Abstract
Energy restriction decreases bone mineral density (BMD), and epidemiological studies suggest that the risk of weight loss-induced bone loss is greater in lean than in heavier individuals. Our goal in this study was to determine how bone density and geometry respond to energy restriction in mature obese rats compared with lean rats. At 6 mo of age, 36 diet-induced obese and lean female Sprague-Dawley rats were allocated to control (CTL; ad libitum; n = 18) and energy-restricted (EnR; 40% restriction; n = 18) diets. After 10 wk of dietary intervention, obese EnR rats lost more weight (−61 ± 14 g) than lean EnR rats (−91 ± 34 g) (P < 0.02), whereas body weight did not change significantly in the 2 CTL groups (14 ± 23 g). Only the lean EnR (and not obese EnR) rats showed lower BMD compared with CTL rats at the tibia, distal, and proximal femur and femoral neck, and trabecular bone volume (P < 0.05). Serum estradiol declined in lean EnR rats compared with baseline (P < 0.05) but not in the obese EnR rats. In addition, the final serum 25-hydroxyvitamin D (25OHD) concentration was higher (P < 0.05) in obese than in lean EnR rats. Serum parathyroid hormone decreased (P < 0.05) from baseline to final in lean and obese CTL, but not EnR rats. These data support the hypothesis that energy restriction in lean rats compared with obese rats is more detrimental to bone, and it is possible that the greater decline in estrogen and lower levels of 25OHD contribute to this effect.
Introduction
Weight loss results in a reduction in bone mass in humans (1–5) and animal models (6–8). In general, body weight has been shown to be a good predictor of bone mineral density (BMD)7 (9–11). Several mechanisms have been proposed for the loss of BMD after body weight reduction, including reduced mechanical loading, altered hormone levels, and dietary factors such as reduced calcium and energy intake (12). Some individuals may be more resistant to bone loss due to weight reduction than others. For example, some studies suggest that younger adults do not have bone loss with moderate weight reduction when micronutrient intake is constant (13,14), and greater bone loss has also been shown in older (10 mo) compared with younger (3 mo) rats that were exposed to 9 wk of energy restriction (7).
Most studies show 1–2% bone loss with 10% weight loss (12). Observational studies show that bone loss may be more substantial in leaner subjects who lose weight (15–17). This may be due to a variety of factors, such as reduced weight bearing, lower estrogen levels, greater frailty, or reduced intestinal calcium absorption (12,18). Understanding how bone is influenced by energy restriction when initial body weight differs is important, because overweight individuals are being told to lose weight to reduce the comorbidities associated with adiposity (19), and there is also a renewed interest in weight loss even in normal-weight populations due to its recent association with longevity (20). To our knowledge, no previous prospective study has addressed how bone is influenced by weight loss when initial body weight differs. In this study, we used an obese and lean mature rodent model to determine how energy restriction, with the recommended intakes of micronutrients, influences bone variables when initial body weight differs. In addition, bone-regulating hormones that may also be influenced by adiposity (12), including serum estradiol (E2), parathyroid hormone (PTH), and 25-hydroxyvitamin D (25OHD), were examined in response to energy restriction in lean and obese rats.
Materials and Methods
Forty-two female Sprague-Dawley rats were obtained from Taconic Farms for this study. Rats were kept in hanging wire cages and exposed to 12-h-light and -dark cycles. The rats were allowed free access to water and were assigned to 1 of 2 diets. Initially, 2-mo-old rats consumed a high-fat diet ad libitum for 2 wk to determine those rats most responsive to it. The 24 rats with the greatest body weight gain during these 2 wk were assigned to the obese group and the remaining diet-resistant rats (n = 18) were assigned to the lean group. Rats were maintained on a purified diet (AIN-93G; 16% fat) or matching high-fat diet (47% fat) for 12 wk until they were 6 mo old, which is considered the age of skeletal maturity (18,21). A power analysis was conducted on mature or aged rats prior to the experiment (7), which showed that 6–8 rats/group were necessary to determine a minimum detectable BMD difference (P < 0.05) at the femur of at least 0.020 g/cm2 and a power of 80% as a result of energy restriction. At 6 mo of age, the rats were obese or lean and switched to the AIN-93M diet for ∼1 wk before baseline blood draws. At 6 mo, each body size group (lean and obese) was divided into 2 weight-matched groups, which were assigned to either ad libitum intake or the 40% energy-restricted diet (EnR) for 10 wk (Supplemental Fig. 1). Weight matching was done so that each rat in the lean group assigned to EnR had a weight-matched lean CTL rat and each EnR obese rat had a weight-matched obese CTL rat. Energy intake was determined by pair feeding with the weight-matched CTL. The rats were weighed on a weekly basis using a XT top-loading balance scale. At the end of the 10-wk ad libitum or EnR feeding period, the 36-wk-old rats were anesthetized by CO2 exposure and killed by decapitation. To control for the effect of the estrus cycle on hormones measured in blood, the cytology of vaginal smears was evaluated daily, according to Salas-Valdes (22), and blood was collected on the day of estrus. If rats did not present the specific estrus smear characteristics (a heavy coarse consistency of the fluid and cornified epithelial cells), hormones were not evaluated. Blood was drawn from the tail vein and serum was separated by centrifugation and frozen at –70°C until determination of hormones. The weight-bearing long bones (femur and tibia) of each rat were removed, cleaned of soft tissue, wrapped in saline-soaked gauze, and stored at –70°C until analysis for bone density and chemical composition. All animal procedures were evaluated and approved by the Rutgers University Institutional Review Board for the Use and Care of Animals.
Diets.
At 6 mo of age, lean and obese rats were weight-matched (within each group) and then randomly assigned to either ad libitum food intake [control (CTL)] (AIN 93-M) (21) or a 40% EnR diet (Research Diets) as described previously (18). There were a total of 4 treatment groups: lean CTL, lean EnR, obese CTL, and obese EnR. The CTL diet was composed of 75.6% carbohydrate, 14.9% protein, and 9.5% fat, whereas the EnR diet was composed of 59.1% carbohydrate, 25.0% protein, and 15.9% fat. These percentages were calculated so that upon pair-feeding between EnR and CTL rats (with a different amount of food consumed by each group), daily intakes of protein, fat, fiber, vitamins, and minerals were the same in both CTL and EnR groups (Supplemental Table 1). For example, the CTL diets had 5.0 and 3.0 mg/g diet for Ca and P, and the EnR diet had 8.1 and 4.9 mg/g for Ca and P, respectively, so that with a 40% restriction, intake did not significantly differ between the treatment groups. In addition, the purified diets for these experiments were designed to be sufficient in all nutrients, including Ca (0.5%) and P (0.3%).
Hormones.
Concentrations of E2 and 25OHD were measured in serum by RIA (double antibody, DPC for E2 and DiaSorin for 25OHD). Serum intact PTH levels were measured in serum by rat-specific RIA (Immutopics). The intra- and inter-assay CV for E2, 25OHD, and PTH were ≤5.5, 12.5, and 8.9%, respectively. Serum hormone levels were assessed at baseline and after 10 wk of consuming CTL or EnR diets.
Bone composition.
Proteoglycan content was estimated by a quantitative determination of total sulfated glycosaminoglycans. Whole femurs were demineralized in 1.7 mol/L glacial acetic acid for 3 d (23). The demineralized tissue was solubilized by papain digestion at 60°C for 18 h. Glycosaminoglycans were analyzed using a binding assay with 1,9 dimethylmethylene blue, chondroitin-6-sulfate standard (ICN Biochemicals), and spectrophotometry at a dual wavelength of 540 and 595 nm.
Hydroxyproline content was analyzed after hydrolysis in 6 mol/L HCl at 110°C for 16 h, drying in a desiccator, and diluting with assay buffer. The solution was centrifuged for 15 min at 1000 × g and the supernatant mixed thoroughly at a 1:2:1 ratio with Chloramine-T and dimethyl-aminobenzaldehyde and then incubated at 60°C for 15 min. Hydroxyproline concentration was measured by spectrophotometry at 550 nm.
Bones were digested using papain buffer containing 0.05 mol/L EDTA and 0.1 mol/L sodium acetate, cysteine hydrochloride (10 mmol cysteine hydrochloride/L papain buffer), and papain enzyme (Sigma Chemical), and incubated at 60°C for 18 h. Digested whole bone was measured for pyridinoline (PYD) and deoxypyridinoline (DPD) concentrations by reverse phase HPLC after subjecting hydrolyzed samples to a prefractionation procedure. Peaks were detected by fluorescence and quantitated by external standards and the CV for PYD and DPD were <8 and <10%, respectively.
Bone X-ray measurements.
BMD was evaluated using dual energy X-ray absorptiometry (GE-Lunar densitometer, PIXImus; software version 2.10.41). Excised bone measurements were obtained by placing the tibia or femur on a Delrin block. The total tibia or femur was scanned and regions of interest included the femoral neck (Supplemental Fig. 2A), entire proximal femur, and distal femur set 20% from distal end (Supplemental Fig. 2B) and according to others (24). The CV for 3 repeated BMD measurements for 4 samples was measured. The CV for whole tibia and femur (whole, neck, distal, and proximal) BMD were 2.2, 1.4, 3.7, 2.4, and 2.0%, respectively. In addition, radiographic images were measured by high density radiograph using a Faxitron MX-20 DC4 (Faxitron X-ray) with an energy of 26 kV, an exposure time of 10 s, and a resolution of 20 linear pixels/mm. Each image included an aluminum alloy step density standard for intensity calibration to estimate trabecular bone and medial and lateral cortical bone density (radiographic intensity). The distal femur was measured using a region that spanned the length of bone within 20% of the distal and proximal ends as the region of interest. Regions of interest were manually adjusted to analyze each site of the bone individually. A single technician performed all the analysis and was unaware of the group designation. Each individual image was calibrated using the density standard steps with intensity units ranging from 1 to 5. All measurements were conducted using Image J software (NIH).
Microcomputed tomography.
In a subset of rats (n = 15), femur bones were embedded in polymethylmethacrylate and then analyzed by microcomputerized tomography (micro-CT; Enhanced Vision Systems) to determine volumetric BMD (vBMD) and geometric variables (trabecular thickness, separation, and number). Femurs were scanned at a resolution of 19 μm voxel resolution and the threshold was determined with GEMS Microview's (GE Medical Systems) auto-thresholding function. In each scan, a calibration phantom including air, water, and a mineral standard material (SB3, Gammex RMI) enabled calibration and conversion of X-ray attenuation such that mineral density was proportional to grayscale values in Hounsfield units. Digital reconstruction of ray projection to CT volume data were accomplished with a modified Parker algorithm. After 3-dimensional reconstruction of the femur volumes, the trabecular region of the femoral neck was analyzed. vBMD, bone volume/tissue volume fraction, trabecular thickness, trabecular separation, and the number of trabeculae were measured in the trabecular bone region of the femoral neck with the region of interest defined as an elliptical cylinder with dimensions 0.45 mm × 0.7 mm × 0.8 mm. Cylinder placement was determined by making measurements beginning 0.49 mm below the inferior edge of the medial femoral head to ensure exclusion of cortical bone from these measurements. The trabecular bone volume fraction was calculated as the number of bone voxels divided by the total number of voxels within the region of interest. Trabecular thickness was determined using the direct thickness measure (25). With this method, the largest sphere that can be contained within each point in a trabecula is determined and reported as the direct thickness. Faxitron and micro-CT measurements were performed in the Musculoskeletal Regeneration and Repair Core Facility at the Hospital for Special Surgery, NY, NY.
Statistical analysis.
Bone variables were analyzed by 2-way ANOVA after the intervention examining the effect of size (obese and lean groups), diet (EnR and CTL), and their interaction. Weight and endocrine factors were also analyzed for their final values and absolute change using 2-way ANOVA (size × diet). When the F test for the model was significant (P < 0.05), further analysis by Tukey's post hoc comparison tests was conducted. Within the same group of rats, a paired student's t test was also used to compare baseline and final values for hormones and body weight. In addition, we calculated the percent difference between weight-matched EnR and CTL rats within a body size group and these values were then compared for lean and obese rats using 1-way ANOVA. Data are means ± SD unless otherwise indicated. All analyses were conducted using the SAS statistical package (SAS Institute, version 9.1.3)
Results
Food intake and body weight.
During the 12-wk growth period of this study, obese rats consumed more energy (234 ± 38 kJ/d) than lean rats (201 ± 38 kJ/d) (P < 0.05). Throughout the experiment, those rats allocated to the obese group were heavier than their lean counterparts (P < 0.05). Over the course of the study, 4 obese and 2 lean rats were eliminated due to sudden death, low food intake, and large unexpected weight fluctuations, leaving 36 rats for the final analysis. Thirty-two rats had vaginal smears characteristic of estrus at baseline and 2 of these rats did not have blood drawn successfully. Hence, serum metabolites are reported for 30 rats at baseline and final measurements (n = 8/group in the lean and 7/group in the obese rats).
During the energy restriction period, CTL rats consumed 40% more energy than EnR rats. Food intake was 15 ± 2 g/d in the CTL rats compared with 9 ± 1 g/d in the EnR rats; intake did not differ between the obese and lean groups.
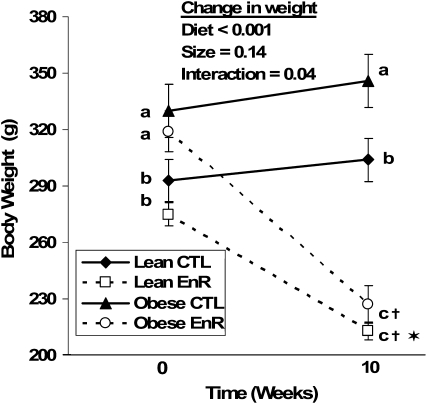
At the time of diet allocation to EnR or CTL diets (baseline), lean rats weighed less (P ≤ 0.001) than the obese rats (Fig. 1). Energy restriction resulted in weight loss and lower final weights (P < 0.001) in both lean and obese rats (Fig. 1). The lean EnR rats lost less (P < 0.02) weight (−61 ± 14 g) than the obese EnR rats (−91 ± 34 g) and the percent weight lost in the lean EnR rats (22 ± 4%) tended (P < 0.07) to be less than in the obese EnR rats (28 ± 9%). Compared with their weight-matched CTL (Fig. 2), the magnitude of weight lost by lean EnR rats was less (P < 0.05) than the weight lost by obese EnR rats.
FIGURE 1 .
Body weight of lean and obese rats that consumed food ad libitum (CTL) or were 40% energy-restricted (EnR) for 10 wk. Data points are the mean ± SEM, n = 9. Within a time point (baseline or final), means without a common letter differ, P < 0.05. †Change from baseline to final differs from CTL, P < 0.001; *change from baseline to final differs from obese EnR, P < 0.02.
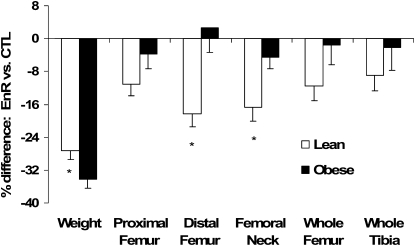
FIGURE 2 .
Percent difference for weight and BMD of the femur (whole, proximal, distal, and neck) and whole tibia of EnR rats compared with their weight-matched CTL in the lean and obese groups. Bars are mean ± SEM, n = 9. *Different from obese, P < 0.05
BMD and geometry.
EnR (diet effect) resulted in a lower BMD at most sites compared with CTL rats (Table 1; P < 0.05), whereas only lean EnR rats had lower BMD compared with their CTL group at the distal femur (interaction; P < 0.02). In addition, post hoc testing showed that EnR in the lean rats (not obese) compared with their CTL had lower BMD at the tibia, distal femur, proximal femur, femoral neck, and the trabecular region of the femoral neck (P < 0.05). There were no significant effects of diet, size, or their interaction on cortical bone. The bone response to EnR was compared with their weight-matched CTL in Figure 2 (i.e. each rat in the lean group assigned to EnR had a weight-matched lean CTL rat, as described in Methods). Energy restriction was associated with a lower BMD in lean but not obese rats compared with their respective CTL (P < 0.05) at the distal femur (−18 ± 3% in lean and +3 ± 6% in obese rats) and femoral neck (−17 ± 3% in lean and −5 ± 4% in obese rats). Micro-CT results were consistent with these findings and showed lower values at the femoral neck for vBMD and bone volume/total volume due to energy restriction in the lean EnR compared with lean CTL rats (P ≤ 0.05; Table 2). In addition, trabecular thickness was lower in all the EnR compared with CTL rats (diet effect; P < 0.05). Other geometric variables assessed by micro-CT (trabecular number and space) were not affected by diet, size, or their interaction (Table 2).
TABLE 1.
BMD of the tibia and femur sites in lean and obese rats that consumed food ad libitum or were 40% energy-restricted for 10 wk1
| Lean |
Obese |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bone site | CTL | EnR | CTL | EnR | Diet | Size | Diet × size | |||
| g/cm2 | ||||||||||
| Whole tibia | 0.168 ± 0.008a | 0.154 ± 0.006b | 0.163 ± 0.007ab | 0.162 ± 0.013ab | 0.031 | —2 | 0.073 | |||
| Whole femur | 0.208 ± 0.022 | 0.188 ± 0.015 | 0.211 ± 0.007 | 0.207 ± 0.017 | 0.096 | 0.072 | — | |||
| Distal femur | 0.322 ± 0.021a | 0.262 ± 0.035b | 0.303 ± 0.017ab | 0.312 ± 0.049a | 0.063 | — | 0.015 | |||
| Proximal femur | 0.186 ± 0.012a | 0.167 ± 0.014b | 0.180 ± 0.004ab | 0.174 ± 0.012b | 0.008 | — | — | |||
| FN3 | 0.254 ± 0.032a | 0.218 ± 0.025b | 0.267 ± 0.016a | 0.249 ± 0.021ab | 0.009 | 0.027 | — | |||
| Trabecular FN3 | 2.51 ± 0.11a | 2.15 ± 0.25b | 2.40 ± 0.29ab | 2.24 ± 0.27b | 0.009 | — | — | |||
| Medial cortical FN3 | 2.41 ± 0.15 | 2.31 ± 0.20 | 2.45 ± 0.14 | 2.34 ± 0.17 | — | — | — | |||
| Lateral cortical FN3 |
2.03 ± 0.24 |
1.88 ± 0.44 |
2.24 ± 0.37 |
2.06 ± 0.48 |
— |
— |
— |
|||
Values are means ± SD, n = 9, unless otherwise noted. Means in a row with superscripts without a common letter differ, P < 0.05.
P > 0.1.
FN, Femoral neck. Measured by Faxitron using a radiographic intensity score of 1 to 3 to estimate BMD, n = 7.
TABLE 2.
vBMD, bone volume fraction, and trabecular thickness, number, and separation of the femoral neck in lean and obese rats that consumed food ad libitum or were 40% energy-restricted for 10 wk1
| Lean |
Obese |
P-value |
|||||
|---|---|---|---|---|---|---|---|
| Bone variable | CTL | EnR | CTL | EnR | Diet | Size | Diet × size |
| vBMD, mg/cm3 | 479.6 ± 45.9a | 329.8 ± 23.6b | 434.9 ± 45.5a | 441.7 ± 30.7a | 0.002 | 0.049 | 0.050 |
| BV/TV, % | 58.3 ± 5.4a | 38.0 ± 6.9b | 55.1 ± 6.8a | 51.4 ± 6.1a | 0.004 | —2 | 0.028 |
| Tb.Th, mm | 0.132 ± 0.025 | 0.092 ± 0.021 | 0.133 ± 0.021 | 0.113 ± 0.022 | 0.026 | — | — |
| Tb.N, mm−1 | 4.224 ± 0.539 | 4.499 ± 0.086 | 4.254 ± 0.239 | 4.556 ± 0.390 | — | — | — |
| Tb.S, mm |
0.100 ± 0.015 |
0.124 ± 0.029 |
0.110 ± 0.018 |
0.107 ± 0.010 |
— |
— |
— |
Values are means ± SD n = 3–4. Means in a row with superscripts without a common letter differ, P < 0.05.
P > 0.1.
BV/TV, bone volume/total volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.S, trabecular separation.
Bone composition.
EnR did not significantly affect the bone content of hydroxyproline, DPD, and glycosaminoglycan. PYD crosslinks tended (P = 0.07) to be lower in EnR lean (10.6 ± 2.8 ng/mg) and obese (11.8 ± 1.6 ng/mg) rats compared with CTL lean (13.7 ± 4.8 ng/mg) and obese (14.0 ± 5.8 ng/mg) rats.
Serum hormones.
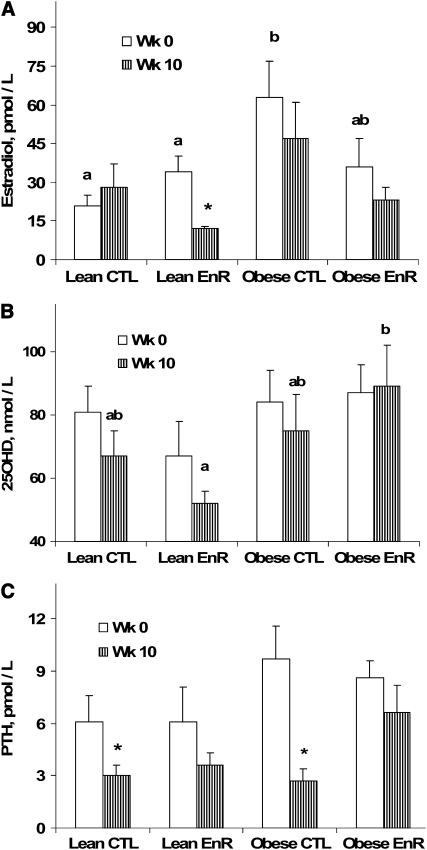
Baseline E2 was higher (P < 0.05) in the obese compared with lean rats (size effect) and post hoc analysis showed higher values in obese CTL compared with lean CTL rats (Fig. 3A). After 10 wk of energy restriction, serum E2 decreased in the lean EnR group from baseline to final (P < 0.02) but not in the lean CTL or either group of obese rats (Fig. 3A). There were no significant effects of diet, size, or their interaction on the absolute change for serum E2, 25OHD, or PTH. Final concentrations of serum 25OHD showed that there were higher levels in obese than lean rats (size effect; P < 0.02). Post hoc testing showed higher (P < 0.01) concentrations of 25OHD in obese EnR than lean EnR rats (Fig. 3B) with a trend (P < 0.09) compared with CTL lean and obese rats. Changes from baseline to final for serum 25OHD were not significant. Serum PTH concentration decreased (P < 0.05) in both lean and obese CTL groups from baseline to final values, but not in the EnR groups (Fig. 3C).
FIGURE 3 .
Serum E2(A), 25OHD (B), and PTH (C) for lean CTL (n = 8) and lean EnR (n = 8), and obese CTL (n = 7) and obese EnR (n = 7) rats at wk 0 and after 10 wk of dietary intervention. Bars are mean ± SEM. Within a time point (baseline or final), means without a common letter differ, P < 0.05. *Different from baseline (same group), P < 0.05.
Discussion
Epidemiological studies show that older women and men who lose weight have an increased risk of fracture (9,26,27), and that bone loss is greater in women with a history of low body weight (9,10,16). These studies suggest that initial body weight is important in predicting how bone responds to either aging or weight reduction. Importantly, an observational study showed that bone loss is up to 5 times greater in thinner elderly women who lose weight than in those who maintain or gain weight (15). In that particular case (15), it was not possible to differentiate between voluntary and involuntary weight loss that may accelerate bone loss, such as a disease-specific condition, but a previous study (27) suggested that a similar bone loss occurs irrespective of intention to lose weight. In addition, observational studies often do not control for differences in nutrient intake or other potential mediators important in the regulation of bone. In this study, we addressed how initial body weight and BMD and content in both trabecular- and cortical-rich regions respond to energy restriction and evaluated whether hormonal changes could explain any differential bone loss between lean or obese rats. Not surprisingly, we found that energy restriction resulted in bone loss despite providing adequate Ca and other nutrients, as has been shown previously (7). However, we have found that initial body weight is a strong predictor for the bone response to weight reduction, especially affecting trabecular-rich regions and that the greater decline in estrogen and lower levels of 25OHD may be factors increasing bone loss in leaner rats.
Complete food deprivation and semistarvation in rats reduces bone formation (28), overall bone turnover, and bone mass (6) and attenuates the normal age-associated increase in serum calcitonin (8). Additionally, the effects of 30% energy restriction for 1 mo in mature rats had a similar reduction in bone formation rate compared with a nonweight-bearing model due to hindlimb suspension (29). Although some studies (6,8,28) were also deficient in micronutrients, other studies have supplemented with micronutrients (7) and found that with adequate calcium, energy restriction resulted in bone loss in old (10 mo) but not younger (3 mo) rats. This is consistent with human weight loss trials showing that despite adequate Ca intake during energy restriction, bone loss occurs in older individuals (1,2) but not in young healthy individuals (12–14). Hence, adequate Ca during weight reduction has been shown to be important in attenuating bone loss, but cannot prevent loss, suggesting that other mechanisms are also important. Chronic food deprivation, beginning at ∼4 mo of age, with adequate mineral supplementation reduced bone mass in aged rats, but did not affect material properties (30). These authors suggest that because of greater bone quantity and maintenance of structural properties when normalized to body weight, chronic energy restriction would be beneficial to bone. Nevertheless, we know from clinical trials that women with a history of low body weight are at greater risk of fracture (9–11), so although correcting BMD for body weight is an important analysis to understand if bone loss is proportional to weight loss (31), it is not a good method for quantifying fracture risk. This higher risk of fracture in leaner individuals may be due to poor bone quality, greater frailty, or less soft tissue padding. In the current study, mature female rats consuming adequate Ca and other micronutrients during energy restriction had reduced tibia and femur BMD only in the absence of obesity. The effect on biomechanical properties to estimate fracture risk should be addressed in a future study.
We found a 4.5% lower BMD at the femoral neck with a 28% weight loss in obese EnR rats compared with CTL, which is consistent with human studies showing a 1–2% decrease in BMD with 10% weight loss (12). In contrast, the lean EnR rats that lost 22% body weight showed lower than anticipated BMD (about −15%) compared with their CTL. Hence, our observations suggest that weight reduction in an already-lean individual may have a more serious impact on bone. Although the rate of weight loss in this study is faster than moderate weight loss diets, it would be equivalent to very low-energy diets (2–3% weight loss/wk). The bone results in rats are relatively consistent with the human weight reduction-induced bone loss (31,32). For example, weight loss of 16.7% over 10 wk in obese adults results in bone loss of 2.5% (total body) (32), and based on our findings for the obese rats, a 16.7% weight loss would result in 2.7% bone loss. The weight loss in severely obese patients due to gastric by-pass results in a greater proportional bone loss (i.e. 9% BMD loss at the femoral neck due to 34% weight loss) but could be attributed to the malabsorption associated with this procedure. Nevertheless, it is still less than the bone loss in the lean EnR rats. In addition, these similar findings of bone loss in ex vivo rodent samples, as in human models, argue against the concern that measurement artifacts in obesity and during weight loss overestimate bone loss (33). Overall, these data suggest that the obese, energy-restricted rat is a good model for bone loss. The absence of exercise in the lean rat in this study makes this model different from healthy normal-weight individuals who lose weight due to both energy restriction and greater physical activity (i.e. military women, anorexia nervosa). The bone loss due to weight reduction in the lean rats might be compared with involuntary weight loss in sedentary individuals or hospitalized patients.
Mechanisms influencing a differential response to energy restriction in those who are initially obese compared with lean can be multiple and possibly related to adipose tissue production of hormones. From clinical trials, we know that serum estrogen levels are important for estimating bone loss associated with weight reduction in women (4,12) and men (34). We found a significant decrease in E2 levels associated with weight loss in lean rats without any change in E2 in the obese, energy-restricted rats. A greater extra-ovarian synthesis of E2 from adipose tissue in the energy-restricted obese than lean rats may have contributed to their higher BMD. In addition, the lean rats may have also experienced compromised ovarian E2 production during the 10 wk of semistarvation (35); however, in the current study, we have no evidence that cycling differed in the lean compared with obese, energy-restricted rats. We found that energy restriction also resulted in a lower level of serum 25OHD in lean compared with obese rats and tended to decrease more in all the lean rats. This could be due to higher vitamin D stores associated with obesity and the release of those depots during food deprivation and fat loss (36,37). Energy restriction tended to prevent a reduction in PTH in both lean and obese rats. This finding is consistent with clinical studies that showed a rise in serum PTH levels during energy restriction (38), which may occur in response to reduced Ca absorption observed in postmenopausal women (39) and in mature rats (18). In addition, because Ca absorption is both a vitamin D- and estrogen-dependent process (40), this could partially explain the greater decrease in BMD in the leaner rats. Overall, the greater decline in serum E2 and lower levels of serum 25OHD in lean than obese rats suggest a potential mechanism regulating the greater bone loss in lean compared with heavier women (15,16). Other hormones and cytokines may be important in regulating bone in lean and obese individuals during energy restriction but were not measured in this study due to limited serum. For example, leptin levels are higher and adiponectin levels are lower in the obese and levels are altered with a decrease in adipose tissue (29,41). In addition, although corticosterone levels did not differ between lean and obese rats in this model (18), there is a rise with energy restriction. Evidence that body fat can regulate bone mass through pathways that are independent of load-bearing is poorly understood and should be addressed in future studies.
In this study, we showed that energy restriction results in bone loss at most sites, but trabecular bone appears to be especially vulnerable. Loss of trabecular bone is not surprising, because this has been found in many conditions, including ovariectomy (42), aging (43), and bone loss induced by gastrectomy (44), although 1 study showed greater cortical bone loss with energy restriction in mice (45). Evidence from our clinical trials (1) and others (4) suggest that trabecular-rich bone sites may be more susceptible to weight reduction in postmenopausal women, suggesting that the level of estrogen during energy restriction is important. Our mature rats were not estrogen deficient, but the change in levels due to energy restriction, especially in the lean group, may have contributed to the lower BMD.
In summary, these studies support the hypothesis that restricting energy consumption in the lean rats compared with obese rats was more detrimental to bone sites rich in trabecular bone, such as the femoral neck. Our results suggest that a greater decline in serum estrogen or lower levels of 25OHD may contribute to the greater loss in bone in lean rats than obese rats due to weight reduction. More studies are necessary to elucidate the mechanisms involved and the preventive measures that need to be taken to avoid the detrimental effects of dieting on bone, particularly in those individuals at greater risk of osteoporosis.
Supplementary Material
Acknowledgments
We thank Dr. Y. Schlussel for the statistical advice on the revisions of this manuscript and D. Sukumar for her editorial assistance. J.H., M.C., and H.A.S. conducted research (hands-on conduct of the experiments and data collection). N.C. conducted research and expertise in the interpretation. S.S. and M.C. designed research. J.H., M.C., and S.S. wrote the paper and S.S. provided oversight for all aspects of the study and had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by NIH-AG12161 to S.A.S. and NIH-AR046121 to N.L.P.
Author disclosures: J. Hawkins, M. Cifuentes, N. L. Pleshko, H. Ambia-Sobhan, and S. A. Shapses, no conflicts of interest.
Supplemental Figures 1 and 2 and Table 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BMD, bone mineral density; CTL, control diet; DPD, deoxypyridinoline; E2, estradiol; EnR, energy restricted; micro-CT, microcomputed tomography; 25OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone; PYD, pyridinoline; vBMD, volumetric bone mineral density.
References
- 1.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/d calcium intake. J Bone Miner Res. 2005;20:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN Jr, Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–50. [DOI] [PubMed] [Google Scholar]
- 3.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab. 2005;90:52–9. [DOI] [PubMed] [Google Scholar]
- 5.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–7. [DOI] [PubMed] [Google Scholar]
- 6.Shires R, Aivioli LV, Bergfeld MA, Fallon MD, Slatopolsky E, Teitelbaum SL. Effects of semistarvation on skeletal homeostasis. Endocrinology. 1980;107:1530–5. [DOI] [PubMed] [Google Scholar]
- 7.Talbott SM, Cifuentes, Dunn MG, Shapses SA. Energy restriction reduces bone density and biomechanical properties in aged female rats. J Nutr. 2001;131:2382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalu DN, Hardin RR, Cockerham R, Yu BP, Norling BK, Egan JW. Lifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 rats. Mech Ageing Dev. 1984;26:103–12. [DOI] [PubMed] [Google Scholar]
- 9.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12:763–8. [DOI] [PubMed] [Google Scholar]
- 10.Finigan J, Greenfield DM, Blumsohn A, Hannon RA, Peel NF, Jiang G, Eastell R. Risk factors for vertebral and nonvertebral fracture over 10 years: a population-based study in women. J Bone Miner Res. 2008;23:75–85. [DOI] [PubMed] [Google Scholar]
- 11.Waugh EJ, Lam MA, Hawker GA, McGowan J, Papaioannou A, Cheung AM, Hodsman AB, Leslie WD, Siminoski K, et al.; Perimenopause BMD Guidelines Subcommittee of Osteoporosis Canada. Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos Int. 2009;20:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapses SA, Reidt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, Sherrell RM, Shapses SA. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr. 2007;85:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E; Pennington Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) Research Team. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13:1458–67. [DOI] [PubMed] [Google Scholar]
- 16.Ravn P, Cizza G, Bjarnason N, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early postmenopausal intervention cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–7. [DOI] [PubMed] [Google Scholar]
- 17.Grinspoon S, Herzog D, Kilbanski A. Mechanisms and treatment options for bone loss in anorexia nervosa. Psychopharmacol Bull. 1997;33:399–404. [PubMed] [Google Scholar]
- 18.Cifuentes M, Morano AB, Chowdhury HA, Shapses SA. Energy restriction reduces fractional calcium absorption in mature obese and lean rats. J Nutr. 2002;132:2660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NIH-N.H.L.B. Obesity education initiative expert panel. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6:S51–209. [PubMed] [Google Scholar]
- 20.Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–51. [DOI] [PubMed] [Google Scholar]
- 21.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 22.Salas-Valdes A. A quick and inexpensive staining method for vaginal smears. Arch Invest Med (Mex). 1979;10:147–50. [PubMed] [Google Scholar]
- 23.Ehrlich H, Koutsoukos PG, Demadis KD, Pokrovsky OS. Principles of demineralization: modern strategies for the isolation of organic frameworks. Micron. 2008;39:1062–91. [DOI] [PubMed] [Google Scholar]
- 24.Binkley N, Dahl DB, Engelke J, Kawahara-Baccus T, Krueger D, Colman RJ. Bone loss detection in rats using a mouse densitometer. J Bone Miner Res. 2003;18:370–5. [DOI] [PubMed] [Google Scholar]
- 25.Hiidebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. [DOI] [PubMed] [Google Scholar]
- 26.Meyer HE, Søgaard AJ, Falch JA, Jørgensen L, Emaus N. Weight change over three decades and the risk of osteoporosis in men: the Norwegian Epidemiological Osteoporosis Studies (NOREPOS). Am J Epidemiol. 2008;168:454–60. [DOI] [PubMed] [Google Scholar]
- 27.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR; Study of Osteoporotic Fractures Research Group. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–7. [DOI] [PubMed] [Google Scholar]
- 28.Ndiaye B, Cournot G, Pélissier MA, Debray OW, Lemonnier D. Rat serum osteocalcin concentration is decreased by restriction of energy intake. J Nutr. 1995;125:1283–90. [DOI] [PubMed] [Google Scholar]
- 29.Baek K, Barlow AA, Allen MR, Bloomfield SA. Food restriction and simulated microgravity: effects on bone and serum leptin. J Appl Physiol. 2008;104:1086–93. [DOI] [PubMed] [Google Scholar]
- 30.Westerbeek ZW, Hepple RT, Zernicke RF. Effects of aging and caloric restriction on bone structure and mechanical properties. J Gerontol A Biol Sci Med Sci. 2008;63:1131–6. [DOI] [PubMed] [Google Scholar]
- 31.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Compston JE, Laskey MA, Croucher PI, Coxon A, Kreitzman S. Effect of diet-induced weight loss on total body bone mass. Clin Sci (Lond). 1992;82:429–32. [DOI] [PubMed] [Google Scholar]
- 33.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8:31–8. [DOI] [PubMed] [Google Scholar]
- 34.Ensrud KE, Lewis CE, Lambert LC, Taylor BC, Fink HA, Barrett-Connor E, Cauley JA, Stefanick ML, Orwoll E. Osteoporotic Fractures in Men MrOS Study Research Group. Endogenous sex steroids, weight change and rates of hip bone loss in older men: the MrOS study. Osteoporos Int. 2006;17:1329–36. [DOI] [PubMed] [Google Scholar]
- 35.Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, et al. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2006;91:1683–90. [DOI] [PubMed] [Google Scholar]
- 36.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. [DOI] [PubMed] [Google Scholar]
- 37.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. [DOI] [PubMed] [Google Scholar]
- 38.Ricci TA, Heymsfield SB, Pierson RN, Stahl T, Chowdury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopuasal women. Am J Clin Nutr. 2001;73:347–52. [DOI] [PubMed] [Google Scholar]
- 39.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr. 2004;80:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, et al. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res. 2003;18:1725–36. [DOI] [PubMed] [Google Scholar]
- 41.Lieben L, Callewaert F, Bouillon R. Bone and metabolism: a complex crosstalk. Horm Res. 2009;71 Suppl 1:134–8. [DOI] [PubMed] [Google Scholar]
- 42.Tromp AM, Bravenboer N, Tanck E, Oostlander A, Holzmann PJ, Kostense PJ, Roos JC, Burger EH, Huiskes R, et al. Additional weight bearing during exercise and estrogen in the rat: the effect on bone mass, turnover, and structure. Calcif Tissue Int. 2006;79:404–15. [DOI] [PubMed] [Google Scholar]
- 43.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207. [DOI] [PubMed] [Google Scholar]
- 44.Surve VV, Andersson N, Lehto-Axtelius D, Håkanson R. Comparison of osteopenia after gastrectomy, ovariectomy and prednisolone treatment in the young female rat. Acta Orthop Scand. 2001;72:525–32. [DOI] [PubMed] [Google Scholar]
- 45.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.