Abstract
Background: Phytosterol supplementation of 2 g/d is recommended by the National Cholesterol Education Program to reduce LDL cholesterol. However, the effects of different intakes of phytosterol on cholesterol metabolism are uncertain.
Objective: We evaluated the effects of 3 phytosterol intakes on whole-body cholesterol metabolism.
Design: In this placebo-controlled, crossover feeding trial, 18 adults received a phytosterol-deficient diet (50 mg phytosterols/2000 kcal) plus beverages supplemented with 0, 400, or 2000 mg phytosterols/d for 4 wk each, in random order. All meals were prepared in a metabolic kitchen; breakfast and dinner on weekdays were eaten on site. Primary outcomes were fecal cholesterol excretion and intestinal cholesterol absorption measured with stable-isotope tracers and serum lipoprotein concentrations.
Results: Phytosterol intakes (diet plus supplements) averaged 59, 459, and 2059 mg/d during the 3 diet periods. Relative to the 59-mg diet, the 459- and 2059-mg phytosterol intakes significantly (P < 0.01) increased total fecal cholesterol excretion (36 ± 6% and 74 ± 10%, respectively) and biliary cholesterol excretion (38 ± 7% and 77 ± 12%, respectively) and reduced percentage intestinal cholesterol absorption (−10 ± 1% and −25 ± 3%, respectively). Serum LDL cholesterol declined significantly only with the highest phytosterol dose (−8.9 ± 2.3%); a trend was observed with the 459-mg/d dose (−5.0 ± 2.1%; P = 0.077).
Conclusions: Dietary phytosterols in moderate and high doses favorably alter whole-body cholesterol metabolism in a dose-dependent manner. A moderate phytosterol intake (459 mg/d) can be obtained in a healthy diet without supplementation. This trial was registered at clinicaltrials.gov as NCT00860054.
INTRODUCTION
Phytosterols are naturally occurring plant sterols that are structurally similar to cholesterol but that reduce intestinal cholesterol absorption (1) and serum LDL-cholesterol concentrations (2, 3). The lipid-lowering benefit of phytosterols was first reported in the 1950s (4, 5) and is the basis for the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (6) recommendation for adults to consume 2 g phytosterols daily to reduce LDL cholesterol and cardiovascular disease risk. Dietary phytosterol intake without supplementation ranges between ≈78 and 500 mg/d (3, 7, 8). Because it is not feasible to obtain 2 g phytosterols in a natural diet, food manufacturers enrich common products (eg, margarine, orange juice, yogurt drinks, and granola bars) with phytosterols to enable Americans to achieve the recommended intake goal.
Despite abundant evidence of the therapeutic benefit of phytosterols, most previous studies have neither eliminated nor quantified phytosterols in the background diet, leaving many questions unanswered regarding the effective dose of phytosterols to derive health benefits and the mechanisms by which such benefits occur. The aim of this study was to evaluate the physiologic effects of 3 levels of phytosterol intake, including a moderate dose that can be obtained naturally in the diet, on whole-body cholesterol metabolism. We used a novel phytosterol-deficient background diet to assess 3 analytically quantified levels of phytosterol intake on cholesterol metabolism in a randomized, crossover, blinded, controlled feeding study.
SUBJECTS AND METHODS
Participants
Volunteers aged 18–80 y were recruited from the greater Baton Rouge, LA, area. Eligible subjects had fasting LDL cholesterol between 100 and 189 mg/dL (based on replicate measures on different days), resting blood pressure <160/95 mm Hg, and body mass indexes between 20 and 35 kg/m2, and were not taking prescription medications except oral contraceptives. Women who were pregnant, nursing, or perimenopausal were excluded. The study was approved by the Institutional Review Board at Pennington Biomedical Research Center (PBRC); all subjects provided written informed consent.
Study design
Participants were enrolled in 4 cohorts between March and May 2006 and randomly assigned to 1 of 6 possible diet sequences. Each sequence contained a phytosterol-deficient background diet plus 3 phytosterol supplement doses (0, 400, and 2000 mg/d) in random order, and subjects were blinded to the dose. Each phytosterol dose was administered for 4 wk, with 1 wk between doses.
Phytosterol-deficient background diet
The phytosterol-deficient background diet (8) was experimentally designed to contain ≤60 mg phytosterols/2000 kcal. The diet was designed initially at Washington University School of Medicine, and a 5-d menu cycle was developed subsequently at the PBRC by using the database ProNutra 3.0 (Viocare, Princeton, NJ). The database was customized by adding the phytosterol content of several foods based on published values (9–12) and analyses performed in the Washington University Mass Spectrometry Resource. To minimize the phytosterol content of the diet, we excluded or minimized foods that were naturally high in phytosterols (eg, vegetable oils, whole grains, seeds, and nuts), incorporated foods that were naturally low in phytosterols (eg, dairy, poultry, meat, fish, and eggs), and developed recipes in which traditional ingredients were replaced with lower-phytosterol ingredients (eg, biscuits made with white rice flour, potato starch, and tapioca flour). In addition, the recipes contained soybean oil that we purified using activated carbon to remove most of the naturally occurring phytosterols (13). The 5-d meal plan is shown in Table 1. A composite of each day's meals was analyzed for phytosterol content at Washington University. As shown in Table 2, this diet was very low in phytosterols but conformed to the macronutrient recommendations of the Dietary Guidelines for Americans (14).
TABLE 1.
Five-day phytosterol-deficient meal plan showing menu items and gram weights1
| Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
||||||
| Quantity | Item | Quantity | Item | Quantity | Item | Quantity | Item | Quantity | Item | |
| Breakfast | ||||||||||
| 42 g | Cornflakes | 2 each | Spice muffin, LP | 85 g | Bagel, white | 2 each | Spice muffin, LP | 42 g | Cornflakes | |
| 227 g | Grape juice | 14 g | Strawberry jam | 10 g | Butter | 5 g | Butter | 227 g | Grape juice | |
| 245 g | Skim milk | 5 g | Butter | 14 g | Strawberry jam | 28 g | Strawberry jam | 490 g | Skim milk | |
| 227 g | Grape juice | 227 g | Grape juice | 227 g | Grape juice | |||||
| 245 g | Skim milk | 245 g | Skim milk | 245 g | Skim milk | |||||
| Lunch | ||||||||||
| 75 g | Turkey breast | 80 g | Tuna salad, LP | 95 g | Cajun roast beef | 80 g | Chicken salad, LP | 100 g | Ham | |
| 1 large | Bun, LP | 20 g | Lettuce, iceberg | 1 large | Bun, LP | 20 g | Lettuce, iceberg | 1 medium | Bun, LP | |
| 30 g | Tomato, sliced | 18 g | Saltine crackers | 12 g | Mayonnaise, fat-free | 18 g | Saltine crackers | 12 g | Mayonnaise, fat-free | |
| 20 g | Lettuce, iceberg | 170 g | Yogurt, light | 5 g | Mustard | 170 g | Yogurt, light | 10 g | Mustard | |
| 12 g | Mayonnaise, fat-free | 368 g | Soda, regular | 110 g | Potato salad, LP | 95 g | Pineapple chunks | 20 g | Lettuce, iceberg | |
| 5 g | Mustard | 245 g | Soda, regular | 245 g | Soda, regular | 30 g | Tomato, chopped | |||
| 85 g | Grapes, green | 70 g | Pears, juice-packed | |||||||
| 368 g | Soda, regular | 368 g | Soda, regular | |||||||
| Snack | ||||||||||
| 198 g | Gelatin | 28 g | Pretzels, tiny twists | 99 g | Pudding, fat-free | 43 g | Pretzels, tiny twists | 198 g | Pudding, fat-free | |
| Dinner | ||||||||||
| 386 g | Rice-crusted meatball pizza, LP | 213 g | Chicken and sausage jambalaya, LP | 124 g | Lemon sage chicken, LP | 145 g | Pork loin, LP | 156 g | Baked catfish, LP | |
| 60 g | Lettuce, iceberg | 50 g | Lettuce, iceberg | 100 g | Rice pilaf, LP | 120 g | Mashed potatoes, LP | 90 g | White rice, instant | |
| 30 g | Tomato, Roma | 30 g | Tomato, chopped | 6 g | Butter | 3 g | Butter | 11 g | Butter | |
| 20 g | Pepperoncini | 24 g | Salad dressing, fat-free | 50 g | Lettuce, iceberg | 50 g | Lettuce, iceberg | 75 g | Green beans | |
| 5 g | Parmesan cheese | 2 each | Dinner biscuit, LP | 30 g | Tomato, chopped | 30 g | Tomato, chopped | 5 g | Butter | |
| 6 g | Olive oil | 10 g | Butter | 24 g | Salad dressing, fat-free | 24 g | Salad dressing, fat-free | 1 each | Dinner biscuit, LP | |
| 24 g | Salad dressing, fat-free | 355 g | Soda, diet | 1 each | Dinner biscuit, LP | 1 each | Dinner biscuit, LP | 5 g | Butter | |
| 355 g | Soda, diet | 5 g | Butter | 5 g | Butter | 355 g | Soda, diet | |||
| 355 g | Soda, diet | 355 g | Soda, diet | |||||||
LP represents a low-phytosterol recipe that was specially formulated to contain low-phytosterol ingredients (eg, soybean oil that was purified to remove the naturally occurring phytosterols, white rice flour, potato starch, tapioca flour, xanthan gum, and instant white rice).
TABLE 2.
Nutrient content of the phytosterol-deficient background diet
| Nutrient | Mean value at the 2000-kcal/d level | Range for the 5-d menu at the 2000-kcal/d level |
| Carbohydrate [g (% of energy)] | 281 (55) | 280–283 (55–56) |
| Protein [g (% of energy)] | 75 (15) | 75–76 (15) |
| Fat [g (% of energy)] | 66 (30) | 66–67 (29–30) |
| Saturated | 20 (8.8) | 19–20 (8.3–9.0) |
| Monounsaturated | 21 (9.3) | 20–21 (9.0–9.5) |
| Polyunsaturated | 21 (9.2) | 20–22 (9.0–9.6) |
| Cholesterol (mg) | 169 | 146–205 |
| Phytosterols (mg) | 50 | 45–60 |
| Fiber (g) | 171 | 16–181 |
Includes 9 g soluble fiber provided daily as a supplement mixed into the phytosterol beverages.
Phytosterol supplements
Phytosterols were added to the phytosterol-deficient diet as a supplement in 2 daily beverages consumed with breakfast and dinner. Each beverage was 118.3 mL (4 oz) and contained 18 g powder drink mix (Carnation Instant Breakfast; Nestlé, Vevey, Switzerland), 120 g skim milk, and 5.25 g fiber powder (Benefiber, Novartis, Parsippany, NJ) to provide 4.5 g soluble fiber and 5 g purified soybean oil containing either no addition or phytosterol esters (ADM, Decatur, IL) in quantities of 328 or 1639 mg (to provide 200 or 1000 mg free phytosterols, respectively). The daily phytosterol supplement doses of 400 and 2000 mg were based on the quantity present in some healthy diets (8) and the NCEP recommendation (6), respectively.
Meal provision
All food and beverages were prepared in the metabolic kitchen. The 5-d menu cycle was repeated during each 4-wk feeding period. Participants ate breakfast and dinner in the PBRC feeding center on weekdays and carried out the remainder of meals and snacks. A multivitamin/mineral supplement (Equate Complete; Perrigo Company, Allegan, MI) was given daily at breakfast. The energy level of the diet was individualized based on each participant's estimated resting metabolic rate (15, 16) and an activity factor of 1.4–1.5; adjustments were made if necessary to ensure weight stability throughout the 12-wk feeding period.
Cholesterol metabolism
Fecal cholesterol excretion and intestinal cholesterol absorption were determined by administering gelatin capsules twice daily for the last 5 d of each 28-d feeding period and collecting stool samples on days 27 and 28. Each capsule contained 450 μg 25,26,26,26,27,27,27-[2H7]cholesterol (CDN Isotopes, Quebec, Canada) and 90 μg 5,6,22,23-[2H4]sitostanol (Medical Isotopes, Pelham, NH) suspended in 125 μL purified soybean oil. The 5-d isotope period was deemed optimal for achieving equilibration in the fecal ratio of labeled cholesterol to labeled sitosterol, before significant recycling of the oral tracers occurs (17). The stool samples were saponified, extracted, and analyzed for cholesterol and sitostanol contents (18) using negative ion chemical ionization gas chromatography/mass spectrometry in the Washington University Mass Spectrometry Resource. The results for days 27 and 28 were averaged, and the average was used in all subsequent analyses. Fecal excretion of cholesterol and metabolites was corrected for fecal recovery of the nonabsorbable marker [2H4]sitostanol. Percentage cholesterol absorption was calculated as 100 × [1-([2H7]cholesterolfeces/[2H4]sitostanolfeces)/([2H7]cholesterolcapsule/[2H4]sitostanolcapsule)]. Dietary cholesterol excretion was calculated as [cholesteroldiet – (cholesteroldiet × percentage cholesterol absorbed)]. Because fecal cholesterol is composed predominantly of biliary cholesterol and dietary cholesterol, we calculated biliary cholesterol excretion as total cholesterol excreted – dietary cholesterol excreted. Concentrations of cholestanol and lathosterol were measured in plasma and expressed relative to the total cholesterol concentration to serve as markers for cholesterol absorption and biosynthesis, respectively (19).
Lipid, glucose, and insulin concentrations
Blood samples were drawn in the morning after a 10-h fast on days 24 and 28 of each feeding period. Total cholesterol and glycerol-blanked triglycerides were measured by using automated enzymatic kits. HDL cholesterol was measured after precipitation of apolipoprotein B–containing lipoproteins by dextran sulfate and magnesium (20). LDL cholesterol was calculated by using the Friedewald equation (21). Non-HDL cholesterol was calculated as the difference between total and HDL cholesterol. Fasting glucose was determined by the glucose oxidase method, and insulin was measured by using an Immulite analyzer in the Washington University Core Laboratory for Clinical Studies. Results for days 24 and 28 were averaged.
Adherence
Adherence to the phytosterol-deficient diet and consumption of the phytosterol-supplemented beverages were assessed in 3 ways: plasma phytosterol concentrations were measured, all food and beverages that were not consumed were weighed, and self-reported intake was computed from daily food logs.
Statistical analysis
Assuming an SD of 10% for lipid measurements, a sample size of 16 subjects gives 80% power to detect a 10% difference between treatments with a significance level of 0.05. To allow for attrition we enrolled 20 participants. Statistical analyses were performed by using SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC). The PROC MIXED procedure was used to analyze the fixed effects of phytosterol intake on cholesterol excretion, absorption, biosynthesis, and serum lipid concentrations, with Tukey adjustments for multiple comparisons. The PROC MIXED procedure was used to analyze carryover effects from one diet period to the next. A carryover effect was not significant for any outcome measure except plasma phytosterol concentration, which was expected based on its prolonged turnover time (22). Significance was accepted at an α level <0.05. Values in the text and tables are expressed as means ± SEMs.
RESULTS
Participants
Twenty participants (14 females, 6 males; 15 whites, 5 African Americans) were randomly assigned; 1 female failed to return to the center after 1 wk of the diet, and 1 male discontinued after 4 wk because of a schedule conflict. Eighteen subjects aged 63 ± 3 y (range: 46–80 y) with a body mass index (in kg/m2) of 27.3 ± 0.8 (range: 22.1–33.1), a systolic blood pressure of 122 ± 4 mm Hg, a diastolic blood pressure of 76 ± 1 mm Hg, a resting pulse rate of 65 ± 3 beats per minute, a fasting glucose concentration of 95 ± 2 mg/dL, and an insulin concentration of 8.3 ± 1.0 μU/mL completed all 3 diet periods. Body weight was stable throughout the 12-wk feeding period, and no changes were observed in blood pressure, pulse, or glucose or insulin concentrations.
Adherence and phytosterol intake
Adherence to the phytosterol-deficient diet and phytosterol-supplemented beverages was excellent, as indicated by a dose-dependent increase in plasma phytosterol concentration (Table 3), observation at the feeding center, and self-report of food and beverages consumed away from the center. Of the 2488 meals that were scheduled to be eaten on site, 6 meals (0.24%) were missed because of excused absences. The prescribed energy level of the diets averaged 2362 ± 136 kcal/d, and the estimated intake was 2368 ± 137 kcal/d. Total phytosterol intake (diet plus beverage supplements) averaged 59, 459, and 2059 mg/d during the 3 diet periods. Cholesterol intake did not differ across diet periods, averaging 199 ± 11, 193 ± 11, and 197 ± 11 mg/d.
TABLE 3.
Cholesterol metabolism and lipid concentrations in response to 3 levels of phytosterol intake1
| Phytosterol intake |
|||
| 59 mg/d (control) | 459 mg/d (moderate) | 2059 mg/d (high) | |
| Cholesterol absorption | |||
| Percentage cholesterol absorbed (%) | 69.9 ± 2.1 | 62.8 ± 2.12 | 52.7 ± 2.123 |
| Dietary cholesterol absorbed (mg/d) | 139 ± 8 | 121 ± 84 | 105 ± 825 |
| Biliary cholesterol absorbed (mg/d) | 1418 ± 167 | 1390 ± 167 | 1244 ± 167 |
| Plasma sterol ratios | |||
| Phytosterols/total cholesterol (μg/mg) | 7.4 ± 1.3 | 14.1 ± 1.32 | 25.8 ± 1.323 |
| Cholestanol/total cholesterol (μg/mg)6 | 1.09 ± 0.08 | 0.95 ± 0.082 | 0.85 ± 0.0825 |
| Lathosterol/total cholesterol (μg/mg)7 | 1.22 ± 0.13 | 1.51 ± 0.142 | 1.71 ± 0.1423 |
| Serum lipid concentrations | |||
| Total cholesterol (mg/dL) | 211 ± 6 | 204 ± 6 | 198 ± 62 |
| LDL cholesterol (mg/dL) | 139 ± 4 | 132 ± 4 | 126 ± 42 |
| HDL cholesterol (mg/dL) | 50 ± 3 | 50 ± 3 | 51 ± 3 |
| Triglycerides (mg/dL) | 112 ± 9 | 112 ± 9 | 104 ± 9 |
| Non-HDL cholesterol (mg/dL) | 161 ± 5 | 154 ± 5 | 146 ± 52 |
| LDL cholesterol/HDL cholesterol | 2.92 ± 0.16 | 2.75 ± 0.164 | 2.58 ± 0.1625 |
All values are means ± SEMs; n = 18 subjects.
Significantly different from control [PROC MIXED (SAS Institute, Cary, NC) with Tukey adjustment]: 2P < 0.01, 4P < 0.05.
Significantly different from moderate (459 mg/d) phytosterol intake (PROC MIXED with Tukey adjustment): 3P < 0.01, 5P < 0.05.
Cholestanol/total cholesterol ratio is an indicator of cholesterol absorption.
Lathosterol/total cholesterol ratio is an indicator of cholesterol biosynthesis.
Cholesterol metabolism
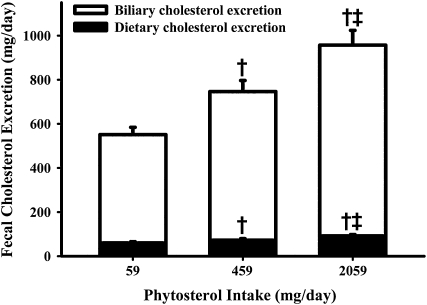
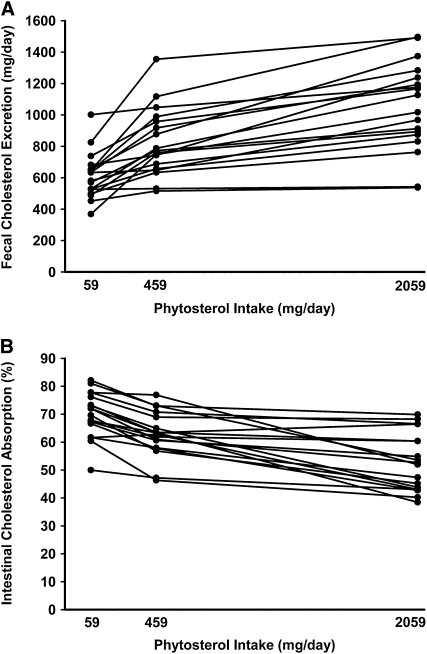
Fecal cholesterol excretion increased 36 ± 6% and 74 ± 10% in response to the moderate and high (ie, 459 and 2059 mg) phytosterol intake levels, respectively. As shown in Figure 1, most of the fecal cholesterol excretion originated from biliary cholesterol, with a smaller proportion from dietary cholesterol. Percentage intestinal cholesterol absorption decreased with increasing phytosterol intake, resulting in a decrease in dietary cholesterol absorbed (Table 3). Individual values for cholesterol excretion and absorption are shown in Figure 2. In agreement with these absorption results, the plasma cholestanol/total cholesterol ratio decreased as the phytosterol dose increased. Cholesterol biosynthesis, estimated from the plasma lathosterol/total cholesterol ratio, increased 31 ± 6% and 50 ± 7% in response to moderate and high phytosterol levels, respectively.
FIGURE 1.
Mean (±SEM) fecal cholesterol excretion in response to 3 levels of phytosterol intake. Open bars represent mean biliary cholesterol excretion, solid bars represent mean dietary cholesterol excretion, and the total height of the bars represents total fecal cholesterol excretion for 18 subjects. Each phytosterol intake period lasted 4 wk, with all food and beverages provided. PROC MIXED procedure with Tukey adjustment in SAS (SAS Institute, Cary, NC): †significantly different from 59 mg/d, P < 0.01; ‡significantly different from 459 mg/d, P < 0.01.
FIGURE 2.
Total fecal cholesterol excretion and percentage intestinal cholesterol absorption in response to 3 levels of phytosterol intake. Individual values for total fecal cholesterol excretion (A) and percentage intestinal cholesterol absorption (B) for 18 subjects. Each phytosterol intake period lasted 4 wk, with all food and beverages provided.
Lipid concentrations
As shown in Table 3, LDL cholesterol decreased significantly only with the highest phytosterol dose (−8.9 ± 2.3%), whereas a nonsignificant trend was observed with the moderate phytosterol intake (−5.0 ± 2.1%; P = 0.077). Similarly, total cholesterol and non-HDL cholesterol decreased with the high phytosterol dose only, whereas the LDL/HDL cholesterol ratio improved with both the moderate and high phytosterol intake levels. Neither HDL cholesterol nor triglycerides was affected by phytosterol intake.
DISCUSSION
We quantified the effects of 3 levels of phytosterol intake on cholesterol metabolism by using a novel phytosterol-deficient background diet. Our principal finding was that dietary phytosterols in moderate (459 mg/d) and high (2059 mg/d) doses significantly enhanced excretion of biliary and dietary cholesterol and reduced the efficiency of intestinal cholesterol absorption relative to a phytosterol-deficient diet. Importantly, the moderate phytosterol intake level studied can be achieved in a healthy diet consisting of whole grains, fruit, vegetables, vegetable oils, nuts, and seeds (8, 23). Accurate evaluation of phytosterol dose effects was not possible previously because of the unknown contribution of naturally occurring dietary phytosterols in previous clinical trials.
Both the moderate and high phytosterol intakes had a large effect on cholesterol excretion. Most of the excreted material was derived from endogenous biliary cholesterol. Because fecal cholesterol excretion is the terminal portion of the reverse cholesterol transport pathway, our data suggest that dietary phytosterols may affect reverse cholesterol transport. Cholesterol is released from peripheral tissues and macrophages lining the arterial wall and are then transported to the liver for excretion into bile (24). If the mechanism by which phytosterols increase cholesterol excretion is, in fact, by facilitating cholesterol efflux from the arterial wall, then phytosterols would be expected to favorably alter the atherosclerotic process. It is also possible that an increase in cholesterol biosynthesis contributed to some of the observed increase in fecal cholesterol excretion. Additional studies are required to elucidate the mechanisms by which phytosterols enhance cholesterol excretion.
The cholesterol absorption values that we obtained are consistent with those reported in the literature (18), and our results further confirm that percentage cholesterol absorption decreases as phytosterol intake increases. Although this effect of phytosterols has been demonstrated previously (1), our results provide novel information that significant but more modest effects can be obtained with a quantity of phytosterols that is achievable naturally in the diet without supplementation. The 10% and 25% reductions in cholesterol absorption that we observed with the moderate and high phytosterol intake levels, respectively, support a dose effect. The dose-dependent increase in cholesterol biosynthesis was likely a compensatory response to the reduced absorption and increased excretion of cholesterol and is consistent with previous results (25).
The effects of phytosterols on LDL cholesterol also appeared to be dose-dependent. The 8.9% reduction in LDL cholesterol observed with the 2059-mg/d dose of phytosterol in the present study is consistent with the 8.8% reduction reported in a meta-analysis (26) of 84 trials in which the mean daily phytosterol supplement dose was 2.15 g (ie, 2150 mg). Both that report (26) and an earlier meta-analysis (27) showed a dose-response relation between phytosterols and LDL cholesterol, with little additional benefit achieved beyond 2.5 g/d, which supports the recommendations of the National Cholesterol Education Program (6) and the American Heart Association (28) for adults to consume 2 g phytosterol supplements daily to significantly lower LDL cholesterol.
The moderate (459 mg/d) phytosterol intake in our study did not reduce LDL cholesterol significantly, although there was a trend. Using a higher phytosterol dose of 740 mg/d in a diet that was high in cholesterol (410 mg/d), Pelletier et al (29) observed a 15% reduction in LDL cholesterol. Similarly, the US Food and Drug Administration (FDA) concluded, based on previous studies (30, 31), that health benefits can be achieved with 800 mg phytosterols/d. Additional studies are needed to determine the threshold for LDL lowering. The FDA requires phytosterol-enriched food products to contain the equivalent of ≥400 mg free phytosterols per serving (ie, 0.65 g plant sterol esters), to be eaten twice daily with meals, for a daily intake of ≥800 mg free phytosterols (ie, 1.3 g plant sterol esters) (32) in order for their labels to contain a health claim. It is important to note, however, that the FDA and NCEP phytosterol dose recommendations are based on studies that neither removed nor controlled for phytosterols already present in the diet. Accurate quantification of dietary phytosterols requires chemical analyses of diet composites because nutrient databases do not contain comprehensive phytosterol values. Therefore, the actual phytosterol intake in most supplementation studies was variable and unknown. Results of the present study provide novel information on the effects of 3 analytically quantified phytosterol intakes, including lower intakes than have been evaluated previously.
Whereas elevated LDL cholesterol is an effective target for treatment because of its association with cardiovascular diseases (33), lipid ratios may be more important than LDL cholesterol alone. Ingelsson et al (34) reported that total/HDL cholesterol and LDL/HDL cholesterol ratios were highly predictive of coronary heart disease events in a 15-y follow-up. In the present study, we observed significant reductions in the LDL/HDL cholesterol ratio with both moderate and high phytosterol doses, although the other lipid values were affected only by the high dose. Another point to consider is that atherosclerotic plaque progression and regression can occur independently of effects on LDL cholesterol (35). Schoenhagen et al (36) showed that the effects of statin therapy on coronary artery wall remodeling in the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering Therapy) trial were not explained by LDL cholesterol, whereas the inflammatory marker C-reactive protein was highly associated with remodeling. Therefore, markers of cardiovascular disease risk other than LDL cholesterol may be important to explore when assessing the health effects of phytosterols.
Limitations of this study included the small sample size, relatively short diet periods, and the provision of phytosterols as a supplemental beverage rather than intact in foods. A small study sample was justifiable because the crossover design controlled for biological variability between the 3 levels of phytosterol intake. The 4-wk feeding and 1-wk washout periods were based on evidence that diet-induced reductions in LDL cholesterol occur within 3 wk (37) with no washout period required. Finally, we used phytosterol supplements so that the background diet would be identical for all 3 phytosterol doses; this enabled us to control all dietary factors that may affect serum lipids (eg, saturated fat, trans fatty acids, and fiber).
In summary, results of this controlled feeding trial indicate that phytosterols consumed in moderate and high doses substantially enhanced fecal cholesterol excretion and reduced percentage intestinal cholesterol absorption relative to a phytosterol-deficient diet, and the effect was dose-dependent. The marked increase in biliary cholesterol excretion may represent an important mechanism by which phytosterols favorably alter lipid metabolism. Importantly, the moderate phytosterol intake of 459 mg/d can be achieved naturally in a healthy diet. Future investigations will be important to elucidate the potential role of phytosterols as a nonpharmacologic means to improve whole-body cholesterol metabolism and potentially reduce cardiovascular disease risk.
Acknowledgments
We are grateful for the skilled assistance of Michele Burton and the staff at the Pennington Biomedical Research Center, the statistical expertise of Kenneth Schechtmann at the Washington University School of Medicine, and the laboratory assistance of Stephen Block at Washington University School of Medicine. We appreciate the dedication of the study participants.
The authors' responsibilities were as follows—REO, ML, SBR CAS, and MMM: developed the study design and diets; XL: performed the oil purification procedure; MF: supervised the data collection; XL, REO, and LM: performed the statistical analyses; REO, SBR, and XL: interpreted the data; and SBR, XL, and REO: wrote the manuscript. REO and Washington University have a financial interest in Lifeline Technologies Inc, a startup company commercializing emulsified phytosterols. Lifeline products were not used in this research. None of the other authors had a personal or financial conflict of interest.
REFERENCES
- 1.Normén L, Dutta P, Lia A, Andersson H. Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am J Clin Nutr 2000;71:908–13 [DOI] [PubMed] [Google Scholar]
- 2.Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric 2000;80:939–66 [Google Scholar]
- 3.Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr 2002;22:533–49 [DOI] [PubMed] [Google Scholar]
- 4.Peterson DW. Effect of soybean sterols in the diet on plasma and liver cholesterol in chicks. Proc Soc Exp Biol Med 1951;78:143–7 [DOI] [PubMed] [Google Scholar]
- 5.Pollak OJ. Reduction of blood cholesterol in man. Circulation 1953;7:702–6 [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 7.Nair PP, Turjman N, Kessie G, et al. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Dietary cholesterol, beta-sitosterol, and stigmasterol. Am J Clin Nutr 1984;40:927–30 [DOI] [PubMed] [Google Scholar]
- 8.Racette SB, Spearie CA, Phillips KM, Lin X, Ma L, Ostlund RE. Phytosterol-deficient and high-phytosterol diets developed for controlled feeding studies. J Am Diet Assoc (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weihrauch JL, Gardner JM. Sterol content of foods of plant origin. J Am Diet Assoc 1978;73:39–47 [PubMed] [Google Scholar]
- 10.Piironen V, Toivo J, Lampi AM. Natural sources of dietary plant sterols. J Food Comp Anal 2000;13:619–24 [Google Scholar]
- 11.Normén L, Bryngelsson S, Johnsson M, et al. The phytosterol content of some cereal foods commonly consumed in Sweden and in the Netherlands. J Food Comp Anal 2002;15:693–704 [Google Scholar]
- 12.Abidi SL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A 2001;935:173–201 [DOI] [PubMed] [Google Scholar]
- 13.Ostlund RE, Jr, Racette SB, Stenson WF. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am J Clin Nutr 2003;77:1385–9 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, US Department of Agriculture Dietary guidelines for Americans. Washington, DC: US Government Printing Office; 2005 [Google Scholar]
- 15.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute of Washington; 1919 [Google Scholar]
- 16.Food and Agricultural Organization/World Health Organization/United Nations University Energy and protein requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Geneva, Switzerland: WHO, 1985:1–206 [Google Scholar]
- 17.Crouse JR, Grundy SM. Evaluation of a continuous isotope feeding method for measurement of cholesterol absorption in man. J Lipid Res 1978;19:967–71 [PubMed] [Google Scholar]
- 18.Lutjohann D, Meese CO, Crouse JR, III, von Bergmann K. Evaluation of deuterated cholesterol and deuterated sitostanol for measurement of cholesterol absorption in humans. J Lipid Res 1993;34:1039–46 [PubMed] [Google Scholar]
- 19.Miettinen TA, Tilvis RS, Kesäniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism 1989;38:136–40 [DOI] [PubMed] [Google Scholar]
- 20.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg 2+ precipitation procedure for quantification of high-density lipoprotein cholesterol. Clin Chem 1982;28:1379–88 [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 22.Salen G, Ahrens EH, Jr, Grundy SM. Metabolism of beta-sitosterol in man. J Clin Invest 1970;49:952–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins DJ, Kendall CW, Popovich DG, et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 2001;50:494–503 [DOI] [PubMed] [Google Scholar]
- 24.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis?. Circulation 2006;113:2548–55 [DOI] [PubMed] [Google Scholar]
- 25.Nestel P, Cehun M, Pomeroy S, Abbey M, Weldon G. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur J Clin Nutr 2001;55:1084–90 [DOI] [PubMed] [Google Scholar]
- 26.Demonty I, Ras RT, van der Knaap HCM, et al. Continuous Dose-Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake. J Nutr 2009;139:271–84 [DOI] [PubMed] [Google Scholar]
- 27.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003;78:965–78 [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 29.Pelletier X, Belbraouet S, Mirabel D, et al. A diet moderately enriched in phytosterols lowers plasma cholesterol concentrations in normocholesterolemic humans. Ann Nutr Metab 1995;39:291–5 [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa M, Masuda Y, Noda M, et al. Minimal effective dose on serum cholesterol concentration and the safety evaluation of dressing containing plant sterol in Japanese subjects. J Oleo Sci 2008;57:23–33 [DOI] [PubMed] [Google Scholar]
- 31.Sierksma A, Weststrate JA, Meijer GW. Spreads enriched with plant sterols, either esterified 4,4-dimethylsterols or free 4-desmethylsterols, and plasma total- and LDL-cholesterol concentrations. Br J Nutr 1999;82:273–82 [PubMed] [Google Scholar]
- 32.US Food and Drug Administration Code of Federal Regulations. Title 21, Volume 2–Food and Drugs, 2008 [Google Scholar]
- 33.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: a population-based study. Atherosclerosis 2006;189:369–74 [DOI] [PubMed] [Google Scholar]
- 34.Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007;298:776–85 [DOI] [PubMed] [Google Scholar]
- 35.Weingärtner O, Lütjohann D, Ji S, et al. Vascular effects of diet supplementation with plant sterols. J Am Coll Cardiol 2008;51:1553–61 [DOI] [PubMed] [Google Scholar]
- 36.Schoenhagen P, Tuzcu EM, Apperson-Hansen C, et al. Determinants of arterial wall remodeling during lipid-lowering therapy: serial intravascular ultrasound observations from the Reversal of Atherosclerosis with Aggressive Lipid Lowering Therapy (REVERSAL) trial. Circulation 2006;113:2826–34 [DOI] [PubMed] [Google Scholar]
- 37.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45 [DOI] [PubMed] [Google Scholar]