Abstract
Recent findings have raised concern about possible associations of high selenium exposure with diabetes and hyperlipidemia in the US, a population with high selenium status. In the UK, a population with lower selenium status, there is little data on the association of selenium status with cardio-metabolic risk factors in the general population. We examined the association of plasma selenium concentration with blood lipids in a nationally representative sample of British adults. A cross-sectional study was conducted among 1042 white participants (aged 19–64 y) in the 2000–2001 UK National Diet and Nutrition Survey. Plasma selenium was measured by inductively coupled-plasma mass spectrometry. Total and HDL cholesterol were measured in nonfasting plasma samples. Mean plasma selenium concentration was 1.10 ± 0.19 μmol/L. The multivariate adjusted differences between the highest (≥1.20 μmol/L) and lowest (<0.98 μmol/L) quartiles of plasma selenium were 0.39 (95% CI 0.18, 0.60) mmol/L for total cholesterol, 0.38 (0.17, 0.59) for non-HDL cholesterol, and 0.01 (−0.05, 0.07) for HDL cholesterol. Higher plasma selenium (i.e., ≥1.20 μmol/L) was associated with increased total and non-HDL cholesterol levels but not with HDL in the UK adult population. These findings raise additional concern about potential adverse cardio-metabolic effects of high selenium status. Randomized and mechanistic evidence is necessary to assess causality and to evaluate the impact of this association on cardiovascular risk.
INTRODUCTION
The role of selenium in chronic disease prevention is the focus of a growing scientific debate and intense investigation (1,2). Selenium is a key component of glutathione peroxidase and of other selenoproteins involved in essential enzymatic functions, such as redox homeostasis, thyroid hormone metabolism, and reproduction (3,4). Whereas the primary emphasis of selenium research has been on evaluating the potential benefits of its antioxidant and anticancer effects (1–3), recent findings from observational studies and randomized clinical trials have suggested an association between moderate to high selenium exposure and adverse cardio-metabolic effects, at least in well-nourished populations (1,5–10). It is therefore of concern that in the UK and other Western countries the use of selenium supplements has increased considerably in recent years as a result of aggressive marketing, despite lack of definitive evidence on their efficacy for cancer and other chronic disease prevention (11–13).
Three independent studies have shown an association between high selenium status or selenium supplementation and increased diabetes risk (5–8). Furthermore, the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX)11 trial showed that long-term supplementation with a daily antioxidant capsule containing 100 μg of selenium, 120 mg of vitamin C, 30 mg of vitamin E, 6 mg of β-carotene, and 20 mg of zinc, adversely affected the lipid profile in a French population with suboptimal dietary selenium intake (9). In addition, a cross-sectional analysis from the US NHANES III found that high selenium status was associated with elevated serum lipids (10). There are, however, few data on the association between selenium status and cardio-metabolic risk factors in the UK general population. Our objective in this study, therefore, was to examine the association of plasma and RBC selenium concentrations and whole-blood glutathione peroxidase activity with blood lipids in a nationally representative sample of British adults, aged 19–64 y, who participated in the 2000–2001 National Diet and Nutrition Survey (NDNS).
METHODS
Study population.
The rationale, design, and methods of the survey have previously been described in detail (13–17). Briefly, the British NDNS is part of a series of cross-sectional surveys conducted at about 3-y intervals to provide a detailed quantitative assessment of nutritional and lifestyle characteristics of the UK general population. Between July 2000 and June 2001, adults aged 19 – 64 y living in private households and, for women, who were not pregnant or breast feeding at the time of the study were randomly selected from 152 areas within mainland Great Britain. The 12-mo fieldwork period was divided into 4 3-mo fieldwork waves to balance seasonal variations in eating behavior and food nutrient content.
Of 3704 potentially eligible participants identified for the study, 37% refused to participate and 2% could not be contacted. The general dietary interview, including socio-demographics and lifestyles, was thus completed by 2251 individuals (61% of the eligible sample). Although the aim was to achieve cooperation with all the components of the survey, the design allowed for partial participation in some components. Of the 1297 participants who provided blood samples, we excluded 68 nonwhites and the following numbers of participants because of missing data: 47 missing biomarkers of selenium status or whole-blood glutathione peroxidase activity; 17 missing a plasma lipid measurement; 20 missing BMI; 71 missing daily physical activity score; 12 missing alcohol intake; 18 missing income data, 1 missing data on educational level, and 1 outlier. The remaining 1042 participants (472 men, 570 women) were included in this analysis.
The potential for selection bias due to nonresponse in the NDNS had previously been evaluated in an independent study carried out by looking at a number of demographic and nutritional variables and their relationship to nonparticipation in the survey (18). Noncontacts and refusals were considered separately. That study concluded that there was no evidence to suggest serious nonresponse bias in the NDNS data (18).
Ethical approval for the survey was obtained from both a Multi-center Research Ethics Committee and from the National Health Service Local Research Ethics Committees covering each of the 152 sampled areas. All subjects gave written informed consent to participate in the study.
Study protocol.
All participants underwent an initial face-to-face interview by trained personnel to assess household characteristics, usual dietary behavior, smoking and drinking habits, general health status, dietary supplement use, socioeconomic characteristics, menopausal status, oral contraceptive use and hormone replacement therapy in women. Diet was further assessed by a 7-d weighed intake diary of all foods and drinks consumed in and out of the home. Duration, intensity, and frequency of physical activity in occupation and outside of work were collected over the same period as the dietary record to calculate a daily activity score as an indicator for energy expenditure.
Standing height, body weight, waist and hip circumferences were measured according to a standardized protocol. BMI was calculated by dividing weight in kilograms by height in meters squared. Blood pressure was measured 3 times at 1-min intervals with a Dinamap 8100 oscillometric monitor.
Laboratory assays.
Trained phlebotomists obtained blood samples by venepuncture. Participants were not asked to fast overnight. The analytical procedures to measure selenium status indices have been described in detail (19). Whole blood and plasma selenium concentrations were measured by inductively coupled plasma mass spectrometry (20). RBC selenium was calculated from whole blood and plasma selenium concentrations, together with the hematocrit (21).
Internal quality control sera were prepared by adding selenium to pools of bovine sera at 0, 0.40 and 1.60 μmol/L. The mean coefficient of variation of 5 different internal quality controls (which were included with every 10 duplicate test samples) was 5.3%, and the assay drift over the 3 mo required to analyze all the samples was <1.3%. Participation in quality assessment programs from the Centre du Toxicologie de Quebec and the Trace Element Quality Assurance Scheme (TEQAS), University of Surrey, provided external quality control. The performance in both external quality assurance schemes was excellent, with 85 and 100% of results within inner and outer target limits for median values for all participating laboratories.
Whole-blood glutathione peroxidase activity was measured with the method of Paglia and Valentine (22). Enzyme activity was measured in diluted whole blood and expressed in nmol·mg hemoglobin (Hb)−1·min−1. Quality assurance was achieved with aliquots of heparinized whole blood from the Cambridge Blood Transfusion Service.
Total cholesterol was measured by the oxidation of cholesterol (liberated by cholesterol esterase) by cholesterol oxidase to 7-hydroxy-cholesterol. The cholesterol assay was calibrated by use of the Roche human calibrator. HDL cholesterol was measured after precipitation of LDL and VLDL cholesterol with magnesium chloride plus phosphotungstic acid. The HDL assay was calibrated by the use of Roche P human calibrator. This precipitation methodology yields results very similar to those of ultracentrifugal separation, the reference method (23). Quality control procedures for the cholesterol assay comprised an internal procedure using heparinized human plasma from the Cambridge Blood Transfusion Service and a double strength Roche N sample. External quality control comprised National External Quality Assessment Scheme (NEQAS) for cholesterol. For HDL cholesterol, an ABX control serum N was used at ×0.5, ×1.0, and ×2.0 concentrations.
Statistical analysis.
Quartiles of plasma selenium were calculated according to the weighted population distribution. Multivariate linear regression was used to estimate the differences (95% CI) in levels of plasma total-cholesterol, HDL cholesterol, and non-HDL cholesterol (total cholesterol – HDL cholesterol), comparing the 3 highest quartiles of plasma selenium to the lowest quartile. The multivariate analyses included the following covariates: age, sex, BMI, smoking status, daily cigarette consumption, daily alcoholic drinking units, daily physical activity score, household income group, educational level group, employment, dietary variables (daily food energy, total fat intake, total cholesterol intake, polyunsaturated-to-saturated fatty acids intake ratio), vitamin/mineral supplement use, oral contraceptive use, and hormone replacement therapy.
To further explore the shape of the relationship between plasma selenium and plasma lipids, we used restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles of the distributions of plasma selenium (24). Tests for interaction between plasma selenium with age, sex, BMI, smoking and drinking status, and vitamin/mineral supplement use showed no statistically significant differences (data not shown). Statistical analyses were performed using the survey package (version 3.6.13) (25) in the statistical program R (version 2.6.1) (26) to account for the survey weights in NDNS.
RESULTS
The mean age of study participants was 40.8 ± 12.8 y and 48.5% of them were men. The mean concentrations of plasma and RBC selenium, and whole-blood glutathione peroxidase activity were 1.10 ± 0.19 μmol/L, 1.65 ± 0.40 μmol/L, and 124.6 ± 29.9 nmol·mg Hb−1·min−1, respectively. Higher plasma selenium was associated with higher age, nonsmoking status, higher income and educational level, use of vitamin/mineral supplements, total cholesterol intake, and polyunsaturated-to-saturated fatty acids intake ratio. Levels of RBC selenium and whole-blood glutathione peroxidase activity increased linearly with higher plasma selenium concentrations (P-trend < 0.001, for both) (Table 1). Indeed, the correlation coefficient of plasma selenium with RBC selenium was 0.45, whereas the correlation coefficients between plasma and RBC selenium with whole-blood glutathione peroxidase activity were 0.16 and 0.17, respectively (all P < 0.001).
TABLE 1.
Sample characteristics by quartiles of plasma selenium: The 2000–2001 UK NDNS1
| Quartile of plasma selenium, interval in μmol/L |
||||||
|---|---|---|---|---|---|---|
| Overall | 1st (0.60 to <0.98) | 2nd (0.98 to <1.08) | 3rd (1.08 to <1.20) | 4th (1.20 to 2.79) | P-trend | |
| n | 250 | 253 | 271 | 268 | ||
| Age, y | 40.8 ± 12.8 | 38.3 ± 12.8 | 38.1 ± 12.6 | 41.8 ± 13.0 | 45.0 ± 11.7 | <0.001 |
| Gender/menopausal status, % | ||||||
| Male | 48.5 | 42.9 | 49.5 | 47.2 | 54.4 | 0.12 |
| Postmenopause female | 16.2 | 15.1 | 13.3 | 15.7 | 20.8 | 0.01 |
| Premenopause female | 35.3 | 42.1 | 37.1 | 37.2 | 24.8 | 0.001 |
| BMI, kg/m2 | 26.7 ± 5.0 | 26.1 ± 5.1 | 26.8 ± 5.6 | 26.7 ± 4.6 | 27.0 ± 4.5 | 0.38 |
| Smoking status, % | ||||||
| Current | 33.1 | 52.5 | 34.3 | 29.2 | 17.5 | <0.001 |
| Former | 39.6 | 27.0 | 41.3 | 39.8 | 49.7 | 0.001 |
| Never | 27.3 | 20.5 | 24.4 | 31.0 | 32.8 | 0.001 |
| Physical activity score | 44.5 ± 8.2 | 44.4 ± 9.1 | 45.6 ± 9.9 | 44.2 ± 6.9 | 43.7 ± 6.3 | 0.09 |
| Income group, (>£600/mo), % | 36.9 | 21.2 | 34.5 | 41.4 | 49.7 | <0.001 |
| Education, (Higher education or degree), % | 31.8 | 21.5 | 30.1 | 32.7 | 42.5 | 0.001 |
| Vitamin/mineral supplements, % | 39.1 | 34.5 | 39.2 | 34.8 | 48.2 | <0.001 |
| Plasma selenium, μmol/L | 1.10 ± 0.19 | 0.88 ± 0.08 | 1.03 ± 0.03 | 1.13 ± 0.04 | 1.34 ± 0.17 | — |
| Red blood cell selenium, μmol/L | 1.65 ± 0.40 | 1.44 ± 0.27 | 1.59 ± 0.32 | 1.69 ± 0.36 | 1.88 ± 0.49 | <0.001 |
| Whole-blood GPx activity,2nmol·mg Hb−1·min−1 | 124.6 ± 29.9 | 117.5 ± 27.2 | 122.6 ± 28.7 | 128.6 ± 32.0 | 129.1 ± 29.8 | <0.001 |
| Daily food energy, kJ | 7768.0 ± 2355.2 | 7469.7 ± 2340.5 | 7947.1 ± 2587.8 | 7714.9 ± 2354.8 | 7928.7 ± 2085.7 | 0.08 |
| Total cholesterol intake, mg/d | 260.7 ± 119.3 | 238.3 ± 118.2 | 258.6 ± 113.5 | 264.9 ± 117.0 | 279.8 ± 125.3 | 0.004 |
| Total fat intake, g/d | 74.9 ± 27.6 | 72.7 ± 26.8 | 77.2 ± 29.1 | 73.3 ± 27.4 | 76.3 ± 26.7 | 0.34 |
| Poly/saturated fatty acids ratio |
0.45 ± 0.17 |
0.42 ± 0.16 |
0.43 ± 0.15 |
0.46 ± 0.16 |
0.49 ± 0.21 |
0.002 |
Values are survey-weighted means ± SD or percentages for continuous or categorical variables; n = 1,042.
GPx, glutathione peroxidase.
The multivariate adjusted mean differences in total plasma cholesterol comparing quartiles 2–4 to quartile 1 of plasma selenium were 0.03 (95% CI −0.16 to 0.21), 0.10 (−0.09 to 0.30), and 0.39 (0.18 to 0.60) mmol/L, respectively (P-trend = 0.001) (Table 2). The corresponding multivariate adjusted mean differences for non-HDL cholesterol levels were 0.03 (−0.16 to 0.22), 0.07 (−0.13 to 0.26), and 0.38 (0.17 to 0.59), respectively (P-trend = 0.001), and for HDL cholesterol levels they were −0.01 (−0.07 to 0.05), 0.04 (−0.02 to 0.10), and 0.01 (−0.05 to 0.07), respectively (P-trend = 0.58).
TABLE 2.
Adjusted differences (95% CI) in lipid fraction concentrations, comparing the 3 highest quartiles to the 1st quartile of plasma selenium, in the 2000–2001 UK NDNS
| Quartile of plasma selenium, interval in μmol/L |
|||||
|---|---|---|---|---|---|
| 1st (0.60 to <0.98) | 2nd (0.98 to <1.08) | 3rd (1.08 to <1.20) | 4th (1.20 to 2.79) | P-trend | |
| n | 250 | 253 | 271 | 268 | |
| Total cholesterol,1mmol/L | 5.08 | 5.07 | 5.23 | 5.63 | |
| Fully adjusted model2 | 0.00 | 0.03 | 0.10 | 0.39 | 0.001 |
| (Reference) | (−0.16, 0.21) | (−0.09, 0.30) | (0.18, 0.60) | ||
| Non-HDL cholesterol,1mmol/L | 3.93 | 3.92 | 4.02 | 4.43 | |
| Fully adjusted model2 | 0.00 | 0.03 | 0.07 | 0.38 | 0.001 |
| (Reference) | (−0.16, 0.22) | (−0.13, 0.26) | (0.17, 0.59) | ||
| HDL cholesterol, 1mmol/L | 1.15 | 1.14 | 1.21 | 1.20 | |
| Fully adjusted model2 | 0.00 | −0.01 | 0.04 | 0.01 | 0.58 |
| (Reference) |
(−0.07, 0.05) |
(−0.02, 0.10) |
(−0.05, 0.07) |
||
Mean lipid levels (survey-weighted).
Differences calculated from fully adjusted models include: age, sex, body mass index, smoking status, daily cigarette consumption, daily alcoholic drinking units, daily physical activity score, household income group, educational level group, employment, daily food energy, total fat intake, total cholesterol intake, polyunsaturated-to-saturated fatty acid ratio, vitamin/mineral supplement use, oral contraceptive use, and hormone replacement therapy.
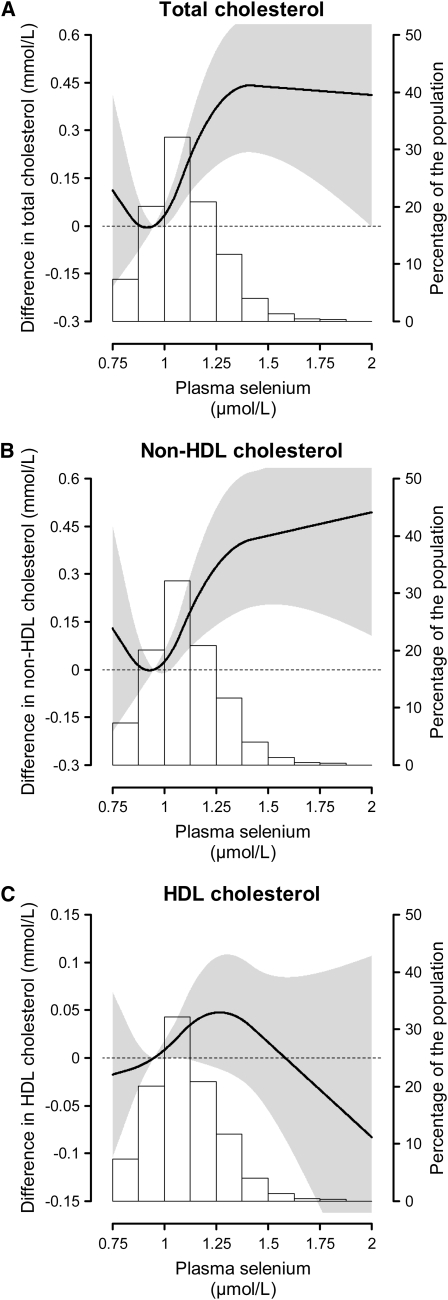
In spline regression models, total and non-HDL cholesterol increased linearly between 0.9 and 1.4 μmol/L of plasma selenium, although there was a near-plateau at high plasma selenium (Fig. 1).
FIGURE 1 .
Adjusted differences (95% CI) in lipid fraction concentrations by plasma selenium, in the 2000–2001 UK NDNS. The curves (read in the scale to the left) represent the adjusted differences (and the gray shading, the 95% CI) in lipids of subjects with any given value of plasma selenium with respect to a subject with 0.95 μmol/L of plasma selenium (plasma selenium at the 20th percentile, which was used as reference). The differences are statistically significant for all the range where the gray shading does not include the dashed reference line that denotes a zero difference. Plasma selenium was modeled as restricted quadratic splines with nodes at the 5th, 50th, and 95th percentiles. Multivariable linear regression models were adjusted for age, sex, BMI, smoking status, daily cigarette consumption, daily alcoholic drinking units, daily physical activity score, household income group, educational level group, employment status, daily food energy, total fat intake, total cholesterol intake, polyunsaturated-to-saturated fatty acid ratio, vitamin/mineral supplement use, oral contraceptive use, and hormone replacement therapy. The histogram (read in the scale to the right) shows the distribution of plasma selenium in the study population.
With respect to RBC selenium and whole-blood glutathione peroxidase activity, there were no consistent, significant associations with any of the lipid variables evaluated (Supplemental Tables 1, 2, Supplemental Fig. 1).
DISCUSSION
The results of our study showed positive associations between plasma selenium concentrations and total and non-HDL plasma cholesterol levels in a nationwide representative sample of British adults. The association with selenium was linear throughout most of the range, although there was a leveling off at plasma selenium concentrations >1.40 μmol/L. The association between plasma levels of selenium and total cholesterol was strong, with a difference of 0.39 mmol/L of cholesterol between the highest and the lowest quartiles of selenium. There was no association of HDL cholesterol with biomarkers of selenium status. To our knowledge, this is the first population-based study examining the association of selenium status with lipid levels in the UK, where a significant proportion of the general population is generally believed to have a suboptimal intake of dietary Se (11). As an indication of their status, 63.8% of NDNS participants had plasma selenium concentrations <1.14 μmol/L (90 μg/L), the level estimated to be required for full expression of glutathione peroxidase activity (27).
Strong, graded, positive associations between serum selenium and serum lipids were recently identified in a cross-sectional analysis from the US NHANES III, a selenium-replete population (10). Our findings, together with the NHANES III data, indicate that serum/plasma selenium is associated with total cholesterol across a very wide range of selenium concentrations. Higher selenium status and elevated total cholesterol levels have also been found in other populations with suboptimal selenium status (28–32), but those studies did not provide detailed dose-response analyses.
Selenium is a trace mineral with a narrow therapeutic window and large interindividual variability in metabolic sensitivity (2,33,34). As selenocysteine, selenium is incorporated into selenoproteins (e.g., glutathione peroxidases, iodothyronine deiodinases, and thioredoxin reductases) that are involved in essential enzymatic functions (4). Above the range of plasma selenium at which selenoprotein concentrations or activities are optimized (27,35,36), selenium is nonspecifically incorporated as selenomethionine into albumin and other plasma proteins replacing methionine, with no further increase in selenoprotein activities (3). The metabolic pathways involving this extra pool of selenium and its urine excretion pathways are incompletely understood, and may be responsible for some of the associations of high selenium exposure with glucose and lipid metabolism.
Potential mechanisms that may explain the association of plasma selenium with lipid levels are unclear. Selenoprotein P, the most abundant plasma selenoprotein, is taken up by the brain and the testes via the apolipoprotein E receptor-2 (37–39), whereas a further apolipoprotein receptor, megalin, mediates the uptake of selenoprotein P by the kidney (40). Additional evidence of a connection between selenoproteins and lipid metabolism comes from experimental animal models. Mouse knock-out models with compromised selenoprotein synthesis showed altered liver Apo E protein concentration, plasma cholesterol, and expression of genes involved in cholesterol biosynthesis, metabolism, and transport, suggesting a role for selenoproteins in the regulation of lipoprotein biosynthesis (41). Furthermore, the activity of the liver protein tyrosine phosphatase 1B (PTP1B), a key enzyme in the stimulation of fatty acid synthesis, was significantly higher in rats supplemented with selenium (75 or 150 μg/kg) than in the placebo group (42). In this study, selenium supplemented rats had higher liver triglyceride concentrations, which may provide a possible further explanation for the lipogenic effect of high selenium exposure. Moreover, selenoprotein and cholesterol synthesis are connected through the common use of isopentenyl pyrophosphate both for the synthesis of Sec-tRNA and for isoprenoid biosynthesis in the mevalonate pathway (43).
In our study, lipid levels were not associated with whole-blood glutathione peroxidase activity. Moreover, the correlations of plasma selenium and RBC selenium with whole-blood glutathione peroxidase activity were relatively weak, consistent with reports showing that above plasma selenium concentrations of 1.0 μmol/L, the correlation with glutathione peroxidase activity becomes progressively weaker because no further enzyme is synthesized (44). In general, concentrations of selenium in plasma or serum are commonly used as biomarkers of selenium status. Whole-blood glutathione peroxidase activity may be a useful index of functional selenium status, although it does not always reflect plasma or serum selenium concentrations (44). Furthermore, development of insulin resistance and obesity has been reported in transgenic mice over-expressing glutathione peroxidase (45,46). Likewise in humans, a strongly positive correlation between glutathione peroxidase activity and insulin resistance has been described in a group of nondiabetic pregnant women (47). This evidence may help to explain the observed associations of high selenium exposure with diabetes risk (5–8). However, the lack of association between whole-blood glutathione peroxidase activity and lipid variables, as reported in this study, suggests that other mechanisms may be involved in the adverse effects of high selenium exposure on lipid metabolism.
Few randomized controlled trials in humans have evaluated the effect of selenium on lipid profile. The SU.VI.MAX trial in a French population with suboptimal dietary selenium intake showed that long-term daily supplementation with a combination of antioxidants including selenium (100 μg/d) increased serum triglyceride levels compared with supplementation with placebo (9). Furthermore, among those in the treatment group, women had higher total cholesterol levels, whereas men were more likely to use lipid lowering medication compared with those on placebo. Likewise, in a randomized trial in a rural Chinese population with a low dietary intake of selenium, long-term combined supplementation with selenium (37.5 μg/d), vitamin C, and vitamin E resulted in small but significant increases in total and LDL cholesterol levels, whereas HDL concentrations were not affected (48). Those trials, however, used selenium in combination with other vitamins or minerals. Only 2 small, short-term intervention studies have examined the effects of supplementation with selenium alone on the lipid profile, but they did not yield significant differences between treatment groups (49,50).
In our cross-sectional study, we were unable to determine whether lipid levels rise as a consequence of increased selenium intake or whether a common metabolic pathway, or common coexposures, might explain the association between selenium status and lipid levels. In our study, the association of high plasma selenium with plasma lipids was actually amplified after multivariable adjustment for BMI and several dietary variables, including cholesterol intake. Besides the cross-sectional design, other limitations of this study deserve mention. Although the results were adjusted for a number of potential confounders, information on preexisting comorbidities or use of lipid-lowering medications was not collected in the survey, and we cannot rule out the possibility that the association could change if we could adjust for those variables. For example, recent findings from the "Etude du Vieillissement Artériel" (EVA) study suggested that long-term use of fibrates (but not statins) increased plasma selenium concentrations in dyslipidemic aged patients (51). However, our findings are consistent with previous studies that were able to adjust for some of these potential confounders (10, 28–32). While the low participation rate could have restricted the generalizability of the findings, an independent study specifically conducted to evaluate selection bias in NDNS concluded that there was no evidence to suggest serious nonresponse bias in the NDNS data (18). Finally, plasma lipids were measured in the nonfasting state, although this is unlikely to alter the concentrations of total and HDL cholesterol (52).
In this study, participants in the highest quartile of plasma selenium (≥1.20 μmol/L) were by far the most likely to use dietary supplements. It is likely that the relatively high selenium status of many of the participants in that quartile was the result of use of dietary supplements containing selenium, rather than from diet alone. Furthermore, the mean plasma selenium of NDNS participants was 1.10 μmol/L (87.0 μg/L), which is higher than that reported (61–79 μg/L) in previous studies of selenium status in the UK population (11,53). Indeed, in the 2000/01 NDNS, 41% of women and 30% of men reported taking dietary supplements, as compared with 17% of women and 9% of men in the 1986/87 Adults Survey (13). However, mean selenium levels in the UK are considerably lower than those reported in the US population (1.10 μmol/L vs. 1.58 μmol/L in NHANES III subjects aged 19–64 y) (10).
Though this study does not allow us to establish causality, our findings reinforce evidence from several sources that raised concern about potential adverse effects of high selenium status on glucose and lipid metabolism (5–10,54). The difference of 0.39 mmol/L (i.e., 15.1 mg/dL) of cholesterol between the bottom and top quartile of selenium levels, as observed in our study, is biologically and clinically relevant, and may have important public health implications. In fact, such a difference in cholesterol levels would translate into a large number of premature deaths from coronary heart disease, which could be prevented by reducing cholesterol levels by that amount at a population level (55–57).
In conclusion, we believe that the widespread use of selenium supplements or of any other strategy that artificially increases selenium status above the level required for optimal selenoprotein concentration/activity (11,58) is unwarranted at the present time. Further evidence from large randomized trials (59,60) and mechanistic studies is needed to provide robust evidence of the full range of health effects, either beneficial or detrimental, of high selenium exposure.
Supplementary Material
Acknowledgments
S. S. and E. G. designed research; M. L. and C. J. analyzed data; S. S. and E. G. wrote the paper; F. P. C., A. N., J. O. and M. R. critically revised the paper; S. S., M. R. and E. G. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by grants 1 R01 ES012673 from the National Institute of Environmental Health Sciences and 0230232N from the American Heart Association. The UK National Diet and Nutrition Survey of adults aged 19–64 years was funded by the Food Standards Agency and the Department of Health and carried out by the Office for National Statistics and the Medical Research Council Human Nutrition Research.
Author disclosures: S. Stranges, M. Laclaustra, C. Ji, F. P. Cappuccio, A. Navas-Acien, J. M. Ordovas, M. Rayman, and E. Guallar, no conflicts of interest.
Supplemental Tables 1 and 2 and supplemental Figure 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: Hb, hemoglobin; NDNS, National Diet and Nutrition Survey; SU.VI.MAX, Supplementation with Antioxidant Vitamins and Minerals.
References
- 1.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk - what is new? Curr Opin Lipidol. 2008;19:43–9. [DOI] [PubMed] [Google Scholar]
- 2.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–68. [DOI] [PubMed] [Google Scholar]
- 3.Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5:75–9. [DOI] [PubMed] [Google Scholar]
- 4.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. [DOI] [PubMed] [Google Scholar]
- 5.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. Adults. Diabetes Care. 2007;30:829–34. [DOI] [PubMed] [Google Scholar]
- 6.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–23. [DOI] [PubMed] [Google Scholar]
- 7.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117:1409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–9. [DOI] [PubMed] [Google Scholar]
- 9.Hercberg S, Bertrais S, Czernichow S, Noisette N, Galan P, Jaouen A, Tichet J, Briancon S, Favier A, et al. Alterations of the lipid profile after 7.5 years of low-dose antioxidant supplementation in the SU.VI.MAX Study. Lipids. 2005;40:335–42. [DOI] [PubMed] [Google Scholar]
- 10.Bleys J, Navas-Acien A, Stranges S, Menke A, Miller, 3rd ER, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayman MP. Dietary selenium: time to act. BMJ. 1997;314:387–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 2004;104:942–50. [DOI] [PubMed] [Google Scholar]
- 13.Henderson L, Irving K, Gregory J, Bates CJ, Prentice A, Swan G, Farron M. The National Diet and Nutrition Survey: Adults aged 19 to 64 years. Volume 3: Vitamin and Mineral Intake and Urinary Analyses. London: The Stationery Office, 2003.
- 14.Henderson L, Gregory J, Swan G. The National Diet and Nutrition Survey: Adults aged 19 to 64 years. Volume 1: Types and Quantities of Foods Consumed. London: The Stationery Office, 2002.
- 15.Henderson L, Gregory J, Irving K, Swan G. The National Diet and Nutrition Survey: Adults aged 19 to 64 years. Volume 2: Energy, Protein, Carbohydrate, Fat and Alcohol Intake. London: The Stationery Office, 2003.
- 16.Ruston D, Hoare S, Henderson L, Gregory J, Bates CJ, Prentice A, Perks J, Swan G, Farron M. The National Diet and Nutrition Survey: Adults aged 19 to 64 years. Volume 4: Nutritional Status (Anthropometry and Blood Analytes), Blood Pressure and Physical Activity. London: The Stationery Office, 2004.
- 17.Hoare J, Henderson L, Bates CJ, Prentice A, Birch M, Swan G, Farron M. The National Diet and Nutrition Survey: Adults aged 19 to 64 years. Volume 5: Summary Report. London: The Stationery Office, 2004.
- 18.Skinner CJ, Holmes D. The 2000–01 National Diet and Nutrition Survey of Adults aged 19–64 years: The impact of non-response. In National Diet and Nutrition Survey Adults Aged 19–64 Years. Appendix E; 2002. Available at: http://www.food.gov.uk/science/101717/ndnsdocuments/ndnsappendices
- 19.Bates CJ, Prentice A, Birch MC, Delves HT. Dependence of blood indices of selenium and mercury on estimated fish intake in a national survey of British adults. Public Health Nutr. 2007;10:508–17. [DOI] [PubMed] [Google Scholar]
- 20.Delves HT, Sieniawska CE. Simple method for the accurate determination of selenium in serum by using inductively coupled plasma mass spectrometry. J Anal At Spectrom. 1997;12:387–9. [Google Scholar]
- 21.Lloyd B, Holt P, Delves HT. Determination of selenium in biological samples by hydride generation and atomic absorption spectroscopy. Analyst. 1982;107:927–33. [DOI] [PubMed] [Google Scholar]
- 22.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 23.Arranz-Pena ML, Tasende-Mata J, Martin-Gil F. Comparison of two homogeneous assays with a precipitation method and an ultracentrifugation method for the measurement of HDL-cholesterol. Clin Chem. 1998;44:2499–505. [PubMed] [Google Scholar]
- 24.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. [DOI] [PubMed] [Google Scholar]
- 25.Thomas LumleySurvey: analysis of complex survey samples. R package version 3.6–13. http://faculty.washington.edu/tlumley/survey/
- 26.R Development Core Team (2007). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://www.R-project.org.
- 27.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70:896–903. [DOI] [PubMed] [Google Scholar]
- 28.Ringstad J, Jacobsen BK, Thomassen Y. The Tromso Heart Study: relationships between the concentration of selenium in serum and risk factors for coronary heart disease. J Trace Elem Electrolytes Health Dis. 1987;1:27–31. [PubMed] [Google Scholar]
- 29.Jossa F, Trevisan M, Krogh V, Farinaro E, Giumetti D, Fusco G, Galasso R, Panico S, Frascatore S, et al. Serum selenium and coronary heart disease risk factors in southern Italian men. Atherosclerosis. 1991;87:129–34. [DOI] [PubMed] [Google Scholar]
- 30.Suadicani P, Hein HO, Gyntelberg F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis. 1992;96:33–42. [DOI] [PubMed] [Google Scholar]
- 31.Gamez C, Ruiz-Lopez D, Artacho R, Navarro M, Puerta A, Lopez C. Serum selenium in institutionalized elderly subjects and relation to other nutritional markers. Clin Chem. 1997;43:693–4. [PubMed] [Google Scholar]
- 32.Coudray C, Roussel AM, Mainard F, Arnaud J, Favier A. Lipid peroxidation level and antioxidant micronutrient status in a pre-aging population; correlation with chronic disease prevalence in a French epidemiological study (Nantes, France). J Am Coll Nutr. 1997;16:584–91. [PubMed] [Google Scholar]
- 33.Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann Clin Lab Sci. 1996;26:99–113. [PubMed] [Google Scholar]
- 34.Institute of MedicineDietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. Washington, DC: National Academy Pr; 2000.
- 35.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low selenium area of China. Am J Clin Nutr. 2005;81:829–34. [DOI] [PubMed] [Google Scholar]
- 36.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–10. [DOI] [PubMed] [Google Scholar]
- 37.Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci. 2007;27:6207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentine WM, Abel TW, Hill KE, Austin LM, Burk RF. Neurodegeneration in mice resulting from loss of functional selenoprotein P or its receptor apolipoprotein E receptor 2. J Neuropathol Exp Neurol. 2008;67:68–77. [DOI] [PubMed] [Google Scholar]
- 39.Burk RF, Hill KE. Selenoprotein P - expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790:1441–7. [DOI] [PMC free article] [PubMed]
- 40.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem. 2008;283:6854–60. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta A, Carlson BA, Hoffmann VJ, Gladyshev VN, Hatfield DL. Loss of housekeeping selenoprotein expression in mouse liver modulates lipoprotein metabolism. Biochem Biophys Res Commun. 2008;365:446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller AS, Klomann SD, Wolf NM, Schneider S, Schmidt R, Spielmann J, Stangl G, Eder K, Pallauf J. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008;138:2328–36. [DOI] [PubMed] [Google Scholar]
- 43.Moosmann B, Behl C. Selenoprotein synthesis and side-effects of statins. Lancet. 2004;363:892–4. [DOI] [PubMed] [Google Scholar]
- 44.Diplock AT. Indexes of selenium status in human populations. Am J Clin Nutr. 1993; 57 (2, Suppl)256S–8S. [DOI] [PubMed] [Google Scholar]
- 45.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–24. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Scholl TO, Leskiw MJ, Donaldson MR, Stein TP. Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab. 2003;88:5963–8. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Gail MH, Wang YQ, Brown LM, Pan KF, Ma JL, Amagase H, You WC, Moslehi R. A randomized factorial study of the effects of long-term garlic and micronutrient supplementation and of 2-wk antibiotic treatment for Helicobacter pylori infection on serum cholesterol and lipoproteins. Am J Clin Nutr. 2006;84:912–9. [DOI] [PubMed] [Google Scholar]
- 49.Luoma PV, Sotaniemi EA, Korpela H, Kumpulainen J. Serum selenium, glutathione peroxidase activity and high-density lipoprotein cholesterol–effect of selenium supplementation. Res Commun Chem Pathol Pharmacol. 1984;46:469–72. [PubMed] [Google Scholar]
- 50.Yu SY, Mao BL, Xiao P, Yu WP, Wang YL, Huang CZ, Chen WQ, Xuan XZ. Intervention trial with selenium for the prevention of lung cancer among tin miners in Yunnan, China. A pilot study. Biol Trace Elem Res. 1990;24:105–8. [DOI] [PubMed] [Google Scholar]
- 51.Arnaud J, Akbaraly TN, Hininger-Favier I, Berr C, Roussel AM. Fibrates but not statins increase plasma selenium in dyslipidemic aged patients - The EVA study. J Trace Elem Med Biol. 2009;23:21–8. [DOI] [PubMed] [Google Scholar]
- 52.Halloran P, Roetering H, Pisani T, van den Berg B, Cobbaert C. Reference standardization and analytical performance of a liquid homogeneous high-density lipoprotein cholesterol method compared with chemical precipitation method. Arch Pathol Lab Med. 1999;123:317–26. [DOI] [PubMed] [Google Scholar]
- 53.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–41. [DOI] [PubMed] [Google Scholar]
- 54.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kannel WB, Neaston JD, Wentworth D, Thomas HE, Stamler J, Hulley SB, Kjelsberg MO. Overall coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for MRFIT. Am Heart J. 1986;112:825–36. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in populations with low cholesterol concentrations. BMJ. 1991;303:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–8. [DOI] [PubMed] [Google Scholar]
- 58.Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, et al. Biofortification of UK food crops with selenium. Proc Nutr Soc. 2006;65:169–81. [DOI] [PubMed] [Google Scholar]
- 59.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr., Kristal AR, Santella RM, Probstfield JL, Moinpour CM, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT). J Natl Cancer Inst. 2005;97:94–102. [DOI] [PubMed] [Google Scholar]
- 60.Marshall JR, Sakr W, Wood D, Berry D, Tangen C, Parker F, Thompson I, Lippman SM, Lieberman R, et al. Design and Progress of a Trial of Selenium to Prevent Prostate Cancer among Men with High-Grade Prostatic Intraepithelial Neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1479–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.