Abstract
Neuregulin-1 (NRG1) is a potential therapeutic agent for the treatment of doxorubicin (Dox)-induced heart failure. NRG1, however, activates the erbB2 receptor, which is frequently overexpressed in breast cancers. It is, therefore, important to understand how NRG1, via erbB2, protects the heart against Dox cardiotoxicity. Here, we studied NRG1-erbB2 signaling in Dox-treated mice hearts and in isolated neonatal rat ventricular myocytes (NRVM). Male C57BL/6 mice were treated with recombinant NRG1 before and daily after a single dose of Dox. Cardiac function was determined by catheterization. Two-week survival was analyzed by the Kaplan-Meier method. Cardiac troponins [cardiac troponin I (cTnI) and cardiac troponin T (cTnT)] and phosphorylated Akt protein levels were determined in mice hearts and in NRVM by Western blot analysis. Activation of caspases and ubiquitinylation of troponins were determined in NRVM by caspase assay and immunoprecipitation. NRG1 significantly improved survival and cardiac function in Dox-treated mice. NRG1 reduced the decrease in cTnI, cTnT, and cardiac troponin C (cTnC) and maintained Akt phosphorylation in Dox-treated mice hearts. NRG1 reduced the decrease in cTnI and cTnT mRNA and proteins in Dox-treated NRVM. Inhibition of erbB2, phosphoinositide 3-kinase (PI3K), Akt, and mTOR blocked the protective effects of NRG1 on cTnI and cTnT in NRVM. NRG1 significantly reduced Dox-induced caspase activation, which degraded troponins, in NRVM. NRG1 reduced Dox-induced proteasome degradation of cTnI. NRG1 attenuates Dox-induced decrease in cardiac troponins by increasing transcription and translation and by inhibiting caspase activation and proteasome degradation of troponin proteins. NRG1 maintains cardiac troponins by the erbB2-PI3K pathway, which may lessen Dox-induced cardiac dysfunction.
Keywords: ErbB2, troponin proteins, signaling
neuregulin-1 (NRG1)-ErbB2 signaling is essential for cardiac development and maintaining adult cardiac function (26, 29). ErbB2 was initially detected as an oncogenic variant that was overexpressed in several tumor types (15). Trastuzumab, a humanized monoclonal antibody that binds to and blocks erbB2, significantly reduces recurrence and early mortality in patients with breast cancer who overexpress erbB2 (27, 34). A significant increase in congestive heart failure was reported, however, when the anti-erbB2 antibody trastuzumab was used in combination with the chemotherapy drug doxorubicin (Dox) (34). Thus inhibition of erbB2 signaling in patients who receive concurrent therapy with Dox causes an increased risk of cardiotoxicity. We hypothesized, accordingly, that activation of erbB2 signaling by NRG1 may mitigate cardiac dysfunction.
Multiple isoforms of NRG1 are synthesized in the endocardium and the endothelium of cardiac vasculature (7, 20, 24). NRG1 activates its receptors erbB2 and erbB4 on cardiomyocytes (11). NRG1 promotes hypertrophy and proliferation of cardiomyocytes through activation of erbB2 and erbB4 and protects cardiomyocytes from apoptosis (20, 24, 38). The soluble recombinant form of a human NRG1 [recombinant human glial growth factor 2 (rhGGF2)] fostered growth and survival of cultured rat ventricular myocytes (38). Administration of recombinant NRG1 improved cardiac function and survival in heart failure (23).
Dox-induced cardiac damage is primarily associated with severe myocyte disarray and myofibrillar protein degradation (3, 16, 19, 21, 33). Dox decreases the expression of myofibrillar genes and cardiac-specific transcriptional factors (1, 16). NRG1 reduced Dox-induced myofibrillar disarray in cultured cardiomyocytes (33). However, little is known whether NRG1 prevents Dox-induced myofibrillar loss and preserves cardiac function in intact animals. Therefore, we studied the cardiac effects of recombinant NRG1 on Dox toxicity and myofibrillar loss in mice and isolated neonatal rat ventricular myocytes.
Whereas the cardiotoxicity in Dox-treated patients is observed clinically long after treatment (months to years), the cardiotoxicity in our rodent model is fairly acute (days). The mechanisms responsible in our preclinical model may be different than those occurring in patients, yet the expediency of the model and new observations may improve our understanding of the effects of NRG1 on Dox-induced cardiotoxicity.
METHODS
Animal model.
Ten- to twelve-week-old male C57BL/6 mice (in total, n = 189; Charles River Laboratory) were treated with a single dose of Dox (20 mg/kg ip; Bedford Laboratories, Bedford, OH). Selection of dose and route of administration was based on a previous study (28).
NRG1 solvent containing 20 mM sodium acetate, 100 mM sodium sulfate, 1% mannitol, and 100 mM l-arginine (pH 6.5) (in total, n = 79) or recombinant human NRG1β (rhGGF2; 0.75 mg/kg sc; in total, n = 80; a gift from Acorda Therapeutics) was administered 1 day before and daily after Dox administration (Dox-NRG1). The timing and dosage of NRG1 administration was based on a previous study on the nervous system (5). They were also based on our preliminary study on a small cohort of Dox-NRG1-treated mice showing beneficial effects on survival and cardiac function. Solvent-treated mice were used as controls (in total, n = 30).
All animal studies were in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, Revised 1996) and approved by Caritas St. Elizabeth's Medical Center Institutional Animal Care and Use Committee.
Left ventricular function.
Left ventricular (LV) function was measured in non-Dox-treated mice (n = 6–8) and in Dox-treated mice 5 days post treatment (n = 8 to 9/group) by LV catheterization using Millar Mikro-Tip Blood Pressure System (ADInstruments, Colorado Springs, CO). In brief, a Millar Mikro-Tip catheter transducer was inserted into the LV via the right carotid artery after mice were anesthetized with urethane (1,000 mg/kg) and α-chloralose (50 mg/kg). LV systolic and diastolic pressures were recorded. LV tissues were frozen in liquid nitrogen for further biochemical analyses.
Serum creatine kinase and cardiac troponin I and cardiac troponin T.
The marker of muscle damage serum creatine kinase (CK) was analyzed in solvent-treated mice (n = 5), NRG1 alone-treated mice (n = 5), Dox-treated mice (n = 6), and Dox-NRG1-treated mice (n = 5) 4 days after Dox administration using a commercially available kit (Diagnostic Chemicals, Oxford, CT) (22). Serum cTnI and cTnT was measured in solvent-treated mice (n = 3), NRG1 alone-treated mice (n = 3), Dox-treated mice (n = 5), and Dox-NRG1-treated mice (n = 5) 3 days after Dox administration. Mice were euthanized, and serum samples were collected after excision of the heart. Serum cardiac troponin I (cTnI) and cardiac troponin T (cTnT) were determined by Western blot analysis using specific antibodies.
Neonatal rat ventricular myocyte culture.
Neonatal rat ventricular myocyte cultures (NRVM) were isolated from 1- to 2-day-old Wistar rats (n = 30–50/experiment) as previously described (30). In brief, rats were anesthetized by isoflurane inhalation followed by cervical dislocation, and ventricles were excised and minced, followed by serial digestions in Hank's solution containing 0.1% Trypsin and 20 U/ml DNase. Nonmyocytes were removed by differential plating for 1 h at 37°C, and myocytes were collected and plated at a density of 580 cell/mm2 and cultured in MEM containing 5% FBS at 37°C. The medium was changed to MEM containing 0.1% BSA for 24 h before stimulation. rhGGF2 (20–50 ng/ml) or equal amount of solvent was added 30 min before Dox (1 μM) stimulation (38). Inhibitors were added 1 h before rhGGF2 or solvent (4 to 5 plates/group from at least 3 independent experiments). Inhibitors include: Z-VAD [100 μM; R&D Systems, (10, 14), Minneapolis, MN], Caspase-Family Inhibitor Set IV [caspase-1 inhibitor: Z-YVAD-FMK, caspase-2 inhibitor: Z-VDVAD-FMK, caspase-3 inhibitor: Z-DEVD-FMK, caspase-4 inhibitor: Z-LEVD-FMK, caspase-5 inhibitor: Z-WEHD-FMK, caspase-6 inhibitor: Z-VEID-FMK, caspase-8 inhibitor: Z-IETD-FMK, caspase-9 inhibitor: Z-LEHD-FMK, caspase-10 inhibitor: Z-AEVD-FMK, caspase-12 inhibitor: Z-ATAD-FMK and caspase-13 inhibitor: Z-LEED-FMK (BioVision, Mountain View, CA), 10 μM; this concentration was used based on manufacture's recommendation], MG-132 (10 μM) (8), cycloheximide (5 μg/ml), AG879 (10 μM), anti-erbB2 antibody (clone 9G6, 6 μg/ml), and AG1478 (10 μM; EMD Chemicals, San Diego, CA) (12) LY294002 (10 μM), PD98059 (50 μM), rapamycin (10 nM; Cell Signaling Technology, Danvers, MA), Akt Inhibitor VIII, Akti-1/2 (5 μM; EMD, Gibbstown, NJ), SB203580 (4 μM; Invitrogen, Carlsbad, CA), PKC inhibitor GF109203X (1 μM; Invitrogen, Carlsbad, CA; the concentration of inhibitors was chosen based on manufacture's recommendation). The mRNA and the protein levels of cTnI and cTnT and signaling molecules mTOR, p70S6K, S6, 4E-BP, and ElF4G were measured 48 h after Dox administration unless otherwise stated.
Western blot analysis.
Mice LV tissue (n = 4 to 5 per group) and NRVM from each group was lysed in a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN). LV tissue was homogenized by PowerGene homogenizer (Fisher Scientific, Pittsburgh, PA), and NRVM were homogenized using a 21-gauge needle. Proteins were quantified using Bradford Assay (Bio-Rad Laboratories, Hercules, CA). LV proteins (50 μg) from controls (n = 6), Dox (n = 4), and Dox-NRG1-treated mice (n = 4) and myocyte proteins (15 μg) from controls, Dox, and Dox-NRG1-treated NRVM (n = 4 to 5 plates/group from at least 3 independent experiments) were separated by SDS page and transferred to Whatman nitrocellulose membrane (pore size, 0.2 μm; Fisher Scientific). Membranes were probed with antibodies against cardiac troponin I (GeneTex, San Antonio, TX), cardiac troponin T, cardiac troponin C, Tropomysin and α-sarcomeric actin, ubiquitin, COX IV and Tubulin (Abcam, Cambridge, MA), α-actinin (Sigma, St. Louis, MO), total erbB2 and erbB4 (Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated erbB2 (Tyr877), phosphorylated and total Akt (Ser473 or Thr308), ERK1/2 (Thr202/Tyr204), mTOR (Ser2448), P70S6K (Thr421/Ser424), S6 (Ser240/244), 4E-BP (Thr37/46), EIF4G (Ser1108), and cytochrome c (Cell Signaling Technology, Danvers, MA).
Caspase assay.
NRVM were collected 16 h after Dox treatment (n = 5–8 of 100-mm plates). Cells were lysed in a buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS), 1 mM DTT, and 0.1 mM EDTA and homogenized using a 21-gauge needle. Proteins were quantified by Bradford Assay (Bio-Rad Laboratories, Hercules, CA). Forty micrograms of proteins were incubated (n = 5–8 of 100-mm plates/group) with caspase-2 substrate (Ac-VDVAD-pNA, 200 μM), caspase-3 substrate (Ac-DEVD-pNA, 200 μM), caspase-9 substrate (Ac-LEHD-pNA, 200 μM; AnaSpec, San Jose, CA), caspase-5 substrate (WEHD-pNA, 200 μM), caspase-6 substrate (Ac-VEID-pNA, 200 μM), and caspase-10 substrate (AEVD-pNA, 200 μM; BioVision, Mountain View, CA) in a reaction buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 0.1 mM EDTA, and 10% glycerol at 37°C for 18 h. The absorbance was recorded at 405 nm using a microplate reader (PerkinElmer, Waltham, MA). The activity of the various caspase substrates was expressed as a percentage of enzyme activity compared with control.
Cell viability measurement.
NRVM were isolated and treated with solvent, Dox, or Dox-NRG1 as described. The suspended cells were collected, washed by PBS, and resuspended in culture media 24 h after the treatment. The attached cells were trypsinized, washed with PBS, and resuspended in culture media. The cells were combined and stained with 0.4% of trypan blue. Stained and nonstained cells were counted using a hemocytometer under the microscope. The percentage of viable cells was determined as the number of nonstained cells divided by the number of total cells × 100.
Immunoprecipitation.
NRVM (5–8 of 100-mm plates/group) were washed with ice-cold PBS and homogenized by a 21-gauge needle in a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and protease inhibitor cocktail tablet (Roche Diagnostics). Cell lysates were centrifuged at 13,500 g for 15 min at 4°C. The supernatant was saved, and protein concentration was measured by the Bradford Assay. One milligram of protein from each group (control, Dox, and Dox-NRG1) was incubated with protein A/G beads (Thermo Fisher Scientific, Rockford, IL) for 2 h at 4°C and centrifuged at 800 g for 5 min at 4°C. The supernatant was incubated with anti-cTnI or anti-cTnT antibody (GeneTex) overnight and then with protein A/G beads for 2 h. Beads were washed and collected by centrifugation (800 g for 5 min at 4°C). Precipitated proteins were released and resuspended in 2× SDS loading buffer and loaded on the SDS-gel. Equal loading of proteins were confirmed by probing the membrane with anti-cTnI (immunoprecipitation: cTnI) or anti-cTnT (immunoprecipitation: cTnT) antibody.
RT-PCR.
RNA was extracted from NRVM (n = 2 to 3 of 100-mm plates/group) using RNeasy Mini kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using QuantiTect Rev. Transcription Kit (Qiagen). cDNA was then quantified and amplified by PCR using specific primers for cTnI (forward, 5′-AAAAAGTCTAAGATCTCCGCCTCCA-3′ and reverse, 5′-GGTTTTCCTTCTCAATGTCCTCCTT-3′), cTnT (forward, 5′-CGTAGAAGAGGTTGGTCCTGATGAA-3′ and reverse, 5′-TGTACCCTCCAAAGTGCATCATGTT-3′), and 18S (Ambion, Austin, TX). cDNA was amplified by 28 cycles of PCR at 94°C for 1 min, 57°C for 45 s, and 72°C for 45 s.
In vitro caspase substrate assay.
In vitro caspase substrate assay was performed as previously described (6). In brief, recombinant human cTnI, cTnT, and cardiac troponin C (cTnC) (GenWay Biotech, San Diego, CA) were reconstituted in a complex (cTnI, cTnT, and cTnC in a ratio of 1:1:1.5) and were incubated with active recombinant caspase-2, caspase-3, caspase-5, caspase-6, caspase-9, or caspase-10 (Biovision, Mountain View, CA), with or without the the pan-caspase inhibitor Z-VAD-FMK (Biovision) in a buffer containing 50 mM HEPES (pH 7.4), 2 mM EDTA, 0.15% (wt/vol) CHAPS, 10% (wt/vol) sucrose, 5 mM DTT, and protease inhibitors at room temperature for 4 h. The reaction was stopped by the addition of Laemmli buffer. Samples were separated by SDS-polyacrylamide gel and followed by Western blot analysis using cTnI or cTnT antibodies.
Statistical analysis.
Results are presented as means ± SE. Statistical analysis was conducted using SigmaStat (Systat Software, San Jose, CA). Comparison between groups was performed by ANOVA followed by Tukey's test. Kaplan-Meier estimates of survival were computed using SAS for Windows v6.12 (SAS Institute, Cary, NC). Differences were considered significant with P < 0.05.
RESULTS
NRG1 improved survival of Dox-treated mice.
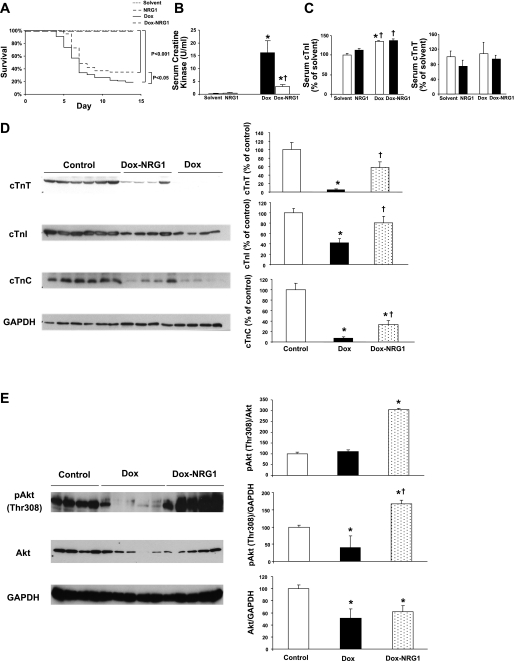
Two-week survival was significantly reduced to 19% (n = 11 of 58 mice survived) in Dox-treated mice compared with solvent-treated or NRG1-treated mice (n = 20/group; P < 0.05). NRG1 significantly improved survival to 35% (n = 21 of 60 mice survived; P < 0.05) compared with Dox-treated mice (Fig. 1A). NRG1 alone did not alter survival rate in non-Dox-treated mice compared with solvent-treated mice.
Fig. 1.
A: survival analysis. Mice were administered a single dose of doxorubicin (Dox; 20 mg/kg ip) with and without cotreatment of neuregulin-1 (NRG1; 0.75 mg/kg sc) 1 day before and daily after Dox administration (Dox-NRG1). Two-week survival was analyzed by the Kaplan-Meier method. Survival was significantly reduced to ∼19% in Dox-treated mice (n = 58, 11 of 58 mice survived; P < 0.05) compared with solvent-treated or NRG1 alone-treated mice (n = 20/group; P < 0.05). NRG1 significantly improved survival to ∼35% (n = 60, 21 of 60 mice survived; P < 0.05) compared with Dox-treated mice. B: serum creatine kinase (CK) levels. Serum CK levels were determined in solvent-treated (n = 5), NRG1 alone-treated (NRG1, n = 5), Dox-treated (n = 6), and Dox-NRG1-treated (n = 5) mice 4 days after Dox administration. Means are ± SE. *P < 0.05 vs. controls; †P < 0.05 vs. Dox. C: serum cardiac troponin I (cTnI) and cardiac troponin T (cTnT). Serum cTnI and cTnT were measured in solvent-treated (n = 3), NRG1 alone-treated (n = 3), Dox-treated (n = 4), and Dox-NRG1-treated (n = 4) mice 3 days after Dox administration by Western blot analysis. *P < 0.05 vs. solvent; †P < 0.05 vs. NRG1. D: NRG1 administration alleviated Dox-induced decrease in cardiac troponins [cTnI, cTnT, and cardiac troponin T (cTnC)] in mice hearts. Mice were treated with Dox (20 mg/kg ip) or with Dox plus NRG1 (0.75 mg/kg sc daily). Protein levels of cTnI, cTnT, and cTnC were determined in controls (n = 6), Dox-treated (n = 4), and Dox-NRG1-treated (n = 4) mice 5 days after Dox administration by Western blot analysis. Means are ± SE. *P < 0.05 vs. controls; †P < 0.05 vs. Dox. E: NRG1 maintained the activation of Akt in Dox-treated hearts. Mice were treated with Dox (20 mg/kg ip) or with Dox plus NRG1 (0.75 mg/kg sc daily). Phosphorylated Akt (Thr308) and total Akt was determined in controls (n = 4), Dox-treated (n = 5), and Dox-NRG1-treated (n = 5) mice 5 days after Dox treatment by Western blot analysis. Means are ± SE. *P < 0.05 vs. controls; †P < 0.05 vs. Dox.
NRG1 improved LV function in Dox-treated mice.
We determined whether NRG1 alone altered cardiac function in non-Dox-treated mice. Mice were treated with either solvent or NRG1, and cardiac function was measured 30 days after treatment. There was no difference in cardiac function between solvent-treated and NRG1 alone-treated mice (Table 1). Solvent-treated mice were used as controls in the subsequent experiments. Cardiac function was measured 5 days after Dox administration when survival declined in Dox-treated mice. Dox significantly reduced body weight, heart weight, and LV weight normalized to tibia length (HW/TL, LVW/TL). Dox-NRG1 treatment did not alleviate these effects of Dox. Dox significantly decreased heart rate (HR), LV systolic pressure (LVSP), maximal change in pressure over time (dP/dtmax), and minimal change in pressure over time (dP/dtmin), whereas these indexes were not significantly different between Dox-NRG1-treated mice and control mice (Table 2). HR and dP/dtmin were significantly higher in Dox-NRG1-treated mice compared with Dox-treated mice.
Table 1.
Cardiac hemodynamic measurements in solvent and NRG1 alone-treated C57BL/6 mice
| Solvent | NRG1 | |
|---|---|---|
| n | 8 | 6 |
| BW, g | 27.7±0.7 | 27.2±0.7 |
| LVW, mg | 99±5 | 90±5 |
| LVW/BW, mg/g | 3.6±0.1 | 3.3±0.1 |
| HR, beats/min | 593±38 | 595±83 |
| LVSP, mmg | 102±10 | 105±24 |
| LVEDP, mmHg | 2.5±0.3 | 3.1±0.2 |
| dP/dtmax, mmHg/s | 13,797±1,057 | 14,938±3,188 |
| dP/dtmin, mmHg/s | 12,063±1,188 | 13,500±3,500 |
Values are means ± SE. NRG1, neuregulin-1; BW, body weight; LVW, left ventricular (LV) weight; HR, heart rate; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; dP/dtmax and dP/dtmin, maximal and minimal change in pressure over time, respectively.
Table 2.
Cardiac hemodynamic measurements in Dox-treated and Dox-NRG1-treated C57BL/6 mice
| Controls | Dox | Dox-NRG1 | |
|---|---|---|---|
| n | 8 | 9 | 8 |
| BW, g | 25±0.4 | 20±0.4* | 21±0.9* |
| TL, mm | 16.7±0.6 | 16.3±0.2 | 16.0±0.0 |
| HW, mg | 110±3 | 83±3* | 89±3* |
| LVW, mg | 88±2 | 70±1* | 75±3* |
| HW/BW, mg/g | 4.5±0.1 | 4.1±0.1* | 4.2±0.1 |
| LVW/BW, mg/g | 3.6±0.1 | 3.5±0.1 | 3.6±0.1 |
| HW/TL, mg/mm | 6.6±0.3 | 5.1±0.1* | 5.7±0.2 |
| LVW/TL, mg/mm | 5.4±0.2 | 4.4±0.1* | 4.8±0.2 |
| HR, beats/min | 646±13 | 420±39* | 598±28† |
| LVSP, mmg | 101±3 | 85±2* | 93±4 |
| LVEDP, mmHg | 5.1±1.7 | 4.0±1.0 | 3.8±1.2 |
| dP/dtmax, mmHg/s | 12,717±928 | 7,582±797* | 10,831±1,058 |
| dP/dtmin, mmHg/s | 8,589±640 | 5,331±568* | 7,968±523† |
Values are means ± SE. Dox, doxorubicin; TL, tibia length; HW, heart weight.
P < 0.05 vs. controls;
P < 0.05 vs. Dox.
CK and serum cTnI and cTnT.
Serum CK was significantly increased in Dox-treated mice (P < 0.05) compared with control mice (solvent; NRG1 alone). Dox-NRG1 significantly reduced serum CK (P < 0.05) compared with Dox-treated mice (Fig. 1B). We also determined the serum cTnI and cTnT in solvent-treated, NRG1 alone-treated, Dox-treated, and Dox-NRG1-treated mice. Serum cTnI was similar between solvent-treated and NRG1 alone-treated mice. There was a trend of reduction in serum cTnT in NRG1 alone-treated mice versus solvent-treated mice. Serum cTnI was significantly increased in both Dox and Dox-NRG1 mice, but there was no difference between Dox and Dox-NRG1-treated mice. There was no difference in serum cTnT between Dox-treated and Dox-NRG1-treated mice (Fig. 1C).
NRG1 attenuated the decrease in cardiac troponin proteins in Dox-treated mice hearts.
Cardiac troponin I, cardiac troponin T, cardiac troponin C, α-sarcomeric actin, α-actinin, and tropomyosin were significantly reduced in Dox-treated mice compared with control mice heart tissue (P < 0.05). Daily dosing with NRG1 significantly attenuated the effects of Dox on cTnI, cTnT, and cTnC (P < 0.05; Fig. 1D) but not on α-sarcomeric actin, α-actinin, and tropomyosin (data not shown). These data indicate that inhibition of the decrease of cardiac troponin proteins in the heart may contribute to the cardiac protective effects of NRG1.
The phosphoinositde 3-kinase (PI3K)-Akt pathway is the central regulator of the NRG-erbB signaling network (37). NRG1 promotes survival and growth of cardiomyocytes by activating the PI3K-Akt pathway (2). We determined whether preservation of the proteins of the troponin complex by NRG1 was associated with an increased activation of cardiac phosphorylated Akt (Thr308) in mice. Dox significantly decreased phosphorylated Akt and total Akt (P < 0.05) compared with controls, whereas daily dosing with Dox-NRG1 significantly increased phosphorylated Akt (P < 0.05) compared with Dox-treated mice but did not increase total Akt in Dox-treated mice hearts (Fig. 1E).
Taken together, these findings demonstrate that NRG1 improved survival and cardiac function, preserved cardiac troponin proteins, and activated the PI3K-Akt pathway in Dox-treated mice hearts.
NRG1 maintained mRNA and protein of cTnI and cTnT in Dox-treated NRVM.
To further investigate mechanisms whereby NRG1 inhibited Dox-induced loss of cardiac troponin proteins, we performed studies in NRVM.
We confirmed our findings of the effects of Dox-NRG1 on cardiac troponin cTnI and cTnT in NRVM using Western blot analysis. Dox decreased both protein (Fig. 2A) and mRNA levels (Fig. 2B) of cTnI and cTnT. Dox-NRG1 abolished these effects.
Fig. 2.
A: NRG1 preserved the Dox-induced decrease in cardiac troponins (cTnI and cTnT) in neonatal rat myocyte (NRVM). NRVM were treated with Dox (1 μM) in the presence or the absence of NRG1 (20 ng/ml). Protein levels of cTnI and cTnT were determined 48 h after Dox treatment by Western blot analysis. The blots are representative of at least 3 independent experiments. B: NRG1 inhibited the Dox-induced decrease in mRNA levels of cTnI and cTnT in NRVM. NRVM were treated with Dox (1 μM) or Dox-NRG1 (20 ng/ml). The mRNA levels of cTnI and cTnT were determined 48 h after Dox treatment by semiquantitative RT-PCR. The images are representative of at least 3 independent experiments. *P < 0.05 vs. control; †P < 0.05 vs. Dox.
NRG1 maintained translation of cTnI and cTnT in Dox-treated NRVM.
We determined whether NRG1 maintained cTnI and cTnT protein levels by increasing translation of both troponin proteins. NRVM were treated with Dox and Dox-NRG1 in the presence of a translation inhibitor cycloheximide. Cycloheximide inhibited cTnI and cTnT proteins in Dox-treated NRVM. It also inhibited the effects of Dox-NRG1 on cTnI and cTnT, suggesting that Dox-NRG1 increased translation of these proteins (Fig. 3A). We then determined the activation of the mTOR pathway, a major signaling pathway downstream of PI3K-Akt, which is important for protein translation (9). Dox decreased the phosphorylation of multiple molecules in the mTOR pathway, including mTOR (Ser2448), P70S6K (Thr421/Ser424), S6 (Ser240/244), 4E-BP (Thr 37/46), and eIF4G (Ser1108), in NRVM (Fig. 3B1). Dox-NRG1 maintained the phosphorylation of these mTOR pathway molecules (Fig. 3B1). Inhibition of PI3K by LY294002 blocked the effects of Dox-NRG1 (Fig. 3B1). LY294002 alone also decreased the phosphorylation of S6 and EIF4G in Dox-treated NRVM (Fig. 3B2). These results indicate that NRG1 maintained the activation of the mTOR pathway via PI3K in Dox-treated cardiomyocytes, which may account for maintaining translation of cTnI and cTnT in Dox-treated NRVM.
Fig. 3.
A: cycloheximide (Cyclo) blocked the effects of NRG1 on cTnI and cTnT in NRVM. NRVM were treated with Dox (1 μM), Dox-NRG1 (20 ng/ml), or Dox-NRG1 in the presence of cycloheximide (5 μg/ml). Protein levels of cTnI and cTnT were determined 48 h after the Dox treatment by Western blot analysis. The blots are representative of at least 3 independent experiments. B1: NRG1 inhibited Dox-induced deactivation of the mTOR pathway in NRVM. NRVM were treated with Dox (1 μM), Dox-NRG1 (20 ng/ml), or Dox-NRG1-LY294002 (LY). Phosphorylated and total mTOR, P70S6K, S6, 4E-BP, and EIF4G were determined 48 h after the Dox treatment by Western blot analysis. The blots are representative of at least 3 independent experiments. B2: LY294002 (10 μM) aggravates Dox-induced dephosphorylation of S6 and EIF4G. NRVM were treated with Dox (1 μM), Dox-NRG1 (20 ng/ml), Dox-LY, or Dox-NRG1-LY. Phosphorylated and total S6 and EIF4G were determined 48 h after the Dox treatment by Western blot analysis. The blots are representative of at least 3 independent experiments. *P < 0.05 vs. control; †P < 0.05 vs. Dox; ‡P < 0.05 vs. Dox-NRG.
NRG1 inhibited caspase activation in Dox-treated NRVM.
Dox activates multiple caspases (13, 17, 36). The existence and the activation of caspases, mainly intrinsic (caspase-3, -6, and -9) and extrinsic (caspase-8) pathways, in apoptosis have been reported in isolated cardiomyocytes and cardiac disease models (10, 14).
To determine whether caspase activation is responsible for the decrease in cTnI and cTnT in Dox-treated cardiomyocytes, we used a pan-caspase inhibitor Z-VAD-FMK and inhibitors for the individual caspases. FMK, used as negative control, was included in each experiment to ensure the specificity of the results. Our findings showed that Z-VAD-FMK abolished the Dox-induced decrease in cTnI and cTnT (data not shown). Inhibitors of caspase-3, caspase-6, caspase-9 (intrinsic pathway), and caspase-13 (inflammatory) blocked the Dox-induced decrease in cTnI and cTnT (Fig. 4A). The decrease in cTnI was also blocked by inhibitors of caspase-2 (DNA damage), caspase-5 (inflammatory), and caspase-10 (extrinsic pathway). Inhibitors of caspase-1, caspase-4 (inflammatory), caspase-8 (extrinsic), and caspase-12 (endoplasmic reticulum stress) did not block the Dox-induced decrease in cTnI and cTnT. FMK, used as a negative control, did not block the effects of Dox (data not shown).
Fig. 4.
A: Dox-induced degradation of cTnI and/or cTnT in NRVM by specific caspases. NRVM were treated with Dox (1 μM) in the presence of individual caspases inhibitors (10 μM). The protein levels of cTnI and cTnT were determined 48 h after the Dox treatment by Western blot analysis. The Western blots are representative of at least 3 independent experiments. B: in vitro caspase activation assay in Dox-treated NRVM. B1: Caspase activation in Dox-treated NRVM. Cells were treated with Dox (1 μM), Dox-NRG1 (20 ng/ml), and Dox-NRG1-LY294002 (LY). The activation of caspase-3, caspase-6, and caspase-9 was determined 16 h after Dox treatment by the caspase activation assay. The results were obtained from at least 3 independent experiments. B2: caspase activation in Dox-treated NRVM. Cells were treated with Dox (1 μM), Dox-NRG1 (20 ng/ml), and Dox-NRG1-LY294002 (10 μM). The activation of caspase-2, caspase-5, and caspase-10 was determined 16 h after Dox treatment by the caspase activation assay. The results were obtained from at least 3 independent experiments. *P < 0.05 vs. control; †P < 0.05 vs. Dox; ‡P < 0.05 vs. Dox-NRG1. C: in vitro caspase substrate assay. Recombinant human cTnI, cTnT, and cTnC proteins were reconstituted into a complex form and incubated with different caspases. The degradation of cTnI and cTnT were determined by Western blot. Lane 1, cTn complex; lane 2, cTn complex + caspase-3; lane 3, cTn complex + caspase-3 + Z-VAD; lane 4, cTn complex + caspase-6 + Z-VAD; lane 5, cTn complex + caspase-6; lane 6, cTn complex; lane 7, cTn complex + caspase-9; lane 8, cTn complex + caspase-9 + Z-VAD; lane 9, cTn complex; lane 10, cTn complex + caspase-10; lane 11, cTn complex + caspase-10 + Z-VAD; lane 12, cTn complex + caspase-5 + Z-VAD; lane 13, cTn complex + caspase-5; lane 14, cTn complex; lane 15, cTn complex + caspase-2; and lane 16, cTn complex + caspase-2 + Z-VAD.
In vitro caspase activation assay showed that Dox significantly increased the activation of caspases-3, -6, and -9, as well as caspases-2, -5, and -10. NRG1 significantly inhibited Dox-induced activation of these caspases (Fig. 4, B1 and B2). PI3K inhibitor LY294002 abolished these effects of NRG1 (Fig. 4, B1 and B2).
We used the in vitro caspase substrate assay to determine whether caspases directly cleave cTnI and cTnT. Incubation of caspases-3, -5, -6, or -10, but not caspases-2 or -9, with the cardiac troponin complex caused degradation of cTnT (Fig. 4C). We did not find degradation of cTnI when the cardiac troponin complex was incubated with these caspases.
These results demonstrate that caspases may directly cause degradation of cTnT protein but may also indirectly cause degradation of cTnI protein. These results also demonstrate that NRG1 inhibited the activation of these caspases via PI3K, which may contribute to the beneficial effects of NRG1 on cTnI and cTnT in Dox-treated NRVM.
NRG1 inhibited proteasome degradation of cTnI in Dox-treated NRVM.
Our result showed that the decrease in cTnI, but not cTnT, by Dox was blocked by the proteasome inhibitor MG-132 (Fig. 5A), suggesting that proteasome degradation was involved in Dox-induced decrease of cTnI, but not cTnT. We further determined the effects of Dox and Dox-NRG1 on ubiquitinylation of cTnI and cTnT. We found that Dox increased the ubiquitinylation of cTnI. Dox-NRG1 abolished the effects of Dox (Fig. 5B). On the other hand, Dox did not increase the ubiquitinylation of cTnT (Fig. 5B).
Fig. 5.
NRG1 inhibited Dox-induced ubiquitinylation and proteasome degradation of cTnI in NRVM. A: the proteasome inhibitor MG-132 blocked the decrease in cTnI, but not cTnT, in Dox-treated NRVM. Cells were treated with Dox (1 μM) in the presence or absence of MG-132 (10 μM). The protein level of cTnI and cTnT was determined 48 h after Dox treatment by Western blot analysis. B: NRG1 decreased Dox-induced ubiquitinylation of cTnI but not cTnT. NRVM were treated with Dox (1 μM) or Dox-NRG1 (20 ng/ml). Cell lysates were immunoprecipitated with a cTnI or a cTnT antibody and Western blotted with an ubiquitin antibody 48 h after Dox treatment. The Western blots are representative of at least 3 independent experiments. IP, immunoprecipitation; WB, Western blot.
NRG1 increased NRVM viability.
We determined whether NRG1 increased the cell viability in Dox-treated NRVM by using typan blue staining. Seventy percent of cells were viable in solvent-treated NRVM (1.6 × 106 out of 2.3 × 106 of cells). Fourty-seven percent of cells were viable in Dox-treated NRVM (8.0 × 105 out of 1.7 × 106 of cells). Fifty-six percent of cells were viable in Dox-NRG1-treated NRVM (1.4 × 106 out of 2.5 × 106 cells).
NRG1 maintained cTnI and cTnT by the ErbB2 and PI3K-Akt pathway in Dox-treated NRVM.
Blockade of the erbB2 receptor impaired cardiac function in chemotherapy-treated patients, suggesting the activation of the erbB2 receptor is important for protecting the heart from chemotherapy-induced cardiotoxicity (34). Here, we studied whether erbB2 receptor activation is required for Dox-NRG1 to maintain cTnI and cTnT levels in NRVM. Inhibition of erbB2 by the tyrosine kinase inhibitor tyrphostin AG879 or by an anti-erbB2 antibody (clone 9G6) abolished the protective effects of Dox-NRG1 on cTnI and cTnT (Fig. 6A), whereas inhibition of the erbB4 receptor by tyrphostin AG1478 had no effects on cTnI and cTnT (Fig. 6A). Moreover, inhibitors of PI3K, Akt, and mTOR blocked the effects of Dox-NRG1 on cTnI and cTnT (Fig. 6B).
Fig. 6.
A: inhibition of the erbB2 receptor abolished the protective effects of NRG1 on cTnI and cTnT in NRVM. NRVM were treated with Dox-NRG1 (20 ng/ml) in the presence of AG879 (10 μM), AG1478 (10 μM), or the anti-erbB2 antibody (Ab; clone 9G6, 6 μg/ml). Protein levels of cTnI and cTnT were determined 48 h after the Dox treatment by Western blot analysis. The Western blots are representative of at least 3 independent experiments. B: inhibitors of phosphatidylinositol 3-kinase, Akt, or mTOR abolished the protective effects of NRG1 on cTnI and cTnT in NRVM. NRVM were treated with Dox-NRG1 (20 ng/ml) in the presence of LY294002 (LY; 10 μM), Akti-1/2 (Akti; 5 μM), or rapamycin (10 nM). Protein levels of cTnI and cTnT were determined 48 h after the Dox treatment by Western blot analysis. The Western blots are representative of at least 3 independent experiments.
These data indicate that the erbB2 receptor and the PI3K-Akt pathway are essential for NRG1 to maintain cTnI and cTnT protein levels in NRVM.
DISCUSSION
We studied Dox cardiotoxicity in mouse hearts and in isolated NRVM and identified cardiac troponins that are preserved by NRG1 in Dox-injured hearts. Our findings in mouse hearts showed that NRG1 improved survival and inhibited Dox-induced cardiotoxicity. We further showed that NRG1 mitigated Dox-induced decrease in cardiac troponin proteins in mouse hearts. Our study in NRVM codified the findings in mice and further demonstrated that NRG1, via the erbB2-PI3K pathway, initiated multiple mechanisms to maintain troponin proteins in Dox-treated cardiomyocytes. These mechanisms include an increase in transcription and translation and a decrease in caspase activation and proteasome degradation of troponin proteins. Whereas the cardiotoxicity in Dox-treated patients is observed clinically long after treatment (months to years), the cardiotoxicity in our rodent model is fairly acute (days). The mechanisms responsible in our preclinical model may be different than those occurring in patients, yet the expediency of the model and new observations may provide important clues regarding cardioprotective mechanisms of NRG1 against Dox-induced cardiotoxicity.
Troponin is a thin filament-associated complex that regulates the formation of actin-myosin cross-bridges during the contraction-relaxation process in the heart (39). Releases of cardiac troponins (cTnI and cTnT) are sensitive and specific markers of myocardial injury (31, 35).
NRG1 activates oncogene erbB2 (11, 37), and it is, therefore, important to determine the mechanisms by which NRG1 protects the heart against chemotherapy-induced cardiotoxicity via erbB2. This may be of clinical relevance especially in patients treated with chemotherapy and erbB receptor antagonists (25).
Loss of myofibrillar proteins (troponin I and troponin T) is considered as one of the major causes of the Dox-induced decrease in cardiac contractile function (16).
Whether and how NRG1 prevents the loss of myofibrillar proteins, however, have not yet been studied.
NRG1 did not reduce Dox-induced release of cTnI into the serum but did maintain troponin cTnI and cTnT in the cardiac myocardium. NRG1 improved cardiac function in Dox-injured hearts. These results suggested to us that the maintenance of troponin in the heart by NRG1 may not be attributed to inhibition of troponin release, prompting us to conduct multiple assays to determine the mechanisms.
Our study shows that NRG1 maintained troponin protein levels in the myocardium by increasing the synthesis of cTnI and cTnT. The mRNA levels of cTnI and cTnT were decreased by Dox but preserved by Dox-NRG1 in NRVM. NRG1 maintained cTnI and cTnT translation. The translation inhibitor cycloheximide blocked translation of cTnI and cTnT proteins in Dox-treated and also in Dox-NRG1-treated NRVM. Activation of the mTOR pathway, which is important for translation of proteins, was decreased by Dox but preserved by Dox-NRG1 in NRVM. The mTOR inhibitor rapamycin inhibited the effect of NRG1 on maintaining cTnI and cTnT proteins in Dox-NRG1-treated NRVM.
Taken together, these findings indicate that NRG1 may maintain cTnI and cTnT by increasing their transcription and translation.
Our findings showed that NRG1 blocked Dox-induced degradation of troponin proteins in NRVM. Our results support other studies, which have shown that caspases are involved in the degradation of cardiac troponins (4, 6, 18, 32). Furthermore, we identified several caspases that were directly responsible for degradation of cTnT and indirectly for degradation of cTnI in NRVM (Fig. 4, A and C).
Our findings provided links between activation of specific caspases and their downstream myofilament targets. We showed that NRG1 inhibited the activation of these caspases. Caspases play an important role in inducing apoptotic cell death. We measured the percentage of viable cells in controls, Dox-treated, and Dox-NRG1-treated NRVM by trypan blue staining to determine whether NRG1 preserved troponin proteins by inhibiting cell death. We found that the percentage of viable cells increased ∼20% in Dox-NRG1-treated NRVM (56% viable cells) versus Dox-treated NRVM (47% viable cells). Yet cTnI and cTnT proteins increased ∼250% and ∼350% in Dox-NRG1 versus Dox-treated NRVM, respectively (Fig. 2A), indicating that inhibition of cell death by NRG1 played a limited role in preserving troponin proteins in NRVM. Our results also showed that Dox increased ubiquitinylation and proteasome degradation of cTnI, but not cTnT. NRG1 inhibited this effect of Dox.
In conclusion, we found that NRG1 appears to be effective in attenuating Dox-induced cardiac dysfunction in mouse hearts. NRG1 maintains cTnI and cTnT through activation of the erbB2 receptor. Our study further indicates that the PI3K-Akt-mTOR pathway is involved in preserving cardiac troponin proteins in Dox-injured isolated ventricular myocytes. These data suggest that NRG1 may be clinically useful to lessen cardiac dysfunction in patients treated with Dox.
GRANTS
This research is funded by American Heart Association Postdoctoral Fellowship Award 0120155T (to X. Yan), American Heart Association Scientist Development Grant 0635549T (to X. Yan), and National Heart, Lung, and Blood Institute Grant HL-52864 (to J. P. Morgan).
DISCLOSURES
M. A. Marchionni is a consultant of Acorda Therapeutics. A. O. Caggiano is a current employee of Acorda Therapeutics. X. Yan receives research material [recombinant NRG1 (rhGGF2)] from Acorda Therapeutics. No conflicts of interest are declared by Y. Bian, M. Sun, M. Silver, K. K. L. Ho, J. R. Stone, I. Amende, T. G. Hampton and J. P. Morgan.
ACKNOWLEDGMENTS
We thank Dr. Beverly H. Lorell and Dr. Lewis C. Cantley for invaluable advice on this work.
REFERENCES
- 1.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA 101: 6975–6980, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol Heart Circ Physiol 277: H2026–H2037, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep 62: 865–872, 1978 [PubMed] [Google Scholar]
- 4.Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol 43: 295–301, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chen LE, Liu K, Seaber AV, Katragadda S, Kirk C, Urbaniak JR. Recombinant human glial growth factor 2 (rhGGF2) improves functional recovery of crushed peripheral nerve (a double-blind study). Neurochem Int 33: 341–351, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99: 6252–6256, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res 311: 135–146, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, Harden NR, Li F, Gerdes AM, Wang X. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol 287: H1417–H1425, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res 61: 143–151, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol 44: 831–854, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol 44: 2231–2238, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta 1588: 94–101, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest 117: 2692–2701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 1198: 165–184, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Miller SC, Billingham ME, Akimoto H, Torti SV, Wade R, Gahlmann R, Lyons G, Kedes L, Torti FM. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc Natl Acad Sci USA 87: 4275–4279, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett 577: 483–490, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ke L, Qi XY, Dijkhuis AJ, Chartier D, Nattel S, Henning RH, Kampinga HH, Brundel BJ. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J Mol Cell Cardiol 45: 685–693, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kurabayashi M, Jeyaseelan R, Kedes L. Doxorubicin represses the function of the myogenic helix-loop-helix transcription factor MyoD. Involvement of Id gene induction. J Biol Chem 269: 6031–6039, 1994 [PubMed] [Google Scholar]
- 20.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem 281: 19469–19477, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH, Morgan JP, Yan X. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol 289: H660–H666, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 48: 1438–1447, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature 378: 386–390, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Miller KD. The role of ErbB inhibitors in trastuzumab resistance. Oncologist 9, Suppl 3: 16–19, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mosesson Y, Yarden Y. Oncogenic growth factor receptors: implications for signal transduction therapy. Semin Cancer Biol 14: 262–270, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Munshi A, Singh P, Jalali R. Trastuzumab: is the new evidence revolutionary? J Cancer Res Ther 2: 144–146, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science 197: 165–167, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res 59: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Okoshi MP, Yan X, Okoshi K, Nakayama M, Schuldt AJ, O′Connell TD, Simpson PC, Lorell BH. Aldosterone directly stimulates cardiac myocyte hypertrophy. J Card Fail 10: 511–518, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Panteghini M. Acute coronary syndrome: biochemical strategies in the troponin era. Chest 122: 1428–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Ruetten H, Badorff C, Ihling C, Zeiher AM, Dimmeler S. Inhibition of caspase-3 improves contractile recovery of stunned myocardium, independent of apoptosis-inhibitory effects. J Am Coll Cardiol 38: 2063–2070, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105: 1551–1554, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wallace KB, Hausner E, Herman E, Holt GD, MacGregor JT, Metz AL, Murphy E, Rosenblum IY, Sistare FD, York MJ. Serum troponins as biomarkers of drug-induced cardiac toxicity. Toxicol Pathol 32: 106–121, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka M, Yamaguchi S, Suzuki T, Okuyama M, Nitobe J, Nakamura N, Mitsui Y, Tomoike H. Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol 32: 881–889, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273: 10261–10269, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Zot AS, Potter JD. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem 16: 535–559, 1987 [DOI] [PubMed] [Google Scholar]