Abstract
HIV protease inhibitor (PI) ritonavir (RTV) may cause vascular injury through oxidative stress. Our purpose in this study was to determine whether equol, a soy isoflavone, could prevent RTV-induced endothelial dysfunction in porcine pulmonary arteries and in human pulmonary artery endothelial cells (HPAEC). Fresh porcine pulmonary artery rings were treated with 15 μmol/L of RTV and/or equol in concentrations of 0.1, 1, and 10 μmol/L for 24 h. A control was set with no amount of equol or RTV administered. Based on myograph tension analysis, RTV significantly reduced endothelium-dependent relaxation in response to bradykinin in the artery rings compared with untreated vessels, whereas the antioxidant equol effectively reversed the RTV effect in a concentration-dependent manner. RTV also reduced the contraction of artery rings in response to thromboxane A(2) analogue U46619 and this reduction was blocked by equol. In addition, RTV treatment significantly reduced endothelial nitric oxide synthase (eNOS) expression in both porcine pulmonary arteries and HPAEC, whereas equol effectively blocked RTV-induced eNOS downregulation. Furthermore, RTV significantly increased superoxide anion production, whereas equol reversed this effect of RTV in porcine pulmonary arteries. Thus, the antioxidant equol effectively protects vascular function from the detrimental effects of HIV PI RTV in both porcine pulmonary arteries and HPAEC via a reduction in the vasomotor dysfunction, eNOS downregulation, and oxidative stress induced by RTV. These novel data suggest that equol may have a clinical application in preventing HIV-associated cardiovascular complications.
Introduction
HIV infection results in progressive immune dysfunction caused by persistent viral replication that destroys the human immune system. The use of HIV protease inhibitors (PI)5 has improved immunologic benefits and survival, and PI have become a key component in the highly active antiretroviral therapy (HAART). Reviews of recent literature reveal that HAART regimens, especially those that include a PI, have been shown to induce metabolic syndrome, which includes dyslipidemia, insulin resistance, and type 2 diabetes, and this may be associated with increased risk for cardiovascular disease (1,2). Pulmonary hypertension, meanwhile, is a rare long-term complication that affects 0.5% of HIV-infected patients; it was first noted over 15 y ago (3) and this has not changed recently despite the use of HAART (4,5). In fact, the use of HAART in developed countries has increased the prevalence of pulmonary hypertension in patients with HIV by 15–20% (6). We have previously shown that ritonavir (RTV), one of the clinically used PI, can cause endothelial dysfunction in human endothelial cells and porcine coronary and carotid arteries (7,8). More recently, we have demonstrated that RTV and other HAART drugs impair endothelium-dependent vasorelaxation in porcine pulmonary arteries (9). This endothelial dysfunction is caused by downregulation of endothelial nitric oxide synthase (eNOS) and a remarkable increase in reactive oxygen species (ROS), such as superoxide anion. As such, these data suggest that antioxidant therapy may be an effective strategy to control HAART-induced endothelial dysfunction in the coronary and pulmonary vascular systems.
Soybeans are the main ingredient in many staple diets in Asian countries. Soybeans are high in active isoflavones belonging to the phytoestrogen class; these molecules are very similar in chemical structure to mammalian estrogens (10). The isoflavones have been shown to have cardiovascular benefits on plasma lipid and lipoprotein concentrations and arterial function (11–13). The primary isoflavones from soy are genistein and daidzein and they both bind to estrogen receptors (ER) (14). After ingestion of the primary isoflavone daidzein, the predominant active metabolite, equol, is produced by the intestinal microflora (15). However, 30–50% of the human population cannot produce equol (16) due to a lack of this specific gut flora (17). This may account for the contradictory results in a review of clinical studies involving isoflavones in humans (18). Direct use of equol may overcome this problem.
Our objective in this study was to determine whether equol could block RTV-induced endothelial dysfunction in the pulmonary vascular system. We used clinically relevant dosages of both the antiviral as well as the equol that is comparable to that found in Asian soy-based diets. Porcine pulmonary arteries were used to study the effect of RTV and equol on vasomotor functions, eNOS expression, and superoxide anion production. Human pulmonary artery endothelial cells (HPAEC) were also used for the eNOS expression study. This study may provide rationale that soybean-rich diets may prove useful as an adjunct therapy to prevent the cardiovascular complications of antiretroviral therapy in HIV-infected patients.
Materials and Methods
Chemicals and reagents.
Equol was obtained from Sigma Chemical. Antibody against human eNOS was obtained from BD Transduction Laboratories. Pure RTV powder was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Tissue harvest and cell culture.
Fresh porcine lungs were harvested from young adult farm pigs (6–7 mo old) at a local slaughterhouse. Porcine pulmonary arteries were carefully dissected and cut into 3-mm rings (9). Several rings from each lung were allocated into groups: controls (DMEM), those treated with 15 μmol/L RTV, those treated with 15 μmol/L RTV plus equol (0.1, 1, and 10 μmol/L), and those treated with equol only. HPAEC were purchased from Cambrex. Once cells grew to 80–90% confluence in 6-well culture plates, they were likewise treated with DMEM, 15 μmol/L RTV, 15 μmol/L RTV plus equol (0.1, 1, and 10 μmol/L), and equol only for 24 h at 37°C. Cells were then harvested and total mRNA was extracted for real-time PCR study.
Myograph analysis.
The myograph tension system used in our laboratory has been previously described (7,9). Briefly, the rings were cultured in the medium for 24 h and then were suspended between the wires of the organ bath chamber (Multi myograph system 700MO; Myo Technology). After equilibration, each ring was precontracted with U46619 (10−7 mol/L) and the relaxation concentration-response curve was generated by the endothelium-dependent vasodilator bradykinin (10−10, 10−9, 10−8, 10−7, and 10−6 mol/L) every 3 min. Lastly, 60 μL of sodium nitroprusside (SNP) (10−6 mol/L) was added to the organ bath and endothelium-independent vasorelaxation was recorded.
Detection of superoxide anion.
Levels of superoxide anion produced by endothelial cells of the porcine pulmonary artery rings were detected using the lucigenin-enhanced chemiluminescence method as previously described in our studies (7,9). Time-based reading of the luminometer was recorded. Final data were represented as relative light unit·(s·mm2). Superoxide anion in the vessel wall was also directly visualized under the fluorescence microscope by using dihydroethidium (DHE) staining (Molecular Probes).
Real-time PCR.
Endothelial cells of porcine pulmonary arteries were collected by scraping the luminal surface with a surgical blade. HPAEC were collected from culture by trypsin digestion. Total cellular RNA was then extracted and eNOS mRNA levels were detected by real-time PCR; these were then normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels 2[Ct(β-GAPDH)−Ct(eNOS)] as previously described (7,9).
Immunohistochemistry of eNOS.
Treated porcine pulmonary artery rings were fixed in 10% formalin and embedded in paraffin. Samples were sectioned at a 5-μm thickness and slides were incubated with monoclonal antibody against human eNOS (1:500) (7,9).
Statistical analysis.
All data are presented as the mean ± SEM. Statistical analysis was completed by comparing multiple groups with a signal control group using a 1-way ANOVA followed by Bonferroni/Dunn post hoc test (Minitab software, Sigma Breakthrough Technologies). P <0.05 was considered significant.
Results
Effects of RTV and equol on vasomotor function in porcine pulmonary arteries.
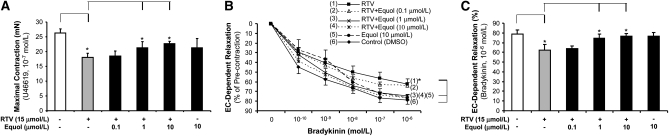
Porcine pulmonary artery rings were cultured for 24 h with DMEM as control or treated with a clinically relevant concentration of RTV (15 μmol/L) with or without equol (0.1, 1, and 10 μmol/L). Vessel contraction was achieved by using the thromboxane analogue U46619 (10−7 mol/L). In response to U46619, the contraction of the vessel rings was reduced by 44% for the RTV-only treatment group compared with the control group (P < 0.05; Fig. 1A). When cultured with equol as well (1 and 10 μmol/L), the contractility increased by 26 and 57%, respectively, compared with the RTV-alone group (P < 0.05; Fig. 1A). The contraction in the equol-alone group was higher than that in the RTV-alone–treated group (P < 0.05; Fig. 1A); however, there was no difference compared with treatment-free controls. Endothelium-dependent vasorelaxation was induced by cumulative concentrations of bradykinin (10−9 – 10−5 mol/L). Equol at 1 and 10 μmol/L blocked RTV-induced impairment in endothelium-dependent relaxation in a concentration-dependent manner compared with RTV-alone–treated vessel rings (P < 0.05; Fig. 1B). For example, in response to bradykinin at 10−6 mol/L, the endothelium-dependent relaxation of vessel rings was reduced by 21% for the RTV-alone group compared with the control group (P < 0.05; Fig. 1C). When cocultured with equol (1 and 10 μmol/L), relaxation increased by 19 and 22%, respectively, in a concentration-dependent manner compared with the RTV-alone group (P < 0.05; Fig.1C). The endothelium-dependent relaxation in the equol-alone group was higher than that in RTV-alone treated group (P < 0.05; Fig. 1B,C); however, again, there was no difference compared with treatment-free controls. Endothelium-independent vasorelaxation was induced by SNP. In response to SNP, treatment with RTV did not significantly affect endothelium-independent vasorelaxation compared with the control group (data not shown). Overall, RTV was found to cause vasomotor dysfunction, which was effectively reversed by equol.
FIGURE 1 .
Effects of RTV and equol on vasomotor reactivity of porcine pulmonary arteries. The vessel rings were cultured with DMEM alone (control) or treated with RTV (15 μmol/L) with or without equol (0.1, 1, and 10 μmol/L) for 24 h. (A) Vessel contraction. Maximal contraction in response to thromboxane A2 analog U-46619 (10−7 mol/L). (B) Vessel relaxation. Endothelium-dependent vasorelaxation in response to cumulative concentrations of bradykinin (10−9 – 10−5 mol/L). (C) Values are means ± SEM, n = 8. *Different from control, P < 0.05.
Effects of RTV and equol on eNOS expression.
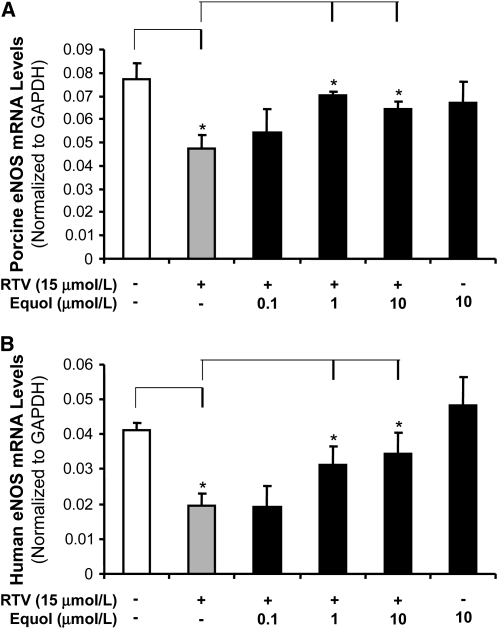
To examine whether the level of eNOS expression corresponded to the changes in the endothelium-dependent relaxation induced by RTV and reversed by equol, we determined the eNOS mRNA level in treated vessels by real-time PCR analysis. Endothelial cells isolated from the porcine pulmonary vessels and HPAEC were used. The eNOS mRNA levels were reduced in the RTV-alone group compared with the control group in both porcine pulmonary artery rings and HPAEC by 38 and 53%, respectively (P < 0.05; Fig. 2A,B). When cocultured with equol (0.1, 1, and 10 μmol/L), the eNOS mRNA levels increased in both porcine pulmonary artery rings and HPAEC compared with the RTV-alone group (P < 0.05; Fig. 2A,B). The eNOS mRNA level in the equol-alone group was higher than that in RTV-alone–treated group (P < 0.05; Fig. 2A,B). However, there was no difference compared with treatment-free controls. Meanwhile, the eNOS protein level was analyzed using immunohistochemistry staining (n = 4 for each group). A representative slide from each group is shown (Fig. 3). The eNOS protein level at the luminal endothelial layer was reduced in the RTV-alone group compared with the control group in porcine pulmonary artery rings, and when cocultured with equol, eNOS immunoreactivity was enhanced to the level in controls. Thus, RTV reduced eNOS expression in both porcine pulmonary arteries and HPAEC, and this detrimental effect was effectively blocked by equol.
FIGURE 2 .
Effects of RTV and equol on eNOS mRNA levels. Porcine pulmonary artery rings and HPAEC were treated with DMEM alone (as control) or treated with RTV (15 μmol/L) with or without equol (0.1, 1, and 10 μmol/L) for 24 h. eNOS mRNA levels were determined by real-time PCR and normalized to GAPDH mRNA. (A) eNOS mRNA levels in porcine artery rings. (B) eNOS mRNA levels in HPAEC. Values are means ± SEM, n = 4. *Different from control, P < 0.05.
FIGURE 3 .
Effects of RTV and equol on eNOS immunoreactivity in porcine pulmonary artery endothelial cells. The vessel rings were treated with DMEM alone (control), RTV (15 μmol/L), or RTV plus equol (10 μmol/L) for 24 h. The eNOS protein expression was determined by immunohistochemistry. Four vessel rings were studied for each group. (A) A representative slide of eNOS immunoreactivity in the control group. (B) A representative slide of eNOS immunoreactivity in the RTV-treated group. (C) A representative slide of eNOS immunoreactivity in the RTV and equol-treated group. Dark brown color represents positive staining at the luminal endothelial layer (arrowheads). Original magnification, ×200. Scale bar = 40 μm.
Effects of RTV and equol on superoxide anion production.
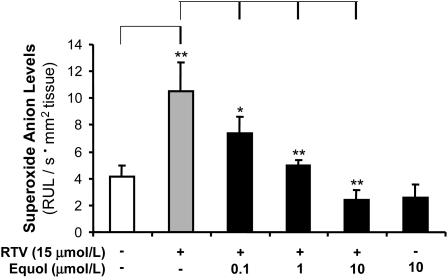
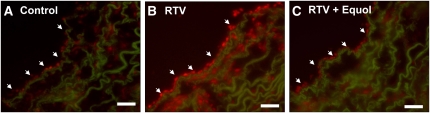
Oxidative stress has been shown to cause endothelial dysfunction and vascular injury. To determine whether this is involved in RTV-induced vasomotor dysfunction, we analyzed superoxide anion production in porcine pulmonary arteries by a lucigenin-enhanced chemiluminescence assay (n = 4). The superoxide anion level of the endothelial layer of vessel rings was substantially increased by 154% for the RTV-alone group compared with the control group (P < 0.01; Fig. 4). When cocultured with equol (0.1, 1, and 10 μmol/L), the superoxide anion level was reduced to the control levels in a concentration-dependent manner (P < 0.05 and P < 0.01, respectively; Fig. 4). The superoxide anion level in the equol-alone group was significantly lower than that in RTV-alone–treated group (P < 0.05; Fig. 4), but there was no difference compared with treatment-free controls. We also investigated the level of superoxide anion using an oxidative fluorescent dye (DHE) staining and directly visualized using fluorescence microscopy. Four vessel rings for each group were studied. A representative slide is shown (Fig. 5). In the RTV-alone group, there was a marked increased in DHE staining (red fluorescence) in both endothelial and smooth muscle cell layers compared with the control group. When cocultured with equol, the DHE staining was reduced compared with the RTV-alone vessels (Fig. 5). Thus, RTV increases superoxide anion production and this effect is effectively reversed by equol.
FIGURE 4 .
Effects of RTV and equol on superoxide anion production in porcine pulmonary arteries. The vessel rings were cultured with DMEM alone (as control) or treated with RTV (15 μmol/L) with or without equol (0.1, 1, and 10 μmol/L) for 24 h. Superoxide anion levels at the endothelial layer were determined with the lucigenin-enhanced chemiluminescence assay. Values are means ± SEM, n = 4. *Different from control, P < 0.05; **P < 0.01.
FIGURE 5 .
Superoxide levels of porcine pulmonary artery rings treated with DMEM alone (control), RTV (15 μmol/L) or RTV plus equol (10 μmol/L) for 24 h. The vessel rings were stained with DHE. One of 4 representative slides of DHE-stained rings are shown for the control group (A), the RTV-treated group (B), and the RTV and equol-treated group (C). Red fluorescence indicates positive staining for superoxide anion at the luminal endothelial layer (arrowheads) and the medium layer. Original magnification, x200. Scale bar = 40 μm.
Discussion
The results of this study demonstrate that a clinically relevant concentration of RTV significantly reduced vasocontractility and endothelium-dependent vasorelaxation in porcine pulmonary arteries. RTV also significantly decreased eNOS mRNA and proteins levels, while increasing superoxide anion production in the vessel rings, which is consistent with our previous studies (9). RTV-induced eNOS downregulation was also confirmed in HPAEC. Importantly, we found that the antioxidant equol can effectively block these detrimental effects of RTV in porcine pulmonary arteries and HAPEC. This study reveals novel therapeutic values of equol in HIV-infected patients.
Compared with other isoflavones, equol (3.5 μmol/L) has a higher affinity for ERβ (400 nmol/L) and ERα (3.5 μmol/L) (19). Equol also has a longer plasma half-life than its parent compound, daidzein; it is detected in the urine for longer following soy challenge (20). It is also more biologically active than daidzein in enhancing cardiac cell function (21) and has more antioxidant effects in arterial segments in vivo and in vitro (22). As such, equol has been predicted to provide more cardiovascular protection than its parent compound daidzein (23); together with the aforementioned variation in gut production in individuals, there is a shift to focus on using equol directly in clinical studies of soy isoflavones. Daidzein, genistein, and equol can achieve plasma concentrations of 50–800 μg/L (0.2–3.3 μmol/L) in adults who consume 50 mg/d of total soy isoflavones (24). These values are similar to the plasma concentrations in Japanese individuals who consume a traditional diet in which soy is a staple (25). Physiologic concentrations of equol were used in this study and we showed that 1 and 10 μmol/L are effective concentrations of equol, which is relevant to potential applications in humans.
In the current study, RTV in a concentration near clinical plasma levels (15 μmol/L) induced vasomotor dysfunction, including decreased vessel contractility and endothelial-dependent vasorelaxation. When the pulmonary artery rings were cultured with RTV and equol (1 or 10 μmol/L) together, there was a notable reversal of the damaging effects of RTV on vasomotor reactivity in a concentration-dependent manner. There was protective effect of equol on relaxation of the vessel walls, and the effect was endothelial dependent. We noticed that equol at 10 μmol/L also reduced vessel contraction. The reason for this observation is not clear. In the cell culture study with HPAEC, we did not observe any changes in cell proliferation in cells treated with 10 μmol/L equol compared with untreated control cells, which suggests that 10 μmol/L equol has no cytotoxity in culture conditions. Vasorelaxant effects of equol have also been shown in vitro in rat aorta (26) and carotid artery and in vivo in basilar artery (27). The effect was noted to be both dependent and independent of eNOS activity in the rat aorta (26). This was independent of eNOS activity in the carotid artery (27). The difference in mechanisms of vasorelaxation could be due to the animal species and vascular bed studied; however, further study is needed.
NO produced from eNOS is a key regulator of vascular homeostasis. Decreased NO availability could be caused by decreased expression of eNOS, inappropriate eNOS activation, inadequate substrate or cofactor for eNOS, and accelerated NO degradation by ROS (28). Using real-time PCR, the current study showed that there was a substantial decrease of eNOS mRNA expression in the RTV-treated porcine pulmonary artery. With immunohistochemistry, there was a considerable decrease in the eNOS protein levels in the endothelium of RTV-treated vessels. However, it could be a limitation that we cannot make reliable, quantitative measurements of eNOS expression with the immunohistochemistry method. Quantum dot deconvolution imaging analysis could produce reliable, quantitative measurement of eNOS expression in the vascular tissues. RTV-induced eNOS downregulation was also confirmed in HPAEC. Equol effectively blocked these effects of RTV on downregulation of eNOS in both mRNA and protein levels, suggesting a potential therapeutic role. Equol may also protect eNOS mRNA and protein from the destructive effect of increased ROS in cells treated with RTV. Indeed, the current study showed that the level of superoxide anion was decreased in the equol-treated samples. Alternatively, equol may be able to increase the synthesis of eNOS mRNA and increase the eNOS protein level. In our cell culture with HPAEC, the equol-alone group had higher levels of eNOS mRNA compared with the control group. Further study is needed to investigate the mechanism of equol in endothelial protection. To our knowledge, this is the first study to show an increased eNOS mRNA level mediated by equol. It is clearly significant that equol increases eNOS expression.
It has been well documented that oxidative stress plays an important role in endothelial dysfunction and cardiovascular disease (29). In the current study, there was a significant increase in superoxide anion production in the endothelial layer of the RTV-treated porcine pulmonary artery rings. The oxidative stress induced by RTV may explain its effects on vascular dysfunction. This study shows that equol can block the effect of RTV-induced production of superoxide anion, leading to improved vasomotor function in porcine pulmonary artery rings. Soy consumers have genistein and daidzein, in addition to equol, circulating in their blood; however, we did not compare these compounds with equol in the current study. It will be interesting to learn in future studies whether other isoflavones also inhibit RTV-induced endothelial dysfunction. Equol has been reported to have antioxidant effects that are superior to those of daidzein or genistein (30), and although equol and daidzein had similar antioxidant activity in vitro in the carotid artery, equol had superior antioxidant activity compared with daidzein in the basilar artery in vivo, which was via decreased NADPH oxidase-derived superoxide anion levels (31).
Previously, we reported that several other natural substances such as capsaicin (50 μmol/L) (31), ginsenoside Rb1 (10 μmol/L), and ginkgolide A (10 μmol/L) (9) can effectively block RTV-induced endothelial dysfunction in porcine pulmonary arteries in vitro. In addition, curcumin (10 μmol/L) and 3 ginsenosides, Rb1, Re, and Rc (10 μmol/L), effectively block RTV-induced endothelial dysfunction in porcine coronary arteries in vivo (32,33). In the current study, even a low concentration of equol (1 μmol/L) had blocking effects on RTV-treated porcine pulmonary arteries, suggesting that the antioxidant effect of equol may be more potent than that of other antioxidants used in our previous studies. The basic structure of isoflavones is the flavone nucleus, which, as mentioned, is very similar to mammalian estrogens (10). Previously, we found that estrogen effectively blocked homocysteine-induced endothelial dysfunction and oxidative stress in porcine coronary arteries (34). Estrogen has been documented to have a variety of positive effects on the endothelium (35,36). However, detailed pharmacologic characterizations, the mechanisms of action, and cytotoxicity of all of these compounds are not fully understood and certainly warrant future investigations in both in vitro and in vivo systems.
In summary, a clinically relevant concentration of RTV (15 μmol/L) can cause vasomotor dysfunction, decreased eNOS expression, and increased superoxide anion production. The soy equol can effectively block the detrimental effects of RTV at concentrations achieved in plasma with a soy diet. As the use of HAART regimens in HIV-infected patients becomes more prevalent, vascular complications continue to be seen, resulting from HIV infection or side effects of antiviral drugs. Also, cardiovascular disease in general is on the rise because of an aging population and high-fat western diet. The data from this study raise the possibility of new therapeutic strategies using soy for cardiovascular protection.
Acknowledgments
RTV was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. C.C., X.W., P.K., P.H.L., Q.Y., and C. Chen designed research; C.C. and X.W. conducted research; C.C., X.W., P.H.L., Q.Y., and C. Chen. analyzed data; C.C., X.W., S.M.W., Q.Y., and C. Chen wrote the paper. C. Chen had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by research grants from the NIH (no. HL076345 to P. H. Lin and nos. HL65916 and HL72716 to C. Chen) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX.
Author disclosures: C. Cheng, X. Wang, S. M. Weakley, P. Kougias, P. H. Lin, Q. Yao, and C. Chen, no conflicts of interest.
Abbreviations used: DHE, dihydroethidium; eNOS, Endothelial nitric oxide synthase; ER, estrogen receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HAART, highly active antiretroviral therapy; HPAEC, human pulmonary artery endothelial cell; PI, HIV protease inhibitor; ROS, reactive oxygen species; RTV, ritonavir; SNP, sodium nitroprusside.
References
- 1.Khunnawat C, Mukerji S, Havlichek D Jr, Touma R, Abela GS. Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol. 2008;102:635–42. [DOI] [PubMed] [Google Scholar]
- 2.Barbaro G. Reviewing the cardiovascular complications of HIV infection after the introduction of highly active antiretroviral therapy. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:337–43. [DOI] [PubMed] [Google Scholar]
- 3.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–71. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–13. [DOI] [PubMed] [Google Scholar]
- 5.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22:S35–40. [DOI] [PubMed] [Google Scholar]
- 6.Barbarinia G, Barbaro G. Incidence of the involvement of the cardiovascular system in HIV infection. AIDS. 2003;17:S46–50. [PubMed] [Google Scholar]
- 7.Chai H, Yang H, Yan S, Li M, Lin PH, Lumsden AB, Yao Q, Chen C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–9. [DOI] [PubMed] [Google Scholar]
- 8.Conklin BS, Fu W, Lin PH, Lumsden AB, Yao Q, Chen C. HIV protease inhibitor ritonavir decreases endothelium-dependent vaso-relaxation and increases superoxide in porcine arteries. Cardiovasc Res. 2004;63:168–75. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setchell KDR, Adlercreutz H. Mammalian lignans and phytoestrogens. Recent studies on their formation, metabolism and biological role in health and diseases. In: Rowland IR, editor. Role of the gut flora in toxicity and cancer. London: Academic Press; 1988. p. 315–45.
- 11.Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:S1390–3. [DOI] [PubMed] [Google Scholar]
- 12.Tikkanen MJ, Wähälä K, Ojala S, Vihma V, Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci USA. 1998;95:3106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestel PJ, Yamashita T, Sasahara T, Pomeroy S, Dart A, Komesaroff P, Owen A, Abbey M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:3392–8. [DOI] [PubMed] [Google Scholar]
- 14.Tikkanen MJ, Adlercreutz H. Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease prevention? Biochem Pharmacol. 2000;60:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol. 2008;58:1221–7. [DOI] [PubMed] [Google Scholar]
- 16.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–9. [DOI] [PubMed] [Google Scholar]
- 17.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J Nutr. 2003;133:2262–7. [DOI] [PubMed] [Google Scholar]
- 18.Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37:423–38. [DOI] [PubMed] [Google Scholar]
- 19.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–5. [DOI] [PubMed] [Google Scholar]
- 20.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40–3. [DOI] [PubMed] [Google Scholar]
- 21.Liew R, Williams JK, Collins P, MacLeod KT. Soy-derived isoflavones exert opposing actions on guinea pig ventricular myocytes. J Pharmacol Exp Ther. 2003;304:985–93. [DOI] [PubMed] [Google Scholar]
- 22.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–63. [DOI] [PubMed] [Google Scholar]
- 23.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 24.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–10. [DOI] [PubMed] [Google Scholar]
- 25.Nagata C, Takatsuka N, Kurisu Y, Shimizu H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J Nutr. 1998;128:209–13. [DOI] [PubMed] [Google Scholar]
- 26.Chin-Dusting JP, Fisher LJ, Lewis TV, Piekarska A, Nestel PJ, Husband A. The vascular activity of some isoflavone metabolites: implications for a cardioprotective role. Br J Pharmacol. 2001;133:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DF. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:II11–7. [PubMed] [Google Scholar]
- 29.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. [DOI] [PubMed] [Google Scholar]
- 30.Rufer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54:2926–31. [DOI] [PubMed] [Google Scholar]
- 31.Dhadwal AK, Wang X, Annambhotla S, Lin PH, Yao Q, Chen C. Capsaicin blocks HIV protease inhibitor ritonavir-induced vascular dysfunction in porcine pulmonary arteries. Med Sci Monit. 2009;15:BR1–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Chai H, Yan S, Lin P, Lumsden AB, Yao Q, Chen C. Curcumin blocks HIV protease inhibitor ritonavir-induced vascular dysfunction in porcine coronary arteries. J Am Coll Surg. 2005;200:820–30. [DOI] [PubMed] [Google Scholar]
- 33.Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2965–71. [DOI] [PubMed] [Google Scholar]
- 34.Spencer TA, Chai H, Fu W, Ramaswami G, Cox MW, Conklin BS, Lin PH, Lumsden AB, Yao Q, et al. Estrogen blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Surg Res. 2004;118:83–90. [DOI] [PubMed] [Google Scholar]
- 35.McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels. Stroke. 2002;33:1685–91. [DOI] [PubMed] [Google Scholar]
- 36.Kawano H, Motoyama T, Hirai N, Yoshimura T, Kugiyama K, Ogawa H, Okamura H, Yasue H. Effect of medroxyprogesterone acetate plus estradiol on endothelium-dependent vasodilation in postmenopausal women. Am J Cardiol. 2001;87:238–40. [DOI] [PubMed] [Google Scholar]