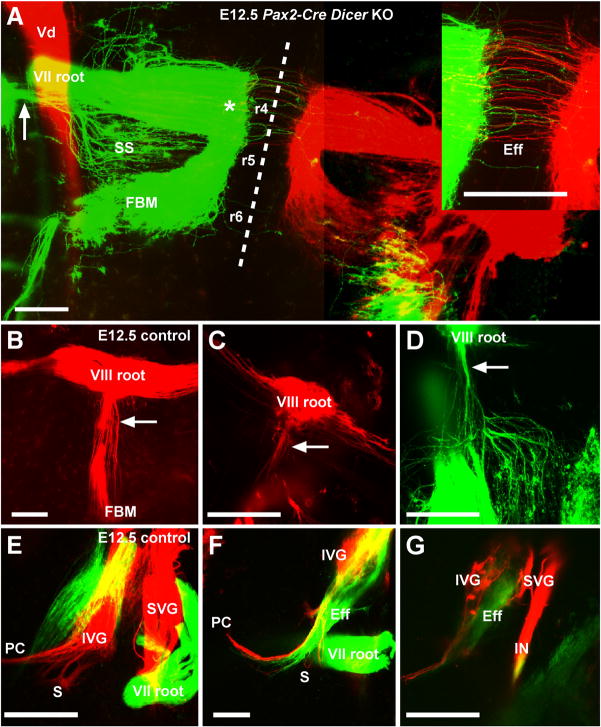
Figure 7.
Efferent and afferent development in the brainstem and ear of E12.5 control and conditional Dicer KO mice. A, Absence of effects on ear-related hindbrain development in Dicer KO mice demonstrated by normal labeling of the facial nerve (VII root) and facial branchial motoneurons (FBM) in rhombomeres 4–6 (r4-6). Left and right sides are labeled green and red, respectively. B–D, Labeling of the auditory nerve afferents (VIII root) and efferents (arrow) in E12.5 control (B) and Dicer KO (C,D) mice. Backfilling from the ear shows a large afferent and efferent component in the control (B), whereas the Dicer KO shows a severe reduction of afferents extending anterior-posterior (horizontally) in the hindbrain (C) and a severe reduction in the efferent fiber bundle (compare arrow in B to those in A,C,D). E–G, Sensory neuron development in E12.5 control (E) and Dicer KO (F,G) inner ear. KO ears show a severe reduction in afferent fibers (red) of the inferior and superior vestibular ganglia (IVG and SVG, respectively) with only some fibers extending toward the posterior crista (PC). There is also a reduction of efferent fibers (green) projecting to the ear, albeit less profound than afferent reduction, and normal labeling of the facial nerve (VII root). Bars represent 200 μm in A and 100 μm in B–G. Vd, descending tract of the trigeminal; SS, superior salivatory nucleus; IN, intermediate nerve.
Figure 8.
Cell death in the brain and ear of an E12.5 conditional Dicer KO mouse. Depicted is ICC detection of caspase 3 and tubulin with Hoechst staining of nuclei in the midbrain (A–C) and ear (D,E). There is a thinning of the midbrain in areas of caspase 3 expression (A,B) where caspase 3-positive cells exhibit multiple pycnotic nuclei (C–C′). The inferior vestibular ganglion (IVG) shows clusters of caspase 3-positive cells that are tubulin-negative (D). Observed throughout the nerve trajectory to the posterior crista (PC) are fragments of caspase 3-positive and tubulin-positive fibers.
In summary, the data demonstrate a near normal initial formation and growth of sensory neurons with rapid and near complete loss of all innervation after the loss of afferent neuronal miRNA expression at E11.5 in the conditional Dicer KO inner ear. Defects in sensory neuron projection and loss of sensory neurons result from either loss of target structures or fibers required for neuronal pathfinding if not directly from conditional Dicer KO. Whether the few remaining afferent neurons at E17.5 (Fig. 5G–K) are impervious to the loss of miRNA expression or retain low levels of some miRNAs remains unclear.
Defects in morphogenesis
Prior work has shown that canal formation hinges on the expression of fibroblast growth factor 10 (Fgf10) and bone morphogenic proteins (BMPs) in canal crista epithelia (Pauley et al., 2003; Chang et al., 2004). Given that canal/crista formation is most affected in conditional Dicer KO inner ears (Figs. 2B and 2F), Fgf10 mRNA expression was examined throughout development by ISH (Fig. 9A). Fgf10 expression in the E11.5 control inner ear is prominent in the delaminating vestibular neurons, utricle, and each canal crista. KO inner ears likewise show expression in the vestibular neurons, utricle, and posterior canal crista. However, there is only limited expression in a poorly defined anterior canal crista, and no expression suggestive of a horizontal canal crista is observed. At E12.5, dramatic differences in Fgf10 expression are seen between KO and control inner ears. Whereas the control shows expression in sensory neurons and all sensory epithelia, the KO demonstrates a loss of expression in sensory neurons and retains only strong expression in the posterior canal crista and utricle, and weak expression in the cochlea. By E14.5, differences in Fgf10 expression are even more pronounced between KO and control inner ears. While the control continues to demonstrate expression throughout sensory neurons and epithelia, the KO shows only strong expression in the posterior crista and weak expression in the utricle. The rapid loss of Fgf10 expression in sensory neurons of the conditional Dicer KO inner ear corresponds well to the early depletion of neuronal miRNAs (Fig. 1C), loss of sensory neurons through apoptosis (Fig. 8), and decline of neuronal differentiation (Fig. 5). Moreover, the respective absence, gradual loss, or persistence of Fgf10 expression in horizontal, anterior, or posterior crista correlates with the observed degree of canal formation driven by epithelial Fgf10 signaling (Fig. 2B). It is also interesting to note that the overall size and morphology of the Dicer KO and control inner ears are somewhat synchronous up to E12.5 in contrast to obvious differences at E17.5 (Fig. 2E), suggesting that cell proliferation and growth are largely affected after E12.5.
Figure 9.
Correlation of Pax2-Cre Dicer KO inner ear morphogenesis with developmental gene expression and residual mature miRNA expression. A, Progressive loss of Fgf10 expression in KO inner ear. At E11.5, the KO inner ear exhibits diminished but discrete expression of Fgf10 in sensory epithelia (PC, posterior crista; AC, anterior crista; HC, horizontal crista; U, utricle; CO, cochlea). At E12.5, Fgf10 expression is observed only in the posterior crista and utricular macula, and expression is entirely lost in vestibular ganglia (VG) and the developing cochlea. By E14.5, Fgf10 expression remains only in the posterior crista with faint expression in the utricular macula, in sharp contrast to extensive expression in all sensory epithelia and delaminating neurons of control littermate inner ear. B, ISH detecting miR-183 and miR-183* in KO and control littermate inner ear. Throughout the control inner ear, miR-183 expression is apparent in statoacoustic ganglia (SAG) and hair cells of developing sensory epithelia, whereas mir-183* does not accumulate as detectible pre-miRNA or mature miRNA. In the KO inner ear, miR-183 is detected primarily in the posterior crista (PC; filled arrowhead) whereas miR-183* is not (open arrowhead), thus suggesting that miR-183 detection results from residual mature miRNA production rather than detection of accumulated pre-miRNA. Labels are as indicated in the legend to Fig. 2.
Residual mature miRNA expression
To understand why there exist such variation between the degree of sensory epithelial and hair cell development in the conditional Dicer KO inner ear, we examined whether Pax2-Cre transgene expression, floxed Dicer deletion, or residual miRNA expression might underlie the observed phenotype. Importantly, R26R expression did not indicate any deviation from the reported expression domain of the Pax2-Cre transgene (Ohyama and Groves, 2004) in conditional Dicer KO or control mice (Fig. S1). We therefore investigated whether any residual mature miRNA expression could explain the variability of the model. Notably, rapid and thorough depletion of miR-124 in the mid-hindbrain and delaminating vestibular ganglia of the KO mouse (Fig. 1C) suggests efficient Dicer deletion and miRNA depletion in prosensory precursors. Accordingly, ISH detecting miR-183, which is normally expressed in all hair cells and sensory neurons of the mouse inner ear (Weston et al., 2006; Pierce et al., 2008), shows absence of miR-183 in remaining sensory neurons of the E17.5 KO inner ear (Fig. S6A). However, some miR-183 is surprisingly observed in KO inner ear sensory epithelia, most prominently in the posterior crista and sometimes weakly in the utricle and cochlea.
To establish whether detection of miR-183 represents mature miRNA expression versus detection of accumulated miRNA precursors, we performed ISH to detect miR-183*, the complementary strand to miR-183 in the precursor miRNA hairpin, reasoning that it should be detected at the same level as miR-183 if detection represents precursor miRNA accumulation. At E14.5, miR-183 detection is robust throughout the sensory neurons and epithelia of the control inner ear, but present only in the posterior crista of the KO inner ear (Fig. 9B). However, miR-183* was undetectable in either control or KO inner ears, consistent with miR-183 detection representing genuine mature miRNA expression. This residual mature miRNA expression persists despite confirmation that the floxed Dicer allele is indeed deleted in the conditional KO inner ear by PCR analysis of DNA isolated from the posterior crista (Fig. S6B).
The data therefore suggest that Dicer mRNA and/or Dicer protein must persist for at least 6 days to generate mature miRNAs in developing hair cells at E14.5 in some sensory epithelia of the Pax2-Cre conditional Dicer KO inner ear, but not sensory neurons. Once formed, mature hair cell miRNAs persist for at least 3 more days until detection at E17.5. Importantly, the differential capacity for residual miRNA expression within and between different ears correlates well with the extent of inner ear neurogenesis and innervation, Fgf10 expression and morphogenesis, sensory epithelial histogenesis, and hair cell development observed in the conditional Dicer KO mouse.
Discussion
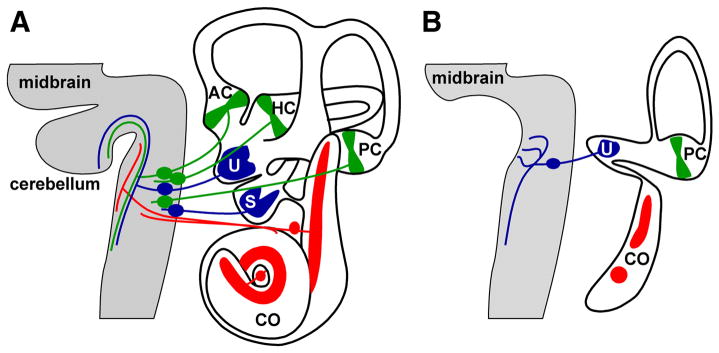
Our data show that progressive reduction of miRNAs through the conditional KO of Dicer using Pax2-Cre results in progressive loss of neurosensory gene expression, arrested neurosensory development and loss of sensory neurons, loss or reduction of sensory epithelia, and associated disruption of morphogenesis (Fig. 10). While delayed and apparently incomplete by E18.5, the oldest viable embryos we could obtain, the depletion of miRNAs demonstrates to a degree unknown in other systems the dependence of neurosensory development on miRNA expression.
Figure 10.
Overview of developmental defects in Pax2-Cre Dicer KO inner ear. A, Normal morphology, histology and innervation of wild type inner ear and brain. B, Morphology, histology and innervation of the KO inner ear and brain. The remaining sensory epithelia of the inner ear are variously affected by a loss of miRNAs, and the only remaining fibers innervating the utricular macula fail to project properly to the brain.
Technical considerations of the conditional KO model
One of the striking features of our conditional KO model is the apparent differential retention of some Dicer in certain hair cell precursors but not in others. It is likely that hair cells up-regulate specific miRNAs (e.g. miR-183) only after exit from cell cycle, which shows specific differences across various epithelia (Ruben, 1967). In contrast, Dicer is likely expressed in all cells of early embryos, as Dicer null mutants exhibit early lethality by E7.5 (Bernstein et al., 2003). Therefore, residual Dicer in our conditional KO model would be required to generate mature forms of hair cell-specific miRNAs. In support of these presumptions, we validated that floxed Dicer is indeed eliminated and showed that miRNA detection represents active mature forms rather than inactive precursor accumulation in remaining hair cells. We were unable to immunohistochemically confirm the presence of Dicer protein, but consider the detection of residual mature miRNAs as proof of residual Dicer protein activity in developing hair cells. Consistent with recent observations regarding the effect of conditional Dicer KO and miRNA depletion in post-mitotic cerebellar Purkinje cells (Schaefer et al., 2007), it appears that there can be substantial differences in the rates at which Dicer protein, Dicer mRNA, and/or mature miRNAs are metabolized. Such issues have to our knowledge not been observed in other conditional Dicer KO models, where progressive loss of miRNAs might contribute to a degree of randomness that characterizes cell fate decision in general (Losick and Desplan, 2008).
Neuronal development
Sensory neurons are among the first cells of the ear to exit cell cycle and initiate differentiation (Ma et al., 1998; Ma et al., 2000; Matei et al., 2005). Analysis shows that these neurons are initially positive for factors such as Fgf10 and send processes toward specific sensory epithelia of the ear and into the brain. However, as early as E12.5 the expression of neuron-specific factors including Fgf10 and miR-124 are lost, extension of both peripheral and central processes stalls, and there is a near-complete loss of neurons by E18.5. Vestibular neurons and their fibers to the end organs exhibit caspase 3 expression, a reliable marker for cell death. At later stages, the few remaining neurons synapse only on utricular hair cells. Interestingly, the most normal developed sensory epithelium of the ear, the posterior crista, shows no trace of innervation at any stage although fibers initially extend toward it.
It remains unclear whether the general loss of neurons and innervation relates exclusively to rapid cell death caused by Dicer KO which is known to have variable effects dependent upon neuron type and/or time of Dicer deletion (Schaefer et al., 2007; Cuellar et al., 2008; Davis et al., 2008). Alternatively, such effects might reflect miRNA-mediated phenotypic differences (Cayirlioglu et al., 2008) or miRNA-mediated lack of sensory epithelial differentiation and loss of epithelial neurotrophins that maintain neurons (Farinas et al., 2001; Fritzsch et al., 2004). It is possible that the earliest neurons to exit the cell cycle at E8.75 retain enough Dicer and miRNAs to complete near normal differentiation and extend peripheral processes to the nearest sensory epithelium (i.e. the utricular macula). In contrast, more distant or later differentiating epithelia receive little to no innervation possibly because the neurons are depleted of miRNAs before they reach their target (e.g. posterior crista) or because the target has not completed differentiation (e.g. organ of Corti). Conditional deletion of Dicer using inducible neuron-specific or prosensory-specific Cre expression driven by promoters for Neurog1 (Raft et al., 2007) or Isl1 (Lin et al., 2006), respectively, is needed to distinguish between direct neuronal effects and sensory epithelia defects. The disorganized central projection that develops after normal onset of projection suggests direct effects as Pax2-Cre is not expressed in rhombomeres 2–6. However, the near complete loss of the cerebellum, which derives from the midbrain and rhombomere 1 (Hatten and Heintz, 1995), could produce secondary effects in the remaining brainstem.
Sensory epithelial development
As with neurons, the initial up-regulation of sensory epithelial specific factors such as Fgf10 is near normal at E11.5 in conditional Dicer KO mice. However, Fgf10 expression is entirely lost within one day in the anterior and horizontal crista and is down-regulated in the utricular macula. It is noteworthy that only the posterior crista retains high levels of Fgf10 expression, and it is the only near normal sensory epithelium developed at E18.5 as evidenced by hair cell and supporting cell specific gene expression (e.g. MyoVIIa, BDNF, Sox2) and cytoarchitecture. As previously demonstrated in Neurog1 null mice (Ma et al., 2000) and neurotrophin and neurotrophin receptor null mice (Fritzsch et al., 1997; Rocha-Sanchez et al., 2007), hair cell differentiation is cell-autonomous and continues in the absence of innervation (Fritzsch et al., 1998).
With similarity to several transcription and growth factor mutant mice (Pirvola et al., 2000; Pauley et al., 2003; Kiernan et al., 2005; Pauley et al., 2006) and notch signaling mutant mice (Daudet and Lewis, 2005; Kiernan et al., 2006), we find in conditional Dicer KO mice that anterior and horizontal cristae do not differentiate despite an initial expression of crista-specific factors such as Fgf10 (Pauley et al., 2003). Certain sensory epithelia are therefore highly sensitive to precise timing and expression levels of factors that are integral to the regulation of normal epithelium development from prosensory domains. Even where sensory epithelia form in conditional Dicer KO mice, overall cellular organization is disrupted as exemplified by the cochlea where discontinuous patches or multiple rows of inner and outer hair cells form as reported for several transcription and growth factors mutants (Pirvola et al., 2002; Kiernan et al., 2005; Pauley et al., 2006). Which one of these factors, or yet to be described factors, is primarily affected by miRNA depletion remains to be demonstrated.
Hair cell development
Consistent with our recent description of the ancestry of transcriptional regulatory machinery in hair cells (Fritzsch et al., 2007), including the ancestry of miRNAs (Pierce et al., 2008), are specific defects of hair cells that include incomplete transformation of microvilli into stereocilia. With similarity to factors that lead to patchy distribution of hair cells or formation of multiple rows, numerous factors are known to affect stereocilia development (Leibovici et al., 2005; Ahmed et al., 2006; Friedman et al., 2007). More work is needed to understand which of these factors requires precise levels of certain miRNAs for normal function. Nevertheless, the fact that hair cells form but cannot fully differentiate underscores the critical role of miRNAs in the normal development of these highly specialized cells. The general requirement for miRNAs to achieve differentiation is supported by other models of small RNA or miRNA depletion in embryonic stem cells (Kanellopoulou et al., 2005; Wang et al., 2007) and conditional Dicer KO tissues (Muljo et al., 2005; Andl et al., 2006; Lynn et al., 2007).
Summary
miRNAs and other small RNAs are among the best understood non-coding functional RNAs produced by the mammalian genome. In recent years, miRNAs have been shown to be involved in multiple aspects of disease, development, and maintenance (Amaral et al., 2008; Couzin, 2008), and they exhibit regulatory functions that parallel the importance of the better known transcription factors (Hobert, 2008; Makeyev and Maniatis, 2008). These regulatory functions combined with the increased number of miRNAs in vertebrates have prompted speculations about the possible involvement of miRNAs in the evolution of complexity (Heimberg et al., 2008). Our data further the appreciation of such known and presumed functions by demonstrating that specific levels of miRNAs are necessary for neurosensory development of the inner ear and, directly or indirectly, morphogenesis of the labyrinth. Importantly, we demonstrate a 3–10 day delay between elimination of Dicer and depletion of specific miRNAs, implying that the extent of inner ear development is reflective of a hypomorphic miRNA condition rather than complete miRNA abrogation. Our data therefore suggest that critical levels of miRNAs are needed to achieve any extent of neurosensory inner ear development. An earlier onset of floxed Dicer deletion through pre-placodal Cre expression at least one day prior to Pax2 (Streit, 2007) is needed to eliminate miRNAs before formation of the otocyst to fully demonstrate the requirement of miRNAs for inner ear development.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service grants P20RR018788 (GAS) and R01DC005590 (BF), and Nebraska State Fund LB692 (BF). The research was conducted in a facility constructed with support from the National Center for Research Resources, National Institutes of Health, Research Facilities Improvement Program grant C06RR017417, and utilized the Nebraska Center for Cell Biology confocal microscope facility supported by the National Science Foundation EPSCoR grant EPS-0346476. We thank Andrew Groves for Pax2-Cre mice, thank Lilian Calisto and George Enninful for assistance with genotyping, and Liqin (Julie) Zhu and Jennifer Kersigo for assistance with ICC.
References
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome is an RNA machine. Science. 2008;319:1787–1789. doi: 10.1126/science.1155472. [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Bok J, Brunet LJ, Howard O, Burton Q, Wu DK. Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev Biol. 2007;311:69–78. doi: 10.1016/j.ydbio.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–692. doi: 10.1016/s0736-5748(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Cayirlioglu P, Kadow IG, Zhan X, Okamura K, Suh GS, Gunning D, Lai EC, Zipursky SL. Hybrid neurons in a microRNA mutant are putative evolutionary intermediates in insect CO2 sensory systems. Science. 2008;319:1256–1260. doi: 10.1126/science.1149483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Couzin J. MicroRNAs make big impression in disease after disease. Science. 2008;319:1782–1784. doi: 10.1126/science.319.5871.1782. [DOI] [PubMed] [Google Scholar]
- Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, McManus MT. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci USA. 2008;105:5614–5619. doi: 10.1073/pnas.0801689105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Avraham KB. Mouse models to study inner ear development and hereditary hearing loss. Int J Dev Biol. 2007;51:609–631. doi: 10.1387/ijdb.072365lf. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Ontogenetic and evolutionary evidence for the motoneuron nature of vestibular and cochlear efferents. In: Berlin CI, editor. The Efferent Auditory System: Basic science and clinical applications. Singular Publishing; San Diego: 1999. pp. 31–59. [Google Scholar]
- Fritzsch B, Barald K, Lomax M. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System, Springer Handbook of Auditory Research. XII. Springer Verlag; New York: 1998. pp. 80–145. [Google Scholar]
- Fritzsch B, Barbacid M, Silos-Santiago I. Nerve dependency of developing and mature sensory receptor cells. Ann NY Acad Sci. 1998;855:14–27. doi: 10.1111/j.1749-6632.1998.tb10543.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51:663–678. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8:277–284. [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola V, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- Heimberg AM, Sempere LF, Moy VN, Donoghue PC, Peterson KJ. MicroRNAs and the advent of vertebrate morphological complexity. Proc Natl Acad Sci USA. 2008;105:2946–2950. doi: 10.1073/pnas.0712259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith H, Gray B, Muirhead K, Ohlsson-Wilhelm B, Fritzsch B. Long-distance three-color neuronal tracing in fixed tissue using NeuroVue dyes. Immunol Invest. 2007;36:763–789. doi: 10.1080/08820130701706711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RH. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- Leibovici M, Verpy E, Goodyear RJ, Zwaenepoel I, Blanchard S, Laine S, Richardson GP, Petit C. Initial characterization of kinocilin, a protein of the hair cell kinocilium. Hear Res. 2005;203:144–153. doi: 10.1016/j.heares.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol. 2006;295:756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes. 2007;56:2938–2945. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008 doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev. 2008;10:106–113. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–685. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Rocha-Sanchez SM, Morris KA, Kachar B, Nichols D, Fritzsch B, Beisel KW. Developmental expression of Kcnq4 in vestibular neurons and neurosensory epithelia. Brain Res. 2007;1139:117–125. doi: 10.1016/j.brainres.2006.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.